Abstract
Background:
Clinical levels of social anxiety disorder (SAD) often appear during childhood and rise to a peak during late adolescence. The temperament trait behavioral inhibition (BI), evident early in childhood, has been linked to increased risk for SAD. Functional and structural variation in brain regions associated with the identification of, and response to, fear may support the BI-SAD relation. While relevant functional studies are emerging, the few extant structural studies have focused on adult samples with mixed findings.
Methods:
A moderated-mediation model was used to examine the relations between BI, SAD symptoms, and brain-volume individual differences in a sample of children at risk for anxiety (ages 9–12; N=130, 52 BI).
Results:
Our findings indicate that at higher levels of BI, children with smaller anterior insula volumes showed stronger correlations between BI and SAD. In addition, larger ventrolateral prefrontal cortex (vlPFC) volumes were associated with fewer SAD symptoms.
Conclusions:
These findings support previous reports linking SAD levels with variations in volume and reactivity in both limbic (insula) and prefrontal (vlPFC) regions. These findings set the foundation for further examination of networks of neural structures that influence the transition from BI to SAD across development, helping further clarify mechanisms of risk and resilience.
The data that support the findings of this study are openly available in clinical trial registration information— http://clinicaltrials.gov/; Attention and Social Behavior in Children (BRAINS); NCT02401282.
Introduction
Animal and human imaging studies suggest that specific brain regions, such as the amygdala, insula, and anterior cingulate cortex (ACC), play a large role in identifying potential fear stimuli in the environment and generating fear responses. In tandem, prefrontal areas such as the orbitofrontal cortex (OFC), ventrolateral- (vlPFC), and dorsolateral-prefrontal (dlPFC) cortices may engage in subsequent cognitive control and emotion modulation (Freitas-Ferrari et al., 2010; Taylor & Whalen, 2015). Over the past two decades an increasing number of studies have reported functional abnormalities in these brain areas (either hyperactivity or hypoactivity) in anxious individuals, compared to controls (Duval, Javanbakht, & Liberzon, 2015). However, relatively few studies have examined neural structures and almost exclusively in clinically anxious adults. The extant literature on structural variation in anxious individuals presents inconsistent findings, making it difficult to draw conclusions regarding any potential relations between neural structure and anxiety. This gap is particularly important as structural variations may be evident early in development and color subsequent variation in function (Schwartz et al., 2010; Sylvester et al., 2016). The current study examines the relation between neural structures associated with anxiety and early symptoms of social anxiety disorder (SAD) in otherwise healthy children characterized for temperamental risk for the disorder. Given that the few available studies have focused on adult samples, this study can address patterns of variation earlier in development and prior to the typical window of diagnosis.
A Developmental Perspective: Behavioral Inhibition as a Risk Factor
Clinical manifestations of anxiety often appear during childhood and early adolescence (Costello, Angold, Burns, & et al., 1996) and rise to a peak at the end of adolescence (Grant et al., 2005; Knappe, Sasagawa, & Creswell, 2015). One possible reason for this trajectory may be that childhood and adolescence are developmental periods marked by rapidly occurring structural and functional brain changes (Alexander-Bloch, Raznahan, Bullmore, & Giedd, 2013; Giedd et al., 1996; Uddin, Supekar, Ryali, & Menon, 2011). These brain changes may leave children vulnerable to excessive anxiety due to an over-reactive alarm system, an under-reactive regulatory system, or possibly, both. Many studies have demonstrated functional abnormalities in central fear circuits in pediatric anxiety (namely social anxiety, generalized anxiety, and separation anxiety; see reviews: (Brühl, Delsignore, et al., 2014; Duval et al., 2015; Freitas-Ferrari et al., 2010). However, relatively few studies have examined structural differences within these brain regions among anxious or at-risk children.
SAD affects 9% of children and 13% of adults, making it the highest lifetime-prevalence anxiety disorder (Gross & Hen, 2004; Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012; Knappe et al., 2015). A theory-driven risk factor for all anxiety disorders centers on early appearing temperament (Kagan, 1989; Rothbart, Ahadi, & Evans, 2000), with behavioral inhibition (BI) as the most widely studied temperamental style (Clauss & Blackford, 2012). BI is defined by a constellation of traits at both the behavioral level (e.g., hypervigilance, social withdrawal, and increased startle response when exposed to unfamiliar people or objects) and psychophysiological level (e.g., pupillary dilation during cognitive tasks, elevated salivary cortisol levels) (Buss & Kiel, 2013; Goldsmith & Lemery, 2000; Kagan, Snidman, Zentner, & Peterson, 1999; Pérez-Edgar & Fox, 2005). BI increases the risk for SAD in children (Ballespí, Jané, & Riba, 2012), adolescents (Lewis-Morrarty et al., 2015; Rapee, 2014), and adults (Mick & Telch, 1998). Prospective longitudinal studies (Clauss & Blackford, 2012) have demonstrated a positive association between BI during childhood and either subclinical social anxiety or SAD in later childhood (Hirshfeld-Becker et al., 2007; Hudson, Dodd, Lyneham, & Bovopoulous, 2011; Muris, Brakel, Arntz, & Schouten, 2011) and adolescence (Chronis-Tuscano et al., 2009; Schwartz, Snidman, & Kagan, 1999).
Even though BI is a strong predictor of latent anxiety disorders (not only SAD; Chronis-Tuscano et al., 2009; Rosenbaum et al., 1993), especially for individuals who displayed consistent signs of BI across childhood (for a review, see: Pérez-Edgar & Fox, 2005), only 40% of BI children develop SAD (Clauss & Blackford, 2012). This discontinuity in outcome is likely due to other individual, social, and environmental factors that moderate the risk of developing SAD, including neural structure variation.
Prior functional imaging work associated several ROIs with both BI levels and risk for SAD. For example, BI is associated with amygdala and insula reactivity to salient stimuli (e.g., emotional faces; Auday, Taber-Thomas, & Pérez-Edgar, 2018; Pérez-Edgar et al., 2007; White, Helfinstein, & Fox, 2010), striatal response to reward (Helfinstein, Fox, & Pine, 2012), prefrontal engagement in emotion regulation (Auday et al., 2018; Fu, Taber-Thomas, & Pérez-Edgar, 2017; Hardee et al., 2013), and variation in resting state functional connectivity (Blackford, Clauss, Avery, Cowan, Benningfield, & VanDerKlok, 2014; Roy et al., 2014; Taber-Thomas, Morales, Hillary, & Pérez-Edgar, 2016). A recent study examining neural correlates in adults who were identified as BI during early childhood reported associations with thinner dACC in adulthood (Sylvester et al., 2016). However, we know very little regarding the relation between the structural characteristics (e.g., volume) of previously implicated ROIs and patterns of anxiety that may lead some at-risk children, but not others, to develop SAD (or other anxiety disorders).
Variations in Neural Structure as a Potential Moderator of Anxiety
Altered region-of-interest (ROI) volume may be a strong marker of psychopathology and risk for disorder. Such associations have been reported in a broad range of studies on cognitive functioning and disorder profiles such as intelligence, attention, schizophrenia, autism, and major depression (Choi et al., 2008; Ehrlich et al., 2012; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007; Koolschijn, van Haren, Lensvelt-Mulders, Hulshoff Pol, & Kahn, 2009; Westlye, Grydeland, Walhovd, & Fjell, 2011). For example, a large meta-analysis found that individuals diagnosed with major depressive disorder showed significant volume reductions in the ACC and OFC (Koolschijn et al., 2009), suggesting perturbed neural circuitry in brain areas proposed to have a role in emotion processing and regulation. In schizophrenia, evidence suggests reduced cortical thickness in frontal, temporal, occipital, and parietal areas, as well as a disruption of structure-function relations, such that patients exhibited an altered neural pattern associated with working memory tasks, compared to healthy comparisons (Ehrlich et al., 2012). Specifically, patients showed associations with temporal-lobe cortical thickness, whereas controls showed associations with prefrontal areas, possibly indicating a compensatory neural network.
A comprehensive literature review of findings linking volumetric patterns to anxiety is beyond the scope of this manuscript, and the reader is encouraged to refer to recent reviews (e.g., Brühl, Delsignore, et al., 2014; Freitas-Ferrari et al., 2010). Table SM1 in Supplementary Material provides a brief view of the inconsistent literature comparing clinically or trait anxious adults with healthy controls (i.e., a categorical rather than dimensional approach), including grey and white matter differences. Studies to date have primarily relied on a categorical approach, comparing clinical samples to healthy controls, overlooking variability in anxiety levels, which may obscure findings or contribute to inconsistent findings. Furthermore, structural abnormalities have been primarily studied in adult populations experiencing clinical levels of one or more anxiety disorders (Baur et al., 2013; Brühl, Delsignore, Komossa, & Weidt, 2014; Irle, 2010; Liao et al., 2011; Machado-de-Sousa et al., 2014; Syal et al., 2012; Talati, Pantazatos, Schneier, Weissman, & Hirsch, 2013), samples heterogeneous in psychopathology (van Tol et al., 2010), or rather small sample sizes (e.g., target group size smaller than 30; Irle, 2010; Liao et al., 2011; Syal et al., 2012), suggesting a few more potential contributors to these inconsistent findings. Therefore, examining neural structural volume in a large sample of children who are at-risk (rather than meeting criteria for clinical levels of anxiety; see next section) for a specific anxiety disorder, while utilizing a continuous measure (again, see next section), could help shed light on the current data.
We anticipated that children in middle childhood would exhibit a range of SAD symptoms, as younger children typically present very few symptoms, risking a floor effect. At the same time, we expected to have few participants meeting diagnostic criteria (Costello, Egger, & Angold, 2005), allowing us to assess risk rather than diagnosis. Older adolescents are more likely to have reached diagnostic cut-offs, making middle childhood a preferred age range for assessing anxiety risk. By examining an early marker of risk, these data may clarify normative structural relations while highlighting points across development that are sensitive for assessment, prevention, and intervention.
The Current Study
This study aimed to examine potential volumetric variations in a large sample of children—temperamentally at-risk for SAD (labeled as BI) and non-yoked age- and sex-matched children not at temperamental risk (labeled non-BI). Our sample examined BI and non-BI children at an age range prior to the typical onset of social anxiety (i.e., 12–16 years age) (Beesdo, Knappe, & Pine, 2009). In line with recent calls to focus on the full range of risk markers (Sanislow, Quinn, & Sypher, 2015), rather than discrete categories of risk, we used continuous measures of both BI and SAD.
Our analysis focused on bilateral ROIs implicated in prior studies, namely: the amygdala, insula, ACC (more specifically, rostral ACC; we will refer to this region as ACC; please see: Etkin, Egner, & Kalishc, 2011; Stevens, Hurley, & Taber, 2011), and OFC. We also included the vlPFC, as it has been implicated in functional studies of SAD (Brühl, Delsignore, et al., 2014) and recent work suggests that changes in vlPFC function may mediate changes in anxiety among BI children (Liu et al., 2018). Despite the inconsistent literature, our working hypotheses were twofold. First, ROI volume would significantly account for variance in the relation between BI and social anxiety. Given the inconsistent literature, we chose to take a conservative approach: rather than specifying directionality, we hypothesized that alterations in ROI volume—limbic (amygdala and insula) and prefrontal (ACC, OFC, and vlPFC) will be associated with a significant BI—anxiety relation. Second, BI levels will moderate the ROI volume—anxiety relation, such that at higher levels of BI, the ROI volume—anxiety relation will be stronger. Therefore, we utilized a moderated-mediation model (for further detail, see ‘Statistical Analyses in a Priori ROIs’ in the Materials and Methods section). As a sensitivity analysis, we also examined two ROIs not expected to vary with our variables of interest, namely the inferior and middle occipital gyri. Finally, we conducted between-group whole-brain exploratory analyses to explore BI vs. non-BI group differences beyond our a-priori ROIs.
Materials and Methods
Participants
Participants were one-hundred forty-five 9–12-year-olds (M=10.81, SD=.99), drawn from a multi-visit study of attention bias to threat and temperamental risk for anxiety (Auday, Taber-Thomas, & Pérez-Edgar, 2018; Fu, Taber-Thomas, & Pérez-Edgar, 2017; Liu, Taber-Thomas, Fu, & Pérez-Edgar, 2018; Morales, Taber-Thomas, & Pérez-Edgar, 2017; Taber-Thomas, Morales, Hillary, & Pérez-Edgar, 2016; Thai, Taber-Thomas, & Pérez-Edgar, 2016). Participants were recruited through a university database of families interested in participating in research studies, community outreach, and word-of-mouth.
Participants were screened based on parent-report using the Behavioral Inhibition Questionnaire (BIQ) (Bishop, Spence, & McDonald, 2003). We used cut-off scores based on previous studies of extreme temperament in children ages 4 to 15 years (Broeren & Muris, 2010) in order to enrich the sample for BI. Sixty-four (64) children met BI criteria, scoring high on both the social novelty subscale and grand total score (N=35), the social novelty subscale only (≥60; N=25), or the grand total score only (≥119; N=4), and participated in the fMRI session. Eighty-one (81) children participated as sex- and age-matched non-BI controls. Data presented here are from the BI children’s first (baseline) visit, as they went on to participate in the large multi-visit study. Non-BI children had only one time point of participation. Although we used categorical cut-offs for identification and recruitment, all analyses used continuous BIQ scores.
Measures
Behavioral inhibition
was assessed using the BIQ (Bishop et al., 2003), a 30-item instrument that measures the frequency of BI-linked behavior in the domains of social and situational novelty (plus a summed total score) on a seven-point scale ranging from 1 (“hardly ever”) to 7 (“almost always”). Questions were edited to be more appropriate for the target age range in the current study (e.g., reference to preschool, kindergarten, and childcare was removed for the question: “Happily separates from parent(s) when left in new situations for the first time (e.g., kindergarten, preschool, childcare). The questionnaire has adequate internal consistency, construct validity, and validity in differentiating inhibited from non-inhibited children (Bishop, Spence, & Mcdonald, 2003), parent report with the BIQ correlates with laboratory observations of BI in social contexts (Dyson, Klein, Olino, Dougherty, & Durbin, 2011), and the BIQ had good internal consistency in the present study (Cronbach’s α = .91).
Social Anxiety symptoms
were assessed using parent-report on the computerized Diagnostic Interview Schedule for Children—Fourth Edition (C-DISC-IV) (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). The C-DISC-IV is an interviewer-administered version of the computerized Diagnostic Interview Schedule for Children used to assess for anxiety symptoms and disorders. The C-DISC-IV is a comprehensive, structured interview that covers 36 mental health disorders for children & adolescents, using DSM-IV criteria. Most questions are worded so that they can be answered “yes”, “no”, and “somewhat” or “sometimes”. Questions reference the 4 weeks, 6 months, and 1 year prior to the interview. The interviews were administered by trained research assistants with the primary caregiver and scored using the software’s algorithm.
Neural Structural Data
Image Acquisition.
Prior to scanning, all participants were placed in a mock MR scanner for approximately 20 minutes and completed practice versions of the tasks administered as part of the larger study (Auday et al., 2018; Fu et al., 2017). This procedure acclimates participants to the scanner environment and minimizes motion artifact and anxiety.
Imaging data were acquired in a 3-Tesla MRI scanner, first with a MAGNETOM Trio with Tim system (N=76). After a scanner upgrade, we used a MAGNETOM Prismafit (N=54), both acquired from Siemens Medical Solutions, Erlangen, Germany. High-resolution T1-weighted structural scans were acquired using a magnetization prepared gradient echo sequence (MP-RAGE) (176 1mm slices, TR=1700, TE=2.01, flip angle=9 degrees, FoV=256mm, voxel size=1×1×1 mm; 256×256 matrix, T1=850ms).
Image Processing.
All images were inspected for loss of image quality, such as blurring, ringing, and ghosting using SPM8 (Wellcome Trust Center for Neuroimaging, London, UK). The structural data were obtained using the standard cortical reconstruction pipeline in FreeSurfer v5.3.0 (http://surfer.nmr.mgh.harvard.edu/), including standardization to the Destrieux cortical atlas (Destrieux, Fischl, Dale, & Halgren, 2010; Fischl et al., 2002), which is a validated approach for use with children (Ghosh et al., 2010). Once the cortical model was completed for each participant, the cerebral cortex was parcellated into units based on gyral and sulcal structure, creating surface-based maps of gyral curvature and sulcal depth (Fischl, Sereno, & Dale, 1999). Calculations of cortical and subcortical volumes using this method are considered comparable to manual segmentation (Lehmann et al., 2010), while other reports highlight potential concerns with the accurate segmentation of subcortical regions (Hanson et al., 2012; Nugent, Luckenbaugh, Wood, Bogers, Zarate, & Drevets, 2013). After the parcellations and surfaces were generated, they were visually reviewed by trained research assistants and, where necessary, manually edited and regenerated. We extracted ROIs volume for each participant in each hemisphere, as well as whole-brain volume.
Statistical Analyses in a Priori ROIs
We normalized individual ROI volumes for each participant using their whole-brain volume (e.g., [region/whole brain volume]*1000). Whole brain volume was calculated as the SupraTentorial volume, excluding ventricles, CSF, and choroid plexus. A priori ROIs were constructed by combining FreeSurfer’s parcellations (see Supporting Information for details). For these ROIs, differences in volume were examined using analysis of covariance (ANCOVA) with continuous BI-score as a fixed-factor and age in months, gender, and scanner model as covariates.
Additionally, we tested a moderated-mediation model using PROCESS version 2.16, a regression macro for SPSS (Hayes, 2013; see Figure 1), where BI score (a continuous measure) exerts an effect on social anxiety symptoms level, both directly and indirectly through each a priori ROI. The model captures the conditional direct effect of BI on Anxiety, the moderating effect of ROI volume on the BI—Anxiety relation, and the conditional indirect effect of BI on Anxiety via ROI volume. This model was run for the following ROIs in each hemisphere: amygdala, posterior insula, anterior insula, ACC, OFC, and vlPFC, as well as our comparison occipital ROIs. Age, sex, and scanner model were covariates in those models (see Participants subsection in Results). Our central analyses present 12 comparisons—6 ROIs in two hemispheres. Comparison models are presented in Supplementary Material. To correct for multiple comparisons, we used a Bonferroni-corrected alpha of 0.05/12=0.0042 for each overall model before proceeding to the individual paths.
Figure 1.
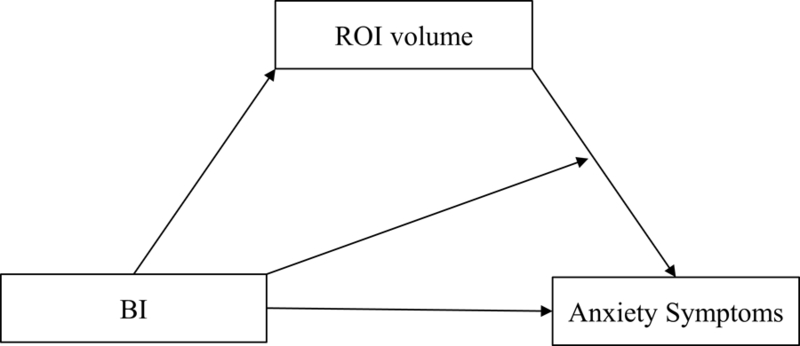
A Moderated-Mediation Model: ROI volume mediating the relation between BI and Anxiety Symptoms.
Cortical Volume Exploratory Analyses
Exploratory analyses in the prefrontal cortex were conducted using general linear modeling programs available through FreeSurfer (Qdec). To correct for multiple comparisons, Monte Carlo simulations available in the FreeSurfer software were used to determine the area of cortex required to meet prefrontal cortex-wide clusterwise significance of p < .05.
Results
Participants
Of our 145 participants, 15 children (12 BI) with poor structural MRI images were excluded from analyses. Excluded children did not differ from included children on Total BIQ score, age, or social anxiety symptoms level (all p’s > .40). Of the included participants, children with high BI (i.e., meeting cutoff criteria) scored significantly higher on the BIQ, t(128)=14.195, p<.0001, d=2.54, and had significantly higher social anxiety symptom levels, measured on the C-DISC, t(128)=5.99, p < .0001, d=1.00, compared to BN. A one-way ANOVA indicated no between-group differences in age, F(1, 128)= .886, p= .35, sex, F(1, 128)= .127, p= .72, or any of the ROI volumes examined (all p’s > .36). We found four correlations between age and ROI volume, including the pInsula (L), r = −0.29, p < 0.01, pInsula (R), r = −0.26, p < 0.01, aInsula (R): r = −0.18, p < 0.05, and ACC (R): r = −0.22, p < 0.05. Age in months was used as a covariate in all analyses. We also controlled for growth/variation in individual size by dividing the ROI volume by total cerebral volume.
Our final sample size included 130 subjects (52 BI). Table 1 presents final-sample Pearson-moment correlations (see Table SM2 in Supplementary Material for whole group, BI, and non-BI descriptive statistics). As noted in Table 1, BI score and SAD symptom levels were moderately correlated, r = 0.50, leaving a substantial amount of variation to be explored.
Table 1.
Inter-correlations of BIQ with anxiety measures and ROI volumes, presented separately by hemisphere.
| Total BIQ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SAD Symptoms | .50***,+ | ||||||||||||
| 2. Amygdala (L) | .09 | .15 | |||||||||||
| 3. pInsula (L) | .18* | −.07 | −.07 | ||||||||||
| 4. aInsula (L) | .08 | −.18* | .01 | .27** | |||||||||
| 5. ACC (L) | .09 | −.10 | .09 | .20* | .04 | ||||||||
| 6. OFC (L) | .10 | .08 | .13 | .12 | .13 | .11 | |||||||
| 7. vlPFC (L) | .08 | −.11 | .09 | .17 | .20* | .06 | .18* | ||||||
| 8. Amygdala (R) | .04 | .19* | .53***,+ | .06 | .09 | .01 | .01 | .12 | |||||
| 9. pInsula (R) | .11 | −.06 | .12 | .44***,+ | .44***,+ | .10 | .10 | .15 | .38***,+ | ||||
| 10. aInsula (R) | .02 | −.15 | −.06 | .26** | .53***,+ | −.05 | −.05 | .03 | .20* | .49***,+ | |||
| 11. ACC (R) | .14 | .02 | .08 | .16 | .06 | .40***,+ | .23** | .07 | .08 | .20* | −.02 | ||
| 12. OFC (R) | .10 | .06 | .02 | .19* | .11 | .20* | .62***,+ | .17 | −.10 | .21* | .06 | .21* | |
| 13. vlPFC (R) | .13 | .02 | .04 | .30***,+ | .19* | −.01 | .25** | .45***,+ | .21* | .28** | .11 | .02 | .19* |
Notes: SAD Sym. = Social Anxiety Symptom Levels (%). aInsula = anterior insula. pInsula = posterior insula. (L) = Left. (R) = Right.
p < .05.
p < .01.
p < .001.
Survived Bonferroni correction for multiple comparisons.
Compared to children scanned in the MAGNETOM Prismafit, children scanned in the MAGNETOM Trio were older, t(128)=4.18, p<.001, d=.73. Data collected with the new scanner showed a significant correlation with Total BIQ for bilateral anterior insula volume, r = .30 and .33, for left- and right-hemisphere, respectively. There were no significant differences between the old and the new scanner in participant sex, Total BIQ score, social anxiety level, or any of the ROI volumes (all p’s were > .08).
Statistical Analyses for a Priori ROIs
Of our 12 a-priori ROIs, only left posterior insula volume was significantly correlated with Total BI score, r = .18, p < .05. As mentioned above, ANCOVA analyses were used to examine volume differences with and age in months, gender, and scanner as covariates. These analyses were not significant for any of the a priori ROI volumes (all p’s > .10).
In the moderated-mediation models, our analyses present 12 comparisons—6 ROIs in two hemispheres. For an overview of model statistics, please refer to Table 2. All models met the Bonferroni-corrected alpha (all p’s <0.001) and BI score was a significant predictor of SAD symptom levels for all ROIs (all p’s < .001).
Table 2.
Significant Findings in Mediation-Moderation Models.
| Model p | F | R | MSE | Main Effects | Interactions | |
|---|---|---|---|---|---|---|
| Left Hemi.: | ||||||
| Amygdala | < .001 | F(6, 123)=8.55 | .5426 | 389.0 | BI, p < .001 | |
| pInsula | < .001 | F(6, 123)=8.63 | .5443 | 387.9 | BI, p < .001 | |
| aInsula | < .001 | F(6, 123)=11.31 | .5963 | 355.3 | BI, p <
.001 aInsula, p < .01 |
BI x aInsula, p < .05 |
| ACC | < .001 | F(6, 123)=9.17 | .5559 | 380.9 | BI, p <
.001 ACC, p = .052 |
|
| OFC | < .001 | F(6, 123)=8.57 | .543 | 388.77 | BI, p < .001 | BI x OFC, p = .08 |
| vlPFC | < .001 | F(6, 123)=8.78 | .5476 | 385.97 | BI, p <
.001 vlPFC, p < .05 |
|
| Right Hemi.: | ||||||
| Amygdala | < .001 | F(6, 123)=9.09 | .5543 | 381.9 | BI, p < .001 | |
| pInsula | < .001 | F(6, 123)=9.41 | .5394 | 390.87 | BI, p < .001 | |
| aInsula | < .001 | F(6, 123)=9.03 | .553 | 382.68 | BI, p < .001 | |
| ACC | < .001 | F(6, 123)=7.92 | .5279 | 397.63 | BI, p < .001 | |
| OFC | < .001 | F(6, 123)=8.02 | .5303 | 396.26 | BI, p < .001 | |
| vlPFC | < .001 | F(6, 123)=8.46 | .5405 | 390.24 | BI, p < .001 |
Note: aInsula = Anterior Insula. pInsula = Posterior Insula. Hemi.=Hemisphere.
These models also revealed a significant, negative main effect for left anterior insula, β = −17.83, p <.01, where smaller ROI-volume was associated with higher SAD symptom levels. Furthermore, the relation between BI scores and SAD symptoms level was moderated by anterior insula volume such that a combination of higher BI and smaller anterior insula volumes was associated with higher levels of SAD symptoms (see Figure 2), β = −0.39, p < .05. A significant, negative main effect was also found for left vlPFC in which larger ROI-volume was associated with lower SAD symptom levels, β = −4.28, p < .05, regardless of BI score.
Figure 2.

Significant Two-Way Interaction of BI-Score by Left Anterior Insula Volume Predicting SAD Symptoms Level.
Two marginally-significant effects were found involving left ACC (main effect: β = −7.09, p = .05) and left OFC (interaction: β = .08, p = .08). For left ACC, there was a negative main effect where smaller ROI-volume was associated with increased SAD symptom levels. Probing the interaction for left OFC revealed a positive relation between BI and SAD symptom levels that was stronger for children with larger OFC volume.
Cortical Volume Exploratory Analyses
To complement the a priori ROI analysis, an exploratory analysis examined the relations among BI and cortical volume in children across prefrontal cortex. In contrast to regional analyses, the prefrontal cortex vertexwise analysis did not detect any regions surviving multiple comparison correction.
Discussion
The extant literature on neural correlates of anxiety consists mostly of studies utilizing adult samples experiencing clinical levels of psychopathology. Moreover, compared to links between childhood BI and adult brain function, little is known concerning the relation between BI and brain structure, at any stage of development. Due to their task neutrality, structural studies may complement functional studies by examining state-independent structural correlates. Examining those relations across varying developmental timepoints could help reveal whether BI is associated with innate structural differences or whether such differences emerge throughout childhood into adulthood. To date, studies examining brain structure in adults who were high-BI children reported thinner left OFC and thicker right vmPFC (Schwartz et al., 2014), larger amygdala and caudate volumes (Clauss et al., 2014), and thinner dACC (Sylvester et al., 2016) in adulthood. With the current study, we aimed to expand the current literature by examining the potential moderating effects of brain volume on the relations between BI, a strong temperamental risk factor, and anxiety symptom severity in a large sample of children (N = 130; 52 high-BI). Our data provide further support for the association of ROIs previously implicated in inhibited temperament and anxiety disorders in the early correlation between BI and SAD.
In line with the extant BI literature, our data suggest a strong link to social anxiety symptom levels in the anterior insula and in vlPFC. Smaller insular volume was associated with greater SAD symptom levels (main effect). Similarly, and regardless of BI score, smaller left vlPFC volumes were associated with greater SAD symptom levels (main effect). Importantly, our findings also showed that the BI-SAD relation was stronger with smaller insular volume (interaction effect; see Figure 2). In contrast, we found no significant relations for the occipital comparisons.
The insula is thought to play a significant role in both fear and anxiety circuitry. It has been argued that the insula is activated during the anticipation of uncertain, aversive events, and initiates and maintains modulation of limbic and frontal structures (for reviews see Freitas-Ferrari et al., 2010; Taylor & Whalen, 2015). Despite mixed findings in studies examining structural correlates of anxiety, functional studies utilizing emotional face tasks more consistently report that anterior insular hyperactivity is associated with higher trait anxiety in clinical and healthy populations (Stein, Simmons, Feinstein, & Paulus, 2007), that anterior insula hyperactivation in SAD is positively associated with symptoms severity (Carré et al., 2014; Klumpp, Post, Angstadt, Fitzgerald, & Phan, 2013; Freitas-Ferrari et al., 2010), and a reduction in insula (both anterior and posterior) activation or regional cerebral blood flow (rCBF) measured during public speaking tasks is evident following pharmacotherapy (Tillfors et al., 2001). A more recent functional-MRI study reported that high social reticence in early childhood (ages 2 to 7) predicted dACC activation as well as mid-to-anterior insula utilizing a social engagement task and anticipated unpredictable social evaluation later in preadolescence—at age 11 (Jarcho et al., 2016), further establishing the insula’s role in social-cognitive contexts.
Findings from a study by Hardee and colleagues (2013) utilizing a threat/angry faces task indicated that negative amygdala-anterior insula functional connectivity was associated with BI in a sample of young adults classified as high-BI in infancy through childhood. They further reported positive insula-dlPFC and negative amygdala-dlPFC functional connectivity, supporting the suggested role of the insula in frontal-limbic modulation, especially in light of impaired frontal-limbic emotion modulation. The vlPFC is thought to modulate amygdala responses to threat in order to maintain goal-directed behavior (Corbetta & Shulman, 2002; Fox & Pine, 2012). Most functional studies of SAD (utilizing an emotional-faces-task in adult samples) reported increased vlPFC activation (Bruhl, Desl, et al., 2014). Similarly, Monk et al (2006) found that youth with generalized anxiety disorder (GAD) displayed greater vlPFC response to angry faces (500ms presentation) compared to healthy adolescents, and that symptom severity was negatively correlated with vlPFC activation. Finally, recent work (Liu et al., 2018) suggests that anxiety symptom decreases in a BI sample may be mediated by increases in vlPFC activation.
Our findings expand the current literature by showing that in addition to functional variations, there are also structural differences in BI in these analog regions. Recognizing that a relation between structure and function is likely not straightforward and may be moderated by other factors, we tentatively suggest that it may be that smaller neural (insular and vlPFC) volume in BI children (and anxious adults) are a result of two compounded processes. First, structural deficiencies exhibited as reduced frontal volume may require compensation for impaired down-regulatory anxiety-processing capabilities by exerting increased activation to allow a greater downregulation of threat-related stimuli, aiming to mitigate limbic hyperactivation (Liao et al., 2011; Phan et al., 2009). Second, in line with findings discussed above (e.g., Hardee et al., 2013), this process may be further exacerbated by reduced frontal-limbic structural connectivity (Baur et al., 2013). Alternatively, it may be that the decreased prefrontal-limbic structural connectivity is the conduit for the altered frontal ROI structure due to lack of practice needed for optimal development of a top-down regulatory mechanism. Future studies need to examine within-group neural-ROI structure and activation patterns in-tandem to help shed more light on these structural-functional relations.
Lastly, approaching significance, we found that reduced left ACC volume was associated with higher anxiety levels, regardless of BI status. Functional studies in SAD and BI reported ACC hyperactivation in response to threatening stimuli, such as angry faces (Amir et al., 2005; Goldin, Manber, Hakimi, Canli, & Gross, 2009; Jarcho et al., 2014). Considering the overall reduced amygdala-insula-ACC functional connectivity in SAD (Duval et al., 2015) and in BI (Taber-Thomas et al., 2016), and our insula main-effect, it may be that a combination of higher BI and smaller paralimbic volume presents higher risk for SAD. The OFC (sometimes referred to as ventral PFC) is thought to take part in avoidance behavior (Clauss, Avery, & Blackford, 2015) and perpetuating negative states (Blackford & Pine, 2012). Although marginally significant, our finding of a stronger BI-SAD association for children with larger OFC volumes provides some support for the OFC as a marker of inhibited temperament.
Conclusion and Future Directions
This study, to our knowledge, is the first to examine the moderating role of neural structural on the relation between BI, an early temperamental risk for developing social anxiety, prior to the typical onset of social anxiety (i.e., 12–16 years age) (Beesdo, Knappe, & Pine, 2009). Overall, our findings extend and provide further support for the extant literature on neural correlates of SAD, showing associations with insular and vlPFC volume, as well as marginally-significant findings in other prefrontal regions, namely ACC and OFC.
These findings should be considered in the context of this study’s limitation. First, the high-BI children in our sample were classified based on concurrent parental reports, rather than continuous behavioral observation throughout childhood. Second, while our findings regarding neural volume associations with the BI-SAD link are in line with the current literature, the exact mechanisms are still unclear. Future studies should employ study designs that incorporate measures of structure, function, and connectivity to further clarify these relations in a large sample. Such studies, over time, could also help inform recent data suggesting that both the strength and directionality of functional connections relevant to anxiety may shift over the course of development (Gee et al., 2013). Furthermore, longitudinal designs that examine these relations across several time-points from early childhood into young adulthood will help delineate if the evident structural differences in childhood and adulthood (i.e., Schwartz et al., 2014; Sylvester et al., 2016) are stable over time or reflect variation in current functional and behavioral patterns.
Of note, test-retest reliability for the parent version of the C-DISC was reported to be .54 for social phobia diagnosis (Shaffer et al., 2000), but the social phobia symptom counts scale reliability was reportedly higher (.63). Nevertheless, we opted to utilize a clinician-administered measure rather than a self-report checklist to allow further clarification for interviewees. Future investigations should consider other clinician-administered measures with better psychometrics (e.g., K-SADS-PL; Kaufman et al., 1997).
In sum, our findings show that several brain regions implicated in fear circuitry and anxiety circuitry moderate the relation between BI and SAD symptom levels in childhood, setting the foundation for larger-scale studies that incorporate multiple layers of variation over time.
Supplementary Material
Acknowledgments:
The study was supported by a grant from the National Institute of Mental Health (BRAINS R01 MH094633).
Footnotes
No conflicts of interest are related to this work.
References
- Alexander-Bloch A, Raznahan A, Bullmore E, & Giedd J (2013). The Convergence of Maturational Change and Structural Covariance in Human Cortical Networks. Journal of Neuroscience, 33(7), 2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, & Miller LS (2005). Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry, 57(9), 975–981. doi: 10.1016/j.biopsych.2005.01.044 [DOI] [PubMed] [Google Scholar]
- Auday ES, Taber-Thomas BC, & Pérez-Edgar KE (2018). Neural correlates of attention bias to masked facial threat cues: Examining children at-risk for social anxiety disorder. NeuroImage: Clinical, 19, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballespí S, Jané MC, & Riba MD (2012). Parent and Teacher Ratings of Temperamental Disposition to Social Anxiety: The BIS 3–6. Journal of Personality Assessment, 94(2), 164–174. doi: 10.1080/00223891.2011.645929 [DOI] [PubMed] [Google Scholar]
- Baur V, Brühl AB, Herwig U, Eberle T, Rufer M, Delsignore A, … Hänggi J (2013). Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: A quantitative fiber tractography study. Human Brain Mapping, 34(2), 437–446. doi: 10.1002/hbm.21447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hänggi J, & Jäncke L (2012). Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC neuroscience, 13(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, & Pine DS (2009). Anxiety and Anxiety Disorders in Children and Adolescents: Developmental Issues and Implications for DSM-V. Psychiatric Clinics of North America, 32(3), 483–524. doi: 10.1016/j.psc.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G, Spence SH, & McDonald C (2003). Can Parents and Teachers Provide a Reliable and Valid Report of Behavioral Inhibition? Child Development, 74(6), 1899–1917. doi: 10.1046/j.1467-8624.2003.00645.x [DOI] [PubMed] [Google Scholar]
- Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, & VanDerKlok RM (2014). Amygdala–cingulate intrinsic connectivity is associated with degree of social inhibition. Biological psychology, 99, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, & Pine DS (2012). Neural Substrates of Childhood Anxiety Disorders A Review of Neuroimaging Findings. Child and adolescent psychiatric clinics of North America, 21(3), 501–525. doi: 10.1016/j.chc.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeren S, & Muris P (2010). A Psychometric Evaluation of the Behavioral Inhibition Questionnaire in a Non-Clinical Sample of Dutch Children and Adolescents. Child Psychiatry and Human Development, 41(2), 214–229. doi:http://dx.doi.org.ezaccess.libraries.psu.edu/10.1007/s10578-009-0162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews, 47, 260–280. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Hänggi J, Baur V, Rufer M, Delsignore A, Weidt S, … Herwig U (2014). Increased cortical thickness in a frontoparietal network in social anxiety disorder: Increased Cortical Thickness in SAD. Human Brain Mapping, 35(7), 2966–2977. doi: 10.1002/hbm.22378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, & Kiel EJ (2013). Temperamental Risk Factors for Pediatric Anxiety Disorders. In Vasa RA & Roy AK (Eds.), Pediatric Anxiety Disorders (pp. 47–68): Springer New York. [Google Scholar]
- Carré A, Gierski F, Lemogne C, Tran E, Raucher-Chéné D, Béra-Potelle C, … Limosin F (2014). Linear association between social anxiety symptoms and neural activations to angry faces: from subclinical to clinical levels. Social Cognitive and Affective Neuroscience, 9(6), 880–886. doi: 10.1093/scan/nst061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Lee JM, … Lee KH (2008). Multiple Bases of Human Intelligence Revealed by Cortical Thickness and Neural Activation. Journal of Neuroscience, 28(41), 10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA (2009). Stable Early Maternal Report of Behavioral Inhibition Predicts Lifetime Social Anxiety Disorder in Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 928–935. doi: 10.1097/CHI.0b013e3181ae09df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, & Blackford JU (2015). The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Progress in Neurobiology, 127-128, 23–45. doi: 10.1016/j.pneurobio.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Seay AL, VanDerKlok RM, Avery SN, Cao A, Cowan RL, … Blackford JU (2014). Structural and functional bases of inhibited temperament. Social cognitive and affective neuroscience, 9(12), 2049–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E, Angold A, Burns BJ, & et al. (1996). The great smoky mountains study of youth: Goals, design, methods, and the prevalence of dsm-iii-r disorders. Archives of General Psychiatry, 53(12), 1129–1136. doi: 10.1001/archpsyc.1996.01830120067012 [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, & Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage, 53(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, & Liberzon I (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and clinical risk management, 11, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson MW, Klein DN, Olino TM, Dougherty LR, & Durbin CE (2011). Social and Non-Social Behavioral Inhibition in Preschool-Age Children: Differential Associations with Parent-Reports of Temperament and Anxiety. Child Psychiatry & Human Development, 42(4), 390–405. doi: 10.1007/s10578-011-0225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Brauns S, Yendiki A, Ho B-C, Calhoun V, Schulz SC, … Sponheim SR (2012). Associations of Cortical Thickness and Cognition in Patients With Schizophrenia and Healthy Controls. Schizophrenia Bulletin, 38(5), 1050–1062. doi: 10.1093/schbul/sbr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron, 33(3), 341–355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage, 9(2), 195–207. doi: 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, … Crippa JAS (2010). Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuropsychopharmacology & Biological Psychiatry, 34(4), 565–580. [DOI] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC, & Pérez-Edgar K (2017). Frontolimbic functioning during threat-related attention: relations to early behavioral inhibition and anxiety in children. Biological psychology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, ... & Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, … Fischl B (2010). Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. NeuroImage, 53(1), 85–93. doi: 10.1016/j.neuroimage.2010.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, … Rapoport JL (1996). Quantitative Magnetic Resonance Imaging of Human Brain Development: Ages 4–18. Cerebral Cortex, 6(4), 551–559. doi: 10.1093/cercor/6.4.551 [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, & Gross JJ (2009). Neural Bases of Social Anxiety Disorder: Emotional Reactivity and Cognitive Regulation During Social and Physical Threat. Archives of General Psychiatry, 66(2), 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, & Lemery KS (2000). Linking temperamental fearfulness and anxiety symptoms: a behavior–genetic perspective. Biological Psychiatry, 48(12), 1199–1209. doi: 10.1016/S0006-3223(00)01003-9 [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, June Ruan W, Goldstein RB, … Huang B (2005). Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine, null(12), 1747–1759. doi: 10.1017/S0033291705006069 [DOI] [PubMed] [Google Scholar]
- Gross C, & Hen R (2004). The developmental origins of anxiety. Nature Reviews Neuroscience, 5(7), 545–552. doi: 10.1038/nrn1429 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, & Tager-Flusberg H (2007). Abnormal activation of the social brain during face perception in autism. Human Brain Mapping, 28(5), 441–449. doi: 10.1002/hbm.20283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Suh J, Nacewicz B, Sutterer M, Cayo A, Stodola D, ... & Essex M (2012). Robust automated amygdala segmentation via multi-atlas diffeomorphic registration. Frontiers in neuroscience, 6, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). An introduction to mediation, moderation, and conditional process analysis: A regression-based approach New York, NY: Guilford Press. [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, … Pérez-Edgar K (2013). Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological psychiatry, 74(4), 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Fox NA, Pine DS, 2012. Approach–withdrawal and the role of the striatum in the temperament of behavioral inhibition. Dev. Psychol 48 (3), 815–826. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, & Rosenbaum JF (2007). Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. Journal of Developmental & Behavioral Pediatrics, 28(3), 225–233. [DOI] [PubMed] [Google Scholar]
- Hudson JL, Dodd HF, Lyneham HJ, & Bovopoulous N (2011). Temperament and Family Environment in the Development of Anxiety Disorder: Two-Year Follow-up. Journal of the American Academy of Child & Adolescent Psychiatry, 50(12), 1255–1264.e1251. doi: 10.1016/j.jaac.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Irle E (2010). Reduced amygdalar and hippocampal size in adults with generalized social phobia. Journal of Psychiatry and Neuroscience, 35(2), 126–131. doi: 10.1503/jpn.090041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Leibenluft E, Shechner T, Degnan KA, … Ernst M (2014). Enduring Influence of Early Temperament on Neural Mechanisms Mediating Attention–Emotion Conflict in Adults. Depression and Anxiety, 31(1), 53–62. doi: 10.1002/da.22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J (1989). Temperamental contributions to social behavior. American Psychologist, 44(4), 668–674. doi: 10.1037/0003-066X.44.4.668 [DOI] [Google Scholar]
- Kagan J, Snidman N, Zentner M, & Peterson E (1999). Infant temperament and anxious symptoms in school age children. Development and Psychopathology, null(02), 209–224. doi:null [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2007). Role of the Primate Orbitofrontal Cortex in Mediating Anxious Temperament. Biological psychiatry, 62(10), 1134–1139. doi: 10.1016/j.biopsych.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, ... & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen HU (2012). Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research, 21(3), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe S, Sasagawa S, & Creswell C (2015). Developmental epidemiology of social anxiety and social phobia in adolescents. In Social Anxiety and Phobia in Adolescents (pp. 39–70): Springer. [Google Scholar]
- Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, & Kahn RS (2009). Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Human Brain Mapping, 30(11), 3719–3735. doi: 10.1002/hbm.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Douiri A, Kim LG, Modat M, Chan D, Ourselin S, … Fox NC (2010). Atrophy patterns in Alzheimer’s disease and semantic dementia: A comparison of FreeSurfer and manual volumetric measurements. NeuroImage, 49(3), 2264–2274. doi: 10.1016/j.neuroimage.2009.10.056 [DOI] [PubMed] [Google Scholar]
- Lewis-Morrarty E, Degnan KA, Chronis-Tuscano A, Pine DS, Henderson HA, & Fox NA (2015). Infant Attachment Security and Early Childhood Behavioral Inhibition Interact to Predict Adolescent Social Anxiety Symptoms. Child development, 86(2), 598–613. doi: 10.1111/cdev.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Xu Q, Mantini D, Ding J, Machado-de-Sousa JP, Hallak JEC, … Chen H (2011). Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Research, 1388, 167–177. doi: 10.1016/j.brainres.2011.03.018 [DOI] [PubMed] [Google Scholar]
- Liu P, Taber-Thomas BC, Fu X, & Pérez-Edgar K (2018). Biobehavioral markers of attention bias modification in temperamental risk for anxiety: A randomized control trial. Journal of the American Academy of Child and Adolescent Psychiatry, 57, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-de-Sousa JP, Osório F. d. L., Jackowski AP, Bressan RA, Chagas MHN, Torro-Alves N, … Hallak JEC (2014). Increased Amygdalar and Hippocampal Volumes in Young Adults with Social Anxiety. PLoS ONE, 9(2), e88523. doi: 10.1371/journal.pone.0088523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick MA, & Telch MJ (1998). Social anxiety and history of behavioral inhibition in young adults. Journal of anxiety disorders, 12(1), 1–20. [DOI] [PubMed] [Google Scholar]
- Milad MR, & Rauch SL (2007). The Role of the Orbitofrontal Cortex in Anxiety Disorders. Annals of the New York Academy of Sciences, 1121(1), 546–561. doi: 10.1196/annals.1401.006 [DOI] [PubMed] [Google Scholar]
- Morales S, Taber-Thomas BC, & Pérez-Edgar KE (2017). Patterns of attention to threat across tasks in behaviorally inhibited children at risk for anxiety. Developmental science [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Brakel A. M. L. v., Arntz A, & Schouten E (2011). Behavioral Inhibition as a Risk Factor for the Development of Childhood Anxiety Disorders: A Longitudinal Study. Journal of Child and Family Studies, 20(2), 157–170. doi: 10.1007/s10826-010-9365-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA Jr, & Drevets WC (2013). Automated subcortical segmentation using FIRST: Test–retest reliability, interscanner reliability, and comparison to manual segmentation. Human brain mapping, 34(9), 2313–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K, & Gross J (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. doi: 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, & Arfanakis K (2009). Preliminary Evidence of White Matter Abnormality in the Uncinate Fasciculus in Generalized Social Anxiety Disorder. Biological psychiatry, 66(7), 691–694. doi: 10.1016/j.biopsych.2009.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, & Fox NA (2005). Temperament and Anxiety Disorders. Child and Adolescent Psychiatric Clinics of North America, 14(4), 681–706. doi: 10.1016/j.chc.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, ... & Ernst M (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage, 35(4), 1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM (2014). Preschool Environment and Temperament as Predictors of Social and Nonsocial Anxiety Disorders in Middle Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 53(3), 320–328. doi: 10.1016/j.jaac.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wright CI, Martis B, Busa E, McMullin KG, Shin LM, … Fischl B (2004). A magnetic resonance imaging study of cortical thickness in animal phobia. Biological Psychiatry, 55(9), 946–952. doi: 10.1016/j.biopsych.2003.12.022 [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, & Kagan J (1993). Behavioral inhibition in childhood: A risk factor for anxiety disorders. Harvard review of psychiatry, 1(1), 2–16. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, & Evans DE (2000). Temperament and personality: Origins and outcomes. Journal of Personality and Social [DOI] [PubMed] [Google Scholar]
- Roy AK, Benson BE, Degnan KA, Perez-Edgar K, Pine DS, Fox NA, & Ernst M (2014). Alterations in amygdala functional connectivity reflect early temperament. Biological psychology, 103, 248–254. Psychology, 78(1), 122–135. doi: 10.1037//0022-3514.78.1.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Quinn KJ, & Sypher I (2015). NIMH Research Domain Criteria (RDoC). In (Cautin RL & Lilienfeld SO, Eds.) The Encyclopedia of Clinical Psychology Hoboken, NJ: Wiley-Blackwell [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Moran LR, Viner JC, Covino JM, ... & Wallace SR (2010). Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Archives of general psychiatry, 67(1), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, & Kagan J (1999). Adolescent Social Anxiety as an Outcome of Inhibited Temperament in Childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 38(8), 1008–1015. doi: 10.1097/00004583-199908000-00017 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. doi: 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Sladky R, Höflich A, Küblböck M, Kraus C, Baldinger P, Moser E, … Windischberger C (2015). Disrupted Effective Connectivity Between the Amygdala and Orbitofrontal Cortex in Social Anxiety Disorder During Emotion Discrimination Revealed by Dynamic Causal Modeling for fMRI. Cerebral Cortex, 25(4), 895–903. doi: 10.1093/cercor/bht279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, & Paulus MP (2007). Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American journal of psychiatry, 164(2), 318–327. [DOI] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, & Taber KH (2011). Anterior cingulate cortex: unique role in cognition and emotion. The Journal of neuropsychiatry and clinical neurosciences, 23(2), 121–125. [DOI] [PubMed] [Google Scholar]
- Syal S, Hattingh CJ, Fouché J-P, Spottiswoode B, Carey PD, Lochner C, & Stein DJ (2012). Grey matter abnormalities in social anxiety disorder: a pilot study. Metabolic Brain Disease, 27(3), 299–309. doi: 10.1007/s11011-012-9299-5 [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Barch DM, Harms MP, Belden AC, Oakberg TJ, Gold AL, … others. (2016). Early childhood behavioral inhibition predicts cortical thickness in adulthood. Journal of the American Academy of Child & Adolescent Psychiatry, 55(2), 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber-Thomas BC, Morales S, Hillary FG, & Pérez-Edgar KE (2016). Altered topography of intrinsic functional connectivity in childhood risk for social anxiety. Depression and Anxiety doi: 10.1002/da.22508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati A, Pantazatos SP, Schneier FR, Weissman MM, & Hirsch J (2013). Grey Matter Abnormalities in Social Anxiety Disorder: Primary, Replication, and Specificity Studies. Biological psychiatry, 73(1), 75. doi: 10.1016/j.biopsych.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, & Whalen PJ (2015). Neuroimaging and Anxiety: the Neural Substrates of Pathological and Non-pathological Anxiety. Current Psychiatry Reports, 17(6), 1–10. [DOI] [PubMed] [Google Scholar]
- Thai N, Taber-Thomas BC, & Pérez-Edgar KE (2016). Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: An ERP study. Developmental Cognitive Neuroscience, 19, 200–210. doi: 10.1016/j.dcn.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, & Fredrikson M (2001). Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. American Journal of Psychiatry, 158(8), 1220–1226. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, & Menon V (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31(50), 18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol M-J, van der Wee NJA, van den Heuvel OA, Nielen MMA, Demenescu LR, Aleman A, … Veltman DJ (2010). Regional brain volume in depression and anxiety disorders. Archives of general psychiatry, 67(10), 1002–1011. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, & Fjell AM (2011). Associations between Regional Cortical Thickness and Attentional Networks as Measured by the Attention Network Test. Cerebral Cortex, 21(2), 345–356. doi: 10.1093/cercor/bhq101 [DOI] [PubMed] [Google Scholar]
- White LK, Helfinstein SM, & Fox NA (2010). Temperamental factors associated with the acquisition of information processing biases and anxiety. Information processing biases and anxiety: A developmental perspective, 233–252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


