Abstract
Background:
Alcohol consumption is associated with poor health outcomes in women living with HIV (WLWH), but whether medication can help to reduce drinking in non-treatment-seeking women, or whether reduction in drinking improves HIV outcomes is unclear. We conducted a randomized clinical trial (RCT) of daily oral naltrexone (50 mg) versus placebo in WLWH who met criteria for current unhealthy alcohol use.
Methods:
WLWH with current unhealthy alcohol use (>7 drinks/week or >3 drinks/occasion) were randomly assigned to daily oral naltrexone 50 mg (n=96) or placebo (n=98) for 4 months. Drinking outcomes, including the proportion of women who reduced (<unhealthy use criteria) or quit drinking, were assessed at baseline, 2 months, 4 months (end of treatment), and 7 months. In a secondary analysis, HIV viral suppression and changes in CD4 counts were compared in women who did or did not reduce/quit drinking, regardless of intervention assignment.
Results:
The participants’ mean age was 48 years, 86% were African American, and 94% were receiving HIV antiretroviral therapy. Among all participants, 89% and 85% completed the 4-month and 7-month follow-ups, respectively. Participants in both groups substantially reduced drinking over time. At 1 month and 3 months, naltrexone was associated with a greater reduction in drinking (p<0.05), but the proportion who reduced/quit drinking at 4 months (52% versus 45%, p=0.36) or 7 months (64% in both groups) was not different. HIV viral suppression at follow-up was significantly better in participants who reduced/quit drinking vs. those continuing unhealthy alcohol use at 4 months (72% vs. 53%, p=0.02) and 7 months (74% vs. 54%, p=0.02).
Conclusions:
Participating in an RCT to reduce drinking was associated with significant drinking reduction regardless of medication assignment, suggesting that non-medication aspects of research study participation (e.g., repeated assessments and support from research staff) could be important interventions to help reduce drinking outside of research studies. Drinking reduction was associated with improved HIV viral suppression, providing evidence to support recommendations to avoid unhealthy alcohol use among WLWH.
Keywords: Alcohol consumption, HIV infection, Randomized clinical trial, Women, Pharmacotherapy
Introduction
Among persons living with HIV infection, antiretroviral therapy (ART) can suppress the level of HIV virus in the blood to undetectable levels, which reduces HIV transmission and improves survival. Despite the availability of ART, many do not achieve HIV viral suppression, with women being less likely than men to achieve HIV viral suppression (Aziz and Smith, 2011; Chakraborty et al., 2015). Women account for approximately 23% of new HIV infections in the U.S. (Centers for Disease Control and Prevention, 2016), and strategies are needed to reduce HIV outcome disparities among women living with HIV/AIDS (WLWH).
Alcohol reduction is one possible strategy to improve health outcomes in WLWH. Unhealthy alcohol use, defined as >7 drinks/week or >3 drinks/occasion for women (National Institute on Alcohol Abuse and Alcoholism, 2008), is reported by approximately 6% – 25% of WLWH (Cook et al., 2013; Theall et al., 2007). Unhealthy alcohol use is associated with poor medication adherence, less HIV viral suppression, more rapid HIV disease progression, and increased hospitalization rates (Deiss et al., 2016; Hahn and Samet, 2010; Kader et al., 2015; Rentsch et al., 2016). Alcohol consumption is also associated with chronic disease outcomes that are more common in persons with HIV, including liver disease, cardiovascular disease, and cancer (Cao et al., 2015; Gao and Bataller, 2011; Kelso et al., 2015; Park et al., 2016; Smith et al., 2014). Longitudinal studies suggest that increases in drinking among WLWH can result in worse HIV-related clinical outcomes (Barai et. al., 2017; Willians et. al., 2018). However, little hard evidence suggests that reductions in drinking will correlate with improved HIV-related health outcomes. Several medications are FDA-approved to help reduce drinking, yet they are not prescribed very often (Chander et al., 2016). Moreover, relatively few clinical trials of alcohol pharmacotherapy included large numbers of women (Canidate et al., 2017), most excluded persons who had comorbid mental health or other substance use behavior, and none examined clinical outcomes related to HIV infection. Therefore, we sought to understand how pharmacotherapy for alcohol consumption might work when offered to a broad, generalizable sample of WLWH who currently exceed recommended drinking levels.
We previously completed a pilot study to demonstrate that WLWH were willing to enroll in a clinical trial of naltrexone versus placebo and that many women could successfully reduce their drinking during the study (Cook et al., 2017). We selected naltrexone rather than other existing medications because it can be taken once daily, is generally well-tolerated (Jonas et al., 2014; Maisel et al., 2013), and because it can be used to support either alcohol reduction or complete cessation (Tidey et al., 2008). However, the pilot study was not powered to determine the overall effect of naltrexone in this population, or whether reductions in drinking could improve HIV-related outcomes.
Therefore, the specific objectives of this study were to determine the effects of naltrexone versus placebo on drinking behavior and clinical outcomes in WLWH. We planned subgroup analyses to compare study outcomes by baseline severity of drinking and by adherence to the study protocol, and we also planned a secondary analysis to compare HIV clinical outcomes in women who did or did not reduce or quit drinking during the trial, regardless of intervention assignment.
Materials and Methods
Overview
The WHAT-IF? (Will Having Alcohol Treatment Improve my Functioning?) study was a double-blind randomized clinical trial in which eligible women received either naltrexone 50 mg orally or placebo for 4 months, allocated in a 1:1 ratio, with follow-up assessments at 2 months, 4 months, and 7 months. The study was approved by IRBs at the University of Miami Miller School of Medicine, Florida International University, and the University of Florida, and was registered on clinicaltrials.gov (). Prior to study initiation, all study staff completed detailed training about the study procedures.
Participants and Setting
Participants were recruited from a range of clinical and community-based settings in Miami, Florida, from 2013–2016. Recruitment strategies included leaving brochures in clinical settings, contacting participants from previous research studies, and referral from other participants. We did not recruit directly from alcohol treatment settings because women receiving other current treatments for alcohol consumption were excluded. WLWH were eligible if they were 18 years or older and met past-month criteria for unhealthy alcohol use (>7 drinks/week or >3 drinks on one single day at least twice) (National Institute on Alcohol Abuse and Alcoholism, 2008). Exclusion criteria included contraindications to using naltrexone (current opiate dependence, current prescription opioid medications, positive urine drug screen for opioids, and/or allergic to naltrexone); elevated liver enzymes, serum creatinine, or blood pressure at time of enrollment; currently pregnant; currently taking a medication for alcohol treatment, tuberculosis, or viral hepatitis; unable to understand English or the study procedures; current prognosis of less than 1 year to live (e.g., metastatic cancer); or recommendation from a study physician based on other information available at the time of enrollment.
Study Procedures
At the enrollment visit, participants learned about the study, signed the informed consent and HIPAA forms, and provided a urine sample to confirm they were not pregnant or using opioids. Eligible participants were then scheduled for a baseline assessment that included a limited clinical assessment by a research nurse (blood pressure, height and weight, and repeated urine test for opioids), completion of study questionnaires, a blood draw, randomization, and initiation of study medication.
The baseline assessment questionnaire was completed by participants using an audio computer-assisted self-interview (ACASI) computer program, in which women entered their own information with assistance from a research assistant if needed. The questionnaire assessed sociodemographic characteristics including age, race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or non-Hispanic other), marital status, employment, and education (less than high school, high school graduate, some college, or college graduate). The baseline questionnaire also inquired about the frequency of use of nine other classes of drugs and tobacco products. Recent drug use was defined as any self-reported use of benzodiazepines, cocaine, amphetamines, marijuana, opioids, or hallucinogens in the past 30 days. Risky sexual behavior was defined as having unprotected sexual intercourse with a male who was HIV-discordant or of unknown HIV status in the past year (Cook et al., 2010). Adherence to HIV ART was self-reported as the percentage of prescribed HIV medication that participants took in the last 30 days (Badiee et al., 2012).
The timeline follow-back (TLFB) (Sobell LC and Sobell MB, 1992) was used to calculate the average number of drinks per week, the number of days of abstinence, and the number of binge-drinking days in the 30 days prior to study initiation. At baseline, participants also completed the Alcohol Use Disorder Identification Test (AUDIT) (Saunders et al., 1993) and the Short Inventory of Problems (SIP), a 15-item measure of consequences of drinking (score 0 – 60) (Miller WR et al., 1985). Craving for alcohol was measured by a single item scored 1–10, with 10 being strongest craving. A computerized version of the Mini International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998) was used to assess for alcohol abuse and/or dependence based on DSM-IV criteria.
Blood samples were obtained and sent to a commercial laboratory to assess CD4+ count, HIV viral load, and a comprehensive metabolic panel. HIV viral suppression was defined as <200 copies/mL (or undetectable). A dried blood spot was sent to a commercial laboratory (USTDL, Des Plaines, IL) to test for PEth (phosphatidylethanol), a biomarker of heavy alcohol use over the previous 3 weeks (Aradottir et al., 2006).
Randomization and Intervention
This clinical trial was double-blinded, so neither the participants nor research staff knew who received naltrexone or placebo. A computer-generated randomization sequence, created by the study biostatistician, assigned each consecutive study ID number to either naltrexone or placebo in blocks of eight ID numbers (four naltrexone and four placebo). Eligible participants were each assigned the next consecutive study ID number when they enrolled, and they received the medication assigned to that ID number.
The study medication was provided by a certified compounding pharmacy, who produced identical-appearing capsules for naltrexone and placebo (WELLHealthrx, Jacksonville, FL). The active medication contained 50 mg naltrexone/pill, and the placebos were identically-appearing pills of inert cellulose. Study medications were stored and distributed by the research pharmacy at the University of Miami Miller School of Medicine, who had access to information linking study ID number and study medication. At the baseline visit, participants took the first pill and remained with the research staff for 30 minutes to make sure they did not have an acute, allergic reaction. The research team then provided a 30-day supply of study medication contained within 30-pill blister packets, and participants were asked to punch out and take one pill daily for 4 months.
Follow-up Procedures
After enrollment, research staff maintained regular contact with study participants to encourage adherence to the study medication and to monitor for adverse events. Participants were assessed weekly for the first month (weeks 1 and 3 by telephone and weeks 2 and 4 in-person) and then in-person monthly until the 4-month visit, at which time participants were asked to stop the study medication. A final follow-up was completed 7 months after enrollment. Participants received incentive payments for each in-person assessment to encourage follow-up and to support the time and inconvenience of completing the assessments.
At each follow-up assessment, research staff used a series of counseling checklists, based on a study manual developed for another naltrexone clinical trial (Pettinati HM and Mattison ME, 2010) to help promote adherence to the study medication. At the in-person visits, research staff also provided refills on study medication and collected updated drinking data by TLFB. Research staff were trained to be neutral in their alcohol assessments to minimize social desirability bias. Research staff also collected data about study medication adherence, assisted by visual inspection of the punch-out pill packs, to determine the proportion of study medication taken (the number of doses taken divided by the number prescribed).
Participants completed follow-up ACASI questionnaires during in-person visits at 2 months, 4 months, and 7 months. The follow-up questionnaires included repeated measures from baseline, including questions about HIV medication adherence, other substance use, and sexual activity. Follow-up laboratory tests for HIV viral load, CD4+ count, and liver and kidney function were obtained at 2 weeks, 2 months, 4 months, and 7 months. Follow-up PEth testing was conducted at 2 months and 7 months.
Safety Monitoring
In addition to regular monitoring of participant liver and kidney function, the research staff collected information about potential adverse events that were reported by participants on their own or after prompting from a specific assessment designed to track potential side effects (Johnson et al., 2005). The research team evaluated each potential adverse event, determined whether the event appeared to be related to the study medication, and tracked events until resolution. Adverse events were defined as any side effect or clinical event that was not present at baseline or became more severe compared to baseline. The research team informed participants about abnormal laboratory results that a study physician deemed to be of potential clinical significance. A data safety and monitoring board (DSMB) consisting of three members independent from the research team reviewed study results and adverse events annually. The DSMB was also consulted to help evaluate persons with serious adverse events during follow-up and to help make recommendations regarding the progress of the study.
Statistical Analysis
We originally proposed to enroll 240 women (160 treatment, 80 control), which would provide 82% power to detect a shift in mean weekly alcohol consumption of 0.4 standard deviations. The study design was changed to a 1:1 allocation before study initiation, and after approximately 100 women had enrolled, the DSMB approved a revised sample size of 200 (100 per treatment group), which would generate a similar effect size, based on better-than-expected retention.
Baseline characteristics were compared between subjects randomized to the naltrexone and placebo groups. T-tests and Wicoxon Mann-Whiteney tests were used to assess differences in means and medians respectively, whereas the chi-square test was used for categorical variables. Primary and secondary outcomes were determined a priori and measured at the 4-month time point, which coincided with completion of study medication. The primary outcome was “reduced/quit drinking,” defined as either reduction of drinking to less than unhealthy amounts (i.e., 7 or fewer drinks per week and 3 or fewer drinks on every day in the past 30 days) or completely stopping drinking. Secondary drinking outcomes included the number of abstinent days and the number of binge drinking days in the past 30 days, the total score on the SIP (range 0–60), and the craving score for alcohol (range 1–10). Clinical and behavioral outcomes included HIV viral suppression (<200 copies/mL), mean CD4+ count, change in CD4+ count (baseline to follow-up), and HIV medication adherence (>95%). We conducted statistical comparisons for each outcome at specific time points, using chi-square and t-tests where appropriate. We compared the proportion of persons with continued unhealthy alcohol use monthly for the 4 months of treatment to explore patterns of changes over time. The primary outcome analyses at 4 months (end of treatment) and 7 months (end of study) used logistic regression to estimate the odds of reducing/quitting drinking in naltrexone vs. placebo, based on intention-to-treat. The primary analyses were unadjusted, but we also did analyses adjusting for baseline alcohol use disorder, since it was significantly different in the two groups at baseline. We also conducted pre-planned subgroup analyses stratified by alcohol dependence at baseline (yes or no), PEth results at baseline (>=8 ng/ml or <8 ng/ml), and adherence to the study medication (>=90% or <90% of doses taken over 4 months). If any results appeared to be significant by subgroup status, we included an interaction term in a regression model to test for statistical significance.
Finally, we conducted an observational analysis to compare the HIV-related outcomes in women who successfully reduced/quit drinking at the end of the study (7 months), compared to those who continued unhealthy alcohol use, regardless of their intervention assignment. For this analysis, we used a propensity weighted logistic regression approach in which we adjusted for treatment assignment (naltrexone or placebo) and baseline variables that were associated with the primary outcome at p<0.02 at the 7-month time point (Brookhart et al., 2006). All analyses were performed in SAS 9.4.
Results
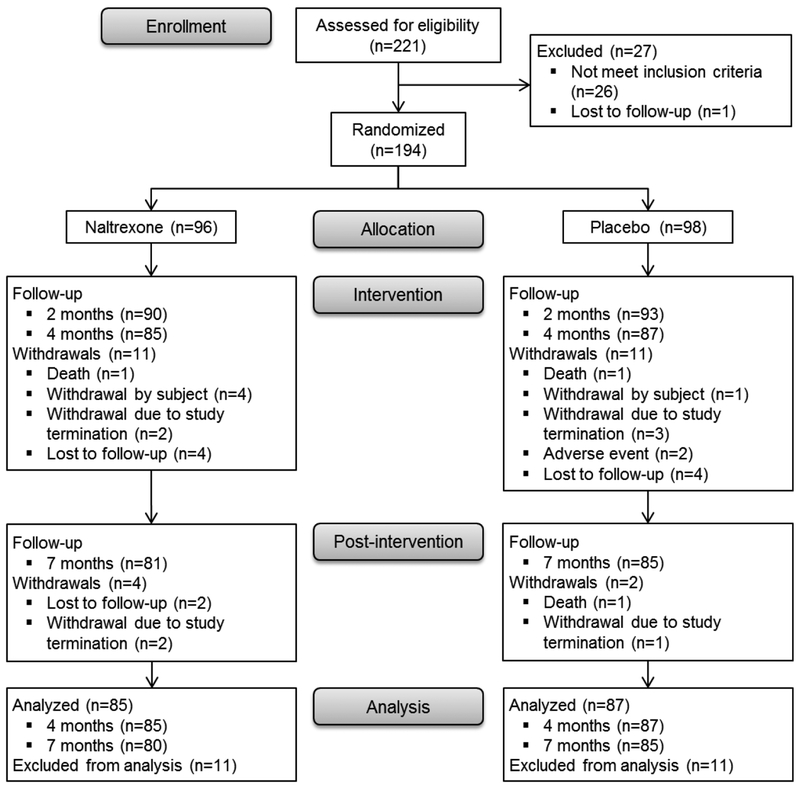
Of 221 women with current alcohol consumption who spoke to clinical or research staff about the study, 27 were either ineligible or lost to follow-up (Figure 1). Of the remaining 194 women, 96 were enrolled and randomized to naltrexone and 98 to placebo. Of those enrolled, 172 (89%) completed the 4-month assessment, and 165 (85%) completed the 7-month assessment. Overall, 112 (66%) women reported taking 90% of the recommended doses of study medication over the 4-month period (60% in naltrexone group versus 72% in placebo group, p=0.08).
Figure 1.
Participant Flow Chart
Baseline characteristics of study participants by randomization assignment are shown in Table 1. The participants were primarily middle-aged (mean age 48 years, SD=9) and African-American (83%). At baseline, participants reported an average of 66 standard drinking units (SDU) per week and an average of 18 binge-drinking days in the 30 days prior to enrollment. All participants met criteria for unhealthy alcohol use; the mean AUDIT score was 17, 62% met criteria for an alcohol use disorder, and 47% had a positive PEth alcohol biomarker at baseline (>8 ng/ml). Only 63% had HIV viral suppression at enrollment and the mean CD4+ count was 556 cells/mL (SD=346). Most participants also reported current smoking (63%) or other drug use (58%), whereas very few reported risky sexual behavior in the past 12 months. No demographic or behavioral characteristics were significantly different by intervention assignment. However, current alcohol use disorder was more common in subjects assigned to naltrexone compared to placebo (71% vs 53%, p<0.05).
Table 1.
Baseline characteristics of 194 women with HIV according to treatment assignment.
| Baseline Characteristics | Total (N=194) |
Naltrexone (n=96) |
Placebo (n=98) |
|---|---|---|---|
| SOCIO-DEMOGRAPHICS | |||
| Age (Mean ± SD) | 48 ± 9 | 48 ± 8 | 49 ± 9 |
| Age group | |||
| 18–39 | 29 (15%) | 16 (17%) | 13 (13%) |
| 40–49 | 67 (35%) | 35 (36%) | 32 (33%) |
| 50–59 | 80 (41%) | 37 (39%) | 43 (44%) |
| ≥60 | 18 (9%) | 8 (8%) | 10 (10%) |
| Race/Ethnicity | |||
| Hispanic | 22 (11%) | 11 (11%) | 11 (11%) |
| Not Hispanic, White | 8 (4%) | 3 (3%) | 5 (5%) |
| Not Hispanic, Black | 161 (83%) | 81 (84%) | 80 (82%) |
| Not Hispanic, Other | 3 (2%) | 1 (1%) | 2 (2%) |
| Married or in a long-term relationship | 31 (16%) | 16 (17%) | 15 (15%) |
| Employed | 20 (10%) | 11 (11%) | 9 (9%) |
| Education | |||
| Less than high school | 84 (43%) | 40 (42%) | 44 (45%) |
| High school graduate | 66 (34%) | 38 (40%) | 28 (29%) |
| Some college or college graduate | 44 (23%) | 18 (19%) | 26 (27%) |
| ALCOHOL CONSUMPTION | |||
| Number of standard drinks per week (Mean ± SD) | 66 ± 78 | 58 ± 51 | 73 ± 97 |
| Abstinent days in the past 30 days (Mean ± SD) | 9 ± 8 | 8 ± 8 | 9 ± 8 |
| Binge drinking days in the past 30 days (Mean ± SD) | 18 ± 9 | 19 ± 9 | 18 ± 10 |
| Alcohol craving score (Mean ± SD) | 5.9 ± 2.9 | 6.0 ± 3.0 | 5.8 ± 2.7 |
| SIP score (Mean ± SD) | 12.9 ± 11.1 | 13.1 ± 11.4 | 12.7 ± 10.9 |
| AUDIT score (Mean ± SD) | 17 ± 8 | 17 ± 8 | 16 ± 8 |
| Alcohol use disorder | |||
| No | 74 (38%) | 28 (29%) | 46 (47%)* |
| Alcohol abuse only | 16 (8%) | 11 (12%) | 5 (5%) |
| Alcohol dependence | 103 (53) | 56 (59%) | 47 (48%) |
| PEth value | |||
| <8 ng/mL | 102 (53%) | 49 (51%) | 53 (54%) |
| 8–20 ng/mL | 18 (9%) | 10 (10%) | 8 (8%) |
| >20 ng/mL | 74 (38%) | 37 (39%) | 37 (38%) |
| HIV CLINICAL AND OTHER BEHAVIORAL FACTORS | |||
| HIV viral suppression (<200 copies/mL) | 119 (63%) | 57 (61%) | 62 (65%) |
| CD4 count (Mean ± SD) | 556 ± 346 | 541 ± 295 | 571 ± 391 |
| Currently on ART | 182 (94%) | 91 (95%) | 91 (93%) |
| ≥95% ART adherence in the past 30 daysa | 98 (61%) | 47 (59%) | 51 (63%) |
| BSI grand total score (Mean ± SD) | 28.6 ± 32.4 | 27.4 ± 32.6 | 29.7 ± 32.2 |
| Tobacco use in the past 30 days | 120 (63%) | 61 (64%) | 59 (61%) |
| Any drug use in the past 30 days | 109 (58%) | 56 (59%) | 53 (58%) |
| Risky sexual behavior in the past 12 months | 13 (7%) | 7 (7%) | 6 (6%) |
SD = Standard deviation, BSI = Brief Symptom Inventory
Among participants who were currently on ART
p < .05 by chi-square test
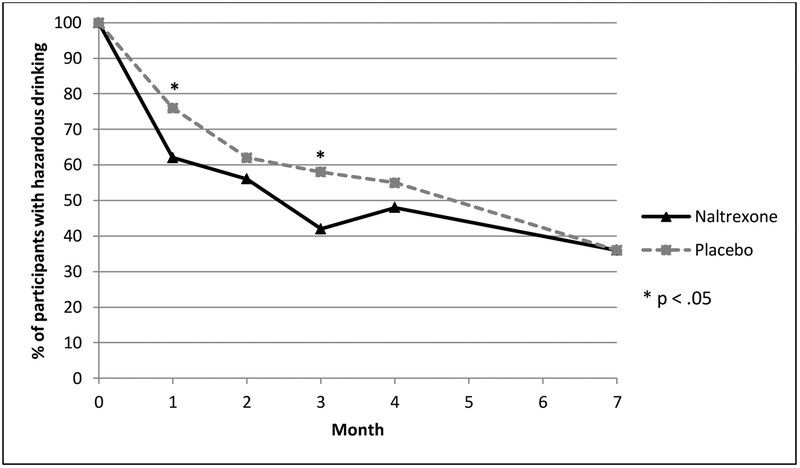
During follow-up, the proportion of subjects with unhealthy alcohol use declined in both groups. Only about 50% of women reported ongoing unhealthy alcohol use at 4 months, and the proportion with unhealthy alcohol use continued to drop even after medication was stopped (Figure 2). While the proportions of women with unhealthy alcohol use were statistically similar at the primary end point of 4 months (p=0.36), the naltrexone group demonstrated significantly lower levels of unhealthy drinking at 1 month (p=0.045) and 3 months (p=0.03) after enrollment (Figure 2). For the main endpoint at 4 months, the unadjusted odds of reducing/quitting drinking for women receiving naltrexone vs. placebo was 1.32 (95% CI 0.73, 2.41, p=0.36). With adjustment for baseline alcohol use disorder, the odds ratio was 1.47 (95% CI 0.79, 2.74, p=0.23).
Figure 2.
Proportions of participants with continued unhealthy drinking in the past 30 days among 194 women with HIV. Participants received treatment (naltrexone or placebo) for 4 months.
Regarding other drinking outcomes, both study groups demonstrated substantial decreases in the average number of drinks per week, the number of binge drinking days per month, and craving for alcohol (Table 2). Consequences related to drinking, as measured by the SIP, were reduced by nearly half in both groups (Table 2). Both groups also demonstrated significant increases in the number of abstinent days per month (Table 2). These drinking outcomes were not statistically different between treatment groups (naltrexone versus placebo). Similarly, the HIV clinical outcomes, including the proportion of women with HIV viral suppression, the mean CD4 count+, or self-reported ART adherence, did not differ during follow-up in women receiving naltrexone versus placebo (Table 2).
Table 2.
Primary and secondary outcomes at Baseline, 4 months, and 7 months
| Naltrexone N=(96) |
Placebo (n=98) |
p value* | |
|---|---|---|---|
| ALCOHOL CONSUMPTION | |||
| Reduced/Quit drinking | |||
| Baseline | 0 (0%) | 0 (0%) | na |
| 4-month | 44 (52%) | 39 (45%) | .36 |
| 7-month | 52 (64%) | 54 (64%) | .93 |
| Number of standard drinks per week (Mean ± SD) | |||
| Baseline | 58 ± 51 | 73 ± 97 | .18 |
| 4-month | 11 ± 20 | 18 ± 42 | .19 |
| 7-month | 7 ± 14 | 11 ± 31 | .24 |
| Binge drinking days in the past 30 days (Mean ± SD) | |||
| Baseline | 19 ± 9 | 18 ± 10 | .20 |
| 4-month | 4 ± 8 | 4 ± 8 | .77 |
| 7-month | 3 ± 6 | 4 ± 8 | .32 |
| Alcohol craving score (Mean ± SD) | |||
| Baseline | 6.0 ± 3.0 | 5.8 ± 2.7 | .57 |
| 4-month | 3.3 ± 3.4 | 3.2 ± 2.9 | .98 |
| 7-month | 2.2 ± 3.0 | 2.6 ± 3.0 | .46 |
| SIP score (Mean ± SD) | |||
| Baseline | 13.1 ± 11.4 | 12.7 ± 10.9 | .77 |
| 4-month | 7.2 ± 9.9 | 6.7 ± 8.4 | .70 |
| 7-month | 5.7 ± 8.9 | 5.9 ± 8.7 | .89 |
| Abstinent days in the past 30 days (Mean ± SD) | |||
| Baseline | 8 ± 8 | 9 ± 8 | .47 |
| 4-month | 23 ± 10 | 22 ± 10 | .57 |
| 7-month | 25 ± 9 | 24 ± 9 | .81 |
| HIV CLINICAL CHARACTERISITCS | |||
| HIV viral suppression (<200 copies/mL) | |||
| Baseline | 57 (61%) | 62 (65%) | .64 |
| 4-month | 56 (68%) | 58 (67%) | .91 |
| 7-month | 53 (67%) | 57 (70%) | .65 |
| CD4+ count (Mean ± SD) | |||
| Baseline | 541 ± 295 | 571 ± 391 | .55 |
| 4-month | 544 ± 311 | 609 ± 384 | .23 |
| 7-month | 527 ± 295 | 592 ± 433 | .26 |
| Change of CD4+ count from baseline (Mean ± SD) | |||
| 4-month | 7 (130) | 25 (184) | .47 |
| 7-month | 3 (138) | 8 (213) | .84 |
| ≥95% ART adherence in the past 30 daysa | |||
| Baseline | 47 (59%) | 51 (63%) | .58 |
| 4-month | 48 (63%) | 51 (70%) | .39 |
| 7-month | 44 (60%) | 50 (66%) | .49 |
SD = Standard deviation
p values by chi-square tests for categorical and t-tests for continuous measures
Among participants who were currently on ART
Subgroup Analyses
In subgroup analyses, naltrexone appeared to be superior to placebo in reducing unhealthy drinking in women with alcohol dependence at baseline (n=92), but not in women without alcohol dependence at baseline (n=79), although the interaction of naltrexone and baseline alcohol dependence was not statistically significant (Table 3). The primary outcome did not differ by treatment group according to the presence of a positive PEth alcohol biomarker at baseline, adherence to the study medication during follow-up, or age (Table 3).
Table 3.
Impact of naltrexone on 4-month unhealthy alcohol use: Subgroup analyses.
| Quit or reduced drinking (%) | |||
|---|---|---|---|
| Naltrexone (n=85) |
Placebo (n=87) |
p value* | |
| Alcohol dependence | |||
| Yes (n=92) | 28 (56%) | 14 (33%) | .03 |
| No (n=79) | 16 (47%) | 25 (56%) | .45 |
| PEth value | |||
| Positive, ≥8 ng/mL (n=80) | 19 (46%) | 13 (33%) | .24 |
| Negative, <8 ng/mL (n=92) | 25 (57%) | 26 (54%) | .80 |
| Adherence to study medication | |||
| ≥90% (n=112) | 26 (52%) | 27 (44%) | .37 |
| <90% (n=58) | 18 (53%) | 11 (46%) | .59 |
| Age | |||
| <50 years old (n=88) | 19 (49%) | 24 (53%) | .67 |
| ≥50 years old (n=84) | 25 (54%) | 15 (36%) | .08 |
p value from bivariate logistic regression within each subgroup
Adverse Events
Adverse events that appeared to be related to the study medications were common. Although the differences were not statistically significant (51% versus 41%, p=0.19), side effects tended to be more common in women receiving naltrexone, especially for gastrointestinal symptoms such as nausea, abdominal pain, diarrhea, and vomiting (Table 4). Severe adverse events occurred in 11 participants in the naltrexone group (1 death and 10 inpatient hospitalizations) and 8 in the placebo group (2 deaths and 6 inpatient hospitalizations). None of these was determined to be related to the study medication. Four participants in the naltrexone group and two in the placebo group stopped taking the study medication due to possible drug-related adverse events (nausea/vomiting, itchiness, elevated liver enzymes, and constipation).
Table 4.
Adverse Events related or possibly related to the study medication
| Naltrexone (n=96) |
Placebo (n=98) |
p value* | |
|---|---|---|---|
| # Participant affected | 49 (51%) | 40 (40.8%) | .19 |
| Severe Adverse Events | 0 | 0 | - |
| Other Adverse Events | |||
| Nausea | 22 (22.9%) | 14 (14.3%) | .12 |
| Increased Appetite | 14 (14.6%) | 11 (11.2%) | .49 |
| Itchiness | 6 (6.3%) | 9 (9.2%) | .44 |
| Fatigue | 8 (8.3%) | 5 (5.1%) | .37 |
| Dizziness | 8 (8.3%) | 4 (4.1%) | .22 |
| Decreased Appetite | 5 (5.2%) | 6 (6.1%) | .78 |
| Sleepiness | 7 (7.3%) | 4 (4.1%) | .33 |
| Abdominal Pain | 7 (7.3%) | 3 (3.1%) | .21 |
| Diarrhea | 8 (8.3%) | 1 (1.0%) | .02 |
| Vomiting | 7 (8.3%) | 2 (2.0%) | .10 |
| Headache | 1 (1.0%) | 6 (6.1%) | .12 |
| Nervousness/Anxiety | 2 (2.1%) | 4 (4.1%) | .68 |
| Insomnia | 2 (2.1%) | 2 (2.0%) | 1 |
| Abnormal liver lab value | 2 (2.1%) | 1 (1.0%) | .62 |
| Depression | 2 (2.1%) | 0 (0.0%) | .24 |
| Constipation | 1 (1.0%) | 0 (0.0%) | .49 |
| Upset stomach | 0 (0.0%) | 1 (1.0%) | 1 |
p value based on chi-square test
Relationship of Drinking Reduction to HIV Clinical Outcomes: Secondary Analysis
HIV viral load and CD4+ counts were compared in subjects who successfully reduced/quit drinking at the final 7-month time point compared to those who continued unhealthy alcohol use at the end of the study. As shown in Figure 3, women who ultimately reduced/quit drinking (n=106, 64%) tended to have improved viral suppression over time, whereas women who continued to drink at unhealthy levels (n=60, 36%) tended to get slightly worse over time. The proportion of women with viral suppression was statistically significantly better in the women who successfully reduced/quit at both the 4-month and 7-month time points, compared to those who continued to drink at unhealthy levels (Figure 3). In a propensity-weighted logistic regression model, the odds of having successful viral suppression at 7 months were significantly greater in women who had reduced/quit drinking compared to those who did not (OR 2.53, 95% CI 1.04–6.18). This model adjusted for treatment assignment (naltrexone or placebo) and other variables that were associated with HIV viral suppression at the 7-month time point, including AUDIT-10 score, number of abstinent days at baseline, ART use, tobacco use, and baseline HIV viral suppression. Overall, the average CD4+ counts were stable during the 7 months of study observation, and no significant changes occurred overall in persons who quit/reduced drinking or in those who continued to drink (data not shown).
Figure 3.
Proportions of participants who had suppressed HIV viral load (<200 copies/mL) among participants who completed the study at 7 months and either had reduced unhealthy alcohol use (n=106) or continued unhealthy alcohol use (n=60).
Discussion
We sought to determine whether oral naltrexone would be effective in reducing drinking in WLWH, many of whom have multiple medical, psychiatric, and substance abuse comorbidities that would typically exclude them from alcohol pharmacotherapy clinical trials. We also sought to determine whether a reduction in drinking would be associated with improved HIV clinical outcomes. Overall, naltrexone was not associated with improvements in the primary endpoint of reduction in unhealthy alcohol use after 4 months of treatment. However, naltrexone was associated with statistically significant reductions in the average number of drinks per week at 1 month and 3 months after enrollment, and naltrexone also appeared to be superior to placebo in women with symptoms of alcohol dependence at baseline.
Overall, the drinking results are similar to those of other studies comparing naltrexone to placebo, which tend to demonstrate mild or modest improvements for naltrexone when compared to placebo (Canidate et al., 2017; Kranzler and Soyka, 2018). However, these modest improvements should be balanced by a relatively high number of persons who experience side effects such as nausea and vomiting (Sinclair et al., 2016). The fact that 94% of study participants were African-American is different from most previous alcohol trials. Previous data suggest that naltrexone may not be as effective in African-Americans than other racial groups (Ray and Olsen, 2009; Bress et al., 2015), and this could have contributed to the relative lack of an effect in our study.
Ultimately, one of the primary goals of drinking reduction is to improve overall health outcomes. Most clinical trials involving naltrexone have only examined its effect on drinking, although reanalysis of least one study demonstrated that reduction in drinking among clinical trial participants is accompanied by improvements in clinical health such as blood pressure, liver enzymes, and quality of life (Witkiewitz et al., 2018). Two recent studies sought to determine whether naltrexone or other alcohol-related therapy can improve HIV outcomes, one of which found that reductions in drinking were associated with improved HIV viral suppression (Edelman et al., 2018; Springer et al., 2018).
The fact that women receiving placebo did so well is striking. Placebo effects have been noted across a range of alcohol clinical trials and could be greater in those with less severe conditions at baseline (Litten et al., 2013). Placebo effects can be biological (e.g., the target receptors are activated) or psychological (e.g., expectancies and/or conditioning) (Finniss et al., 2010). The reduction in drinking in both groups also suggests that other aspects of research study participation could be equally or more important than the specific pharmacologic medication for overall drinking reduction. Women in this study may have chosen to participate in a clinical trial related to drinking because they were already interested in quitting. Although we did not directly inquire whether participants were treatment-seeking, many participants may have been interested in reducing or stopping their drinking and waiting for the right opportunity to attempt to change their drinking (Cook et al., 2017). All women received multiple assessments related to alcohol consumption and its consequences, and reliable evidence suggests that repeated assessments alone can have an impact on overall drinking behavior (McCambridge and Kypri, 2011). This study had consistent research staff throughout the study, and many participants commented that the study staff seemed to care about them as persons, beyond being just a participant in a research study. Such social support could also be a mechanism to help women succeed in changing their drinking behavior.
Our data suggest that a reduction in drinking to less than unhealthy levels can result in improved HIV-related clinical outcomes for women, regardless of the specific medication used. While other observational data from clinical trials or longitudinal cohorts have shown increases in drinking to be associated with worse HIV clinical outcomes, little evidence suggests that reductions in drinking are associated with improved HIV-related outcomes such as HIV viral suppression (Barai et al., 2017; Williams et al., 2018). One challenge with traditional longitudinal alcohol studies is that persons may reduce drinking because they have become sicker, causing it to appear that quitting drinking may be more harmful than beneficial (Williams et al., 2018). Of note, a recently reported clinical trial of stepped integrated alcohol treatment within the VA healthcare system reported that persons receiving an alcohol intervention did have improved HIV viral suppression, compared to usual care, although that study sample was 98% male (Edleman et al., 2019).
One of the major limitations in this and nearly every other randomized clinical trial to change alcohol consumption is that the primary outcome is based on self-report. We attempted to maximize our self-reported outcomes by training our staff to conduct a detailed TLFB. Although we encouraged research staff to be neutral, it is always possible that women reported reductions in drinking to please study staff. The study recruited slightly fewer women than originally intended, which could have affected the ability to detect statistically-significant changes in drinking. However, the decision to stop the study with slightly less than the originally planned enrollment was supported by review of an updated power analysis by the study DSMB, who noted that retention was better than predicted. Notably, only about 50% of participants had a positive PEth biomarker at enrollment, which suggests that some women could have exaggerated their drinking behavior at baseline to enroll in a research study that paid cash incentives to participants. While PEth is clearly associated with drinking behavior in women at the aggregate level (Wang et al., 2018), some other studies have reported inconsistent results for PEth versus self-reported drinking in women (Littlefield et al., 2017; Papas et al., 2016). It remains unclear how sensitive or specific a PEth result is for an individual woman, or how its test characteristics may vary according to the time between the last drinking episode and the PEth assessment (Moore et al., 2018). It was also notable that the average AUDIT score was 17, which should correlate strongly with alcohol use disorder, yet only 62% of women met criteria for alcohol use disorder.
Summary and Conclusions
This study showed that it is feasible to provide oral naltrexone to WLWH who also have a range of other medical and substance use comorbidities and that participating in a clinical trial with repeated assessments appears to have an impact on drinking overall. While our study did not find that naltrexone provided superior results than placebo, reductions in drinking were associated with improved HIV viral suppression. These data support recommendations to reduce drinking in WLWH infection and also suggest that continued research is needed to identify new medications or other alcohol interventions that can more effectively help women reduce or stop undesired alcohol consumption.
Acknowledgements
This investigators greatly appreciate the input and assistance from the study recruitment team (Diego Bueno, Gabrielle Barquin, and Diana Harwood), study coordinators (Ximena Levy, Allison Trainor, and Jennifer Steshyn), DSMB (Robert Mallow, Bob Kolb, and Adam Gordon), research pharmacy support (Khemraj Hirani and Eric Scott Zetka), and all of the women who participated in the study.
Support:
NIAAA U01AA020797 and U24AA02002, NCATS and NIMHD 1UL1TR000460
Sources of Support
The authors confirm they have no conflicts of interest with this publication. This study was supported by the SHARC Center for Translational HIV Research (NIAAA U01AA020797 and U24AA02002) and the Miami Clinical and Translational Science Institute (NCATS and NIMHD 1UL1TR000460). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C (2006) Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol Oxf Oxfs 41:431–437. [DOI] [PubMed] [Google Scholar]
- Aziz M, Smith KY (2011) Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am 52 Suppl 2:S231–237. [DOI] [PubMed] [Google Scholar]
- Badiee J, Riggs PK, Rooney AS, Vaida F, Grant I, Atkinson JH, Moore DJ, Hiv Neurobehavioral Research Program (HNRP) Group (2012) Approaches to identifying appropriate medication adherence assessments for HIV infected individuals with comorbid bipolar disorder. AIDS Patient Care STDs 26:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barai N, Monroe A, Lesko C, Lau B, Hutton H, Yang C, Alvanzo A, McCaul ME, Chander G. The Association Between Changes in Alcohol Use and Changes in Antiretroviral Therapy Adherence and Viral Suppression Among Women Living with HIV. AIDS Behav. 2017. July;21(7):1836–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress A, Kittles R, Wing C, Hooker SE Jr, King A. Genetic ancestry as an effect modifier of naltrexone in smoking cessation among African Americans: an analysis of a randomized controlled trial. Pharmacogenet Genomics. 2015. June;25(6):305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T (2006) Variable selection for propensity score models. Am J Epidemiol 163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canidate SS, Carnaby GD, Cook CL, Cook RL (2017) A Systematic Review of Naltrexone for Attenuating Alcohol Consumption in Women with Alcohol Use Disorders. Alcohol Clin Exp Res 41:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL (2015) Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ 351:h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2016) HIV Among Women. Available at: http://www.cdc.gov/hiv/group/gender/women/ Accessed July 24, 2016.
- Chakraborty H, Iyer M, Duffus WA, Samantapudi AV, Albrecht H, Weissman S (2015) Disparities in viral load and CD4 count trends among HIV-infected adults in South Carolina. AIDS Patient Care STDs 29:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G, Monroe AK, Crane HM, Hutton HE, Saag MS, Cropsey K, Eron JJ, Quinlivan EB, Geng E, Mathews WC, Boswell S, Rodriquez B, Ellison M, Kitahata MM, Moore RD, McCaul ME. HIV primary care providers--Screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend. 2016. April 1;161:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, McGinnis KA, Samet JH, Fiellin DA, Rodriguez-Barradas MC, Rodriquez-Barradas MC, Kraemer KL, Gibert CL, Braithwaite RS, Goulet JL, Mattocks K, Crystal S, Gordon AJ, Oursler KK, Justice AC (2010) Erectile dysfunction drug receipt, risky sexual behavior and sexually transmitted diseases in HIV-infected and HIV-uninfected men. J Gen Intern Med 25:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Weber KM, Mai D, Thoma K, Hu X, Brumback B, Karki M, Bryant K, Rathore M, Young M, Cohen M (2017) Acceptability and feasibility of a randomized clinical trial of oral naltrexone vs. placebo for women living with HIV infection: Study design challenges and pilot study results. Contemp Clin Trials 60:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Zhu F, Belnap BH, Weber KM, Cole SR, Vlahov D, Cook JA, Hessol NA, Wilson TE, Plankey M, Howard AA, Sharp GB, Richardson JL, Cohen MH (2013) Alcohol consumption trajectory patterns in adult women with HIV infection. AIDS Behav 17:1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss RG, Mesner O, Agan BK, Ganesan A, Okulicz JF, Bavaro M, Lalani T, O’Bryan TA, Bebu I, Macalino GE (2016) Characterizing the Association Between Alcohol and HIV Virologic Failure in a Military Cohort on Antiretroviral Therapy. Alcohol Clin Exp Res 40:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Moore BA, Holt SR, Hansen N, Kyriakides TC, Virata M, Brown ST, Justice AC, Bryant KJ, Fiellin DA, Fiellin LE (2018) Efficacy of Extended-Release Naltrexone on HIV-Related and Drinking Outcomes Among HIV-Positive Patients: A Randomized-Controlled Trial. AIDS Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Maisto SA, Hansen NB, Cutter CJ, Dziura J, Deng Y, Fiellin LE, O’Connor PG, Bedimo R, Gibert CL, Marconi VC, Rimland D, Rodriguez-Barradas MC, Simberkoff MS, Tate JP, Justice AC, Bryant KJ, Fiellin DA. Integrated stepped alcohol treatment for patients with HIV and alcohol use disorder: a randomised controlled trial. Lancet HIV. 2019. May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Bataller R (2011) Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141:1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JA, Samet JH (2010) Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep 7:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Roache JD (2005) The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl 157–167; discussion 140. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311:1889–1900. [DOI] [PubMed] [Google Scholar]
- Kader R, Govender R, Seedat S, Koch JR, Parry C (2015) Understanding the Impact of Hazardous and Harmful Use of Alcohol and/or Other Drugs on ARV Adherence and Disease Progression. PloS One 10:e0125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso NE, Sheps DS, Cook RL (2015) The association between alcohol use and cardiovascular disease among people living with HIV: a systematic review. Am J Drug Alcohol Abuse 41:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M (2018) Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 320:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Castle IJ, Falk D, Ryan M, Fertig J, Chen CM, Yi HY. The placebo effect in clinical trials for alcohol dependence: an exploratory analysis of 51 naltrexone and acamprosate studies. Alcohol Clin Exp Res. 2013. December;37(12):2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Brown JL, DiClemente RJ, Safonova P, Sales JM, Rose ES, Belyakov N, Rassokhin VV (2017) Phosphatidylethanol (PEth) as a Biomarker of Alcohol Consumption in HIV-Infected Young Russian Women: Comparison to Self-Report Assessments of Alcohol Use. AIDS Behav 21:1938–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW (2013) Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addict Abingdon Engl 108:275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, Kypri K (2011) Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PloS One 6:e23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R (1985) THE DRINKER INVENTORY OF CONSEQUENCES (DrInC) - match04.pdf. Available at: http://pubs.niaaa.nih.gov/publications/ProjectMatch/match04.pdf Accessed July 24, 2016.
- Moore KE, Santiago Rivera OJ, Anderson B, Johnson JE, Hahn JA, Kurth ME, Reddy MK, Schonbrun YC, Stein MD (2018) Phosphatidylethanol Levels Among Incarcerated Women: The Influence of Pre-incarceration Alcohol Consumption and Length of Abstinence. Alcohol Clin Exp Res 42:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2008) Helping Patients Who Drink Too Much: A Clinician’s Guide | National Institute on Alcohol Abuse and Alcoholism (NIAAA). Available at: https://www.niaaa.nih.gov/guide Accessed July 24, 2016.
- Papas RK, Gakinya BN, Mwaniki MM, Keter AK, Lee H, Loxley MP, Klein DA, Sidle JE, Martino S, Baliddawa JB, Schlaudt KL, Maisto SA (2016) Associations Between the Phosphatidylethanol Alcohol Biomarker and Self-Reported Alcohol Use in a Sample of HIV-Infected Outpatient Drinkers in Western Kenya. Alcohol Clin Exp Res 40:1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R (2016) Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS Lond Engl 30:273–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Mattison ME (2010) Medical Management Treatment Manual - MMManual.pdf. Available at: http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf Accessed July 25, 2016. [Google Scholar]
- Ray LA, Oslin DW. Naltrexone for the treatment of alcohol dependence among African Americans: results from the COMBINE Study. Drug Alcohol Depend. 2009. December 1;105(3):256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch C, Tate JP, Akgün KM, Crystal S, Wang KH, Ryan Greysen S, Wang EA, Bryant KJ, Fiellin DA, Justice AC, Rimland D (2016) Alcohol-Related Diagnoses and All-Cause Hospitalization Among HIV-Infected and Uninfected Patients: A Longitudinal Analysis of United States Veterans from 1997 to 2011. AIDS Behav 20:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addict Abingdon Engl 88:791–804. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sinclair JMA, Chambers SE, Shiles CJ, Baldwin DS (2016) Safety and Tolerability of Pharmacological Treatment of Alcohol Dependence: Comprehensive Review of Evidence. Drug Saf 39:627–645. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Kowalska JD, de Wit S, Law M, el Sadr W, Kirk O, Friis-Moller N, d’Arminio Monforte A, Phillips AN, Sabin CA, Lundgren JD, D:A:D Study Group (2014) Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet Lond Engl 384:241–248. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline Follow-back: A technique for assessing self-reported ethanol consumption In: Measuring Alcohol Consumption: Psychosocial and Biological Methods, pp 41–72. Totowa, NJ, Humana Press. [Google Scholar]
- Springer SA, Di Paola A, Barbour R, Azar MM, Altice FL (2018) Extended-release Naltrexone Improves Viral Suppression Among Incarcerated Persons Living with HIV and Alcohol use Disorders Transitioning to the Community: Results From a Double-Blind, Placebo-Controlled Trial. J Acquir Immune Defic Syndr 1999 79:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall KP, Clark RA, Powell A, Smith H, Kissinger P (2007) Alcohol consumption, ART usage and high-risk sex among women infected with HIV. AIDS Behav 11:205–215. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA (2008) Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res 32:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen X, Hahn JA, Brumback B, Zhou Z, Miguez MJ, Cook RL (2018) Phosphatidylethanol in Comparison to Self-Reported Alcohol Consumption Among HIV-Infected Women in a Randomized Controlled Trial of Naltrexone for Reducing Hazardous Drinking. Alcohol Clin Exp Res 42:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, McGinnis KA, Bobb JF, Rubinsky AD, Lapham GT, Skanderson M, Catz SL, Bensley KM, Richards JE, Bryant KJ, Edelman EJ, Satre DD, Marshall BDL, Kraemer KL, Blosnich JR, Crystal S, Gordon AJ, Fiellin DA, Justice AC, Bradley KA. Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort. Drug Alcohol Depend. 2018. August 1;189:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Kranzler HR, Hallgren KA, O’Malley SS, Falk DE, Litten RZ, Hasin DS, Mann KF, Anton RF. Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder. Alcohol Clin Exp Res. 2018. December;42(12):2453–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]