Abstract
Background
The efficacy of intraoperative corticosteroids to improve outcomes following congenital cardiac operations remains controversial.
Objective
To determine whether intraoperative methylprednisolone improves postoperative recovery in neonates undergoing cardiac surgery.
Methods
Neonates undergoing cardiac surgery with cardiopulmonary bypass at 2 centers were enrolled in a double-blind randomized controlled trial of methylprednisolone (30 mg/kg) or placebo after the induction of anesthesia. The primary outcome was a previously validated morbidity-mortality composite that included any of the following events following surgery before discharge: death, mechanical circulatory support, cardiac arrest, hepatic injury, renal injury, or rising lactate level (>5 mmol/L).
Results
Of the 190 subjects enrolled, 176 (n=81 methylprednisolone, n=95 placebo) were included in this analysis. A total of 27 (33%) subjects in the methylprednisolone group and 40 (42%) in the placebo group reached the primary study endpoint (OR=0.63; 95% CI, 0.31–1.3; p=0.21). Methylprednisolone was associated with reductions in vasoactive inotropic requirements and in the incidence of the composite endpoint in subjects undergoing palliative operations (OR=0.38; 95% CI 0.15–0.99; p=0.048). There was a significant interaction between treatment effect and center. In this analysis, methylprednisolone was protective at 1 center with an OR=0.35 (95% CI, 0.15–0.84; p=0.02) and not so at the other center with OR=5.13 (95% CI, 0.85–30.9; p=0.07).
Conclusions
Intraoperative methylprednisolone failed to show an overall significant benefit on the incidence of the composite primary study endpoint. There was, however, a benefit in patients undergoing palliative procedures and a significant interaction between treatment effect and center suggesting there may be center or patient characteristics which make prophylactic methylprednisolone beneficial.
Keywords: congenital heart disease, cardiopulmonary bypass, pediatric
Condensed Abstract
The efficacy of perioperative corticosteroids to improve outcomes following congenital cardiac surgery has been debated for 5 decades. This study enrolled neonates undergoing cardiac surgery with cardiopulmonary bypass at 2 centers in a double-blind randomized controlled trial of methylprednisolone or placebo to determine whether intraoperative methylprednisolone improves postoperative recovery. Intraoperative methylprednisolone failed to show an overall benefit on the incidence of the composite primary study endpoint. There was, however, a benefit in patients undergoing palliative procedures and a significant interaction between treatment effect and center suggesting there may be center or patient characteristics which make prophylactic methylprednisolone beneficial.
Introduction
Due to the pioneering work of Drs. Gibbon, Lillehei, and Kirklin open heart surgery became a reality in the 1950s and revolutionized lifesaving treatment options for those with congenital heart disease. Although cardiopulmonary bypass (CPB) is a necessary component of most congenital cardiac operations, it is not without consequences. CPB is a complex pathophysiologic environment in which exposure to non-physiologic surfaces in the circuit, hemolysis, and systemic and myocardial ischemia/reperfusion combine to initiate complex cascades that culminate in a systemic inflammatory response (1–3). This inflammatory response is believed to contribute to postoperative morbidity including cardiovascular, pulmonary, renal, hepatic, and neurologic dysfunction (1–4). As a result, the empiric use of steroids as prophylactic anti-inflammatory therapy dates back to the 1960s and has become common practice in pediatric cardiac surgery (5–8).
In addition to their anti-inflammatory properties steroids may also be given to supplement a relative adrenal insufficiency. However a lack of accepted normal ranges for steroid levels, variations in the dosing and interpretation of the corticotropin stimulation test, and the use of concomitant exogenous steroids has resulted in the basic lack of understanding of the typical response of the neonatal hypothalamic-pituitary-adrenal axis to CPB (9). As such, there is currently no blood test or assay that can identify patients who might benefit from steroid supplementation.
Over the last 2 decades, several small single center pediatric randomized controlled trials of perioperative steroids demonstrated a reduction in inflammatory markers albeit with variable improvement in clinical outcomes (10–12). More recently, the literature has given way to slightly larger trials, meta-analysis, and large observational studies that suggest no benefit with prophylactic steroids and possibly an association with increased morbidity particularly in lower surgical risk groups (8,13–15). Given these conflicting results, the routine use of intraoperative steroids remains controversial.
The aim of this study was to assess whether a single intraoperative dose of methylprednisolone benefited neonates undergoing cardiac surgery with cardiopulmonary bypass.
Methods
Study Design
The Corticosteroid Therapy in Neonates Undergoing Cardiopulmonary Bypass trial is a doubleblind, randomized, placebo-controlled trial of intraoperative methylprednisolone in neonates undergoing cardiac surgery with cardiopulmonary bypass. Infants ≤ 1 month (31 days) of age at two North American centers scheduled to undergo cardiac surgery with CPB were eligible for this study. Exclusion criteria included prematurity (defined as <37 weeks post gestational age) at the time of surgery, treatment with steroids in the 2 days prior to surgery, participation in research studies involving the evaluation of investigational drugs or vaccines within 30 days of randomization, suspected infection that would contraindicate steroid use (e.g. herpes), known hypersensitivity to methylprednisolone or other contraindication to steroid therapy (e.g. gastrointestinal bleeding), preoperative use of mechanical circulatory support or active resuscitation at the time of proposed randomization. The study was approved by the institutional review board at each institution, and written informed consent was obtained from parents or legal guardians. All authors vouch for the accuracy of the data and the fidelity of the study to the protocol, which is available with the full text of this article in the Online Appendix.
Randomization And Masking
Study participants were randomly assigned in the ratio of 1:1 to either receive intravenous methylprednisolone (30 mg/kg of body weight) after the induction of anesthesia or placebo. Patients were assigned by permuted block randomization within strata according to the planned procedure being palliative or corrective and by the surgeon. Study drug was prepared and masked by the local investigational pharmacy and delivered to the operating room. The study drug was infused intravenously after the induction of anesthesia prior to skin incision. All patients, caregivers, health-care providers, and investigation personnel were blinded to the treatment allocation until the close of the study. In all other respects, subjects were managed according to the usual practices at each center. At site 1, general perfusion strategies included full-flow bypass at 2.6 l/min/m2 at 32°C, or low flow bypass at 1.3 l/min/m2 between 20–25°C. Regional antegrade cerebral perfusion was utilized during aortic arch reconstructions, generally carried out at a temperature of 20°C and a flow of 50 mL/kg/min, with monitoring of cerebral near-infrared spectroscopy (NIRS) with a target goal of > 90%. Cold-blood cardioplegia was given at 20 minute intervals during periods of aortic cross-clamping. Deep hypothermic circulatory arrest was performed at 20°C, when necessary. Acid-base management was by a pH stat strategy with a hematocrit goal of 28% while on CPB. Conventional and modified ultrafiltration were used in all cases. At site 2, full-flow bypass was considered 200 mL/kg/min at 36°C and flow is decreased as patient temperature decreases to meet mean arterial pressure goals of 30–35 mmHg. Generally this resulted in a flow of 80–100 mL/kg/min at 18°C. Regional antegrade cerebral perfusion was utilized during arch reconstructions either at 18°C at a flow of 30 mL/kg/min with monitoring of cerebral NIRS with a target goal of >90%; or at 25°C at a flow of 60–80 mL/kg/min with monitoring of cerebral NIRS, but without a specific target depending on the surgeon. Circulatory arrest was performed at moderate hypothermia (around 25°C), typically for very brief periods, when needed. Cold-blood cardioplegia was given at 45–90 minute intervals during periods of aortic cross-clamping. Acid-base management was by a pH-stat strategy with a hematocrit goal of 30% while on CPB at 20°C or lower. Alpha-stat management was used for periods of warming. Conventional ultrafiltration was used in all cases. Modified ultrafiltration was used in the vast majority of cases.
Outcomes
The primary study outcome was a composite of death and objective signs of inadequate cardiac output that has been validated in neonates undergoing cardiac surgery with CPB (16). The composite morbidity-mortality outcome was met if any of the following occurred after surgery but before hospital discharge: death, cardiac arrest, extracorporeal membrane oxygenation (ECMO), renal injury (defined as creatinine more than two times normal), hepatic injury (defined as aspartate aminotransferase or alanine aminotransferase more than two times normal > 36 hrs post-op), or rising lactate level (>5 mmol/L) (16).
Secondary outcomes include the incidence of the composite morbidity-mortality outcome at 30 days post cardiac surgery regardless of hospital discharge status, the number of postoperative days of mechanical ventilation, the length of stay in the cardiac intensive care unit (ICU), the length of stay in the hospital, and the number of days alive and out of the hospital at 90 days. Additional outcomes of cardiac dysfunction/inadequate cardiac output included serum lactate levels, the occurrence of low cardiac output syndrome (LCOS) and the vasoactive inotropic score (VIS) in the first 36 hours postoperatively (17–19). LCOS was determined to be present if there were clinical signs and symptoms (e.g., tachycardia, oliguria, cold extremities) which required one or more of the following interventions during the initial 36 hours post admission from the operating room: mechanical circulatory support, escalation of existing pharmacological circulatory support to ≥100% over baseline, or initiation of new pharmacological circulatory support (17). VIS was recorded upon arrival to the ICU, 4, 8, 12, 24, and 36 hours postoperatively. The highest VIS and serum lactate values as well as the treatment with open label steroids and reason for administration during this 36 hour timeframe were recorded. Secondary outcomes of renal function were urine output and total fluid balance over the first 36 hours postoperatively and the incidence of acute kidney injury, defined by the Acute Kidney Injury Network’s criteria (20). Safety outcomes included the number of subjects with poor wound healing that required surgical exploration and number of health care-associated infections (ventilator-associated pneumonia, catheter associated blood stream infection, catheter associated urinary track infection, and surgical site infections, as defined by the Centers for Disease Control and Prevention). All infections were adjudicated by local infection-control clinicians who were unaware of the study-group assignments. The highest blood glucose level and treatment with insulin within the first 36 hours postoperatively were also recorded.
Statistical Analysis
We hypothesized that methylprednisolone administration would reduce the incidence of the primary outcome. Based on prior studies, the incidence of the primary outcome was estimated to occur in 33% (16). To detect an absolute difference of 20% between treatment groups with a power of 80% at a 2-sided 0.05 significance level, allowing for 1 interim analysis, 190 subjects were required for enrollment.
This study began as a single center study. After 1 year of being active, a second site was added to improve the generalizability of the results and ensure the timely completion of enrollment. The original statistical plan included analysis of the primary outcome variable using generalized linear model adjusting for planned corrective or palliative operation and by surgeon (as there was only 1 center in the original protocol) on a modified intention to treat analysis. The modification of the intention to treat was determined, a priori, that subjects whose surgery plan changed and did not receive CPB were not included in the analysis. With the addition of a second site the final statistical plan was revised to adjust for site rather than surgeon and an unanticipated interaction effect between treatment and site was found. The surgeons within centers had similar outcomes but the distribution of outcomes was very different between centers. Therefore additional primary and secondary outcomes analyses by center were performed. Patient follow-up was until 1 year from initial cardiac surgery by study protocol. Herein, we report the primary study endpoint together with secondary outcomes through initial hospital discharge or 90 days after cardiac surgery, whichever occurred later. Additional analyses were performed on a per-protocol basis (no open label steroids given in the postoperative period). Preplanned subgroup analyses for the primary outcome included palliative vs corrective procedures, age ≤ 7 days vs > 7days, and body weight ≤ 2.5 kg vs >2.5kg.
Descriptive statistics were used to characterize the two treatment groups with respect to demographics, baseline clinical, and operating room characteristics. Where model assumptions of normality were valid, appropriate statistical models, such as logistic regression models, student T-tests, and chi-square tests were used to compare the two treatment groups. Where the distributional assumptions did not hold, non-parametric analogs to the parametric tests were used, as appropriate, to compare the groups.
Blinded data were presented to an independent data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute at 6-month intervals throughout the study. A single preplanned interim analysis on the primary study endpoint was performed at 50% enrollment to consider early termination of the study for safety, efficacy, or futility. To preserve an experiment-wise 2-sided alpha level of 0.05, the threshold for significance of the primary endpoint in the final analysis was 0.049 (21).
Results
Study Participants
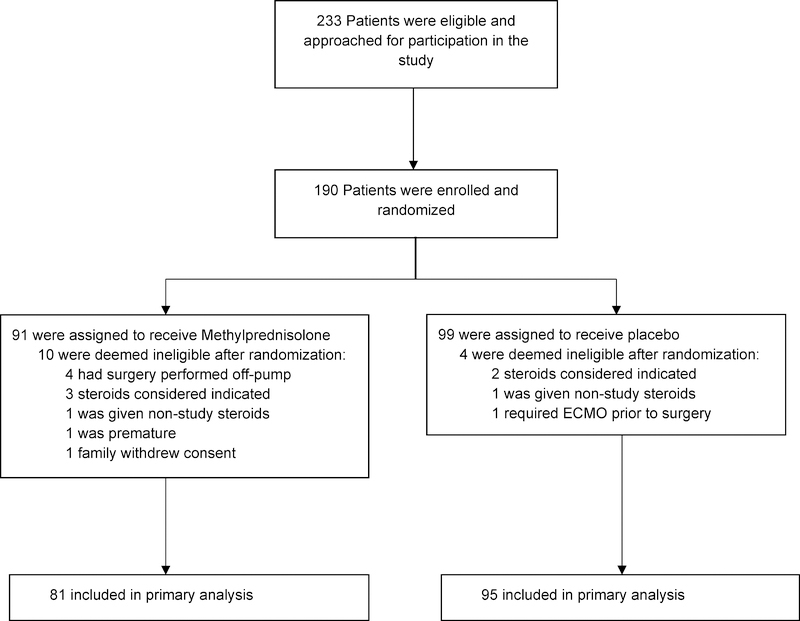
Enrollment began in June 2012 and ended in November 2017. A total of 190 neonates were randomized; 91 were assigned to methylprednisolone and 99 to placebo. Fourteen (10 methylprednisolone, 4 placebo) were withdrawn before cardiac surgery and were not included in the analyses (Figure 1). Complete data during initial hospitalization and through 90 days following cardiac surgery were available on all 176 subjects in the intention-to-treat analysis.
Figure 1: Enrollment Flowchart.
The trial enrolled and randomized 190 neonates; 91 were assigned to methylprednisolone and 99 to placebo. Fourteen (10 methylprednisolone, 4 placebo) were withdrawn before cardiac surgery and not included in analyses. Abbreviations: ECMO, extracorporeal membrane oxygenation.
The treatment groups were similar with regard to demographic variables, cardiac diagnoses, surgical complexity and intraoperative techniques (Table 1). Mean age at the time of surgery was 8.6 days, weight was 3.3 kg, and there was a slight male predominance of 60%.
Table 1.
Demographic, Clinical, and Surgical Characteristics of the Methylprednisolone and Placebo Groups.
| Characteristic | Methylprednisolone (N=81) | Placebo (N=95) |
|---|---|---|
| Demographics | ||
| Gestational age at birth, weeks | 38.6 (1.2) | 39.0 (1.1) |
| Female sex | 35 (43%) | 35 (36%) |
| Race | ||
| White | 55 (67%) | 60 (63%) |
| Black | 22 (27%) | 27 (28%) |
| Asian | 0 (0%) | 2 (2%) |
| American Indian or Alaska Native | 0 (0%) | 1 (1%) |
| Other | 4 (4%) | 5 (5%) |
| Ethnicity Hispanic | 4 (4%) | 8 (8%) |
| Procedure and Diagnoses - no. (%) | ||
| Corrective | 50 (62%) | 60 (63%) |
| Transposition of the great arteries | 20 (25%) | 24 (25%) |
| Aortic arch hypoplasia with ventricular septal defect | 10 (12%) | 18 (19%) |
| Other | 13 (16%) | 13 (14%) |
| Truncus arteriosus | 6 (8%) | 5 (5%) |
| Tetralogy of Fallot | 1 (1%) | 0 (0%) |
| Palliative | 31 (38%) | 35 (37%) |
| Hypoplastic left heart syndrome | 18 (22%) | 18 (19%) |
| Other single ventricle lesions | 7 (9%) | 11 (12%) |
| Tetralogy of Fallot with pulmonary atresia | 4 (5%) | 2 (2%) |
| Other | 2 (3%) | 4 (4%) |
| Intraoperative Characteristics | ||
| Age at surgery, d | 9.1 (5.4) | 8.2 (5.6) |
| Weight at surgery, kg | 3.2 (0.5) | 3.4 (0.5) |
| STAT mortality risk category | ||
| 1 | 1 (1%) | 0 (0%) |
| 2 | 2 (2%) | 2 (2%) |
| 3 | 16 (19%) | 17 (17%) |
| 4 | 40 (49%) | 49 (51%) |
| 5 | 22 (27%) | 27 (28%) |
| Cardiopulmonary bypass duration, min | 185 (64) | 176 (64) |
| Aortic cross clamp duration, min | 83 (38) | 81 (36) |
| Use of deep hypothermic circulatory arrest | 18 (22%) | 25 (26%) |
| Deep hypothermic circulatory arrest duration, min | 11 (6–28) | 6 (5–12) |
| Modified ultrafiltration | 81 (100%) | 93 (97%) |
Abbreviations: STAT, Society of Thoracic Surgery-European Association for Cardio-Thoracic Surgery mortality risk category. Data are shown as No. (%),mean (SD), or median (interquartile range) as appropriate.
Primary Composite Endpoint
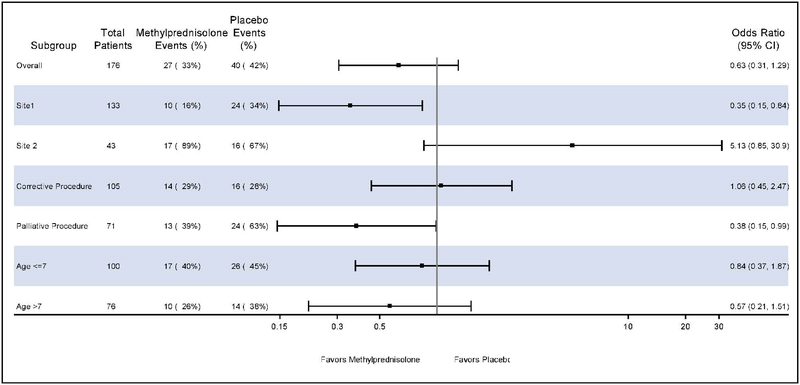
The primary composite outcome occurred in 27 of 81 neonates (33%) allocated to methylprednisolone and in 40 of 95 neonates (42%) allocated to placebo. Methylprednisolone did not significantly reduce the odds of meeting the primary composite endpoint (OR, 0.63, 95% CI, 0.31–1.3; P=0.21; Table 2, Central Illustration).
Table 2.
Primary Study Endpoint and Subgroup Analyses of Primary Outcome in the Methylprednisolone and Placebo Groups
| Methylprednisolone | Placebo | |||||
|---|---|---|---|---|---|---|
| No. Subjects | No. Events | No. Subjects | No. Events | Odds Ratio (95% CI) | P Value | |
| Primary outcome* | ||||||
| Primary Composite Outcome | 81 | 27(33) | 95 | 40(42) | 0.63 (0.31 – 1.29) | 0.21 |
| Site 1 | 62 | 10(16) | 71 | 24(34) | 0.35 (0.15 – 0.84) | 0.02 |
| Site 2 | 19 | 17(89) | 24 | 16(67) | 5.13 (0.85 – 30.93) | 0.07 |
| Subgroups | ||||||
| Corrective procedure | 48 | 14(29) | 57 | 16(28) | 1.01 (0.45 – 2.47) | 0.90 |
| Palliative procedure | 33 | 13(39) | 38 | 24(63) | 0.38 (0.15 – 0.99) | 0.048 |
| Age ≤ 7 | 42 | 17(40) | 58 | 26(45) | 0.84 (0.37 – 1.87) | 0.66 |
| Age > 7 | 39 | 10(26) | 37 | 14(38) | 0.57 (0.21 – 1.51) | 0.26 |
Primary study endpoint was a composite of death, extracorporeal membrane oxygenation, cardiac arrest, renal injury, liver injury, or rising lactate following cardiac surgery through hospital discharge. Data are shown as No. (%).
Central Illustration: Methylprednisolone Therapy in Neonates Undergoing Cardiac Operations with Cardiopulmonary Bypass and Morbidity-Mortality.
Forest plots of primary and subgroup analyses of methylprednisolone and the morbidity-mortality composite outcome. Methylprednisolone failed to show an overall benefit but there may be center or patient characteristics which make prophylactic methylprednisolone beneficial.
Secondary Endpoints
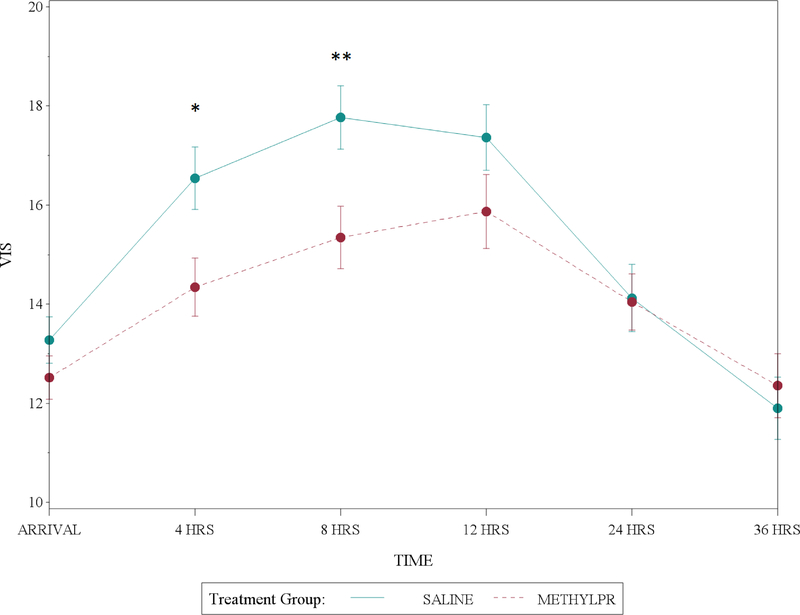
Individual components of the primary composite outcome and secondary outcomes of fluid balance, acute kidney injury, low cardiac output syndrome, duration of mechanical ventilation, length of intensive care unit and hospital stay, and days alive and out of the hospital at 90 days did not differ between the methylprednisolone and placebo groups (Table 3). Methylprednisolone was associated with a reduction in the VIS at 4 and 8 hours postoperatively as well as the highest VIS in the first 36 hours postoperatively (Figure 2). This corresponded to a trend in open label steroids being given more often in the first 36 hours after surgery, typically for catecholamine resistant hypotension, in the placebo group (Table 3). The safety outcomes of peak postoperative serum glucose, insulin treatment, infection, or poor wound healing were not different between treatment groups.
Table 3.
Secondary Endpoints in the Methylprednisolone and Placebo Groups
| Secondary outcomes | Methylprednisolone (n=81) | Placebo (n=95) | P Value |
|---|---|---|---|
| Components of primary outcome | |||
| Death | 5(6%) | 6(6%) | 0.97 |
| Cardiac arrest | 4(4%) | 8(8%) | 0.54 |
| Extracorporeal membrane oxygenation | 4(4%) | 11(11%) | 0.19 |
| Renal injury | 2(2%) | 7(7%) | 0.26 |
| Hepatic injury | 19(23%) | 26(27%) | 0.68 |
| Elevated lactate | 14(17%) | 24(25%) | 0.27 |
| Secondary Outcomes | |||
| Composite outcome at 30 days | 24(30%) | 40(42%) | 0.09 |
| Duration of mechanical ventilation, d | 4(3–7) | 5(3–8) | 0.44 |
| Length of ICU stay, d | 11(7–18) | 11(7–20) | 0.87 |
| Length of hospital stay, d | 20(13–35) | 22(12–35) | 0.98 |
| Days alive and out of the hospital at 90 days | 64(46–76) | 61(39–75) | 0.48 |
| Low cardiac output syndrome | 37(46%) | 55(58%) | 0.11 |
| Highest vasoactive inotropic score | 18.2(6.9) | 20.4(6.9) | 0.03 |
| Highest lactate | 4.2(2.8) | 4.1(2.8) | 0.80 |
| Postoperative steroids | 22(27%) | 38(40%) | 0.07 |
| Total fluid in at 36 hours, mL | 558(480–667) | 635(523–724) | 0.52 |
| Total urine output at 36 hours, mL | 438(299–580) | 468(306–630) | 0.45 |
| Total fluid out at 36 hours, mL | 587(434–723) | 579(444–768) | 0.36 |
| Acute kidney injury | 35(43%) | 42(44%) | 0.89 |
| Safety Outcomes | |||
| Infection | 4(5%) | 8(8%) | 0.36 |
| Poor wound healing | 1(1%) | 4(4%) | 0.38 |
| Highest glucose, mg/dL | 155 (55) | 151(42) | 0.64 |
| Insulin therapy | 2(2%) | 2(2%) | 0.99 |
Abbreviations: ICU, intensive care unit.
Data are shown as No. (%), mean (SD), or median (interquartile range) as appropriate.
Figure 2: Vasoactive Inotropic Score over Time by Treatment Group.
Methylprednisolone treatment resulted in lower vasoactive inotropic score at 4 and 8 hours postoperatively. Circles represent the mean and error bars represent the standard deviation. They were generated by estimating the mean and variance of the VIS measure over time separately for each group and each time point. Abbreviations: VIS, vasoactive inotropic score; Hrs, hours. *p=0.01, **p<0.01.
Subgroup Analysis
In prespecified subgroup analysis, methylprednisolone was protective for the primary composite endpoint in palliative operations (OR 0.38, 95% CI, 0.15–0.99; P=0.048) but not corrective operations (Table 2, Figure 2). There was no difference in the effects of methylprednisolone on the primary outcome in those ≤7 days of age or >7 days. Only 7 neonates were ≤2.5 kg at the time of surgery so this subgroup was not explored. In the per protocol analysis, there was no difference in the effects of methylprednisolone on the primary outcome when those that received open label steroids in the postoperative period were removed (OR 0.67, 95% CI, 0.26–1.73; P=0.40).
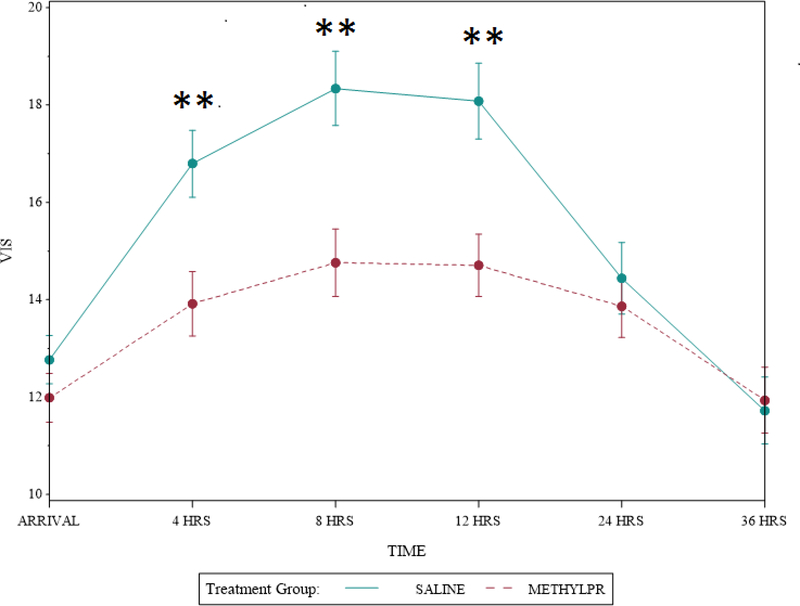
In the a priori analysis adjusting for underlying corrective versus palliative procedure and center, there was a significant interaction between treatment effect and center (point estimate 0.087, 95% CI, 0.04–0.21; P=0.02). Therefore additional primary and secondary outcomes analyses by center were performed. At site 1, the center enrolling the majority of patients (133/176, 76%) methylprednisolone was protective for the primary composite endpoint overall (OR 0.35, 95% CI, 0.15–0.84; P=0.02), for palliative operations OR 0.22 (95% CI, 0.06–0.74; P=0.02) but not corrective operations OR 1.06 (95% CI, 0.45–2.47; P=0.90), and reduced the occurrence of the primary composite outcome at 30 days 13% (8/62) vs 34% (24/71), P=0.005. Methylprednisolone also reduced the incidence of LCOS 42% (26/62) vs 63% (45/71), P=0.01, VIS at 4, 8, and 12 hours, postoperatively (Figure 3), peak VIS (17.3 ± 5.2 vs. 20.8 ± 6.2, P=0.0008) and reduced treatment with postoperative open label steroids 26% (16/62) vs 49% (35/71), P = 0.005. There was no additional treatment effect on outcomes at this center and no difference in any outcomes between treatment groups at the other center.
Figure 3: Vasoactive Inotropic Score over Time by Treatment Group at Site 1.
Methylprednisolone treatment resulted in lower vasoactive inotropic score at 4, 8, and 12 hours at site 1. Circles represent the mean and error bars represent the standard deviation. They were generated by estimating the mean and variance of the VIS measure over time separately for each group and each time point. Abbreviations: VIS, vasoactive inotropic score; Hrs, hours. **p<0.01
Discussion
In neonates undergoing cardiac surgery with cardiopulmonary bypass, administration of intraoperative methylprednisolone failed to show an overall statistically significant benefit on the incidence of the primary study composite endpoint of death and objective signs of inadequate cardiac output (Central Illustration). There was, however, a benefit in patients undergoing palliative procedures and a significant interaction between treatment effect and center. In this analysis, methylprednisolone was protective for the primary composite endpoint overall and at 30 days at 1 center. Methylprednisolone also reduced the incidence of LCOS, vasoactive/inotropic requirements, and treatment with postoperative open label steroids at one center but not the other.
To the best of our knowledge, this trial is the first multi-centered randomized placebo-controlled trial on the highly debated topic of intraoperative steroid use during cardiac surgery with cardiopulmonary bypass in neonates. Previous single center pediatric randomized controlled trials and retrospective studies have demonstrated improvement in some inflammatory markers, hemodynamic, and clinical variables (10–13,22,23). Conversely, large database trials have demonstrated no benefit with steroids and possible harm, especially in lower surgical risk groups (8,15). A strength of this study is the sole inclusion of neonates. The morbidity factors of smaller patient size, greater hemodilution, hypothermia, and longer CPB times combined with the biological immaturity of all organ systems create a post-bypass recovery period that is longer and more complex than similar operations performed in older infants and children. This high level of severity itself provides a substrate for identifying the positive effects as well as the greatest potential for benefit in this vulnerable population.
Although the effect of methylprednisolone on the primary endpoint was negative, the beneficial effect at 1 center was impressive. Methylprednisolone resulted in a reduction in the incidence of the composite endpoint of death and objective signs of inadequate cardiac output at 30 days and hospital discharge, the incidence of LCOS, and resulted in a lower inotropic requirement. Both the primary outcome measure and the VIS have been validated and associated with clinical outcomes in this population (16,18,19). LCOS was used as the primary endpoint in one of the largest pediatric cardiac perioperative drug trials to date (17). Similar to other studies, further support for improved hemodynamics with methylprednisolone came from blinded clinicians ordering less “rescue” steroids according to their own subjective criteria in the early postoperative period (22).
Just as intriguing as the positive effects methylprednisolone had at 1 center, is the lack of an effect and trend in the opposite direction at the other center. Interestingly, the primary composite outcome and all components of the outcome occurred 3–4 times more often at this center (primary composite outcome 26% vs. 76%, death 3% vs 13%, cardiac arrest 4% vs 13%, ECMO 6% vs 16%, renal injury 3% vs 9%, hepatic injury 16% vs 53%, and elevated lactate 13% vs 46% of total subjects at site 1 and 2 respectively). One could hypothesize that methylprednisolone may provide a small benefit that is easily abolished by patient characteristics or perioperative management strategies that are larger contributors to these outcome discrepancies. Indeed there were some differences between patient demographics and perfusion strategies between the centers. However, it is recognized that clinical center often remains an independent predictor of outcomes in pediatric cardiac clinical trials even when details of potential risk factors including operative and perfusion techniques are included in analysis (24–26).
This trial should be viewed in light of its limitations. Nearly one third of the subjects received additional steroids in the early postoperative period. While that offers an additional opportunity to evaluate the perceived hemodynamic state of the subjects by blinded clinicians, it potentially diminishes the ability to detect both beneficial and adverse effects (27). The trial may not have been sufficiently powered to detect important differences in some of the secondary outcomes. Finally, it only included 2 centers and although the differing treatment effects by center are compelling, a detailed determination of how patient and center specific perioperative factors contribute to the treatment effect was beyond the scope of this work. Examining these factors as well as inflammatory markers and 12 month neurodevelopmental assessment will be part of future analyses.
In conclusion, these findings suggest that technical factors or support strategies may overwhelm the impact of steroids in the grand scheme and provides insight into why it has been so difficult to determine if steroids are beneficial in this population despite over a half a century of investigation. Although our trial does not support the routine use of intraoperative methylprednisolone for neonates undergoing cardiac surgery with cardiopulmonary bypass, it does suggest there may be center or patient characteristics which make prophylactic methylprednisolone beneficial. Further analysis is ongoing to elicit these factors. This trial, with differing treatment effects by center, does provide important equipoise for the ongoing large multicenter trial Steroids to Reduce Systemic Inflammation after Neonatal Heart Surgery (NCT03229538).
Supplementary Material
Clinical perspectives.
Competency in Patient Care and Procedural Skills
Intraoperative administration of methylprednisolone does not uniformly enhance postoperative recovery of neonates undergoing cardiopulmonary bypass but may be beneficial in certain patients or under certain circumstances.
Translational Outlook
Future studies should define patient or hospital characteristics associated with beneficial responsiveness to prophylactic methylprednisolone.
Acknowledgments
Funding: This work was supported by grant HL112968 from the National Heart, Lung, and Blood Institute (NHLBI). This work is solely the responsibility of the authors and does not necessarily represent the official views of NHLBI or NIH.
ABBREVIATIONS
- CPB
cardiopulmonary bypass
- ECMO
extracorporeal membrane oxygenation
- ICU
intensive care unit
- LCOS
low cardiac output syndrome
- NIRS
near-infrared spectroscopy
- STAT
Society of Thoracic Surgery-European Association for Cardio-Thoracic Surgery
- VIS
vasoactive inotropic score
Footnotes
Disclosures: Dr. Graham is a research consultant to Bayer. The authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham EM, Atz AM, McHugh KE et al. Preoperative steroid treatment does not improve markers of inflammation after cardiac surgery in neonates: results from a randomized trial. J Thorac Cardiovasc Surg 2014;147:902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarnok A, Emmrich F. Immune consequences of pediatric and adult cardiovascular surgery: report of the 7th Leipzig workshop. Cytometry B Clin Cytom 2003;54:54–7. [DOI] [PubMed] [Google Scholar]
- 3.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 2003;75:S715–20. [DOI] [PubMed] [Google Scholar]
- 4.Mahle WT, Matthews E, Kanter KR, Kogon BE, Hamrick SE, Strickland MJ. Inflammatory response after neonatal cardiac surgery and its relationship to clinical outcomes. Ann Thorac Surg 2014;97:950–6. [DOI] [PubMed] [Google Scholar]
- 5.Replogle RL, Gazzaniga AB, Gross RE. Use of corticosteroids during cardiopulmonary bypass: possible lysosome stabilization. Circulation 1966;33:I86–92. [DOI] [PubMed] [Google Scholar]
- 6.Checchia PA, Bronicki RA, Costello JM, Nelson DP. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: an international survey of 36 centers. Pediatr Crit Care Med 2005;6:441–4. [DOI] [PubMed] [Google Scholar]
- 7.Elhoff JJ, Chowdhury SM, Zyblewski SC, et al. Intraoperative Steroid Use and Outcomes Following the Norwood Procedure: An Analysis of the Pediatric Heart Network’s Public Database. Pediatr Crit Care Med 2016;17:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquali SK, Hall M, Li JS et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation 2010;122:2123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham EM, Bradley SM. First nights, the adrenal axis, and steroids. J Thorac Cardiovasc Surg 2017; 153:1164–1166. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder VA, Pearl JM, Schwartz SM, Shanley TP, Manning PB, Nelson DP. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation 2003;107:2823–8. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg L, Forsell C, Jogi P, Olsson AK. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. Br J Anaesth 2003;90:728–32. [DOI] [PubMed] [Google Scholar]
- 12.Bronicki RA, Backer CL, Baden HP, Mavroudis C, Crawford SE, Green TP. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg 2000;69:1490–5. [DOI] [PubMed] [Google Scholar]
- 13.Graham EM, Atz AM, Butts RJ et al. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: results from a randomized trial. J Thorac Cardiovasc Surg 2011;142:1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson-Malt S, Afrane B, El Barbary M. Prophylactic steroids for pediatric open heart surgery. Cochrane Database Syst Rev 2007:CD005550. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali SK, Li JS, He X et al. Perioperative methylprednisolone and outcome in neonates undergoing heart surgery. Pediatrics 2012;129:e385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butts RJ, Scheurer MA, Zyblewski SC et al. A composite outcome for neonatal cardiac surgery research. J Thorac Cardiovasc Surg 2014;147:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman TM, Wernovsky G, Atz AM et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107:996–1002. [DOI] [PubMed] [Google Scholar]
- 18.Gaies MG, Jeffries HE, Niebler RA et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014;15:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butts RJ, Scheurer MA, Atz AM et al. Comparison of maximum vasoactive inotropic score and low cardiac output syndrome as markers of early postoperative outcomes after neonatal cardiac surgery. Pediatr Cardiol 2012;33:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–56. [PubMed] [Google Scholar]
- 22.Robert SM, Borasino S, Dabal RJ, Cleveland DC, Hock KM, Alten JA. Postoperative Hydrocortisone Infusion Reduces the Prevalence of Low Cardiac Output Syndrome After Neonatal Cardiopulmonary Bypass. Pediatr Crit Care Med 2015;16:629–36. [DOI] [PubMed] [Google Scholar]
- 23.Clarizia NA, Manlhiot C, Schwartz SM et al. Improved outcomes associated with intraoperative steroid use in high-risk pediatric cardiac surgery. Ann Thorac Surg 2011;91:1222–7. [DOI] [PubMed] [Google Scholar]
- 24.Newburger JW, Sleeper LA, Bellinger DC et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation 2012;125:2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabbutt S, Ghanayem N, Ravishankar C et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg 2012;144:882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs JP, Mayer JE Jr., Mavroudis C et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2016 Update on Outcomes and Quality. Ann Thorac Surg 2016;101:850–62. [DOI] [PubMed] [Google Scholar]
- 27.Mastropietro CW, Barrett R, Davalos MC et al. Cumulative corticosteroid exposure and infection risk after complex pediatric cardiac surgery. Ann Thorac Surg 2013;95:2133–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.