Abstract
Children on dialysis have a cardiovascular mortality risk equivalent to older adults in the general population, and rapidly develop medial vascular calcification, an age-associated pathology. We hypothesized that premature vascular ageing contributes to calcification in children with advanced chronic kidney disease (CKD). Vessels from children with Stage 5 CKD with and without dialysis had evidence of increased oxidative DNA damage. The senescence markers p16 and p21 were also increased in vessels from children on dialysis. Treatment of vessel rings ex vivo with calcifying media increased oxidative DNA damage in vessels from children with Stage 5 CKD, but not in those from healthy controls. Vascular smooth muscle cells cultured from children on dialysis exhibited persistent DNA damage, impaired DNA damage repair, and accelerated senescence. Under calcifying conditions vascular smooth muscle cells from children on dialysis showed increased osteogenic differentiation and calcification. These changes correlated with activation of the senescence-associated secretory phenotype (SASP), an inflammatory phenotype characterized by the secretion of proinflammatory cytokines and growth factors. Blockade of ataxia-telangiectasia mutated (ATM)-mediated DNA damage signaling reduced both inflammation and calcification. Clinically, children on dialysis had elevated circulating levels of osteogenic SASP factors that correlated with increased vascular stiffness and coronary artery calcification. These data imply that dysregulated mineral metabolism drives vascular “inflammaging” by promoting oxidative DNA damage, premature senescence, and activation of a pro-inflammatory SASP. Drugs that target DNA damage signaling or eliminate senescent cells may have the potential to prevent vascular calcification in patients with advanced CKD.
Keywords: aging, calcification, dialysis, senescence, vascular smooth muscle cells
Age is the dominant risk factor for cardiovascular disease, and medial vascular calcification is a prevalent, age-associated pathology. Medial calcification is also a prominent pathology in patients with chronic kidney disease (CKD) and progresses rapidly in patients on dialysis.1–3 Medial calcification is associated with increased vascular stiffening and cardiac workload, poor coronary perfusion, and sudden cardiac death and is thought to be responsible for the high cardiovascular mortality observed in CKD patients.4 Significantly, even children and adolescents on dialysis develop vascular calcification and have a vastly elevated risk for cardiovascular mortality when compared with the normal age-matched population. Strikingly, the risk in adolescence is equivalent to that of the very elderly in the general population.2,5 The clear association between aging and vascular calcification in the general population has led to the suggestion that CKD patients may exhibit accelerated vascular aging; however, so far, there is little direct molecular evidence to support this notion.6,7
A key event leading to cellular aging is the accumulation of unrepairable or persistent DNA damage.8,9 DNA damage increases with age and factors such as oxidative stress can accelerate aging in part, by promoting oxidative DNA damage.10–12 Persistent DNA damage signaling, via key transducers such as the kinase ataxia-telangiectasia mutated (ATM), promotes upregulation of the checkpoint cell cycle inhibitors p16 (CDKN2A/INK4a) or p21 (CDKN1A) or both leading to cell cycle arrest and ultimately to cellular senescence.13 Senescent cells are viable but can no longer contribute to repair processes. Importantly, they display an inflammatory phenotype termed the senescence-associated secretory phenotype (SASP) characterized by the secretion of an array of proinflammatory cytokines and growth factors as well as proteases that can act in a paracrine fashion to influence remote cells and tissues.14 DNA damage and cellular senescence have recently been reported to promote osteogenic differentiation of vascular smooth muscle cells (VSMCs), suggesting there may be a direct link between aging and calcification, however, the mechanisms involved as well as evidence for this process in vivo are still limited.15,16
In CKD nontraditional risk factors such as dysregulated calcium (Ca) and phosphate (P) metabolism accelerate vascular calcification by promoting VSMC death and osteogenic differentiation.17,18 There is also evidence to suggest that dysregulated mineral metabolism, and in particular elevated P, can drive premature aging.19 Fibroblast growth factor 23 and its obligate coreceptor Klotho are major physiological regulators of Ca and P metabolism.20 Mice deficient in either of these proteins develop an array of age-associated pathologies including osteoporosis, vascular calcification, and premature death in the context of hypercalcemia, hyperphosphatemia, and vitamin D dysregulation.21,22 Importantly normalization of mineral metabolism can alleviate premature aging in these models. Elevated P is also associated with increased cardiovascular calcification and mortality in aging populations;23 however, the molecular events linking aging and calcification with dysregulated mineral metabolism are not understood.
Children make an ideal model for studying accelerated aging. They are not confounded by long-term exposure to environmental stresses that complicate the interpretation of aging measures in adults. In the context of CKD, vascular damage and calcification occurs almost exclusively due to the complications of renal failure, rather than smoking, dyslipidemia, or preexisting cardiovascular disease that are prevalent in adults with CKD. Dysregulated mineral metabolism is a key cause of vascular calcification in children on dialysis,24 and we recently reported accumulation of the aging biomarker prelamin A in the calcified arteries of these children.15,25 Prelamin A interferes with DNA damage repair leading to accelerated VSMC senescence and activation of the SASP.15,26 This toxic nuclear protein also accumulates in the calcified vasculature of aged adults and is causal in the induction of accelerated vascular calcification and stiffening in children with the premature aging disorder Hutchinson-Gilford progeria syndrome.27,28
We hypothesized that vessels from children with CKD are prematurely aged and that persistent DNA damage leading to premature senescence may be a key event in driving accelerated calcification. We examined evidence for vascular aging and senescence in children both in vitro and in vivo and correlated these with clinical vascular measures. We found direct evidence for premature VSMC aging and define a potential role for DNA damage signaling and “inflammaging” in driving vascular calcification in children with CKD.
Results
Vessels from CKD children show premature vascular aging
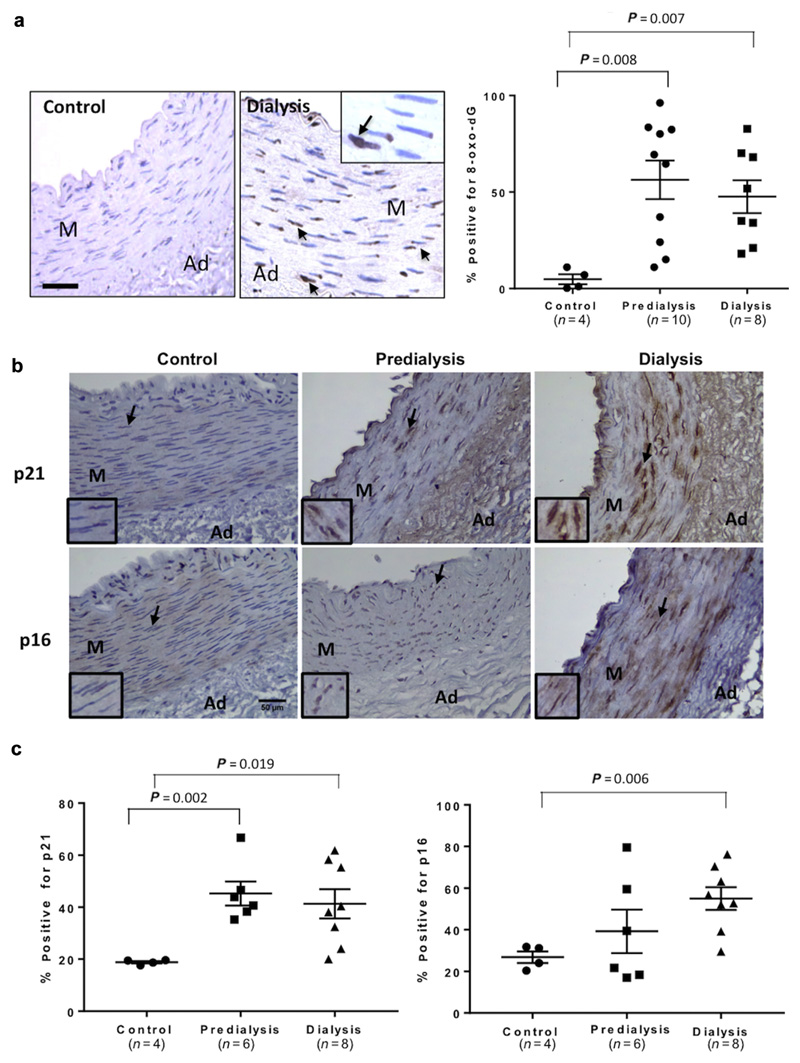
Medium-sized muscular arteries were harvested from children in predialysis CKD stage 5 (CKD5) and on dialysis (CKD5D), as well as healthy control subjects (Supplementary Table S1). An antibody to 8-oxo-dG that recognizes oxidatively modified DNA showed that vessels from control patients had low levels of oxidative DNA damage as expected in young children. In contrast, vessels from children with CKD5-5D showed Significantly elevated levels of oxidative DNA damage (Figure 1a). Compared with control subjects, vessels from CKD5-5D patients also showed elevated levels of the senescence marker p21. However, p16 was Significantly increased only in dialysis vessels with highly variable levels in CKD5 patients (Figure 1b and c).
Figure 1. Vessels from children with chronic kidney disease (CKD) show elevated levels of oxidative DNA damage and senescence markers.
(a) Immunohistochemistry and quantification for 8-oxo-dG showed a significantly increased percentage of vascular smooth muscle cells with oxidative DNA damage in vessels from predialysis (CKD5) and dialysis (CKD5D) patients compared with control subjects. Positive nuclei are indicated by arrows, and the boxed inset shows enlargement. Bar = 100 μm. (b) Immunohistochemistry showing increased p21 and p16 nuclear staining in CKD stage 5 predialysis (CKD5) and dialysis (CKD5D) vessels compared with control vessels. Note the nuclear staining (arrowed and insets). Bar = 50 μm. (c) The percentage of positively stained cells and nuclei for p21 was significantly higher in both CKD5 and CKD5D when compared with control vessels, while p16 was only significantly elevated in dialysis vessels. Graphs show mean ± SE (analysis of variance.) Ad, adventitia; M, media. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
VSMCs from children on dialysis show elevated levels of DNA damage and premature senescence in vitro
VSMCs were explanted from control and CKD5-5D vessels and compared during serial passaging in vitro. There was marked variability in growth potential between different CKD5-5D isolates (Supplementary Table S2 and Supplementary Figure S1) but commonly, VSMCs explanted from CKD patients grew more slowly and senesced at earlier passages than control cells did (control; passage >30 vs. CKD; passage 16.4 ± 10.1) (Figure 2a and b). Senescence was demonstrated using senescence-associated β-galactosidase staining and observation of an enlarged, flattened, cell morphology (Figure 2b). On Western blot, VSMCs approaching senescence showed elevated levels of p16 with variable levels of p21, suggesting senescence was mediated by p16 in vitro (Figure 2c).
Figure 2. Vascular smooth muscle cells (VSMCs) from dialysis vessels senesce early and show elevated levels of DNA damage in vitro.
(a–c) VSMCs grown from dialysis patients showed premature senescence compared with cells from control subjects as shown by (a) slower growth rates, (b) senescence-associated β galactosidase staining, and (c) elevated levels of p16 at equivalent passage number (P). Representative data from 3 control and 3 dialysis isolates are shown. Bar = 50 μm. (d) Immunofluorescence staining for γH2AX and phosphorylated ataxia-telangiectasia mutated (pATM) and ataxia telangiectasia and Rad3-related protein (ATR) substrate shows dialysis VSMCs have increased DNA damage signaling when compared with control cells at equivalent early (P6) passage (positive nuclei are indicated by arrows). Bar = 10 μm. (e) The percentage of γH2AX– and pATM/ATR–positive nuclei was significantly higher in dialysis VSMCs compared with both control and predialysis (CKD5) cells. Each bar represents mean ± SE (***P < 0.001, dialysis vs. control and dialysis vs. predialysis cells, analysis of variance). (f) Western blot showing increased γH2AX and pATM/ATR protein levels in dialysis (n = 3, patients [pt] 04, 34, 51) VSMCs compared with chronic kidney disease stage 5 predialysis (n = 2, patients 07, 40) and control (n = 2, patients 20, 39) VSMCs at equivalent passage number. (g) Comet assays confirm increased DNA damage in dialysis VSMCs with an increased percentage of cells with comet tails (indicated by arrow in inset showing dialysis VSMCs) compared with control cells at equivalent passage (****P < 0.001, n = 3, Student’s t-test). Bar = 10 μm. DAPI, 4′,6-diamidino-2-phenylindole. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
To assess whether the limited growth potential of CKD VSMCs was associated with DNA damage, immunofluorescence staining for the DNA damage response signaling markers γH2AX and phosphorylated ataxia-telangiectasia mutated (pATM)–ataxia telangiectasia and Rad3-related protein substrate (pATM/ATR) was performed. VSMCs cultured from dialysis patients showed increased DNA damage compared with CKD5 or control subjects at equivalent passage numbers (Figure 2d and e). Elevated levels of DNA damage signaling in dialysis VSMCs was confirmed by Western blot performed on a limited number of cell isolates that had not been subjected to freeze-thaw cycles during culture, in order to limit any potential bias toward selection of healthy cells. Levels in control and CKD5 VSMCs were low and equivalent (Figure 2f). Comet assays were used to determine whether the elevated DNA damage signaling observed in dialysis VSMCs was due to unrepaired DNA double-strand breaks. This was confirmed as comet tails were observed in dialysis VSMCs and were mainly absent in control cell nuclei (3.5 ± 1.3% control vs. 37.1 ± 1.2% dialysis) (Figure 2g).
Ca and P induce oxidative stress and DNA damage in VSMCs
To test whether dysregulated mineral metabolism might contribute to DNA damage, control VSMCs were treated with calcifying media containing elevated levels of calcium and phosphate (CaP). Increased levels of DNA damage, shown by γH2AX nuclear foci and protein on Western blot were observed at levels equivalent to those induced by hydrogen peroxide and doxorubicin, both of which induce oxidative DNA damage (Figure 3a–c). Lucigenin assays demonstrated that calcifying media induced hydrogen peroxide production in VSMCs suggesting oxidative DNA damage may be responsible for the increased levels of DNA damage signaling observed (Supplementary Figure S2).
Figure 3. Elevated calcium and phosphate (CaP) caused oxidative stress and increased oxidative DNA damage in chronic kidney disease (CKD) vessels.
(a) Immunofluorescence staining for γH2AX and phosphorylated ataxia-telangiectasia mutated (pATM) or ataxia telangiectasia and Rad3-related protein (ATR) substrate or both indicates that CaP promoted DNA damage (arrows indicate positive nuclei). Bar = 10 μm. (b) The percentage of γH2AX– and pATM/ATR–positive nuclei in response to CaP media is similar to doxorubicin and hydrogen peroxide treatments and significantly higher compared with baseline vascular smooth muscle cells. Each bar represents mean ± SE (****P < 0.001 compared with baseline). Bar = 10 μm. (c) Western blotting shows an increase in γH2AX and pATM/ATR protein levels in calcifying media equivalent to doxorubicin (Dox) and hydrogen peroxide treatments. (d) Immunohistochemistry for 8-oxo-dG shows that calcifying media promotes an increase in 8-oxo-dG staining in CKD stage 5 predialysis (CKD5) and dialysis (CKD5D) vessels. (e) Quantification shows that calcifying media significantly increases 8-oxo-dG positive nuclei in CKD5 (P = 0.04; n = 4) and dialysis (P = 0.001; n = 4) vessels with a greater increase in dialysis vessels despite similar baseline levels of oxidative DNA damage. The effect of CaP on control vascular smooth muscle cells was not significant (P = 0.08; n = 4). M, media. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
To examine further the roles of Ca and P in the induction of DNA damage, vessel rings from control and CKD patients were exposed to calcifying medium for 7 days ex vivo and levels of 8-oxo-dG staining were quantified. Vessel rings from control patients were relatively resistant to the induction of oxidative DNA damage and did not show a significant elevation in staining. In contrast, VSMCs in CKD5-5D showed significantly elevated 8-oxo-dG staining after CaP treatment, with dialysis vessels showing the greatest accumulation over the time period tested (Figure 3d and e).
To explore the mechanism of increased susceptibility to DNA damage observed in dialysis VSMCs, we treated control and dialysis VSMCs with an acute dose of doxorubicin and examined levels of DNA damage before and after washout, to quantify the efficiency of DNA damage repair. The DNA damage response was similar in control and dialysis VSMCs after 2 hours; however, 24 hours after washout control cells showed a greater efficiency of repair evidenced by fewer γH2AX foci (Figure 4a). At this stage, dialysis VSMCs also showed evidence for persistent DNA damage with a higher percentage of cells retaining >5 53BP1 nuclear foci, which is consistent with a reduced repair capacity in these cells (Figure 4b).
Figure 4. Dialysis vascular smooth muscle cells (VSMCs) showed impaired DNA damage repair.
(a,b) Immunofluorescence showing elevated levels of DNA damage in dialysis VSMCs (arrows) 24 hours after washout of the DNA damage agent doxorubicin (Dox). In contrast, control VSMCs are mostly repaired at this stage. Quantification shows both γH2AX and 53BP1 foci are elevated. A high percentage of cells with >5 53BP1 foci is indicative of persistent DNA damage and failure of repair. n = 3 isolates/group. Analysis of variance (*P < 0.05, **P < 0.01). Bar = 10 μm. DMSO, dimethylsulfoxide. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
DNA damage and senescence drive increased calcification and the proinflammatory SASP in dialysis VSMCs
Given the clear differences in the DNA damage response between control and dialysis VSMCs, we next used quantitative reverse transcriptase–polymerase chain reaction to compare gene expression at equivalent early passage numbers between these 2 cell types. At baseline in normal media, levels of the senescence marker p16 were not different. However, VSMCs from dialysis patients had decreased levels of the differentiation marker α-smooth muscle actin and elevated expression of the osteogenic morphogen bone morphogenetic protein 2 (BMP2), as well as Runx2 and bone sialoprotein, although these did not reach significance (Figure 5a). In response to calcifying media, VSMCs from dialysis patients showed greater mineralization (Figure 5b). This was associated with increased expression of the osteogenic marker Runx2 and dramatically elevated BMP2 expression compared with expression in control subjects (Figure 5c).
Figure 5. Vascular smooth muscle cells (VSMCs) from dialysis patients show increased osteogenic differentiation and calcification.
(a) Quantitative reverse transcriptase–polymerase chain reaction shows that VSMCs from dialysis patients have reduced expression of SM markers and increased expression of osteogenic markers when compared with control VSMCs. Each bar represents mean ± SE, Student’s t-test. (b) Alizarin red staining shows VSMCs from dialysis vessels are more prone to calcify than VSMCs from control vessels in response to calcifying medium for 7 days. Each bar represents mean ± SE (*P < 0.05, analysis of variance). (c) Quantitative reverse transcriptase–polymerase chain reaction shows that VSMCs from dialysis patients have significantly elevated mRNA expression of the osteogenic markers Runx2 and bone morphogenetic protein-2 (BMP2) in response to calcifying media when compared with control media. Each bar represents mean ± SE (*P < 0.05, **P < 0.01, ***P < 0.001). Ca, calcium; CaP, calcium and phosphate; P, phosphate; SMA, smooth muscle actin.
We previously identified BMP2 as a SASP factor in adult aortic VSMCs,15 so we next tested whether elevated Ca or P or both might accelerate senescence in dialysis VSMCs. Senescence-associated β-galactosidase staining showed a small but significant increase in the number of senescent cells after short-term treatment (16 hours) with calcifying media in dialysis but not in control VSMCs. Of note, treatment with P alone was sufficient to increase the number of senescent cells (Figure 6a and b). Western blot showed that CaP media consistently increased the expression of p16 in dialysis VSMCs, in contrast to control VSMCs where no pattern was observed (Figure 6c). Western blot also confirmed elevated BMP2 in dialysis compared with control VSMCs in both normal and calcifying conditions (Supplementary Figure S3).
Figure 6. Dialysis vascular smooth muscle cells (VSMCs) show increased senescence in response to calcifying media.
(a,b) VSMCs were incubated in control (Con), calcium (Ca) (2.7 mmol/l), phosphate (P) (2.5 mmol/l), or CaP supplemented serum-free medium for 16 hours and stained for senescence-associated β galactosidase (SA-βG). Increased staining was observed in response to elevated CaP or P alone in dialysis VSMCs (***P < 0.001, analysis of variance). Bar = 100 μm. (c) Western blot shows that in response to elevated calcium and phosphate (CaP) p16 is increased in dialysis VSMCs but not in control VSMCs. The p21 levels did not consistently change in either group. Blots representative of n = 3 isolates/group. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
To examine in more detail the inflammatory SASP profile of control and dialysis VSMCs, antibody arrays were performed comparing conditioned media from equivalent early passage cells. A number of previously identified SASP factors were found to be abundantly secreted by VSMCs including the cytokines interleukin (IL)-6, IL-8, osteoprotegerin (OPG), and monocyte chemoattractant protein-1 and the protease inhibitors tissue inhibitor of metalloproteinase-1 and -2 (Supplementary Figure S4A and B). Dialysis VSMCs secreted elevated levels of a number of these factors compared with control cells including IL-6, IL-8, and OPG and also secreted elevated levels of the chemokines colony stimulating factor-2 and CXC chemokine ligand-1 and -3 although of these, only CXC ligand-3 was relatively abundant (Supplementary Figure S4B and C). Quantitative reverse transcriptase–polymerase chain reaction analysis was used to verify that BMP2, IL-6, and OPG were all more highly expressed by dialysis VSMCs than by control VSMCs and increased secretion of IL-6 and OPG was confirmed using enzyme-linked immunosorbent assay (Figure 7c and Supplementary Figure S5).
Figure 7. Inhibition of DNA damage signaling reduces calcification and expression of senescence-associated secretory phenotype (SASP) factors in dialysis vascular smooth muscle cells (VSMCs).
(a,b) Control and dialysis VSMCs were treated with osteogenic media in the presence or absence of the ataxia-telangiectasia mutated inhibitor (ATMi) Ku55933. Alizarin red staining shows that after 5 days, dialysis but not control VSMCs had calcified and calcification of dialysis cells was decreased by ATM inhibition. (*P < 0.05, analysis of variance.) (c) Quantitative reverse transcriptase–polymerase chain reaction showing levels of expression of the SASP factors bone morphogenetic protein-2 (BMP2), interleukin-6 (IL-6), and osteoprotegerin (OPG) in control and dialysis VSMCs. Note the higher levels of these factors in dialysis VSMCs at baseline. ATM inhibition using small, interfering RNA (siRNA) decreased elevated levels of the SASP factors BMP2, OPG, and IL-6 in dialysis VSMCs (pink) with little or no effect on control VSMCs (green). Effectiveness of ATM siRNA knockdown compared with control siRNA was equivalent for both control and dialysis VSMCs. (Mean ± SE, analysis of variance, *P < 0.05.) CaP, calcium and phosphate. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
To determine whether DNA damage signaling acting upstream of the inflammatory SASP was responsible for the increased calcification observed in dialysis VSMCs, both dialysis and control cells were treated with either a small, interfering RNA or a chemical inhibitor (Ku55933) targeting ATM, a key signaling kinase in the DNA damage response. ATM inhibition reduced the accelerated calcification observed in dialysis VSMCs (Figure 7a and b). Importantly the reduced calcification observed in dialysis VSMCs was mirrored by the pattern of expression of the SASP markers BMP2, IL-6, and OPG; ATM inhibition significantly decreased their expression in dialysis cells consistent with their activation in response to persistent DNA damage (Figure 7c).
CKD children show elevated levels of circulating SASP factors correlating with vascular aging measures
Three of the SASP factors elevated in dialysis VSMCs—BMP2, OPG, and IL-6—have previously been shown to play a role in regulating calcification.15 Therefore we quantified the serum levels of these factors in children with CKD5-5D, correlating these biochemical measures with vascular scans. Serum BMP2, OPG, and IL-6 levels did not depend on age or sex in children. However, both IL-6 and BMP2 levels were significantly higher in dialysis versus in the CKD5 group (Figure 8a and b), and IL-6 levels increased with increasing time on dialysis (Supplementary Figure S6A). The circulating levels of BMP2 and OPG correlated with arterial stiffness measured by aortic pulse wave velocity (PWV), a surrogate marker of medial calcification (Figure 8c and d). Moreover, levels of IL-6 correlated with both BMP2 and OPG, suggesting increases in these cytokines may occur concomitantly (Supplementary Figure S6B and C). Importantly, comparing CKD children with coronary artery calcification measured by multislice computed tomography scan (n = 8) against those who did not show calcification (n = 16), there was an increase in these secreted SASP factors in patients with overt calcification (Figure 8e), suggesting that the paracrine release of these factors may be clinically relevant.
Figure 8. Children with chronic kidney disease (CKD) show elevated senescence-associated secretory phenotype factors correlating with vascular stiffness.
(a,b) Serum levels of bone morphogenetic protein-2 (BMP2) and interleukin-6 (IL-6) in children with CKD stage 5 predialysis (n = 11) and those on dialysis (n = 24) showing significantly higher levels of both markers in children on dialysis. In children with CKD stage 5 and those on dialysis who were older than 5 years of age (n = 31), BMP2 and osteoprotegerin (OPG) correlated with aortic pulse wave velocity (PWV) measured by applanation tonometry (c,d), showing significant correlation between these markers and PWV. (e) Coronary artery calcification (CAC) was measured by multislice computed tomography scan and patients were divided into 2 groups: those with calcification (n = 8) and those who did not have calcification (n = 16).The median (range) CAC score was 149 (43 to 2019) in the calcification group and 7 of 8 calcified patients were on dialysis. Children with CAC had significantly higher levels of BMP2, OPG, and IL-6 compared with those without calcification (P < 0.0001; multiple analysis of variance). Comparing individual factors both BMP2 and IL-6 were significantly elevated in the calcified group while OPG was not (P values for unpaired t-test are indicated on legend).
Discussion
In this study, we show that vessels from children with CKD exhibited features of premature aging in vivo including oxidative DNA damage and elevated senescence markers, and these aging indices persisted on the culture of VSMCs in vitro. CaP treatment was shown to increase DNA damage in CKD vessel rings ex vivo and to drive senescence of dialysis VSMCs in vitro. The persistent DNA damage observed in dialysis VSMCs was associated with increased osteogenic differentiation and calcification driven in part, by activation of the proinflammatory SASP. Importantly, a number of these SASP factors, previously identified as procalcific, correlated with increased PWV and were significantly increased in the serum of children with clinically measurable calcification. Taken together, these data suggest that the paracrine secretion of inflammatory or osteogenic factors or both by aged VSMCs may be an important driver of arterial stiffening and vascular calcification in CKD. We suggest that the onset of VSMC senescence may be a key “set point” driving the rapid progression of this age-associated pathology in young CKD patients. Importantly, it may be that only a few senescent VSMCs are required to initiate this cascade.
VSMCs from children with CKD show hallmarks of premature aging
We demonstrated that CKD and in particular, the dialysis environment, is associated with increased oxidative DNA damage and premature senescence of VSMCs in vivo. While studies have shown that oxidative DNA damage and the senescence markers p16 and p21 are elevated in atherosclerotic plaque and adult vessels from CKD patients,11,29,30 this is the first study to show increased levels of these markers in vessels from young children, who are free of confounding factors including atherosclerosis, providing compelling evidence that the vascular pathology observed in these patients is closely associated with premature aging.31,32 Our data suggest that oxidative DNA damage accrual begins in predialysis CKD5, as levels of 8-oxo-dG and p21 were significantly elevated at this stage, but is accelerated in the dialysis milieu leading to unrepaired DNA damage and stress-induced premature senescence a hallmark of which is p16 accumulation.29,31
Dysregulated mineral metabolism promotes oxidative DNA damage and premature senescence in dialysis VSMCs
A major signal driving oxidative DNA damage was Ca and P stress. Osteogenic media induced oxidative DNA damage in CKD5-5D vessel rings cultured ex vivo, with control vessels showing some resistance to the same stimulus. This is consistent with our previous studies showing that dialysis vessels, and to a lesser extent CKD5 vessels, undergo VSMC death, osteogenic differentiation, and calcification when cultured ex vivo in Ca or P or CaP media, providing further support for a link between premature aging and calcification.24,33 There are a number of potential mechanisms, which are not mutually exclusive, that may explain why dialysis patient VSMCs show increased susceptibility to CaP stress. We showed that osteogenic media induced DNA damage and accelerated senescence of dialysis VSMCs in vitro and that these cells showed delayed DNA damage repair compared with control cells. Delayed repair may be due in part to the reported increased levels of prelamin A in dialysis VSMCs as this nuclear toxin delays DNA damage repair and accelerates senescence15,26 by sequestering and compromising nuclear import of essential DNA repair factors.26,34 Dialysis VSMCs also display decreased oxidant defenses35 and this, as well as mitochondrial damage,36 could lead to further elevations in reactive oxygen species. Of note, P alone was able to promote senescence of dialysis VSMCs and a recent study showed that P may interfere with the expression of a key longevity gene Sirtuin1, which deacetylates and inactivates p53, allowing cells to survive DNA damage.37 In addition, key longevity genes expressed in VSMCs such as Klotho are intimately linked with P metabolism and oxidative stress38 and these proteins are downregulated in response to aging and disease, suggesting further studies on the regulation and role of these factors in VSMCs are warranted.39,40
Aged VSMCs show increased inflammation, osteogenic differentiation, and calcification
The SASP is a storm of inflammatory cytokines released by cells in response to persistent DNA damage signaling,41,42 and previous studies have shown that senescent adult aortic VSMCs in vitro upregulate and secrete potent osteoinductive and inflammatory factors such as BMP2 and IL-6 as part of the SASP response.16,43,44 In this study, we showed that VSMCs from children on dialysis, without additional stimulation, expressed and secreted elevated levels of these same SASP osteoinductive factors and that CaP stimulation increased levels further. Furthermore, elevated levels of SASP inflammatory markers correlated with increased osteogenic differentiation and calcification of dialysis VSMCs while blocking ATM-mediated DNA damage signaling reduced inflammation and calcification. SASP factors including IL-6 and OPG have been reported to be elevated in both adult and pediatric dialysis patient cohorts31,45,46 while in adult CKD populations increased circulating levels of BMP2 have been reported correlating with increased serum 8-oxo-dG levels and vessel wall stiffness.47 It is plausible that the systemic release of cytokines from prematurely aged vascular cells may induce a vicious cycle of inflammation and calcification in CKD patients.42 In support of this hypothesis, elevated serum IL-6 levels in hemodialysis patients were found to associate with aortic calcification and to predict cardiovascular death.48,49 Moreover, IL-6 was a Significantly better predictor of mortality risk than other inflammation markers such as C-reactive protein, albumin, or tumor necrosis factor-α.50 The presence of SASP factors in the serum of patients on dialysis may also explain studies in vitro where dialysis serum promotes calcification of VSMCs and this is independent of serum Ca and P levels.51
Limitations and clinical implications
This study has a number of limitations that need to be addressed before taking any findings forward to clinical interventions. Although children provide the best model for investigating aging processes, tissue retrieval is difficult, hence, the study was limited by small sample size. A key question that was not fully addressed was whether the factors driving premature aging were present in predialysis CKD5 or at even earlier stages of CKD. Although the findings suggest that DNA damage does begin predialysis, this needs to be confirmed using a larger patient cohort. Moreover, the origin of the cells from different vascular beds, children of different ages and dialysis vintage, the inherent heterogeneity of the CKD population, and the phenotypic modulation that takes place in vitro were all reflected in the behavior of the VSMCs studied, making it impossible to draw further conclusions. The clinical studies were also limited by small sample size and the lack of a control group. The correlations with PWV and coronary artery calcification suggest that inflammation closely follows the onset of vessel stiffening and worsens when overt calcification is present. However, extrapolating from peripheral arteries to coronary arteries should be viewed with caution, although it is likely that in children, who are free of atherosclerosis, coronary calcification is also likely to be medial.
Despite these limitations, these findings may have important clinical implications because they do point to a mechanism whereby elevated oxidative DNA damage in vessels, probably starting in predialysis CKD, promotes a cascade of senescence, inflammation, and calcification. Studies have shown that oxidant stress occurs in CKD before dialysis therapy is initiated52 and increases according to duration of dialysis treatment,53,54 and it is likely that in addition to Ca and P, other factors present in the dialysis environment contribute to oxidative stress.55,56 These agents are likely to have a cumulative effect over time as evidenced by the increased senescence markers found in dialysis vessels, thus highlighting the need to monitor and maintain Ca and P levels within the age-appropriate range starting from CKD5.57 It also highlights that conventional clinical interventions, aimed at correcting circulating risk factors, must be complemented by alternate techniques and that drugs targeting alternate pathways such as the DNA damage response or senescent cells (senolytics) may be potential therapies to slow the progression of vascular calcification and associated “inflammaging” in this patient group.58,59
Materials and methods
See the extended methods in Supplementary Methods.
Patient samples
The study was conducted at Great Ormond Street Hospital, London, with full ethical approval from the Institutional Review Board (12/LO/1186). Medium-sized muscular arteries—omental, mesenteric, or inferior epigastric—routinely removed and discarded in the course of planned surgery were collected from CKD5-5D patients at the time of catheter insertion or renal transplantation and compared with disease-free, age-matched children (control subjects) undergoing intra-abdominal surgery. The demographic details and baseline calcium load in the vessel wall are shown in Supplementary Table S1. Freshly isolated vessel rings were paraffin embedded or cultured ex vivo in control or calcifying media for 7 days as previously described.33 VSMCs were grown by explant culture as previously described.60 Cells were counted in triplicate at each passage to ensure equivalent plating densities and to determine growth rates and used between passages 4 and 10 for comparisons. All experiments, except where indicated, were performed on at least 3 independent VSMC isolates from each patient group (Supplementary Table S2).
Antibody array
Antibody arrays (RayBio, human cytokine antibody array VI and VII, catalog no. AAH-CYT-6/-7; RayBiotech, Norcross, GA) were performed using conditioned media from control (n = 2) and dialysis (n = 2) patient VSMCs. Signals were normalized and quantified using densitometry and the signal ratio of control versus dialysis calculated. Enzyme-linked immunosorbent assay or quantitative reverse transcriptase–polymerase chain reaction for verification was performed as previously.15
Clinical studies
Simultaneous vascular imaging and blood tests were performed in children with CKD5 (n = 11) and those on dialysis (n = 24). Vascular measures were performed in all children above 5 years of age (n = 8 CKD5 and n = 23 dialysis patients). Applanation tonometry for aortic PWV and 64-slice spiral computed tomography scan were performed using methods previously described.28 Vascular scans were performed by a single blinded operator before a mid-week session of hemodialysis or after overnight cycling peritoneal dialysis. Serum levels of BMP2, IL-6, and OPG were determined using enzyme-linked immunosorbent assay (R & D Systems, Minnesota, MN) according to the manufacturer’s instructions.
Statistical analysis
Shapiro-Wilk test was used to test for normalcy of data before 1-way analysis of variance or Kruskal-Wallis tests were used to determine the significance of differences among groups as appropriate. Student’s t or Mann-Whitney U tests were used to assess differences between means as appropriate. Spearman correlation tests were used for correlation analyses. Statistical Package for Social Sciences Software (SPSS, IBM, Armonk, NY) and GraphPad software (GraphPad, San Diego, CA) were used for statistical computations. A P value < 0.05 was considered to indicate a significant difference.
Supplementary Material
Supplementary information is linked to the online version of the paper at www.kidney-international.org.
Acknowledgments
This work was supported by grants from the British Heart Foundation (RG/11/14/29056 and RG/17/2/32808) to CMS and a British Heart Foundation Clinical Research Fellowship to RS. PS acknowledges support from the European Renal Association—European Dialysis and Transplant Association for European Renal Association—European Dialysis and Transplant Association fellowships. RS holds a Career Development Fellowship with the National Institute for Health Research. A part of this work took place in the Biomedical Research Centre at Great Ormond Street Hospital for Children National Health Service Foundation Trust and University College London. DAL holds a Medical Research Council New Investigator Award (MR/J003638/1).
Footnotes
Disclosure
All the authors declared no competing interests.
References
- 1.Goodman WG. Vascular calcification in end-stage renal disease. J Nephrol. 2002;15(suppl 6):S82–S85. [PubMed] [Google Scholar]
- 2.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 3.Goodman WG, London G, Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14:525–531. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 6.Kovacic JC, Moreno P, Nabel EG, et al. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 7.Kovacic JC, Moreno P, Hachinski V, et al. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011;123:1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinet W, Knaapen MW, De Meyer GR, et al. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 11.Sedelnikova OA, Redon CE, Dickey JS, et al. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40:634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 14.Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Drozdov I, Shroff R, et al. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res. 2013;112:e99–e109. doi: 10.1161/CIRCRESAHA.111.300543. [DOI] [PubMed] [Google Scholar]
- 16.Burton DGA, Matsubara H, Ikeda K. Pathophysiology of vascular calcification: pivotal role of cellular senescence in vascular smooth muscle cells. Exp Gerontol. 2010;45:819–824. doi: 10.1016/j.exger.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Shroff RC, Donald AE, Hiorns MP, et al. Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol. 2007;18:2996–3003. doi: 10.1681/ASN.2006121397. [DOI] [PubMed] [Google Scholar]
- 18.Shanahan CM, Crouthamel MH, Kapustin A, et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Painer J, Kuro OM, et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol. 2018;14:265–284. doi: 10.1038/nrneph.2017.169. [DOI] [PubMed] [Google Scholar]
- 20.Kuro-o M. Overview of the FGF23-Klotho axis. Pediatr Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 21.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson TE, Olauson H, Hagstrom E, et al. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30:333–339. doi: 10.1161/ATVBAHA.109.196675. [DOI] [PubMed] [Google Scholar]
- 24.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 25.Olive M, Harten I, Mitchell R, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobb AM, Larrieu D, Warren DT, et al. Prelamin A impairs 53BP1 nuclear entry by mislocalizaing NUP153 and disrupting the Ran gradient. Aging Cell. 2016;15:1039–1050. doi: 10.1111/acel.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhard-Herman M, Smoot LB, Wake N, et al. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension. 2012;59:92–97. doi: 10.1161/HYPERTENSIONAHA.111.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga R, Eriksson M, Erdos MR, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews C, Gorenne I, Scott S, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P, Luttropp K, McGuinness D, et al. CDKN2A/p16INK4a expression is associated with vascular progeria in chronic kidney disease. Aging (Albany NY) 2017;9:494–507. doi: 10.18632/aging.101173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobi C, Hömme M, Melk A. Is cellular senescence important in pediatric kidney disease? Pediatr Nephrol. 2011;26:2121–2131. doi: 10.1007/s00467-010-1740-6. [DOI] [PubMed] [Google Scholar]
- 32.Ragnauth CD, Warren DT, Liu Y, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 33.Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cobb AM, Murray TV, Warren DT, et al. Disruption of PCNA-lamins A/C interactions by prelamin A induces DNA replication fork stalling. Nucleus. 2016;7:498–511. doi: 10.1080/19491034.2016.1239685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 36.Zhao MM, Xu MJ, Cai Y, et al. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011;79:1071–1079. doi: 10.1038/ki.2011.18. [DOI] [PubMed] [Google Scholar]
- 37.Takemura A, Iijima K, Ota H, et al. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2054–2062. doi: 10.1161/ATVBAHA.110.216739. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltese G, Psefteli PM, Rizzo B, et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. 2017;21:621–627. doi: 10.1111/jcmm.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao D, Zuo Z, Tian J, et al. Activation of SIRT1 attenuates Klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68:1191–1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakano-Kurimoto R, Ikeda K, Uraoka M, et al. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol. 2009;297:H1673–H1684. doi: 10.1152/ajpheart.00455.2009. [DOI] [PubMed] [Google Scholar]
- 44.Burton DG, Giles PJ, Sheerin AN, et al. Microarray analysis of senescent vascular smooth muscle cells: a link to atherosclerosis and vascular calcification. Exp Gerontol. 2009;44:659–665. doi: 10.1016/j.exger.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Shroff RC, Shah V, Hiorns MP, et al. The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant. 2008;23:3263–3271. doi: 10.1093/ndt/gfn226. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein SL, Leung JC, Silverstein DM. Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin J Am Soc Nephrol. 2006;1:979–986. doi: 10.2215/CJN.02291205. [DOI] [PubMed] [Google Scholar]
- 47.Dalfino G, Simone S, Porreca S, et al. Bone morphogenetic protein-2 may represent the molecular link between oxidative stress and vascular stiffness in chronic kidney disease. Atherosclerosis. 2010;211:418–423. doi: 10.1016/j.atherosclerosis.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Lee CT, Chua S, Hsu CY, et al. Biomarkers associated with vascular and valvular calcification in chronic hemodialysis patients. Dis Markers. 2013;34:229–235. doi: 10.3233/DMA-130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pecoits-Filho R, Barany P, Lindholm B, et al. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 50.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 51.Chen NX, Duan D, O’Neill KD, et al. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 52.Ward RA, McLeish KR. Oxidant stress in hemodialysis patients: what are the determining factors? Artif Organs. 2003;27:230–236. doi: 10.1046/j.1525-1594.2003.07170.x. [DOI] [PubMed] [Google Scholar]
- 53.Descamps-Latscha B, Drueke T, Witko-Sarsat V. Dialysis-induced oxidative stress: biological aspects, clinical consequences, and therapy. Semin Dial. 2001;14:193–199. doi: 10.1046/j.1525-139x.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen-Khoa T, Massy ZA, De Bandt JP, et al. Oxidative stress and haemodialysis: role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant. 2001;16:335–340. doi: 10.1093/ndt/16.2.335. [DOI] [PubMed] [Google Scholar]
- 55.Kose K, Dogan P, Gunduz Z, et al. Oxidative stress in hemodialyzed patients and the long-term effects of dialyzer reuse practice. Clin Biochem. 1997;30:601–606. doi: 10.1016/s0009-9120(97)00100-8. [DOI] [PubMed] [Google Scholar]
- 56.Guo Z, Kozlov S, Lavin MF, et al. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 57.Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. 2017;92:26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Yamada S, Taniguchi M, Tokumoto M, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res. 2012;27:474–485. doi: 10.1002/jbmr.539. [DOI] [PubMed] [Google Scholar]
- 59.Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shanahan CM, Cary NR, Salisbury JR, et al. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.