Abstract
Scope:
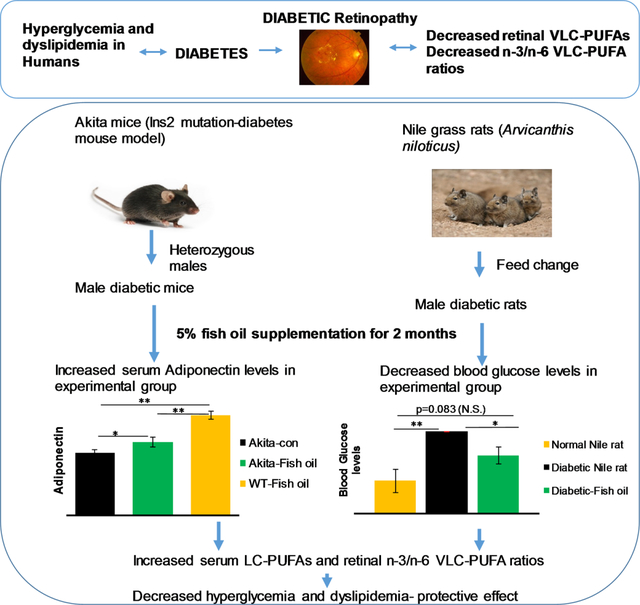
Long chain (LC)-PUFAs act as precursors for special class of retinal lipids known as Very-long-chain (VLC)-PUFAs and the effect of diabetes on retinal VLC-PUFA levels has been unexplored. In order to understand the supplemental effect of n-3 PUFAs, on decreasing levels of VLC-PUFAs due to diabetes, we chose Nile rat which develops diabetes spontaneously and Akita mouse, a genetic diabetes model.
Methods and results:
Human retinal punches from donors were collected from eye bank, lipids were extracted and analyzed to study the alterations in VLC-PUFAs and their n-3/n-6 ratios. Nile rats were fed high fat diet to induce hyperglycemia, following which an n-3 PUFA rich diet was fed to the experimental group for two months. Diabetic male Akita mice and WT mice were fed with 5% fish oil mixed in with their chow for two months to observe the effect of n-3 PUFAs. Results indicate that VLC-PUFA levels were lower in human diabetic and retinopathic retinal punches compared to age-matched controls. With supplementation of n-3 PUFAs, there was a significant increase in n-3/n-6 VLC-PUFA ratios in both animal models compared to diabetic controls.
Conclusion:
Dietary supplementation with n-3 LC-PUFAs help to prevent progression of diabetes and associated retinopathy.
Keywords: VLC-PUFAs, n-3/n-6 VLC-PUFA ratio, diabetic retinopathy, Nile grass rats, n-3/n-6 LC-PUFA ratio
Graphical Abstract
Layman Summary:
VLC-PUFAs are a special class of non-dietary fatty acids present in the vertebrate retina and testes. We used diabetic animal models to observe the effect of n-3 LC-PUFAs on replenishing VLC-PUFAs and observed that n-3 PUFAs not only help to improve n-3 VLC-PUFAs but also decrease blood glucose levels and improve diabetic conditions in these animal models.
1. Introduction:
Diabetes mellitus (DM), a growing public health concern, reportedly affected nearly 422 million people worldwide in 20141. Diabetic retinopathy (DR) is a major complication of diabetes and the leading cause of blindness in 30–60 year-olds. DR results from chronic diabetic damage to small blood vessels in the retina caused by above normal blood sugar levels2. DR has two phases: a non-proliferative phase characterized by increased vascular permeability and hemorrhages, and a proliferative phase characterized by retinal neovascularization, both of which are accompanied by vision loss3.
The role of lipids in the progression of DR began to be documented in the 1950s. Later studies involving diabetes control and complication trials also confirmed that dyslipidemia (altered serum lipid levels) is associated with the progression of diabetes4. Dyslipidemia is a complex disorder involving abnormal levels of lipids and altered fatty acid compositions in the plasma that arise due to metabolic disproportion partly caused by the imbalance of insulin levels. Dyslipidemia is also an established risk factor for cardiovascular diseases and is strikingly common in patients with DM, affecting almost 50% of this population5. In addition to hyperglycemia and hypertension, dyslipidemia is a modifiable cardiovascular risk factor that remains largely uncontrolled in patients with DM. Although a few studies have shown a correlation between dyslipidemia and diabetes, the association is not well understood. Most clinical guidelines recommend tight control of dyslipidemia, especially in high risk patients6. Both systemic and retina-specific fatty acid profiles are affected by diabetes and have been suggested to contribute to the progression of DR. Hence, we deemed it important to study the alterations of the fatty acid compositions in both retina and serum to understand the disease process and to facilitate development of effective therapeutics.
Among the fatty acids, long-chain polyunsaturated fatty acids (LC-PUFAs) have been gaining the interest of researchers and supplement companies with regard to inflammation and human health. Omega-3 (n-3) LC-PUFAs (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) are anti-inflammatory, while omega-6 (n-6) LC-PUFAs such as arachidonic acid (AA) are proinflammatory in nature. The balance between these two fatty acids plays a pivotal role in disease progression and health. Mouse model studies, human clinical trials, and epidemiological studies have demonstrated that intake of n-3 LC-PUFAs such as DHA, EPA, or fish (a major source of both DHA and EPA) has protective effects against the progression of many lifestyle-related retinal diseases like age-related macular degeneration (AMD), DR and glaucoma2, 7, 8.
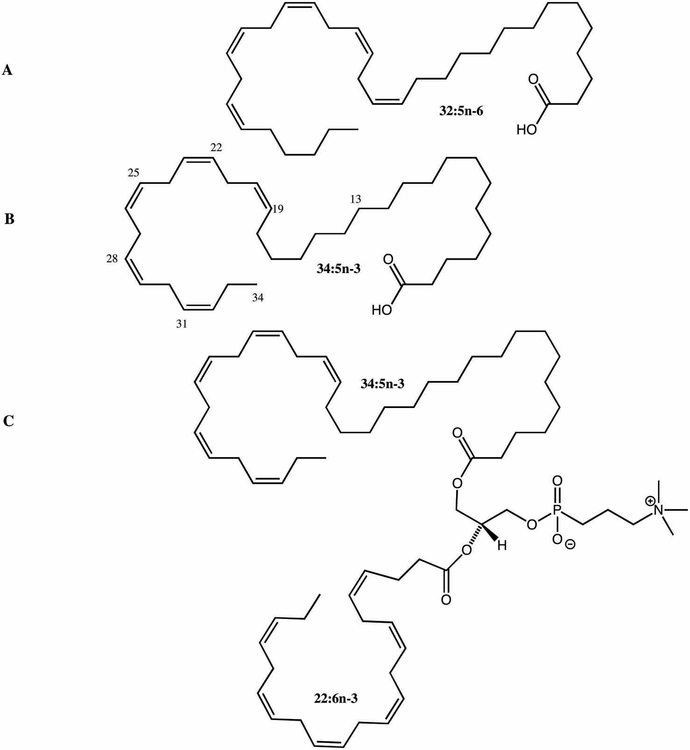
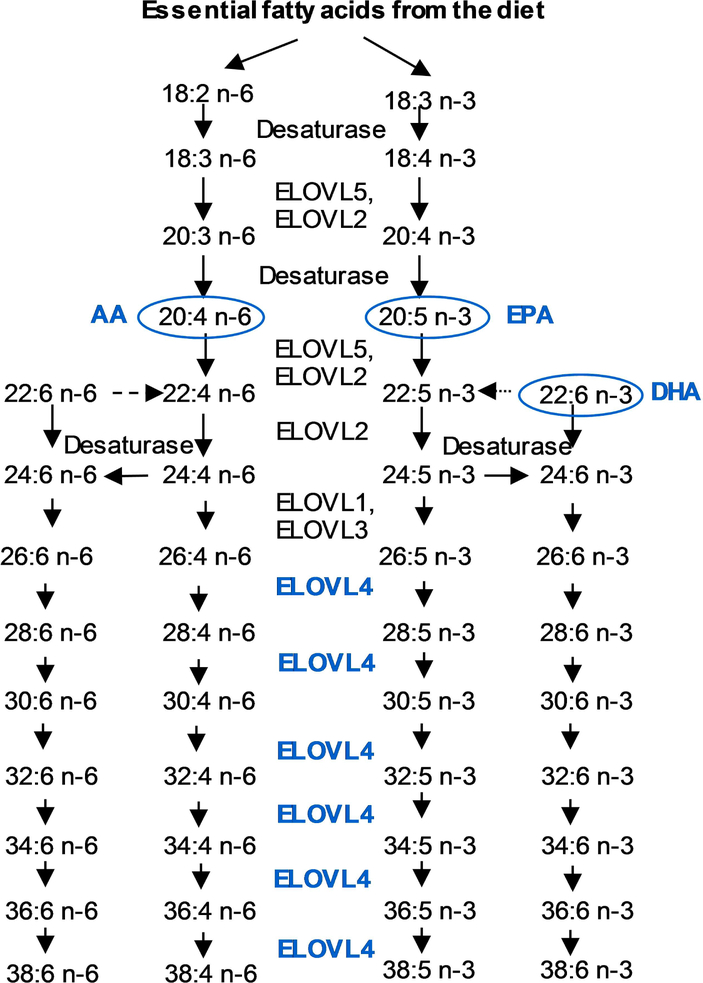
Very-long-chain polyunsaturated fatty acids (VLC-PUFAs) are a recently recognized new class of fatty acids that are considered important for retinal membrane fluidity and maintenance of the highly curved membrane disks of the photoreceptor outer segments (Figure 1). VLC-PUFAs are non-dietary fatty acids with chain lengths greater than 24 carbons; they have been identified in the vertebrate retina and a few other tissues such as testes. Until recently, very little attention had been paid to these rare C26-C38 retinal lipids due to their low abundance (<2% of all retinal fatty acids) and technical difficulties in measuring their retinal levels by standard GC methods. These lipids exhibit a unique hybrid structure, combining a proximal end with a typical saturated fatty acid character and a distal end more characteristic of common PUFAs (Figure 1). These rare fatty acids cannot be synthesized de novo in vertebrates and are rarely consumed in normal diets. They are synthesized in vivo from specific precursors such as α-linolenic acid (18:3 n-3), EPA (20:5 n-3), linoleic acid (18:2 n-6), and AA (20:4 n-6) through the action of an enzyme known as elongase 4 (ELOVL4) (Figure 2).
Figure 1:
VLC-PUFA structures (A. 32:5 n-6; B. 34:5 n-3; C. Phospholipid with 34:5 n-3 at sn-2 position and DHA at sn-1 position).
Figure 2:
Biosynthetic pathway of VLC-PUFAs in the mammalian retina.
In a recently published study from our laboratory, we observed that diet can profoundly influence retinal lipid (VLC-PUFA) composition9. For example, one subject who consumed 7g of fish oil/day showed a six-fold increase in EPA/AA and n-3/n-6 LC-PUFA ratios in serum, and a subsequent increase in n-3/n-6 VLC-PUFA ratios in retina, indicating a strong impact of diet on n-3/n-6 VLC-PUFA ratios in retina9. Moreover, significant reductions in retinal VLC-PUFA levels and n-3/n-6 ratios were noted in donated eyes from individuals with histories of AMD relative to age-matched controls. With this background, we sought to explore whether retinal VLC-PUFA levels and their n-3/n-6 ratios were also altered in diabetes and DR.
We hypothesized that diabetes and DR affects the retinal lipid profile in the eyes and that these alterations can be ameliorated by dietary supplementation of n-3 PUFAs. For this investigation, we chose human donor eyes to study the differences in retinal lipid profiles and animal models (spontaneous diabetes and genetic diabetes models) for n-3 LC-PUFA rich fish oil supplementation. We studied the LC-PUFA, VLC-PUFA levels and their n-3/n-6 ratios, glucose levels, serum adiponectin and gene expression levels of AdipoR1, a regulatory switch for DHA10 and ELOVL4, an elongase required for the biosynthesis of VLC-PUFAs11.
2. Materials and Methods:
2.1. Chemicals:
All chemical reagents, such as methanol, hydrochloric acid, isopropanol, n-hexane and diethyl ether, were of gas chromatography mass spectrometry (GC-MS) grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). All standards, including the internal standards such as tridecanoic acid (13:0), and Supelco-37 (a commercial mixture of fatty acid methyl esters (FAMEs)) were purchased from Matreya (Pleasant Gap, PA, USA). Silica gel, glass-encased solid phase extraction cartridges (500 mg/6 ml) were purchased from Sorbent Technology (Atlanta, GA, USA). A glucometer was purchased from Bayer HealthCare LLC (Pittsburgh, PA, USA). Serum adiponectin levels were measured by Mouse Adiponectin/Acrp30 Immunoassay (R&D Systems, Minneapolis, MN, USA). All assays were performed according to the manufacturers’ protocols. Prolab RMH 2000 diet, Custom made-fish oil mouse diet and control diet were purchased from Lab Supply, TX, USA and Labdiet, MO, USA respectively. Fish oil (1,560 mg of PUFA/5mL) for Nile rat supplementation was purchased from Nordic Naturals, Inc. (Watsonville,CA, USA).
2.2. Human Donor Eye Samples:
All human donor eyes and respective blood were collected from the Utah Lions Eye Bank by the Steele Center for Translational Medicine under an IRB-approved protocol. Donor eyes with large drusen, severe macular atrophy, macular hemorrhage, or any grossly visible chorio-retinal pathologic abnormalities were excluded. 6 mm punches of extramacular retina and serum samples were collected. All punched human retinal tissues were stored in tubes and kept at −80°C. We collected diabetic human donor eyes (n=17) between the ages of 76–90. As controls, age-matched control-eyes (n=22) and serum were collected using the same procedures.
2.3. Nile Rat Model:
Nile rats (also known as Nile grass rats) (Arvicanthis niloticus) are diurnal animals with cone-rich retinas, and they are a validated model of spontaneous diabetes which, in many ways, mimics the human form of diabetic condition and better than streptozotocin-induced mouse or rat models of diabetes. In general, Nile rats were fed with guinea pig diet in a special room set up with 37°C temperature and 70% humidity. Male Nile rats with a blood glucose level of 250 mg/dL on guinea pig chow were fed with Prolab RMH 2000 diet (low fiber and high fat) for 2 months to develop hyperinsulinemia and hyperglycemia leading to diet-induced DM. After confirmation of diabetes(blood glucose levels more than 500 mg/dL), all diabetic rats were divided into two groups one group fed RMH 2000 as control and experimental group was fed RMH 2000 plus fish oil (n=6/group). After two months of supplementation, all rats were sacrificed, serum, eyes, and other organs were collected. Eyes were dissected under a light microscope to separate retinal pigment epithelium from retinas, and collected samples were frozen at −80 °C for future analysis of LC-PUFAs and VLC-PUFAs.
2.4. Akita Mouse Model:
Akita mice (C57BL/6J) have an Ins2 mutation which results in a single amino acid substitution in the insulin 2 gene which causes misfolding of the insulin protein. Akita mice develop diabetic symptoms, such as hyperglycemia, polydipsia, and polyuria soon after weaning. 3-month-old Akita mice were fed with fish oil diet for two months. After supplementation, all mice were sacrificed, and organs were harvested. Retinas were separated from retinal pigment epithelium and used for LC-PUFA and VLC-PUFA analysis. Mice were genotyped by PCR amplification of genomic DNA from tail biopsy by specific primer pairs for C57BL/6-Ins2Akita/J (fwd: 5’-TGC TGA TGC CCT GGC CTG CT-3’ ; rev: 5’- TGG TCC CAC ATA TGC ACA TG-3’). All the procedures used in this study were approved by appropriate Institutional Animal Care and Use Committees and were carried out according to National Institutes of Health guidelines.
2.5. Dosage Administration:
For Nile rats, the above mentioned fish oil (5%) was mixed with pulverized RMH 2000 diet and fed to the experimental group for two months. For Akita mice, a 5% fish oil mouse chow (AIN-93G), custom-made by Test Diet was used for feeding to the experimental Akita and wild type (WT) mice. On average, the Nile rats and Akita mice were given 1.2 g/kg body weight/day and 3.13 g/kg body weight/day of n-3 PUFA, respectively.
2.6. Extraction of Lipids and Analysis
Retina samples were homogenized with a Biospec Beadbeater, and internal standards (50 μg of tridecanoic acid) were added along with 4 ml hexane-isopropanol (3:2 v:v). After centrifugation at 3,000 rpm for 5 min, the supernatant was transferred to a clean vial and then dried under a stream of nitrogen. To the dried film, 4% HCl in methanol was added and incubated at 80°C for 4 hr to form FAMEs. The FAME mixture was extracted with hexane and dried under nitrogen gas. Silica gel, glass-encased solid phase extraction cartridges were subsequently used to clean the FAMEs extracts. The cartridge was initially washed with hexane, and the eluate was discarded after which the FAMEs were eluted with hexane:ether (8:2) and dried. The dry film was dissolved in hexane, and 1 μl of sample was injected into the GC-MS instrument for LC-PUFA analysis. For VLC-PUFA analysis, the sample was dried with nitrogen again and re-dissolved in 20 μl of nonane, and 5 μl samples were injected into the GC-MS instrument. Analyses employed a Thermo Trace single quadrupole GC-MS DSQ II system (Thermo Fisher Scientific, Waltham, MA, USA). The chromatographic separation was carried out with a Rxi-5MS coated 5% diphenyl/95% dimethyl polysiloxane capillary column (30 m × 0.25 mm i.d, 0.25 μm film thickness) (Restek, Bellefonte, PA, USA). The GC-MS analyses protocol is detailed in an earlier publication12.
2.7. Statistical Analysis:
Differences between groups were evaluated by one-way ANOVA or Student’s t-test with p<0.05 as significant. All statistical analyses were conducted using Graph Pad Prism version 7 software. Data are reported as mean ± SEM.
3. Results:
3.1. Effect of Diabetes on Retinal Lipid Profiles in Human Eyes:
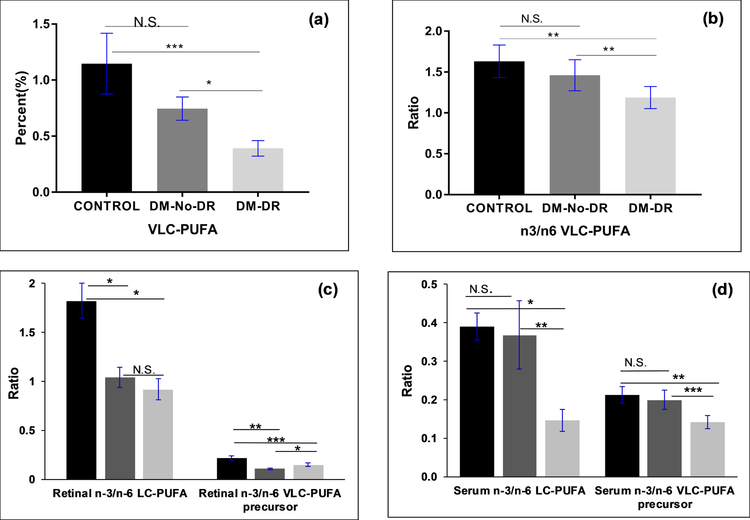
Seventeen diabetic and twenty-two age-matched control human retinal punches (age between 76–90) and their respective serum were extracted for lipids using the procedure elsewhere described, and analyzed by GC-MS9. The retinal VLC-PUFA levels and n-3/n-6 VLC-PUFA ratios in human age-matched control versus diabetic retinal punches are shown in Figure 3a and 3b. The VLC-PUFA levels were significantly lower in retinal punches from samples derived from donors with diabetic retinopathy (DM-DR) (n=8), as compared to those derived from diabetic donors without retinopathy (DM-No-DR) (n=9) and age-matched controls (n=22).
Figure 3:
Comparison of (a) retinal VLC-PUFA levels; (b) n-3/n-6 VLC-PUFA ratios (c) retinal LC-PUFAs (d) serums lipid biomarkers in age-matched controls (n=22), diabetic-No-Diabetic retinopathy (DM-No-DR) (n=9) and diabetic retinopathy (DM-DR) (n=8) donor retinal punches. ( -Age-Matched controls;
-Age-Matched controls;  -DM-No-DR;
-DM-No-DR;  -DM-DR) (P values: ***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
-DM-DR) (P values: ***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
The n-3/n-6 VLC-PUFA ratios were significantly reduced in DM-DR retinas compared to DM-No-DR and age-matched control retinas. The present data also showed a decreasing trend in n-3/n-6 retinal LC-PUFA ratios in DM-DR compared with DM-No-DR and age-matched controls. As shown in Figure 3c, the retinal n-3/n-6 LC-PUFA and n-3/n-6 VLC-PUFA precursor ratios dropped in DM-DR. The major VLC-PUFA precursors (LC-PUFAs responsible for synthesis of VLC-PUFAs in the presence of ELOVL4) for retinal VLC-PUFAs were as follows: 18:3n-3, 18:4n-3, 20:4n-3, 20:5n-3, and 22:5n-3 for n-3 VLC-PUFAs and 18:3n-6, 20:3n-6, 20:4n-6, and 22:4n-6 for n-6 VLC-PUFAs. We compared the n-3/n-6 VLC-PUFA precursor ratios in retina, and observed a decrease in the ratios, indicating the loss of n-3 LC-PUFAs during retinopathy. We compared the serum lipid profiles of diabetics and age-matched controls. The serum biomarkers like EPA/AA, DHA/AA and n-3/n-6 LC-PUFA also dropped in a similar trend to retinal LC-PUFA ratios. The n-3/n-6 LC-PUFA and n-3/n-6 VLC-PUFA precursor ratios in DM-DR serum samples were significantly lower than those in age-matched control serum (p<0.05) (Figure 3d).
We investigated whether the loss of VLC-precursors or lack of the enzyme ELOVL4 are responsible for decreased VLC-PUFA levels in the retinopathy retinas by genotyping the retinas for ELOVL4 variants (responsible for elongation of LC-PUFAs to form VLC-PUFAs). We did not observe any significant relationship between ELOVL4 variants and retinal lipid profiles. We also genotyped genetic variations in the AdipoR1 gene (responsible for transport of n-3 LC-PUFAs to the retina) and again did not observe any significant associations with abnormal lipid profiles.
3.2. Effects of Fish Oil Supplementation on Diabetic Nile Rats:
Due to the limited availability of diabetic human donor eyes, we chose to investigate the effect of fish oil supplementation in diabetic animal models. For this experiment, we chose Nile rat because they develop DM in a similar progression to humans and are a good model for spontaneous diabetes. As mentioned in the Methods section, we supplemented a group (n=6) of diabetic male Nile rats with 5% fish oil mixed in diet, with the other group (n=6) receiving no fish oil as control.
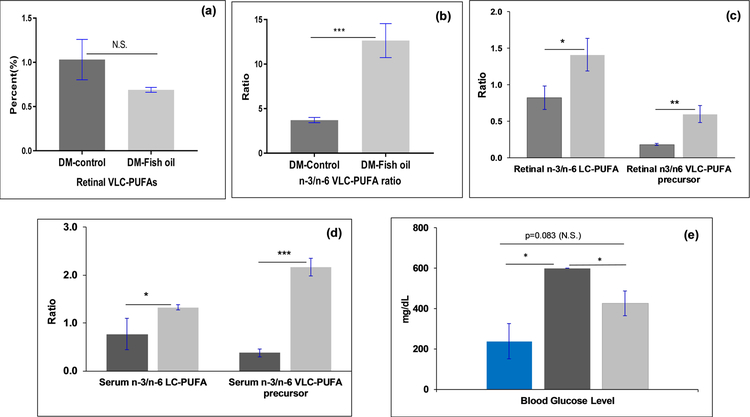
Even though the VLC-PUFA levels did not change significantly (Figure 4a) with fish oil feeding, the n-3/n-6 VLC-PUFA ratio improved in the retina (Figure 4b), indicating a slow recycling of VLC-PUFAs and an improved n-3 VLC-PUFAs and n-3 LC-PUFAs in the retina, which may be a key factor in decreasing dyslipidemia in the retina. The fish oil supplemented group had remarkably higher retinal ratios of EPA/AA, DHA/AA, n-3/n-6 LC-PUFA and retinal n-3/n-6 VLC-PUFA precursor ratios in comparison to the diabetic control group (p<0.05) (Figure 4c). Increased n-3/n-6 LC-PUFA levels in the retina shows that in spite of being diabetic, the transport process of EPA and DHA from fish oil diet to the retinas is functional in these rats.
Figure 4:
Comparison of (a) retinal VLC-PUFA levels; (b) n-3/n-6 VLC-PUFA ratios; (c) retinal LC-PUFA ratiosratios; (d)serum lipid biomarkers; (e) blood glucose levels in n-3 PUFA rich fish oil supplemented diabetic Nile rats group (n=6) in comparison to control diabetic Nile rat group (n=6) ( -DM-control nile rats;
-DM-control nile rats;  -DM-Fish oil supplemented Nile rats;
-DM-Fish oil supplemented Nile rats;  - non diabetic control Nile rats) (***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
- non diabetic control Nile rats) (***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
In our earlier study, we indicated that serum EPA/AA and n-3/n-6 LC-PUFA ratios serve as important biomarkers that correlate with the n-3/n-6 VLC-PUFA ratios in the retina, and this was likewise true in this experiment. Thus, dietary fish oil supplementation improved serum n-3 VLC-PUFA precursor levels (Figure 4d) and retinal n-3/n-6 VLC-PUFA ratios (Figure 4b), indicating that diet could play a significant role in improving dyslipidemia. We also tested the glucose levels of Nile rats and found that the serum glucose levels of the fish oil supplemented group were significantly reduced in comparison to the control group, consistent with beneficial systemic effects of fish oil on diabetes (Figure 4e).
3.3. Effects of Fish Oil Supplementation on Diabetic Mouse Model:
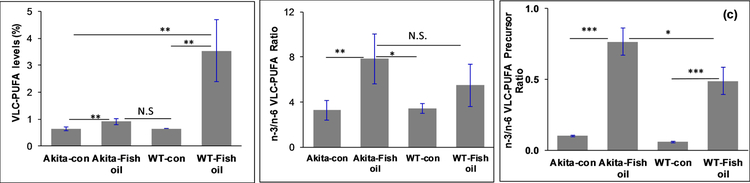
The effects of fish oil supplementation on Akita mice (a genetic model for type 1 diabetes with a Ins2 gene mutation) were also assessed. Male Akita mice develop type 1 diabetes as early as 2 months old. In the present study, one group of Akita mice (n=6) was fed with fish oil chow (5%) for 2 months, and the other group (n=6) was fed a control chow (without added fish oil) to study the influence of fish oil supplementation on n-3/n-6 VLC-PUFA ratios, dyslipidemia, blood glucose and serum adiponectin. As a control, age-matched WT mice (n=6) were also fed with fish oil chow for 2 months. The results of the study indicate that the n-3/n-6 VLC-PUFA ratios improved significantly in fish oil fed Akita mice in comparison to the control diet fed Akita mice, although VLC-PUFA levels were not altered significantly in the former group compared to the latter (Figure 5a). As shown in Figure 5b, the n-3/n-6 VLC-PUFA ratio in fish oil fed WT mice was insignificantly lower than supplemented Akita mice, although the VLC-PUFA levels were higher in the former group compared to the latter group.
Figure 5:
Effect of fish oil supplementation on (a) retinal VLC-PUFA levels; (b) n-3/n-6 VLC-PUFA ratios (c) n-3/n-6 VLC-PUFA precursor ratios of Akita mice (n=6) and WT mice (n=6) in comparison to control Akita mice (n=6) and WT control mice (n=6) respectively (***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
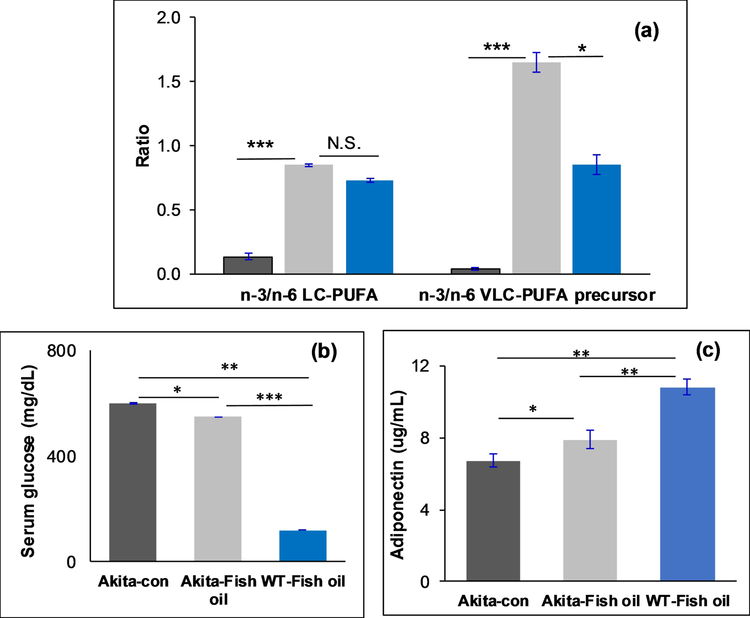
The retinal n-3/n-6 LC-PUFA ratios improved approximately threefold in the fish oil fed Akita mouse group in comparison to the control diet group. The n-3/n-6 VLC-PUFA precursor ratios in retina also improved significantly in the fish oil fed Akita mice compared to the control diet group (Figure 5c). Since DHA levels are quite constant in the retina, we did not see any significant differences between the groups. In the serum, we observed significantly higher n-3/n-6 LC-PUFAs and VLC-PUFA precursor ratios in the fish oil supplemented group compared to the control diet group (Figure 6a)(P<0.05). These results indicate that an increase in n-3 PUFAs in diet significantly decreases the levels of dyslipidemia in diabetes.
Figure 6:
Comparison of serum (a) lipid biomarkers; (b) Adiponectin levels (c) glucose levels in fish oil supplemented Akita mice group (n=6), fish oil supplemented WT mice (n=6) compared to Akita control group (n=6) ( - Akita-control;
- Akita-control;  - Akita-fish oil supplemented;
- Akita-fish oil supplemented;  - WT mice-fish oil supplemented) (***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
- WT mice-fish oil supplemented) (***P<0.005; **P<0.01; *P<0.05; N.S., not significant).
We also observed that serum adiponectin levels improved significantly in the fish oil supplemented groups in comparison to the control group (p<0.05). Higher serum adiponectin levels in WT mice are consistent with the previous results of serum adiponectin levels being lower in both mice and humans with diabetes (Figure 6b). We also tested serum glucose levels in all groups before sacrificing the animals, and observed that serum glucose levels improved in the fish oil supplemented group significantly in comparison with the control group (Figure 6c).
4. Discussion:
Even with the unique presence and specificity of VLC-PUFAs in the human retina, there are controversies regarding their beneficial effects and the clinical significance of their role in health and disease. There is considerable evidence that mutations in the ELOVL4 gene, required for the biosynthesis of VLC-PUFAs in retina, result in Stargardt-3 (STGD3) disease, a dominant juvenile macular dystrophy10, 13. However, the mechanistic process by which STGD3 disease occurs, either by protein aggregation or by VLC-PUFA deficiency, is not well characterized14, 15. Our clinical trials with STGD3 patients have indicated that consuming fish16 and possibly fish oil supplements17 improved the disease phenotype, which suggests that fish oil supplementation with n-3 PUFA supplementation can actually reverse VLC-PUFA deficiency and improve vision. Epidemiological studies generally support the recommendation that consuming foods rich in n-3 LC-PUFAs may lower the risk of developing retinal degenerative diseases like AMD7, 8, glaucoma18 and DR19, but prospective clinical intervention studies with n-3 PUFA supplements have been either negative or equivocal20.
In the present study, the lipid profiles of seventeen diabetic donor retinal punches were compared with twenty-two age-matched control donor retinal punches. This is the first report to measure VLC-PUFA levels in human diabetic retinas, and we observed a decreasing trend in VLC-PUFA levels and n-3/n-6 VLC-PUFA ratios in diabetic retinas in comparison to age-matched control retinas. In correlation with our study, Kady et al21 reported that intravitreal delivery of human ELOVL4 reduced the diabetes-induced increase in vascular permeability, thereby increasing VLC-ceramides in diabetes. Tikhonenko et al22 also observed a decrease in retinal VLC-PUFA levels and a decrease in ELOVL4 expression levels as well as ELOVL4 protein levels in early diabetic animals in comparison to control animals. These studies indicate that diabetes has a profound effect on VLC-PUFA levels even before retinopathy symptoms are detected. Since seven of the donors had DR, we compared diabetic donor eyes having no retinopathy (DM-No-DR) with those of the diabetic retinopathy donors and also with age-matched controls. Even though the numbers were small, we observed that retinal VLC-PUFA levels in diabetic retinopathy are lower than than the DM-No-DR group (Figure 3a) indicating that DR affects retinal VLC-PUFAs as well as membrane permeability.
We also observed changes in serum LC-PUFAs and other lipid biomarkers. As shown in Figure 2, the fatty acids which elongate to give rise to n-3 VLC-PUFAs are considered to be n-3 VLC-PUFA precursors, and the same terms are used for the n-6 series. Compared to the age-matched control,subjects with diabetic retinopathy has reduced retinal and serum n-3/n-6 LC-PUFA and n-3/n-6 VLC-PUFA precursor ratio indicating dyslipidemia (Figure 6). AdipoR1, a receptor for adiponectin in the eye known to control the delivery of fatty acids to the retina, is also known to be associated with retinal degeneration11, 23. According to the present results, we did not observe any differences between AdipoR1 and ELOVL4 gene expressions in these diabetic patients and age-matched controls. These results from the human retinal punches indicate that impaired insulin metabolism causes dyslipidemia, which affects n-3/n-6 VLC-PUFA ratios and VLC-PUFA levels as well as other serum biomarkers in humans.
n-3 PUFAs have long been studied for their therapeutic potential in the context of type 2 diabetes, insulin resistance and glucose homeostasis. Crochemore et al.24 and Ebbesson et al.25 demonstrated the influence of n-3 PUFAs in DM women and Alaskan Eskimos and suggested that n-3 PUFAs in serum can improve insulin sensitivity and glucose tolerance. Thorsdotter et al.26 also correlated the amount of n-3 PUFAs from cow’s milk to the prevalence of diabetes in Nordic populations. In spite of all these population studies and mouse studies, there is a lack of concrete knowledge about the practical application of n-3 PUFAs as nutritional therapeutics against insulin resistance in humans. Given the above studies, we conclude that supplementation with fish oil to diabetic animal models will improve glucose tolerance, n-3/n-6 LC-PUFA ratios in serum, and most importantly, counteracts the decreasing levels of VLC-PUFAs in the retina. Adiponectin is known to be the major regulator of lipid and glucose homeostasis through its insulin-sensitizing properties27, and lower adiponectin contributes to vascular complications and diabetic retinopathy. Hence in this study, we also considered adiponectin as a biomarker for diabetes.
The Nile grass rat is a diurnal rodent which is a becoming popular for studying circadian rhythms. It has a cone-rich retina with 30% cones in comparison to 3% cones in mice or rats28, making it is a very useful model to study eye diseases, and it is also a good model to study metabolic syndromes and spontaneous diabetes29. Diabetic Nile grass rats in the early phase of the disease develop hyperinsulinemia and show a strong inverse correlation between plasma adiponectin and HbA1C levels29. We hypothesized that the Nile grass rat would make a good model to study diabetes and its effects on VLC-PUFAs. As discussed in the methods section, we changed their regular guinea pig chow to an RMH 2000 diet, which altered their blood glucose levels and made all the male rats diabetic.
As depicted in Figure 4, the VLC-PUFA levels did not show any significant difference between the fish oil supplemented groups and control diet groups, but the n-3/n-6 VLC-PUFA ratio altered significantly. The retinal n-3/n-6 LC-PUFA ratio improved from 4.5 to 11 in the fish oil supplemented group, indicating decreased levels of dyslipidemia and improved n-3 VLC-PUFA precursor levels. There is a significant increase in the retinal EPA/AA and n-3/n-6 VLC-PUFA precursor ratios in the supplemented groups compared to the control diabetic group. To observe any differences in retinal VLC-PUFA levels, we may have to supplement fish oil for periods longer than two months. We also observed that fish oil supplementation improved serum lipid biomarkers like EPA/AA, n-3/n-6 LC-PUFA ratios and n-3/n-6 VLC-PUFA presursor ratios ,, which shows the improvement in dyslipidemia in these animals. As discussed in the methods section, the glucose levels of normal guinea pig chow fed male Nile rats were approximately 250 mg/dL, which after feeding high fat RMH 2000 diet for 2 months increased to above 600 mg/dL ratsduring the course of the experiment, while supplementation with fish oil decreased serum glucose levels to 400 mg/dL, consistent with the positive effects of 5% fish oil (containing 1360 mg/5 mL oil) supplementation on glucose tolerance (Figure 4e).
Furthermore, we studied the effect of n-3 PUFAs in Akita mice which is an Ins2 genetic diabetes (Ins2) mouse model. Male heterozygous KO (Ins2−/+) mice develop hyperglycemia in less than 2 months, while homozygous KO mice die before birth. Akita mice show various retinal pathologies characteristic of early non-proliferative diabetic retinopathy30 and also exhibit increased frequency of apoptic retinal neurons, increased vascular permeability31 and decreased retinal blood flow32. These properties could answer the question of whether or not availability of substrate or lack of enzyme leads to loss of VLC-PUFAs in diabetes. After supplementation of 3-month-old Akita mice with fish oil (5%), we observed that the serum and retinal lipid profiles improved in comparision to control Akita mice. The retinal VLC-PUFAs improved in fish oil supplemented Akita mice compared to the control Akita mice (Figure 5). The n-3/n-6 VLC-PUFA ratios improved significantly in fish oil fed groups (WT and Akita mice) in comparision to the control groups ..We also observed that retinal n-3/n-6 LC-PUFAs increased significantly in supplemented Akita mice compared to control Akita mice . In correlation with our study, earlier studies suggested that n-3 PUFA supplementation effectively reduced pathological retinal neovascularization and protected retinal neurons and retinal ganglion cells in various animal models33, 34. Our results are consistent with a clinical study in India that lower n-3/n-6 ratios in dietary lipids increase the prevalence of type 2 diabetes and that higher n-3/n-6 ratios may restore normal insulin action35. The serum n-3/n-6 LC-PUFA and n-3/n-6 VLC-PUFA precursor ratios improved significantly in the PUFA supplemented group compared to control Akita mice (Figure 6a).
Even though the differences in VLC-PUFA ratios in supplemented groups were not significantly different than control diabetic groups of Akita mice and Nile rats, we observed that n-3/n-6 VLC-PUFA ratios improved significantly in supplemented groups. Moreover, the serum lipid profile of diabetic supplemented groups had significantly higher n-3/n-6 LC-PUFA ratios compared to control groups. Previous experiments have shown that adding n-3 LC-PUFAs to the diet reduces the progression of retinopathy compared with controls36, 37. These animal studies found that a 2% change in dietary intake of n-3 LC-PUFA (n-3 vs n-6 FA) resulted in a twofold increase in retinal LC-PUFAs, which is in correlation with our studies. Also, we observed that serum adiponectin significantly improved in the fish oil supplemented group in comparison to the control group (Figure 6b), indicating the beneficial effects of n-3 PUFA supplementation in diabetic mice. Because of type 1 diabetes and hyperglycemia, the blood glucose levels for Akita mice were above 600 mg/dL before the experiment, but the supplemented group experienced a significant reduction in serum glucose compared with control diabetic animals (Figure 6c). This indicates that improvement in the lipid profile decreases dyslipidemia which in turn reduces hyperglycemia. Further studies with histopathological observations and adiponectin levels, AdipoR1 and ELOVL4 mRNA expression, and assessment of genetic variations in these genes will give us better knowledge of the benefits of fish oil supplementaion in diabetic models.
In conclusion, these data confirm our prior findings that retinal VLC-PUFA levels and n-3/n-6 VLC-PUFA ratios are lower in degenerated retinas in comparison with age-matched controls38, which indicates the need to understand the physiology of these special fatty acids. Humans have relatively less VLC-PUFAs compared to mice12, and further loss of these active molecules may indeed induce physiological and structural disruption in the photoreceptors as seen in DR. In the present study, we observed that a decrease in serum and retinal n-3 PUFAs is a plausible reason for the loss of retinal VLC-PUFAs and decreased n-3/n-6 ratios. Therefore, we studied the importance of fish oil supplementation in diabetic animal models with respect to retinal VLC-PUFAs. The retinal and serum n-3/n-6 LC-PUFA ratios increased in both the Nile rat and Akita mouse groups supplemented with fish oil, indicating reduced dyslipidemia. We also observed that fish oil supplementation reduces hyperglycemia and improves blood glucose and serum adiponectin levels in diabetic animal models. Diet plays an important role in altering the serum n-3/n-6 LC-PUFA ratios which in turn influence the n-3/n-6 LC-PUFA and VLC-PUFA ratios and levels in retina with possible beneficial effects on retinal physiology and protection against degeneration. Our results clarify the biological mechanisms underlying the epidemiological studies which have shown that diets rich in n-3 fatty acids are protective against retinal degenerative diseases. Future studies involving diabetic retinopathy should seek to discover the advantages of VLC-PUFAs and their role in degeneration, as well as the effect of hyperglycemia on these special fatty acids.
ACKNOWLEDGEMENTS
This work was supported by grants from the Research to Prevent Blindness and the National Institutes of Health (EY-14800). The invaluable assistance of Christopher Pappas, Preejith Vachali, Binxing Li and the donor eye acquisition staff members of the Utah Lions Eye Bank and the Steele Center for Translational Medicine Donor Eye Repository is gratefully acknowledged.
Abbreviations:
- AA
Arachidonic acid
- AMD
Age-related macular degeneration
- DHA
Docosahexaenoic acid
- DM
Diabetes mellitus
- DR
Diabetic retinopathy
- ELOVL4
Elongase 4
- EPA
Eicosapentaenoic acid
- LC
Long-chain
- n-3
Omega-3
- n-6
Omega-6
- STGD3
Stargardt-3 disease
- VLC
Very-long-chain
- WT
Wild type
Footnotes
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest. GH is a founder and shareholder of Voyant Biotherapeutics LLC.
5 References:
- [1].Mathers CD,Loncar D, Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006, 3, e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Klein BE, Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007, 14, 179. [DOI] [PubMed] [Google Scholar]
- [3].Lechner J, O’Leary OE,Stitt AW, The pathology associated with diabetic retinopathy. Vision Res. 2017, 139, 7. [DOI] [PubMed] [Google Scholar]
- [4].Hammer SS,Busik JV, The role of dyslipidemia in diabetic retinopathy. Vision Res. 2017, 139, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saydah SH, Fradkin J,Cowie CC, Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004, 291, 335. [DOI] [PubMed] [Google Scholar]
- [6].Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA,Soran H, Diabetes Dyslipidemia. Diabetes Therapy. 2016, 7, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].SanGiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris FL 3rd, Gensler GR, Kurinij N, Lindblad AS, Milton RC, Seddon JM,Sperduto RD, The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol 2007, 125, 671. [DOI] [PubMed] [Google Scholar]
- [8].Tan JS, Wang JJ, Flood V,Mitchell P, Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009, 127, 656. [DOI] [PubMed] [Google Scholar]
- [9].Gorusupudi A, Liu A, Hageman GS,Bernstein PS, Associations of human retinal very long-chain polyunsaturated fatty acids with dietary lipid biomarkers. J Lipid Res. 2016, 57, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Agbaga MP, Brush RS, Mandal MN, Henry K, Elliott MH,Anderson RE, Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc Natl Acad Sci U S A. 2008, 105, 12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rice DS, Calandria JM, Gordon WC, Jun B, Zhou Y, Gelfman CM, Li S, Jin M, Knott EJ, Chang B, Abuin A, Issa T, Potter D, Platt KA,Bazan NG, Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat Commun 2015, 6, 6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu A, Terry R, Lin Y, Nelson K,Bernstein PS, Comprehensive and sensitive quantification of long-chain and very long-chain polyunsaturated fatty acids in small samples of human and mouse retina. J Chromatogr A. 2013, 1307, 191. [DOI] [PubMed] [Google Scholar]
- [13].Vasireddy V, Wong P,Ayyagari R, Genetics and molecular pathology of Stargardt-like macular degeneration. Prog Retin Eye Res. 2010, 29, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Logan S, Agbaga MP, Chan MD, Kabir N, Mandal NA, Brush RS,Anderson RE, Deciphering mutant ELOVL4 activity in autosomal-dominant Stargardt macular dystrophy. Proc Natl Acad Sci U S A. 2013, 110, 5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barabas P, Liu A, Xing W, Chen CK, Tong Z, Watt CB, Jones BW, Bernstein PS,Krizaj D, Role of ELOVL4 and very long-chain polyunsaturated fatty acids in mouse models of Stargardt type 3 retinal degeneration. Proc Natl Acad Sci U S A. 2013, 110, 5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hubbard AF, Askew EW, Singh N, Leppert M,Bernstein PS, Association of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutation. Arch Ophthalmol. 2006, 124, 257. [DOI] [PubMed] [Google Scholar]
- [17].Choi R, Gorusupudi A,Bernstein PS, Long-term follow-up of autosomal dominant Stargardt macular dystrophy (STGD3) subjects enrolled in a fish oil supplement interventional trial. Ophthalmic Genet. 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Renard JP, Rouland JF, Bron A, Sellem E, Nordmann JP, Baudouin C, Denis P, Villain M, Chaine G, Colin J, de Pouvourville G, Pinchinat S, Moore N, Estephan M,Delcourt C, Nutritional, lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: an exploratory case-control study. Acta Ophthalmol. 2013, 91, 505. [DOI] [PubMed] [Google Scholar]
- [19].Sala-Vila A, Diaz-Lopez A, Valls-Pedret C, Cofan M, Garcia-Layana A, Lamuela-Raventos RM, Castaner O, Zanon-Moreno V, Martinez-Gonzalez MA, Toledo E, Basora J, Salas-Salvado J, Corella D, Gomez-Gracia E, Fiol M, Estruch R, Lapetra J, Fito M, Aros F, Serra-Majem L, Pinto X,Ros E, Dietary Marine omega-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals With Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142. [DOI] [PubMed] [Google Scholar]
- [20].Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. Jama. 2013, 309, 2005. [DOI] [PubMed] [Google Scholar]
- [21].Kady NM, Liu X, Lydic TA, Syed MH, Navitskaya S, Wang Q, Hammer SS, O’Reilly S, Huang C, Seregin SS, Amalfitano A, Chiodo VA, Boye SL, Hauswirth WW, Antonetti DA,Busik JV, ELOVL4-Mediated Production of Very Long Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability. Diabetes. 2018, 67, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, Renis RL, Kern T, Jump DB, Reid GE,Busik JV, Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010, 59, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kaarniranta K, Paananen J, Nevalainen T, Sorri I, Seitsonen S, Immonen I, Salminen A, Pulkkinen L,Uusitupa M, Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci Lett. 2012, 513, 233. [DOI] [PubMed] [Google Scholar]
- [24].Crochemore IC, Souza AF, de Souza AC,Rosado EL, omega-3 polyunsaturated fatty acid supplementation does not influence body composition, insulin resistance, and lipemia in women with type 2 diabetes and obesity. Nutr Clin Pract. 2012, 27, 553. [DOI] [PubMed] [Google Scholar]
- [25].Ebbesson SO, Risica PM, Ebbesson LO, Kennish JM,Tejero ME, Omega-3 fatty acids improve glucose tolerance and components of the metabolic syndrome in Alaskan Eskimos: the Alaska Siberia project. Int J Circumpolar Health. 2005, 64, 396. [DOI] [PubMed] [Google Scholar]
- [26].Thorsdottir I, Hill J,Ramel A, Omega-3 fatty acid supply from milk associates with lower type 2 diabetes in men and coronary heart disease in women. Prev Med. 2004, 39, 630. [DOI] [PubMed] [Google Scholar]
- [27].Frankenberg ADV, Reis AF,Gerchman F, Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review. Arch Endocrinol Metab. 2017, 61, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gilmour GS, Gaillard F, Watson J, Kuny S, Mema SC, Bonfield S, Stell WK,Sauve Y, The electroretinogram (ERG) of a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus). Vision Res. 2008, 48, 2723. [DOI] [PubMed] [Google Scholar]
- [29].Noda K, Melhorn MI, Zandi S, Frimmel S, Tayyari F, Hisatomi T, Almulki L, Pronczuk A, Hayes KC,Hafezi-Moghadam A, An animal model of spontaneous metabolic syndrome: Nile grass rat. Faseb j. 2010, 24, 2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Han Z, Guo J, Conley SM,Naash MI, Retinal angiogenesis in the Ins2(Akita) mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013, 54, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW,Bronson SK, The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005, 46, 2210. [DOI] [PubMed] [Google Scholar]
- [32].Wright WS, Yadav AS, McElhatten RM,Harris NR, Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2(Akita) mouse. Exp Eye Res. 2012, 98, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ, Tang J, Kern TS, Akula JD,Smith LEH, Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutrition &Amp; Diabetes. 2012, 2, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N Jr, Serhan CN,Smith LEH, Increased dietary intake of ω−3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature Medicine. 2007, 13, 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Raheja BS, Sadikot SM, Phatak RB,Rao MB, Significance of the N-6/N-3 ratio for insulin action in diabetes. Ann N Y Acad Sci. 1993, 683, 258. [DOI] [PubMed] [Google Scholar]
- [36].Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K,Smith LE, 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med 2011, 3, 69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr., Serhan CN,Smith LEH, Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007, 13, 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu A, Chang J, Lin Y, Shen Z,Bernstein PS, Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 2010, 51, 3217. [DOI] [PMC free article] [PubMed] [Google Scholar]