Abstract
Purpose of Review
Excessive sugar and particularly fructose consumption has been proposed to be a key player in the pathogenesis of metabolic syndrome and kidney disease in humans and animal models. However, besides its dietary source, fructose can be endogenously produced in the body from glucose via the activation of the polyol pathway. In this review, we aim to describe the most recent findings and current knowledge on the potential role of endogenous fructose production and metabolism in disease.
Recent Findings
Over the recent years, the activation of the polyol pathway and endogenous fructose production has been observed in multiple tissues including the liver, renal cortex and hypothalamic areas of the brain. The activation occurs during the development and progression of metabolic syndrome and kidney disease and results from different stimuli including osmotic effects, diabetes and ischemia. Even though the potential toxicity of the activation of the polyol pathway can be attributed to several intermediate products, the blockade of endogenous fructose metabolism either by using fructokinase deficient mice or specific inhibitors resulted in marked amelioration of multiple metabolic diseases.
Summary
New findings suggest that fructose can be produced in the body and that the blockade of tis metabolism could be clinically relevant for the prevention and treatment of metabolic syndrome and kidney disease.
Keywords: aldose reductase, polyol pathway, fructokinase, endogenous fructose, metabolic syndrome
Introduction
Fructose, a monomeric sugar with unique metabolic properties
Metabolic syndrome is a common condition characterized by one or several of the following features: obesity, insulin resistance, dyslipidemia, fatty liver and high blood pressure. It is estimated that over one third of the population meet the criteria for having metabolic syndrome and excessive consumption of sugar has been closely linked to the development of multiple of these features in humans and animal models (1–4). Dietary sugar contains both glucose and fructose, either as disaccharides (sucrose) or monosaccharides in solution (high fructose corn syrup, HFCS). Although both glucose and fructose have been implicated in the pathogenesis of metabolic syndrome, fructose seems to be particularly relevant due to its unique metabolism. While glucose has been postulated to increase the risk for metabolic syndrome through the actions of hyperinsulinemia to drive glycogen and fat storage; fructose is thought to work primarily by inducing insulin resistance and triggering lipogenesis and blocking fat oxidation(5–7). This is now recognized to be linked with the unique and rapid metabolism via the enzyme fructokinase -also known as ketohexokinase or khk- in the gut and liver that acutely reduces intracellular ATP levels and increasing nucleotide turnover into uric acid(8). In this regard, liquid fructose but not glucose elevate plasma uric acid levels in overweight subjects.
Fructose-dependent uric acid accumulation is considered to be a major mechanism whereby this sugar induces its metabolic effects. Excessive production of uric acid in the liver and other organs has been shown to induce mitochondrial dysfunction and inactivation of the enzyme AMP-activated protein kinase (AMPK) (9) leading to reduced fat oxidation, increased lipogenesis and de novo gluconeogenesis which underlie the development and progression of fatty liver, dyslipidemia and insulin resistance. The metabolic effects of fructose on nucleotide turnover and uric acid generation have been documented not only in animal models but also in humans(10).
Of interest, even though glucose metabolism itself does not cause an immediate significant nucleotide turn-over or uric acid generation, recent evidence suggest that some of this sugar is directly converted to fructose, and that the metabolism of this endogenously produced fructose could be an important deleterious step in the pathogenesis of metabolic syndrome.
The polyol pathway and the endogenous production of fructose
Fructose and glucose are structural isomers. However, the natural conversion of each other does not naturally occur and thus require complex enzymatic reactions. For example, it is well documented that in the liver, and most recently in the gut, fructose metabolism produces glucose by providing direct gluconeogenic substrates (dihydroxyacetonephosphate and glyceraldehyde) as well as lactate (11). In contrast, fructose can be enzymatically produced through three different enzymatic routes. Fructose is obtained by isomerization of either glucose, mannose or xylose as well as by dehydrogenation of sorbitol. Glucose isomerization through glucose isomerase is part of the processing of HFCS. Of interest, while isomerization of glucose, mannose and xylose are important substrates of fructose production in plants, as a means to produce either sucrose (in fruits) and inulin (fructose polymers in roots and stalks), these metabolic routes are not documented in most mammals. Therefore, the only mechanism for endogenously producing fructose known to date in humans is from sorbitol as part of the polyol pathway.
The polyol pathway is a metabolic route constituted by two enzymes, aldose reductase that converts glucose into sorbitol and sorbitol dehydrogenase which further metabolizes it to fructose (figure 1). Of interest, while sorbitol dehydrogenase is ubiquitously expressed in the majority of tissues and organs, constitutive aldose reductase expression is limited to hypertonic areas of the inner medulla and papilla of the kidney. Sorbitol dehydrogenase is nearly absent in the inner medullar and papilla of the kidney and thus, the high levels of aldose reductase in this part of the kidney results in sorbitol accumulation which is an important osmolyte for a proper urinary concentrating mechanism. Based on this, it is thought that under normal conditions, the polyol pathway is mostly inactive in the majority of tissues and organs. This is supported by the observation that circulating fructose levels both fasting and post-prandial are markedly lower than glucose levels (12).
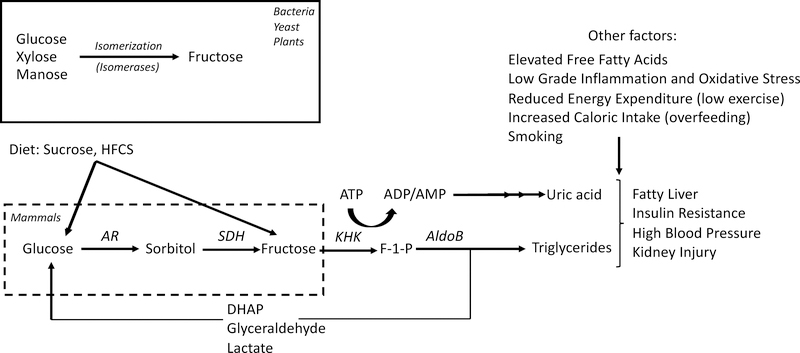
Figure 1:
Schematic of endogenous fructose production and metabolism in mammals. While bacteria, plants and yeast can produce fructose by isomerization of other polyols. In humans and most mammals, fructose can only be endogenously produced from glucose and sorbitol by the polyol pathway. While fructose can also be converted back into glucose, further metabolism of fructose stimulates triglyceride and uric acid accumulation which in turn contribute to the pathogenesis of features of metabolic syndrome and kidney disease along with other important factors including elevated free fatty acids, inflammation, oxidative stress, low energy expenditure, overfeeding or smoking among others. Abbreviations: AR: aldose reductase, SDH sorbitol dehydrogenase, KHK: fructokinase, AldoB: Aldolase B, DHAP: Dihydroacetonephosphate.
However, newly evidence now suggest that this pathway can be activated in vivo. Francey et al recently were able to determine in healthy individuals ingesting a glucose/fructose solution, rates of endogenous fructose appearance in plasma (12). Also, and consistent with the presence of an active polyol pathway, fructokinase expression is not only limited to tissues involved in metabolizing dietary fructose as it has been reported to be present in brain, pancreas and heart among other tissues (13). In this regard, Hwang et al, demonstrated the activation of the polyol pathway in the human brain with concomitant production of fructose from glucose (14)and Oppelt et al. showed that specific regions of the brain are capable of fructose metabolism (15). Consistently, and as shown by Song et al, the activation of the polyol pathway in the hypothalamus leads to endogenous fructose generation and metabolism which stimulate the production and release of the antidiuretic hormone, vasopressin (13).
To date, three major mechanisms have been shown to stimulate aldose reductase expression, and the polyol pathway: hypertonic stress, hypoxia and hyperglycemia. Of these, high tonicity is probably the most established stimulant of aldose reductase expression (16). Osmotic effects on aldose reductase expression have been shown in multiple tissues besides the papilla of the kidney and include the nucleus pulposus, lens and retina. More recently, our group has shown in mice that slightly hypertonic solutions markedly raise the tonicity of the portal vein and the systemic circulation and activate aldose reductase and the polyol pathway in the liver and brain during the development of features of metabolic syndrome including fatty liver, insulin resistance, obesity and high blood pressure (17). The effects of ischemia in activating the polyol pathway expression are also well-documented. Expression of aldose reductase is markedly repressed by vasodilators like nitric oxide (18).
The regulation of aldose reductase expression is mediated at the transcriptional level. Nuclear Factor of activated T cells 5 (NFAT5, also known as tonicity enhancer binding protein or TonEBP) activates the transcription of aldose reductase upon stimuli. Of interest, expression and activity of NFAT5 has been reported in multiple organs and tissues even at isoosmolar conditions (19–21) further supporting the concept that the polyol pathway can be potentially activated anywhere in the body. It has been shown that oxidative stress is the main driver in the activation of aldose reductase in cells and, in these settings, aldose reductase would act to detoxify lipid peroxides produced under oxidative conditions. In this regard, the role of uric acid in the activation of the polyol pathway has gained much interest recently. Uric acid is produced from ATP depletion as a by-product of fructose metabolism and even though it has important anti-oxidant properties, we and others have shown that intracellularly, uric acid can act as a prooxidant at high concentrations by stimulating NADPH-dependent superoxide production in the cytosol and mitochondria (9). Huang et al first reported that uric acid could stimulate aldose reductase expression in endothelial cells and that its blockade in hyperuricemic mice protected against endothelial cell dysfunction (22). Our group has recently proved a critical role for hepatic uric acid in activating NFAT5 and the polyol pathway thus producing endogenous fructose during the pathogenesis of fatty liver (21). Interestingly, uric acid also stimulates the expression of fructokinase in mice and human hepatocytes thus exacerbating the metabolism of endogenous and dietary fructose (23).
Endogenous fructose metabolism and its role in disease
The toxicity of the polyol pathway can be attributed to multiple factors. On one hand, an exacerbated flux through the polyol pathway results in the expenditure of cofactors for aldose reductase (NADPH) and sorbitol dehydrogenase (NAD+) leading to a redox state change and metabolic imbalances. Also, increased flux causes a significant decrease in the activity of glutathione reductase secondary to NADPH depletion. Sorbitol accumulation has been proposed to be an important mediator in cataracts and while important in the inner medulla of the kidney and the papilla, it can cause hyperosmotic swelling elsewhere deranging the cell membrane, and causing leakage of amino acids and glutathione.
High Glycemic Diets and Endogenous Fructose
To determine the relative importance of endogenous fructose production and metabolism in metabolic syndrome, our group first analyzed the response of fructokinase knockout mice to products with a high glycemic index. By feeding mice high glucose/no fructose-containing solutions, we identified the activation of the polyol pathway in the liver of mice. This activation was characterized by the up-regulation of aldose reductase, the production of sorbitol and the accumulation and metabolism of endogenous fructose. Of interest, not only aldose reductase knockout mice showed a marked protection against high-glucose induced metabolic syndrome but also similar protection was observed in fructokinase-deficient animals. Fructokinase knockout mice fed glucose demonstrated significantly reduced fatty liver, hyperinsulinemia, hyperleptinemia and body weight gain compared to wild type counterparts (24). This observation would suggest that the polyol pathway is involved in the pathogenesis of metabolic syndrome by producing and metabolizing endogenous fructose. The initial work developed with high glucose solution was then expanded to other organs and conditions. In diabetes for example, hyperglycemia has been shown to induce the expression of aldose reductase in multiple organs and tissues including the lens, retina, peripheral nerves and the renal mesangium. It has been reported that while under normal conditions a minimal amount of glucose enter into the polyol pathway, in diabetes approximately 30 % of glucose flux into this metabolic route (25). Of interest, in diabetic tissues, the blockade of aldose reductase significantly ameliorated inflammation and oxidative stress suggestive of an important role of the polyol pathway in the pathogenesis of diabetic complications.
High Salt Diets, Blood Pressure and Metabolic Syndrome
High salt diets are strongly linked with hypertension in epidemiological studies, and there is strong support that modest salt restriction is important in the control of blood pressure and reduction of cardiovascular events, especially in older individuals and African Americans with hypertension. While the association with blood pressure is well known, more recently it has been reported that high salt diets may also predict the development of obesity, metabolic syndrome and diabetes (17, 26). We recognized that a high salt diet would transiently increase osmolality, especially in the portal vein and liver, and that this could be a mechanism for inducing AR and the polyol pathway. We therefore performed studies in which mice were administered high salt diets, especially by giving drinking water that was slightly hypertonic (17). This resulted in the induction of AR and the production of fructose in the liver. While initially animals appeared mildy catabolic, over time they developed marked leptin resistance with the development of many features of metabolic syndrome including obesity, fatty liver and insulin resistance. Mice lacking fructokinase were completely protected despite drinking similar amounts of salt. Furthermore, we found that western diet with added salt also showed increased metabolic features compared to western diet alone. Finally, we documented that a high salt diet was epidemiologically linked with increased risk for fatty liver and diabetes in a cohort of healthy Japanese adults. Thus, these studies suggest that a high salt diet could contribute risk for obesity through a mechanism that involves endogenous fructose production.
Diabetic Nephropathy and Chronic Kidney Disease
Recently, our group demonstrated that in mice, and during the progression of diabetic nephropathy, the proximal tubule of the kidney activates the polyol pathway possibly secondary to both high glucose and hyperosmolar conditions resulting in endogenous fructose production and metabolism (27). Similar degree of activation was observed in wild type and fructokinase deficient mice. However, the blockade of endogenous fructose metabolism in the kidney cortex of fructokinase knockout mice resulted in substantial improvement in both diabetes-associated renal injury and dysfunction. Furthermore, a key role for endogenous fructose production and metabolism in renal proximal tubular cells has been proposed as a potential mechanism in the pathogenesis of Mesoamerican nephropathy (28), a idiopathic type of chronic kidney disease associated with exposure to severe heat and recurrent dehydrating conditions. It has been hypothesized that under hot and dehydrating conditions, increased plasma osmolality induce the activation of aldose reductase in the proximal tubule of the kidney which is one of the major sites where sorbitol dehydrogenase and fructokinase are expressed, and therefore fructose is produced endogenously from glucose and metabolized thus causing the inflammation and tubular injury that characterize this condition.
Acute Kidney Injury
Besides chronic kidney disease, the activation of the polyol pathway and endogenous fructose production has also been observed in models of acute kidney injury. In this regard, increased urinary fructose has been observed in pediatric patients undergoing ischemic acute kidney injury following cardiac by-pass and the blockade of fructokinase either by using fructokinase knockout mice or specific inhibitors was shown to prevent and even be relevant to treat kidney injury after an ischemic insult (8). Furthermore, blockade of fructokinase has also been reported to be beneficial in another model of acute kidney injury, contrast-induced nephropathy, a particular case of renal damage associated with the three main stimulants of aldose reductase expression, hypertonicity (radiocontrast agents are hyperosmolar), ischemia and diabetes (8).
Hereditary Fructose Intolerance: A State of Fructose Sensitivity
A critical role for endogenous fructose metabolism has also been proposed in hereditary fructose intolerance (HFI). HFI is an orphan genetic disease characterized for the deficiency of aldolase B. People with HFI are asymptomatic. However, after fructose ingestion, the lack of aldolase B results in the accumulation of fructose-1-phosphate and the depletion of ATP and phosphate pools leading to cell death mainly in gut and liver. Consistent with marked depletion of ATP pools following fructose exposure, hyperuricemia is commonly found in subjects with HFI. Of interest, high levels of uric acid following a fructose challenge have also been recently reported in heterozygous carriers (29) suggesting that a reduced expression of aldolase b could be associated with metabolic changes despite not presenting the characteristic clinical manifestations of HFI. The most important clinical sign following fructose intake in HFI, is acute and severe hypoglycemia (30). Even though the blockade of fructokinase has been shown to be effective, no pharmacological treatment has been clinically tested or approved to date for HFI and the only recommended approach is to avoid the intake of sugar. However, even with little or no ingestion of fructose, individuals with HFI often develop episodes of hypoglycemia, show signs of general ill health and exhibit symptoms of chronic fructose intoxication. One possibility that could explain these episodes relates to the fact that fructose is heavily substituted in the diet by foods with high glycemic index like dextrose. Excessive glucose consumption in aldolase b deficient subjects could lead to the activation of the polyol pathway and endogenous production of fructose.
Conclusion
In summary, over the last 5 years, and as depicted in Figure 1, mounting evidence support the relevance for endogenous fructose production and metabolism in the pathogenesis of metabolic syndrome and associated conditions including kidney disease. Although the majority of evidence has been obtained from animal models, the presence of an active polyol pathway has also been described in humans. However its relative importance in human metabolic disease still remains to be elucidated and warrants further studies.
Key points.
Endogenous fructose production is occurring in multiple tissues in the pathogenesis of metabolic syndrome and renal disease.
Endogenous fructose production is mediated by the up-regulation of aldose reductase and the activation of the polyol pathway
Blockade of endogenous fructose metabolism could be clinically relevant for the prevention and treatment of metabolic syndrome and kidney disease.
Acknowledgments
Financial Support and sponsorship: This work was supported by grants from the National Institute of Health (NIH) R01 DK109408-01A1
Footnotes
Conflicts of Interest: The authors disclose no conflicts of interest related to the manuscript. MAL and RJJ are members of Colorado Research Partners, LLC that is developing inhibitors of fructose metabolism for the treatment of metabolic syndrome and kidney disease.
References
- 1.Mortera RR, Bains Y, Gugliucci A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front Biosci (Landmark Ed). 2019;24:186–211. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RJ, Perez-Pozo SE, Lillo JL, Grases F, Schold JD, Kuwabara M, et al. Fructose increases risk for kidney stones: potential role in metabolic syndrome and heat stress. BMC Nephrol. 2018;19(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan W, Smith B, Stegall M, Borrows R. Obesity and Metabolic Syndrome in Kidney Transplantation: The Role of Dietary Fructose and Systemic Endotoxemia. Transplantation. 2019;103(1):191–201. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Jiang ZH, Li CG, Zhu YJ, Li Z, Tang YZ, et al. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed Pharmacother. 2018;105:1283–90. [DOI] [PubMed] [Google Scholar]
- 5.Nair J, Velpandian T, Das US, Sharma P, Nag T, Mathur SR, et al. Molecular and Metabolic Markers of Fructose Induced Hepatic Insulin Resistance in Developing and Adult Rats are Distinct and Aegle marmelos is an Effective Modulator. Sci Rep. 2018;8(1):15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu WC, Wu CW, Tain YL, Fu MH, Hung CY, Chen IC, et al. Oral pioglitazone ameliorates fructose-induced peripheral insulin resistance and hippocampal gliosis but not restores inhibited hippocampal adult neurogenesis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):274–85. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez-Lara EJ, Navarrete-Vazquez G, Sanchez-Lopez A, Centurion D. Pharmacological evaluation of metformin and N-benzylbiguanide, a novel analogue of metformin, on the vasopressor responses to adrenergic system stimulation in pithed rats with fructose-induced insulin resistance. Eur J Pharmacol. 2017;814:313–23. [DOI] [PubMed] [Google Scholar]
- 8.Andres-Hernando A, Li N, Cicerchi C, Inaba S, Chen W, Roncal-Jimenez C, et al. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat Commun. 2017;8:14181.*This paper demonstrate the importance of endogenous fructose production and metabolism in acute kidney injury.
- 9.Maarman GJ, Andrew BM, Blackhurst DM, Ojuka EO. Melatonin protects against uric acid-induced mitochondrial dysfunction, oxidative stress, and triglyceride accumulation in C2C12 myotubes. J Appl Physiol (1985). 2017;122(4):1003–10. [DOI] [PubMed] [Google Scholar]
- 10.Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, et al. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A (31)P MRS study. Clin Nutr. 2016;35(3):645–9. [DOI] [PubMed] [Google Scholar]
- 11.Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018;27(2):351–61 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francey C, Cros J, Rosset R, Creze C, Rey V, Stefanoni N, et al. The extra-splanchnic fructose escape after ingestion of a fructose-glucose drink: An exploratory study in healthy humans using a dual fructose isotope method. Clin Nutr ESPEN. 2019;29:125–32.** This is the first paper to evidence the presence of endogenous fructose in the circulation in humans.
- 13.Song Z, Roncal-Jimenez CA, Lanaspa-Garcia MA, Oppelt SA, Kuwabara M, Jensen T, et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J Neurophysiol. 2017;117(2):646–54.* This study described for the first time a role for endogenous fructose metabolism in the brain. Endogenous fructose metabolism was found to be important for vasopressin production and release.
- 14.Hwang JJ, Jiang L, Hamza M, Dai F, Belfort-DeAguiar R, Cline G, et al. The human brain produces fructose from glucose. JCI Insight. 2017;2(4):e90508.** This paper shows for the first time the activation of the polyol pathway and endogenous fructose production in the human brain.
- 15.Oppelt SA, Zhang W, Tolan DR. Specific regions of the brain are capable of fructose metabolism. Brain Res. 2017;1657:312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata T, Minucci S, McGowan M, Carper D. Gene regulation of aldose reductase under osmotic stress. Adv Exp Med Biol. 1997;414:507–14. [DOI] [PubMed] [Google Scholar]
- 17.Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A. 2018;115(12):3138–43.*This paper described f the importance of endogenbous fructose production and metabolism in liver and brain in the pathogenesis of emtabolic syndrome induced by salt.
- 18.Chandra D, Jackson EB, Ramana KV, Kelley R, Srivastava SK, Bhatnagar A. Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes. 2002;51(10):3095–101. [DOI] [PubMed] [Google Scholar]
- 19.Yang XL, Wang X, Peng BW. NFAT5 Has a Job in the Brain. Dev Neurosci. 2018;40(4):289–300. [DOI] [PubMed] [Google Scholar]
- 20.Arnold C, Feldner A, Zappe M, Komljenovic D, De La Torre C, Ruzicka P, et al. Genetic ablation of NFAT5/TonEBP in smooth muscle cells impairs flow- and pressure-induced arterial remodeling in mice. FASEB J. 2018:fj201801594R. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Lozada LG, Andres-Hernando A, Garcia-Arroyo FE, Cicerchi C, Li N, Kuwabara M, et al. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J Biol Chem. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z, Hong Q, Zhang X, Xiao W, Wang L, Cui S, et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun Signal. 2017;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7(10):e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan LJ. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model Exp Med. 2018;1(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwabara M, Hisatome I, Roncal-Jimenez CA, Niwa K, Andres-Hernando A, Jensen T, et al. Increased Serum Sodium and Serum Osmolarity Are Independent Risk Factors for Developing Chronic Kidney Disease; 5 Year Cohort Study. PLoS One. 2017;12(1):e0169137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol. 2014;25(11):2526–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71(6):851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debray FG, Damjanovic K, Rosset R, Mittaz-Crettol L, Roux C, Braissant O, et al. Are heterozygous carriers for hereditary fructose intolerance predisposed to metabolic disturbances when exposed to fructose? Am J Clin Nutr. 2018;108(2):292–9. [DOI] [PubMed] [Google Scholar]
- 30.Lanaspa MA, Andres-Hernando A, Orlicky DJ, Cicerchi C, Jang C, Li N, et al. Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J Clin Invest. 2018;128(6):2226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]