Abstract
Context
Modern management of differentiated thyroid cancer requires individualized care plans that tailor the intensity of therapy and follow-up to the estimated risks of recurrence and disease-specific mortality.
Design
This summary is based on the authors’ knowledge and extensive clinical experience, supplemented by review of published review articles, thyroid cancer management guidelines, published staging systems, and original articles identified through a PubMed search, which included terms such as risk stratification, staging, clinical outcomes, and differentiated thyroid cancer.
Main Outcome Measures
In the past, risk stratification in differentiated thyroid cancer usually referred to a static estimate of disease-specific mortality that was based on a small set of clinicopathological features available within a few weeks of completing initial therapy (thyroidectomy, with or without radioactive iodine). Today, risk stratification is a dynamic, active process used to predict the appropriateness for minimalistic initial therapy, disease-specific mortality, risk of recurrence, and the most likely response to initial therapy. Rather than being a static prediction available only after initial therapy, modern risk stratification is a dynamic, iterative process that begins as soon as a suspicious nodule is detected and continues through final follow-up.
Conclusions
Dynamic risk assessment should be used to guide all aspects of thyroid cancer management, beginning before a definitive diagnosis is made and continuing through the final follow-up visit.
This review examines the critical role of ongoing risk stratification from the time of detection through final follow-up in differentiated thyroid cancer.
Risk stratification in differentiated thyroid cancer has traditionally used a relatively small set of clinical and pathological factors to create models that predict disease-specific mortality or overall survival (1–7). Although clinically useful, these models provided static estimates of risk with information available within the first few months of initial therapy and demonstrated suboptimal, long-term outcome predictions for any individual patient (1, 6).
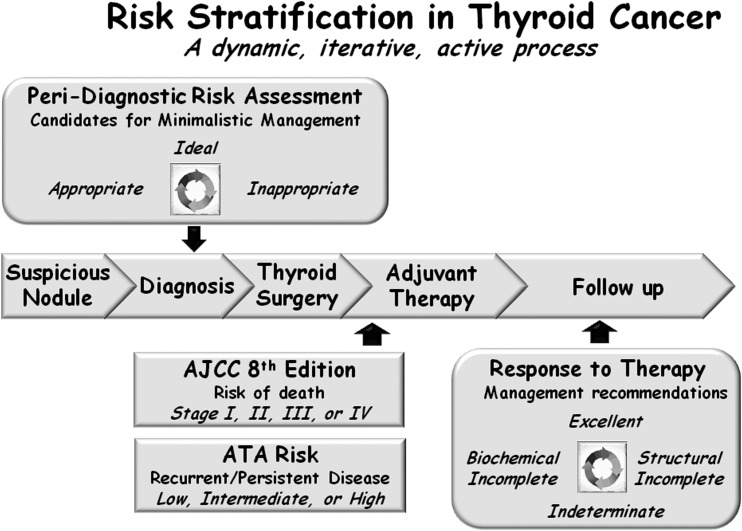
Over the last decade, additional models have been developed that provide predictive information with regard to other clinically relevant outcomes, such as the risk of having persistent disease after initial therapy, the risk of structural or biochemical disease recurrence, and the likelihood of going into remission following initial therapy in adult patients with thyroid cancer (6, 8–14). Furthermore, rather than using information that is only available at one particular point in time, these new models emphasize the importance of dynamic risk assessment, where the initial risk assessment is modified over time as new data become available. These dynamic risk assessments allow us to integrate response to therapy assessments with the underlying individual tumor biology to provide real-time risk assessments at any point in the course of the patient’s disease. Thus, the modern view of risk stratification begins with the identification of a suspicious nodule (peri-diagnostic period) and continues through the phases of diagnosis, treatment, adjuvant therapy, and follow-up (Fig. 1). Whereas the general concepts of risk-adapted management and follow-up are applicable to pediatric thyroid cancer (15), anaplastic thyroid cancer (16), and medullary thyroid cancer (17, 18), we will focus this review specifically on differentiated thyroid cancer that has been studied more extensively.
Figure 1.
Risk stratification in thyroid cancer is best viewed as a dynamic, iterative, active process that begins in the peri-diagnostic period and extends through final follow-up. AJCC, American Joint Committee on Cancer; ATA, American Thyroid Association.
From a practical standpoint, postoperatively, we use the eighth edition of the American Joint Committee on Cancer/tumor node metastasis (AJCC/TNM) staging system to predict disease-specific mortality and the American Thyroid Association (ATA) risk stratification system to predict the risk of recurrent or persistent disease (Fig. 1) (19, 20). These initial risk estimates are then modified over time using the descriptions from the ATA guidelines to define the patient’s response to therapy at any point during follow-up, as excellent (no evidence of persistent/recurrent disease), biochemically incomplete [abnormal thyroglobulin (Tg) or rising Tg antibodies in the absence of identifiable structural disease], structurally incomplete (structural evidence of persistent/recurrent disease), or indeterminate (nonspecific findings that cannot be confidently classified as benign or malignant) (21). These modified risk estimates are then used to plan ongoing management.
Recently, the move toward deferred intervention (active surveillance) of very low-risk thyroid cancers and a more minimalistic approach to thyroid surgery has expanded the risk-stratification horizon to include not only the intraoperative and postoperative time periods but also the peri-diagnostic time frame that begins with the detection of a suspicious thyroid nodule (Fig. 1) (21–25). In this peri-diagnostic period, it is important to identify low-risk thyroid cancers that may be eligible for either an active surveillance management approach (with or without cytological confirmation) or for a minimalistic surgical intervention, such as thyroid lobectomy without neck dissection (23, 25, 26). Conversely, it is equally important to identify, in the peri-diagnostic period, those patients who would be most likely to benefit from more aggressive initial interventions that could include total thyroidectomy, with or without prophylactic or therapeutic neck dissection, radioactive iodine treatment, external beam radiation, or upfront systemic therapy.
It is also important to recognize that highly sensitive disease-detection tools can often detect small foci of papillary thyroid cancer that may not require immediate diagnosis and therapy. The 2015 ATA guidelines provided several specific examples where an observational management approach, often without cytologic confirmation of disease, is recommended as the preferred or alternative management approach to small-volume disease (21–25). For example, an active surveillance observational management approach is allowed for carefully selected patients with either highly suspicious subcentimeter asymptomatic thyroid nodules without the need for cytologic confirmation or biopsy-proven, very low-risk thyroid cancers, such as intrathyroidal papillary microcarcinomas, in locations not adjacent to trachea or neurovascular structures without evidence of lymph node metastasis (21). Furthermore, an observational management approach is also allowed for patients with persistent/recurrent small abnormal cervical lymph nodes, asymptomatic stable or slowly growing distant metastasis, and stable or declining abnormal Tg or Tg antibodies (21).
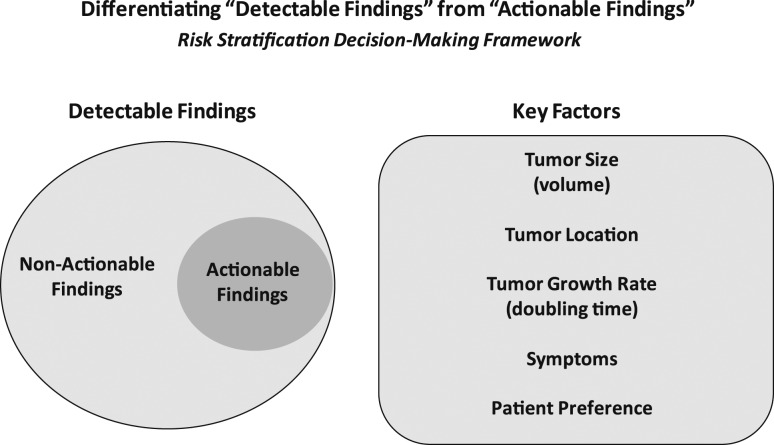
As it is clear that not all detectable findings require immediate diagnostic or therapeutic intervention, it is imperative that we develop a risk-stratification decision-making framework to differentiate actionable findings from non-actionable findings (Fig. 2). Whether we are considering a highly suspicious subcentimeter thyroid nodule without cytologic confirmation of disease, a biopsy-proven thyroid nodule with low-risk thyroid cancer, or persistent/recurrent disease in the neck or elsewhere, we find it useful to consider five key factors that when taken together, allow us to predict the likelihood that a specific tumor focus represents clinically important disease that may require additional evaluations, ongoing observation, or therapeutic intervention (Fig. 2). Both tumor size and tumor location are the major factors that determine whether a tumor focus is likely to cause clinically substantial invasion into local structures, such as the recurrent laryngeal nerve, airway, gastrointestinal tract, major vessels, or other important structures (27–29). A third important factor is the tumor growth rate (measured as tumor volume doubling time), with an observational management approach being much more appropriate for tumors either anticipated to have a slow tumor growth rate or with actual documented slow growth rates over time (29–33). Obviously, tumors that are either symptomatic or likely to have symptomatic progression would be considered actionable. Finally, patient preference plays a key role when deciding whether a particular lesion is actionable or non-actionable, as it is important to integrate the patient’s understanding of the risks and benefits of intervention vs observation with their value system and goals (34, 35). In addition to providing initial guidance as to whether the detectable lesion is actionable at the time of detection, ongoing re-evaluation of these same factors, using the basic concepts of dynamic-risk stratification, can also assist the clinician in the determination of when it is time to transition from an observational management approach to active therapeutic intervention (22).
Figure 2.
Highly sensitive detection tools often detect small-volume disease that may or may not require action. Key factors that differentiate actionable from non-actionable findings include tumor volume, location, growth rate, symptoms, and patient preference.
Thus, risk stratification has moved from a single postoperative static assessment of the risk of disease-specific mortality to an all-encompassing evaluation of the patient that is continually modified over time, beginning from the first detection of a suspicious thyroid nodule and continuing throughout the life of the patient.
Risk Stratification in Highly Suspicious Thyroid Nodules or Cytologically Confirmed Primary Papillary Thyroid Cancer
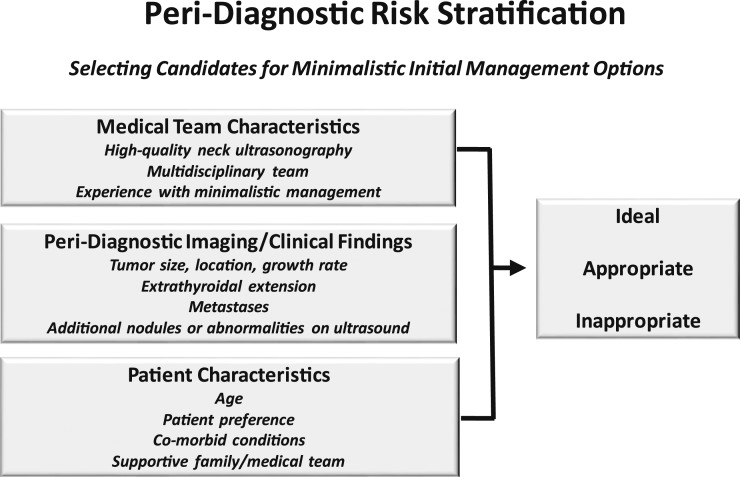
Risk stratification begins immediately upon identification of a suspicious thyroid nodule. In the absence of a validated peri-diagnostic risk-stratification system, we use a clinical framework that incorporates tumor imaging characteristics, medical team characteristics, and patient preferences to risk stratify patients as ideal, appropriate, or inappropriate for minimalistic initial management options, such as active surveillance or thyroid lobectomy (Fig. 3) (22, 23, 25). This clinical framework address the key factors that differentiate actionable from non-actionable disease (Fig. 2).
Figure 3.
Peri-diagnostic risk stratification considers medical team characteristics, imaging/clinical findings, and patient characteristics to classify patients as ideal, appropriate, or inappropriate for a minimalistic initial management approach.
Risk stratification in active surveillance of papillary microcarcinoma
Asymptomatic, small thyroid nodules (usually ≤1 cm maximal diameter, 1 cm3, or 1 mL volume) confined to the thyroid and surrounded by normal thyroid parenchyma can be followed with active surveillance, with or without cytologic confirmation, in patients who value their normal thyroid function and who desire avoidance of thyroid surgery (21–23, 25). Patients who demonstrate tumors larger than 1.5 to 2.0 cm; tumors in subcapsular locations adjacent to important structures, such as the trachea and recurrent laryngeal nerve; or tumors with documented growth rate doubling times of <2 years are generally considered inappropriate for observation and would be considered to have actionable disease. If the tumor growth rate is unknown at the time of nodule detection, then this can be established with serial ultrasound evaluations done approximately every 6 months for 1 to 2 years. The frequency of ultrasound evaluations and long-term follow-up depends on the tumor size, location, and established growth rate (25). With the use of this paradigm, active surveillance continues until there is a 3-mm increase in tumor diameter (which corresponds to a 100% increase in tumor volume), identification of metastatic disease, direct invasion into surrounding structures of the thyroid, or a decision to discontinue active surveillance based on patient preference (23, 25).
This risk-stratified, minimalistic management approach to very low-risk thyroid cancers has been shown to be safe and effective over 5 to 10 years of follow-up in studies from Japan, Korea, and the United States (23, 29, 36–39). In the first 10 years of active surveillance follow-up, only 2% to 8% of papillary microcarcinomas increase ≥3 mm in maximum diameter, 12% to 14% demonstrate an increase in tumor volume of >50% (the smallest change in nodule volume that can be reproducibly measured), and novel lymph node metastases are detected in 2% to 4% (23, 29, 36–39). The likelihood of disease progression is higher in younger patients than in older patients (40). Importantly, at the time of disease progression, deferred surgical intervention is quite effective with excellent outcomes and no disease-specific mortality (23, 29, 36–39).
Risk-stratification considerations for thyroid lobectomy
The 2015 ATA guidelines now accept a minimalistic surgical approach (thyroid lobectomy without neck dissection) to treat intrathyroidal papillary thyroid carcinomas <4 cm in properly selected patients (21). Careful peri-diagnosis, preoperative, and intraoperative risk stratification are the keys to successful use of thyroid lobectomy without having to perform an unacceptable rate of early-completion thyroidectomies. As described in detail in our previous review (25), patients classified as being ideal for lobectomy would have papillary microcarcinomas that appeared to be confined to the thyroid in the setting of an otherwise normal thyroid ultrasound and clinical N0 neck. We classify patients as appropriate for lobectomy if the tumor is 1 to 4 cm in maximum dimension, if the contralateral lobe is normal, or if there are other abnormalities on the ultrasound, such as thyroiditis or benign-appearing nodules (again, in the setting of the clinical N0 neck). Patients with extrathyroidal extension, clinical N1 disease, or distant metastasis would be considered inappropriate for thyroid lobectomy as initial therapy.
In addition to the relevance of peri-diagnostic and preoperative risk stratification with respect to the selection of thyroid lobectomy as initial therapy, it is important to recognize that there are intraoperative findings that should alter that recommendation and lead to an immediate total thyroidectomy (25, 26). We encourage patients to find a surgeon who they trust and to empower the surgeon to make a final decision in the operating room regarding the extent of initial surgery that should be performed, which can vary from lobectomy to total thyroidectomy, with or without neck dissection. However, even with appropriate preoperative and intraoperative risk stratification, as many as 6% to 20% of patients will have unexpected findings on the final pathology report that may lead to a completion thyroidectomy and usually, radioactive iodine (41–45). An additional 5% to 10% may require completion thyroidectomy at some later point during follow-up for diagnostic or therapeutic purposes (41–45).
The rate of early-completion thyroidectomy, performed following review of the initial pathology report, will vary, depending on how aggressive each management team is with regard to the use of radioactive iodine for either remnant ablation or adjuvant treatment. If minor factors, such as minor extrathyroidal extension, very small-volume lymph node metastasis, or small tumors with aggressive histologic features usually lead to radioactive iodine therapy, then the completion thyroidectomy rate may be as high as 20% (41–45). In our hands, the completion thyroidectomy rate is much lower, as we have a much more restricted use of radioactive iodine (43–45). The most common reason for completion thyroidectomy in our hands is unanticipated, extensive vascular invasion documented on the pathology report that obviously could not be visualized preoperatively or intraoperatively.
Thus, patients need to understand that the final determination of whether a thyroid lobectomy is the appropriate initial therapy can only be achieved by the integration of preoperative, intraoperative, and postoperative risk stratification (25). Patients who are uncomfortable with this approach will often choose a total thyroidectomy as initial therapy. Patients motivated to keep part of the thyroid will often accept that uncertainty, recognizing that the final decision regarding the completeness of initial therapy cannot be completely known until several weeks after the surgery is completed when more complete risk stratification can be accomplished.
Predicting Survival Outcomes: The Updated AJCC/TNM Staging System
In October 2016, the AJCC (www.cancerstaging.org) published the eighth edition of the AJCC/TNM cancer staging system, which replaced the seventh edition that had been used by clinicians, cancer registries, and researchers since 2009 (46). On 1 January 2018, tumor registries officially began using the eighth edition for tumor staging. Whereas the staging tables for medullary thyroid cancer and anaplastic thyroid cancer showed only minimal changes, the rules for the staging of well-differentiated thyroid cancer underwent substantial modifications (46). These included the following: (i) an increase of the age cutoff from 45 years to 55 years of age at diagnosis; (ii) removal of microscopic extrathyroidal extension as a key component of the staging system; (iii) no longer mandating assignment of stage III to older patients with microscopic extrathyroidal extension or lymph node metastases; and (iv) establishment of a new T3b category for tumors of any size that demonstrate gross extrathyroidal extension involving only the surrounding strap muscles (19, 20).
The AJCC Differentiated Thyroid Cancer Committee carefully considered the possibility of inclusion of molecular markers (specifically, BRAFV600E and TERT promoter mutations) in the AJCC prognostic staging definitions (19, 20, 46). Whereas both of these mutations, particularly when present together, have been shown to be predictors of poor clinical outcomes, they appeared to add only marginal benefit to the traditional anatomic staging factors (47–52). Thus, molecular characterization of differentiated thyroid cancers, although providing some prognostic information, were not powerful enough factors to merit upstaging tumors to prognostic stages above those mandated by TNM risk factors. Nonetheless, similar to the approach used in the ATA risk-stratification system, molecular results can be used to refine further and individualize risk within risk categories or stages (21).
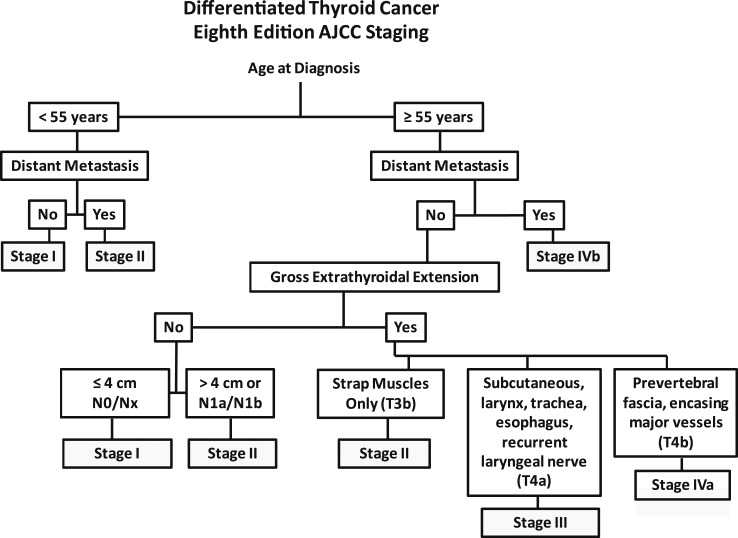
The three critical factors that determine the prognostic stage groups of the eighth edition AJCC/TNM cancer staging system include the age at diagnosis, the presence or absence of distant metastases, and the presence or absence of gross extrathyroidal extension (19, 20). Rather than the use of the standard TNM staging tables provided in the AJCC/TNM manual (19, 20), we find it easier to use the flow diagram in Fig. 4 to stage patients rapidly based on the key clinical risk factors (age at diagnosis, distant metastasis, gross extrathyroidal extension, and lymph node metastases). In patients age <55 years, this figure rapidly classifies patients as either stage I (any T, any N, M0) or stage II (any T, any N, M1). In the older patients, additional factors, such as the presence or absence of distant metastasis, invasion of strap muscles, and extent of gross extrathyroidal extension, are also used to define the prognostic stage groups. In the eighth edition of the AJCC/TNM cancer staging system, it was anticipated that the majority of patients would be classified as stage I or stage II, reflecting the excellent outcomes expected in the majority of thyroid cancer patients. A smaller number of patients, particularly the older patients with either distant metastases or gross extrathyroidal extension, were anticipated to do worse and are therefore classified as stage III or IV (19, 20, 53).
Figure 4.
A simplified approach to AJCC staging in differentiated thyroid cancer, emphasizing the critical decision nodes, which include age at diagnosis, distant metastasis, and gross extrathyroidal extensions.
Multiple publications have demonstrated that the eighth edition of the AJCC/TNM cancer staging system moved a substantial number of patients into lower prognostic stage groups without affecting the overall survival of those lower-stage groups (7, 54–58). The patients who remained in the higher-stage groups had poorer prognoses, as expected. This resulted in a much better separation of the four prognostic stage groups with respect to survival, such that 5- to 10-year disease-specific survival (DSS) was 99% in stage I patients, 88% to 97% in stage II patients, 72% to 85% in stage III patients, and 67% to 72% in stage IV patients (56, 57). Unlike previous editions of the AJCC/TNM staging system in which there was substantial overlap in survival in patients with stage I, II, and III disease, the eighth edition provides meaningful separation among the prognostic stage groups that appear to be clinically relevant (19, 20). The differences in predicted and published ∼10-year survival rates are best seen when analyzed based on age group (age <55 years vs age ≥55 years) as shown in Table 1. The predicted 10-year DSS has been validated for all age and stage groups, with only the younger (age <55 years) stage II patients appearing to do more poorly than anticipated. The lower-than-anticipated 10-year DSS in the younger patients (age <55 years) with stage II disease was the result of the stage migration of patients in the 45- to 55-year age group from seventh edition AJCC stage IV to eighth edition AJCC stage II (see Predicting Response to Therapy regarding integration of AJCC/TNM and ATA risk stratification in individual patients for further discussion of these patients).
Table 1.
Predicted and Published 10-Year DSS for the Eighth Edition AJCC/TNM Staging System
| Patients | Stage | Eighth Edition Description | Eighth Edition Predicted 10-Year DSS | Eighth Edition Published 10-Year DSS |
|---|---|---|---|---|
| Younger | I | Age <55 y | 98%–100% | ≈99% |
| All patients without distant metastases regardless of tumor size, lymph node status, or extrathyroidal extension | ||||
| II | Age <55 y | 85%–95% | ≈65% | |
| Distant metastases | ||||
| Older | I | Age ≥55 y, ≤4 cm tumor | 98%–100% | ≈99% |
| Confined to the thyroid | ||||
| II | Age ≥55 y, tumors >4 cm | 85%–95% | ≈95% | |
| Or: | ||||
| Tumors of any size with central or lateral neck lymph nodes | ||||
| Or: | ||||
| Gross extrathyroidal extension into strap muscles | ||||
| III | Age ≥55 y | 60%–70% | ≈60% | |
| Tumors of any size with gross extrathyroidal extension into subcutaneous tissue, larynx, trachea, esophagus, or recurrent laryngeal nerve | ||||
| IV | Age ≥55 y | <50% | ≈30% | |
| Tumors of any size or lymph node status with gross extrathyroidal extension into prevertebral fascia, encasing major vessels | ||||
| Or: | ||||
| Distant metastasis |
Predicting Response to Therapy: The Updated ATA Risk-Stratification System
Unlike many cancers, the risk of recurrence does not parallel the risk of mortality in differentiated thyroid cancer (59). In most patients, the risk of recurrence far exceeds the risk of disease-specific mortality, and thus staging systems designed to predict mortality in thyroid cancer would not be anticipated to be predictive of disease recurrence. To address this issue, a risk-stratification system was developed and validated to predict the risk of structural disease recurrence based on information obtained around the time of initial therapy (6, 8–14, 60–62). A modified version of this original risk-stratification system was endorsed in the 2009 ATA guidelines and subsequently modified in the 2015 ATA guidelines (21).
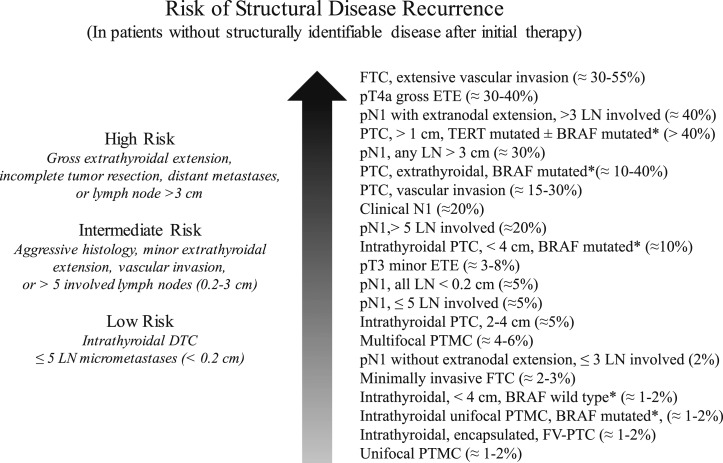
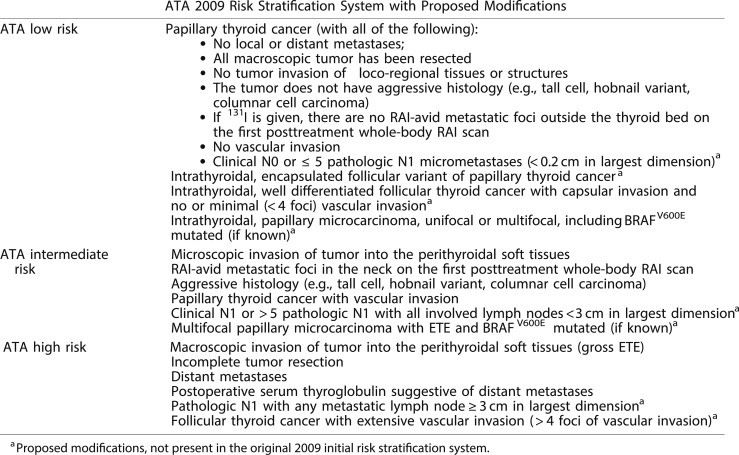
Whereas initially conceived as a three-category model of risk assessment (low, intermediate, or high risk) (12, 63), the ATA risk-stratification system is now visualized as a continuum of risk, ranging from very low to very high risk of structural disease recurrence (Fig. 5) (21). Nonetheless, the three-category model was proven to be very useful and reproducible across multiple studies (6, 8–14, 60–62). The 2015 ATA guidelines (21) expanded the low-risk category to include not only intrathyroidal papillary thyroid cancer but also patients with very small-volume lymph node micrometastases (<0.2 cm in largest dimension), intrathyroidal well-differentiated follicular thyroid cancer with capsular invasion and fewer than four foci of vascular invasion, intrathyroidal encapsulated follicular variant of papillary thyroid carcinoma (now known as noninvasive follicular thyroid neoplasm with papillary-like nuclear features), and either unifocal or multifocal intrathyroidal papillary microcarcinoma, even if they have known BRAFV600E mutations. The high-risk category was also expanded to include follicular cancer with more than four foci of vascular invasion and pathologic lymph node metastasis with any metastatic lymph node ≥3 cm in largest dimension. The remaining tumors were classified as intermediate risk based on the data available at the time the guidelines were written (Fig. 6).
Figure 5.
As described in the ATA guidelines, individualized risk stratification is best visualized as a “continuum of risk” rather than as three discrete risk categories that predict the risk of structural disease recurrence. [Adapted with permission from Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.]
Figure 6.
The 2015 ATA guidelines expanded the inclusion criteria for ATA low-risk and ATA high-risk disease categories as described in this table. [Adapted with permission from Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.]
The last several years have seen an abundance of published data confirming the association among specific molecular alterations, histological subtypes, and clinical outcomes in follicular cell-derived thyroid cancer (64, 65). Point mutations in BRAFV600E are associated with increased risk of recurrence, radioactive iodine refractoriness, extrathyroidal extension, lymph node metastases, and disease-specific mortality. Likewise, oncogenic genetic alterations in TERT promoter, TP53, EIF1AX, and β-catenin are associated with more aggressive tumor behavior and poorer clinical outcomes. Furthermore, mutational combinations (such as BRAFV600E + TERT promoter mutations or RAS + TERT promoter mutations) are associated with significantly increased risk beyond that associated with either mutation in isolation. As shown in the ATA continuum of risk figure (Fig. 5), appropriate molecular risk stratification requires integration of the genetic abnormality into the proper clinical context, as the presence of a specific mutation does not always portend a poor prognosis (e.g., BRAFV600E mutations are found in >50% of papillary microcarcinomas, which usually display an indolent clinical course). Although not yet proven, it seems reasonable to consider either more careful follow-up or potentially more aggressive therapies for tumors with the highest risk mutational profiles (particularly those with mutational combinations associated with the poorest clinical outcomes) (65–68). It is important to remember that there is no guarantee that more aggressive surgery, radioactive iodine therapy, thyroid-stimulating hormone suppression, or other systemic therapies will necessarily provide therapeutic benefit simply because we can identify a patient at high risk for poorer outcomes on the basis of clinic-pathological presentation or molecular profiling. Prospective studies evaluating the impact of more aggressive surgical and systemic therapies in the setting of high-risk mutational profiles are needed.
The ATA risk-stratification system performs well in clinical practice, with low-risk patients demonstrating no evidence of disease 80% to 90% of the time, biochemical incomplete responses 15% of the time, and structural incomplete responses 3% to 5% of the time. Intermediate-risk patients achieve excellent response ∼60% of the time, have a biochemical incomplete response ∼15% to 20% of the time, and have a structural incomplete response ∼20% of the time. High-risk patients seldom achieve no evidence of disease status (<30%) and usually demonstrate a structural incomplete response (50% to 75%) or biochemical incomplete response (10% to 15%). The studies contributing to these approximations are extensively reviewed in the ATA guidelines (21). Interestingly, age is a major determinant of response to therapy. In ATA high-risk patients, the proportion of excellent responders was found to be significantly higher among younger patients (age <55 years) than among older patients (age ≥55 years; 40.3% vs 27.5%, P = 0.02), and the proportion of structural incomplete responders was significantly larger among older patients than among younger patients (53% vs 33%, P = 0.002). Moreover, ATA high-risk younger patients with a structural incomplete response to therapy had a significantly better DSS than older patients (74% vs 12%, respectively, P < 0.001) (69).
Integrating AJCC/TNM and ATA Risk Stratification in Individual Patients
Whereas the eighth edition of the AJCC/TNM cancer staging system and the ATA risk-stratification system were independently developed to address different clinical outcomes, a recent publication suggests that the ATA risk-stratification system can provide valuable survival prognostic information when integrated into the AJCC staging system (54). In older patients (age ≥55 years at diagnosis), the ATA risk parallels the AJCC stage groups, with ATA low-risk patients being AJCC stage I, ATA intermediate-risk patients being AJCC stage II, and the majority of ATA high-risk patients classified as either AJCC stage III or IV (except ATA high-risk patients based on gross invasion into strap muscles, who are classified as AJCC stage II).
In younger patients (age <55 years at diagnosis), however, AJCC stage I includes all patients in this age group unless they present with distant metastasis (19, 20). As a result, stage I patients, age <55 years, can range from papillary microcarcinomas that may not require any therapy at all to locally invasive, unresectable disease invading major neck structures. However, because the vast majority of stage I patients are low risk, the 10-year DSS in this entire cohort is still ≈99% (Table 1) (7, 54–58). However, within this large group of patients is a smaller group of patients who may be expected to do more poorly because of their locally aggressive features or poor histology (70, 71).
The ATA risk-stratification system can be used to identify the few patients, age <55 years, who were classified as stage I and likely to demonstrate a significantly worse outcome than the entire cohort of stage I patients (54). In a cohort of 4881 differentiated thyroid cancer patients, age <55 years at diagnosis, 98% were classified as AJCC stage I, and 2% were classified as AJCC stage II. Whereas the entire cohort of stage I patients demonstrated a 98% 10-year DSS, ATA high-risk stage I patients (manifest by gross extrathyroidal extension, incomplete tumor resection, large-volume N1 disease, or follicular thyroid cancer with extensive vascular invasion) exhibited a 10-year DSS of 92%. Among the ATA high-risk stage I patients, prognosis also varied by age at diagnosis, with a 95%, 10-year DSS seen in patients age 18 to <45 years compared with only an 87%, 10-year DSS in patients age 45 to 55 years. As expected, patients with ATA low or intermediate risk have excellent survival of 98% or better. Although representing only 2% of this cohort, the stage II patients were all ATA high risk (M1 disease) and had a 10-year DSS of 68%. Stage II ATA high-risk patients also demonstrated the importance of age at diagnosis, with 78%, 10-year DSS seen in younger patients (age 18 to <45 years at diagnosis) and 61%, 10-year DSS seen in older patients (age 45 to 55 years at diagnosis).
From a practical application standpoint, the vast majority of patients, age <55 years at diagnosis, will be classified as stage I (98%) and can be expected to have DSS in excess of 98% (54). However, the small group of AJCC stage I patients who are also ATA high risk may exhibit less optimistic outcomes, particularly in those patients in the 45- to 55-year age group, where DSS may be as low as 87%. As expected, the AJCC stage II patients who all presented with distant metastasis had poor DSS; not surprisingly, this was age dependent, with the lowest DSS of 61% seen in patients in the 45- to 55-year age group.
Use of Dynamic Risk Stratification to Modify and Refine Initial Risk Estimates
Although both the AJCC/TNM staging system and the ATA risk-stratification system provide valuable information with regard to initial risk stratification, they are both static risk assessments that can only incorporate information available in the peri-diagnostic, preoperative, intraoperative, and early postoperative periods. However, all of the static staging systems published provide suboptimal long-term predictions for individual patients, as demonstrated by the proportion of variance explained, ranging from 20% to 30% across a wide range of studies (a measure of how well a predictive model correlates with the final outcome of interest) (6). However, when these initial risk estimates are refined and modified over time as a response to therapy and as a reflection of the underlying biology of a particular patient’s thyroid cancer, risk estimates become more reliable and can achieve a proportion of variance explained as high as 70% to 80% (6).
Over the last decade, several groups have developed and validated the general concept of dynamic risk stratification in which the baseline initial risk estimates are continually modified over time as new data become available (8, 12). Initially, dynamic risk stratification was validated only in the setting of total thyroidectomy and radioactive iodine and only in response to initial therapy (8, 12). Over the last several years, it has become readily apparent that the concept of dynamic risk stratification should not be restricted to response to initial therapy but should rather be used to reclassify each patient when they return for their follow-up visits (6, 72). Furthermore, definitions for response to therapy outcomes have been published and validated for patients receiving total thyroidectomy without radioactive iodine and even for low-risk patients treated with lobectomy alone (73, 74).
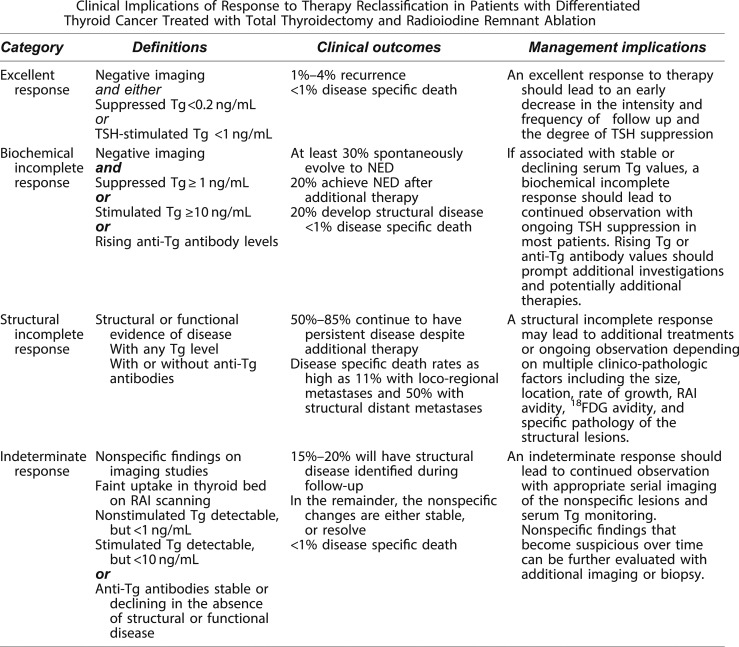
In patients treated with total thyroidectomy and radioactive iodine, the ATA guidelines provided a set of definitions, clinical outcomes, and management implications for the use of dynamic risk stratification. In this paradigm, at each follow-up visit, the patient is classified as having an excellent, biochemically incomplete, structurally incomplete, or indeterminate response to therapy (see Fig. 7 for definitions) (21). Unlike the AJCC/TNM staging and the ATA risk-stratification systems, the response-to-therapy category can change over time as new data become available at each visit. As can be seen from Fig. 7, patients who have an excellent response to therapy are expected to have essentially a normal overall survival and a very low risk of disease recurrence and therefore may not require intensive follow-up. Patients with biochemical incomplete response have an abnormal Tg value but no structurally identifiable disease and are usually followed with observation unless the Tg or Tg antibodies are rising, in which case additional imaging and evaluations are warranted to try to identify the source of the abnormal Tg (21).
Figure 7.
Definitions, clinical outcomes, and management implications of the ATA response to therapy categories. NED denotes a patient as having no evidence of disease at final follow-up. Tg value cutoffs used in the definition of excellent, biochemical, and incomplete response categories assume the absence of anti-Tg antibodies. [Adapted with permission from Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.]
The indeterminate response category, initially described as acceptable response, was designed to be a temporary holding area for patients with nonspecific findings that could not be confidently described as benign or malignant. Over time, ∼15% to 20% of these patients will develop structural disease that may require additional therapy. In the remainder, the nonspecific changes are either stable or resolve, and many of these patients can be reclassified as having an excellent response over time (21). Whereas patients with rising anti-Tg antibodies are classified as having a biochemical incomplete response, patients with stable or declining anti-Tg antibodies are categorized as having an indeterminate response to therapy. Although Tg assays that use a liquid chromotography-tandem mass spectroscopy (LC-MS/MS) methodology (75) can identify some Tg antibody-positive patients as having detectable Tg in the setting of known structural disease, up to 20% to 40% of patients with structural disease will have undetectable Tg measurements on LC-MS/MS (76–79). Thus, in the setting of antithyroglobulin antibodies, an undetectable Tg obtained on the current LC-MS/MS assays is insufficient evidence to classify a patient as having an excellent response.
Patients with a structural incomplete response are particularly challenging in that the majority of them will continue to have persistent disease despite additional therapies, and this is the category of patients from which nearly all of the disease-specific mortality arises (21). These patients are likely to need additional imaging, ongoing thyroid-stimulating hormone suppression, and additional therapies over time. From a practical standpoint, we use the same risk-stratification decision-making framework that we use to evaluate primary thyroid cancers for either active surveillance or minimalistic treatment options. Whether this structural disease is a highly suspicious or cytologically proven cervical lymph node metastasis or distant metastasis, the same five key factors can drive decision-making with regard to whether a lesion represents an actionable finding: the volume, the location, the rate of growth of the disease, the presence or absence of symptoms, and patient preference (Fig. 2). It is important to note, particularly in the metastatic disease setting, that observation to determine the tumor volume change rate over time is not always required in patients who have a specific histology, molecular profile, or functional imaging study that predicts a high likelihood of rapid tumor growth.
Conclusions
The dramatic increase in the prevalence of low-risk thyroid cancer over the last 10 to 20 years has demanded a re-evaluation of the traditional “one-size-fits-all” approach to differentiated thyroid cancer. This transition to a more individualized approach to patient management has led to a much more risk-adapted approach to the diagnosis, initial therapy, adjuvant therapy, and follow-up of patients with differentiated thyroid cancer (21). This has required a comprehensive re-evaluation of our approach to the prediction of disease-specific mortality and the risk of structural/biochemical disease recurrence. The last 10 years have also seen substantial modifications to the AJCC/TNM staging system, the development and validation of the ATA risk-stratification system for prediction of disease recurrence, and the recognition and implementation of dynamic risk stratification to allow real-time, ongoing re-evaluation of risk from initial detection to final follow-up. While much more work needs to be done to refine each of these models, we have certainly made substantial strides in more closely tailoring the intensity of our initial therapy and follow-up evaluations to realistic ongoing, individualized risk estimates that reflect disease-specific morbidity and mortality.
Acknowledgments
Financial Support: This work was funded, in part, by US National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 to Memorial Sloan-Kettering Cancer Center and by Special Program of Research Excellence, SPORE in Thyroid Cancer, Grant P50 CA172012-01A1.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AJCC
American Joint Committee on Cancer
- ATA
American Thyroid Association
- DSS
disease-specific survival
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- Tg
thyroglobulin
- TNM
tumor node metastasis
References
- 1. Brierley JD, Panzarella T, Tsang RW, Gospodarowicz MK, O’Sullivan B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer. 1997;79(12):2414–2423. [PubMed] [Google Scholar]
- 2. Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Contr. 2000;7(3):229–239. [DOI] [PubMed] [Google Scholar]
- 3. Gillanders SL, O'Neill JP. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC). Eur J Surg Oncol. 2018;44(3):286–296. [DOI] [PubMed] [Google Scholar]
- 4. Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79(3):564–573. [DOI] [PubMed] [Google Scholar]
- 5. Glikson E, Alon E, Bedrin L, Talmi YP. Prognostic factors in differentiated thyroid cancer revisited. Isr Med Assoc J. 2017;19(2):114–118. [PubMed] [Google Scholar]
- 6. Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014;43(2):401–421. [DOI] [PubMed] [Google Scholar]
- 7. Shaha AR, Migliacci JC, Nixon IJ, Wang LY, Wong RJ, Morris LGT, Patel SG, Shah JP, Tuttle RM, Ganly I. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery. 2018;8(18):30578–30576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165(3):441–446. [DOI] [PubMed] [Google Scholar]
- 9. Lee YM, Cho JW, Hong SJ, Yoon JH. Dynamic risk stratification in papillary thyroid carcinoma measuring 1 to 4 cm. J Surg Oncol. 2018;118(4):636–643. [DOI] [PubMed] [Google Scholar]
- 10. Ozkan E, Soydal C, Nak D, Kucuk NO, Kir KM. Dynamic risk stratification for predicting the recurrence in differentiated thyroid cancer. Nucl Med Commun. 2017;38(12):1055–1059. [DOI] [PubMed] [Google Scholar]
- 11. Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid. 2013;23(11):1401–1407. [DOI] [PubMed] [Google Scholar]
- 12. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, Vaisman M, Tuttle RM. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). 2012;77(1):132–138. [DOI] [PubMed] [Google Scholar]
- 14. Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid. 2011;21(12):1317–1322. [DOI] [PubMed] [Google Scholar]
- 15. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. [DOI] [PubMed] [Google Scholar]
- 17. Lindsey SC, Ganly I, Palmer F, Tuttle RM. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid. 2015;25(2):242–249. [DOI] [PubMed] [Google Scholar]
- 18. Tuttle RM, Ganly I. Risk stratification in medullary thyroid cancer: moving beyond static anatomic staging. Oral Oncol. 2013;49(7):695–701. [DOI] [PubMed] [Google Scholar]
- 19. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;1(10):21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): what changed and why? Thyroid. 2017;27(6):751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26(1):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito Y, Miyauchi A. Active surveillance as first-line management of papillary microcarcinoma. Annu Rev Med. 2019;70:369–379. [DOI] [PubMed] [Google Scholar]
- 24. Ito Y, Miyauchi A, Kudo T, Oda H, Yamamoto M, Sasai H, Masuoka H, Fukushima M, Higashiyama T, Kihara M, Miya A.. Trends in the implementation of active surveillance for low-risk papillary thyroid microcarcinomas at Kuma Hospital: gradual increase and heterogeneity in the acceptance of this new management option. Thyroid. 2018;28(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuttle RM, Zhang L, Shaha A. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab. 2018;13(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carty SE, Doherty GM, Inabnet WB III, Pasieka JL, Randolph GW, Shaha AR, Terris DJ, Tufano RP, Tuttle RM; Surgical Affairs Committee Of The American Thyroid Association. American Thyroid Association statement on the essential elements of interdisciplinary communication of perioperative information for patients undergoing thyroid cancer surgery. Thyroid. 2012;22(4):395–399. [DOI] [PubMed] [Google Scholar]
- 27. Robenshtok E, Fish S, Bach A, Dominguez JM, Shaha A, Tuttle RM. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 2012;97(8):2706–2713. [DOI] [PubMed] [Google Scholar]
- 28. Rondeau G, Fish S, Hann LE, Fagin JA, Tuttle RM. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011;21(8):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, Untch B, Ganly I, Shaha AR, Shah JP, Pace M, Li D, Bach A, Lin O, Whiting A, Ghossein R, Landa I, Sabra M, Boucai L, Fish S, Morris LGT. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angell TE, Vyas CM, Medici M, Wang Z, Barletta JA, Benson CB, Cibas ES, Cho NL, Doherty GM, Doubilet PM, Frates MC, Gawande AA, Heller HT, Kim MI, Krane JF, Marqusee E, Moore FD Jr., Nehs MA, Zavacki AM, Larsen PR, Alexander EK. Differential growth rates of benign vs. malignant thyroid nodules. J Clin Endocrinol Metab. 2017;102(12):4642–4647. [DOI] [PubMed] [Google Scholar]
- 31. Sabra MM, Sherman EJ, Tuttle RM. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer. 2017;123(15):2955–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuttle RM, Zhang L, Shaha A. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab. 2018;13(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tuttle RM, Brose MS, Grande E, Kim SW, Tahara M, Sabra MM. Novel concepts for initiating multitargeted kinase inhibitors in radioactive iodine refractory differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017;31(3):295–305. [DOI] [PubMed] [Google Scholar]
- 34. D'Agostino TA, Shuk E, Maloney EK, Zeuren R, Tuttle RM, Bylund CL. Treatment decision making in early-stage papillary thyroid cancer. Psychooncology. 2018;27(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Groopman J, Hartzband P.. Your Medical Mind. How to Decide What Is Right for You. New York, NY: Penguin Books. [Google Scholar]
- 36. Ito Y, Miyauchi A. Prognostic factors and therapeutic strategies for differentiated carcinomas of the thyroid. Endocr J. 2009;56(2):177–192. [DOI] [PubMed] [Google Scholar]
- 37. Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, Kim WB, Shong YK, Song DE, Baek JH, Chung KW, Kim TY. Active surveillance for patients with papillary thyroid microcarcinoma: a single center’s experience in Korea. J Clin Endocrinol Metab. 2017;102(6):1917–1925. [DOI] [PubMed] [Google Scholar]
- 38. Sugitani I, Fujimoto Y. Does postoperative thyrotropin suppression therapy truly decrease recurrence in papillary thyroid carcinoma? A randomized controlled trial. J Clin Endocrinol Metab. 2010;95(10):4576–4583. [DOI] [PubMed] [Google Scholar]
- 39. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222–1231. [DOI] [PubMed] [Google Scholar]
- 40. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calcatera NA, Lutfi W, Suman P, Suss NR, Wang CH, Prinz RA, Winchester DJ, Moo-Young TA. Concordance of preoperative clinical stage with pathological stage in patients ≥45 years with well-differentiated thyroid cancer. Endocr Pract. 2017;16(10):2017–0095. [DOI] [PubMed] [Google Scholar]
- 42. Kluijfhout WP, Pasternak JD, Drake FT, Beninato T, Shen WT, Gosnell JE, Suh I, C L, Duh QY. Application of the new American Thyroid Association guidelines leads to a substantial rate of completion total thyroidectomy to enable adjuvant radioactive iodine. Surgery. 2017;161(1):127–133. [DOI] [PubMed] [Google Scholar]
- 43. Nixon IJ, Ganly I, Patel SG, Palmer FL, Whitcher MM, Tuttle RM, Shaha A, Shah JP. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151(4):571–579. [DOI] [PubMed] [Google Scholar]
- 44. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, da Cruz MD, Dias F, Corbo R, Vaisman M, Tuttle RM. Thyroid lobectomy is associated with excellent clinical outcomes in properly selected differentiated thyroid cancer patients with primary tumors greater than 1 cm. J Thyroid Res. 2013;2013:398194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vaisman F, Shaha A, Fish S, Michael Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 2011;75(1):112–119. [DOI] [PubMed] [Google Scholar]
- 46. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 47. Jin A, Xu J, Wang Y. The role of TERT promoter mutations in postoperative and preoperative diagnosis and prognosis in thyroid cancer. Medicine (Baltimore). 2018;97(29):e11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim TH, Ki CS, Kim HS, Kim K, Choe JH, Kim JH, Kim JS, Oh YL, Hahn SY, Shin JH, Jang HW, Kim SW, Chung JH. Refining dynamic risk stratification and prognostic groups for differentiated thyroid cancer with TERT promoter mutations. J Clin Endocrinol Metab. 2017;102(5):1757–1764. [DOI] [PubMed] [Google Scholar]
- 49. Vuong HG, Altibi AMA, Duong UNP, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):411–417. [DOI] [PubMed] [Google Scholar]
- 50. Vuong HG, Duong UN, Altibi AM, Ngo HT, Pham TQ, Tran HM, Gandolfi G, Hassell L. A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocr Connect. 2017;6(3):R8–R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yin DT, Yu K, Lu RQ, Li X, Xu J, Lei M, Li H, Wang Y, Liu Z. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2016;85(2):299–305. [DOI] [PubMed] [Google Scholar]
- 53. Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, Puxeddu E, Morelli S, Torlontano M, Massa M, Bellantone R, Pontecorvi A, Montesano T, Pagano L, Daniele L, Fugazzola L, Ceresini G, Bruno R, Rossetto R, Tumino S, Centanni M, Meringolo D, Castagna MG, Salvatore D, Nicolucci A, Lucisano G, Filetti S, Durante C. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr Relat Cancer. 2018;25(3):L7–L11. [DOI] [PubMed] [Google Scholar]
- 54. Ghaznavi SA, Ganly I, Shaha AR, English C, Wills J, Tuttle RM. Using the American Thyroid Association risk-stratification system to refine and individualize the American Joint Committee on Cancer eighth edition disease-specific survival estimates in differentiated thyroid cancer. Thyroid. 2018;28(10):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ito Y, Miyauchi A, Hirokawa M, Yamamoto M, Oda H, Masuoka H, Sasai H, Fukushima M, Higashiyama T, Kihara M, Miya A. Prognostic value of the 8(th) edition of the tumor-node-metastasis classification for patients with papillary thyroid carcinoma: a single-institution study at a high-volume center in Japan. Endocr J. 2018;65(7):707–716. [DOI] [PubMed] [Google Scholar]
- 56. Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA, Sosa JA. Projecting survival in papillary thyroid cancer: a comparison of the Seventh and Eighth Editions of the American Joint Commission on Cancer/Union for International Cancer Control Staging Systems in two contemporary national patient cohorts. Thyroid. 2017;27(11):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tam S, Boonsripitayanon M, Amit M, Fellman BM, Li Y, Busaidy NL, Cabanillas ME, Dadu R, Sherman S, Waguespack SG, Williams MD, Goepfert RP, Gross ND, Perrier ND, Sturgis EM, Zafereo ME. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid. 2018;28(10):1301–1310. [DOI] [PubMed] [Google Scholar]
- 58. van Velsen EFS, Stegenga MT, van Kemenade FJ, Kam BLR, van Ginhoven TM, Visser WE, Peeters RP. Comparing the prognostic value of the eighth edition of the American Joint Committee on Cancer/tumor node metastasis staging system between papillary and follicular thyroid cancer. Thyroid. 2018;28(8):976–981. [DOI] [PubMed] [Google Scholar]
- 59. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1447–1463. [DOI] [PubMed] [Google Scholar]
- 60. Pitoia F, Abelleira E, Tala H, Bueno F, Urciuoli C, Cross G. Biochemical persistence in thyroid cancer: is there anything to worry about? [published correction appears in Endocrine 2014;46(3):538]. Endocrine. 2014;46(3):532–537. [DOI] [PubMed] [Google Scholar]
- 61. Sung TY, Cho JW, Lee YM, Lee YH, Kwon H, Jeon MJ, Kim WG, Choi YJ, Song DE, Chung KW, Yoon JH, Hong SJ. Dynamic risk stratification in stage I papillary thyroid cancer patients younger than 45 years of age. Thyroid. 2017;27(11):1400–1407. [DOI] [PubMed] [Google Scholar]
- 62. Tarasova VD, Tuttle RM. A risk-adapted approach to follow-up in differentiated thyroid cancer. Rambam Maimonides Med J. 2016;7(1):1–10. https://www.rmmj.org.il/userimages/556/0/PublishFiles/556Article.pdf. Accessed 9 April 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165(3):441–446. [DOI] [PubMed] [Google Scholar]
- 64. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375(23):2307. [DOI] [PubMed] [Google Scholar]
- 65. Xing M. Genetic-guided risk assessment and management of thyroid cancer. Endocrinol Metab Clin North Am. 2019;48(1):109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ 3rd, Ganly I, Hodak SP, Kebebew E, Patel KN, Shaha A, Steward DL, Tufano RP, Wiseman SM, Carty SE. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015;25(7):760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nikiforov YE. Role of molecular markers in thyroid nodule management: then and now. Endocr Pract. 2017;23(8):979–988. [DOI] [PubMed] [Google Scholar]
- 68. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381(9871):1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shah S, Boucai L.. Effect of age on response to therapy and mortality in patients with thyroid cancer at high risk of recurrence. J Clin Endocrinol Metab. 2018;103(2):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosario PW. Eighth edition of AJCC staging for differentiated thyroid cancer: is stage I appropriate for T4/N1b patients aged 45-55 years? Endocrine. 2017;56(3):679–680. [DOI] [PubMed] [Google Scholar]
- 71. Shteinshnaider M, Muallem Kalmovich L, Koren S, Or K, Cantrell D, Benbassat C.. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: a comparative study. Thyroid. 2018;28(2):201–209. [DOI] [PubMed] [Google Scholar]
- 72. Krajewska J, Chmielik E, Jarząb B. Dynamic risk stratification in the follow-up of thyroid cancer: what is still to be discovered in 2017? Endocr Relat Cancer. 2017;24(11):R387–R402. [DOI] [PubMed] [Google Scholar]
- 73. Momesso DP, Vaisman F, Yang SP, Bulzico DA, Corbo R, Vaisman M, Tuttle RM. Dynamic risk stratification in patients with differentiated thyroid cancer treated without radioactive iodine. J Clin Endocrinol Metab. 2016;101(7):2692–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Park S, Kim WG, Song E, Oh HS, Kim M, Kwon H, Jeon MJ, Kim TY, Shong YK, Kim WB. Dynamic risk stratification for predicting recurrence in patients with differentiated thyroid cancer treated without radioactive iodine remnant ablation therapy. Thyroid. 2017;27(4):524–530. [DOI] [PubMed] [Google Scholar]
- 75. Hoofnagle AN, Roth MY. Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab. 2013;98(4):1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Azmat U, Porter K, Senter L, Ringel MD, Nabhan F. Thyroglobulin liquid chromatography-tandem mass spectrometry has a low sensitivity for detecting structural disease in patients with antithyroglobulin antibodies. Thyroid. 2017;27(1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN, Spencer CA, Turcu AF, Algeciras-Schimnich A. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab. 2015;100(8):E1074–E1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Spencer C, Petrovic I, Fatemi S, LoPresti J.. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications. J Clin Endocrinol Metab. 2014;99(12):4589–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wheeler SE, Liu L, Blair HC, Sivak R, Longo N, Tischler J, Mulvey K, Palmer OMP. Clinical laboratory verification of thyroglobulin concentrations in the presence of autoantibodies to thyroglobulin: comparison of EIA, radioimmunoassay and LC MS/MS measurements in an urban hospital. BMC Res Notes. 2017;10(1):725. [DOI] [PMC free article] [PubMed] [Google Scholar]