Abstract
Disease surveillance in wildlife is rapidly expanding in scope and methodology, emphasizing the need for formal evaluations of system performance. We examined a syndromic surveillance system for respiratory disease detection in Gombe National Park, Tanzania, from 2004 to 2012, with respect to data quality, disease trends, and respiratory disease detection. Data quality was assessed by examining community coverage, completeness, and consistency. The data were examined for baseline trends; signs of respiratory disease occurred at a mean frequency of less than 1 case per week, with most weeks containing zero observations of abnormalities. Seasonal and secular (i.e., over a period of years) trends in respiratory disease frequency were not identified. These baselines were used to develop algorithms for outbreak detection using both weekly counts and weekly prevalence thresholds and then compared retrospectively on the detection of 13 respiratory disease clusters from 2005 to 2012. Prospective application of outbreak detection algorithms to real-time syndromic data would be useful in triggering a rapid outbreak response, such as targeted diagnostic sampling, enhanced surveillance, or mitigation.
Keywords: Surveillance, Respiratory disease, Wildlife epidemiology, Wildlife health
Introduction
Increasing anthropogenic pressures such as habitat loss and human population growth have increased connectivity between human, domestic animal and wildlife populations, increasing the risk of disease emergence across species. Consequently, there is a call for increased wildlife disease surveillance where high biodiversity overlaps with human populations (Woolhouse and Gowtage-Sequeria 2005; Jones et al. 2008; Keesing et al. 2010). Nonhuman primates have been prioritized for surveillance of novel pathogens of risk to humans (Woolhouse and Gowtage-Sequeria 2005; Jones et al. 2008; Calvignac-Spencer et al. 2012), while potential benefits of surveillance to these populations are also recognized due to infectious disease threats from humans (Fruth et al. 2008; Oates et al. 2008; Robbins and Williamson 2008; Walsh et al. 2008).
Although health surveillance in free-ranging primates is complicated, populations closely monitored for research and tourism present the most feasible opportunity to design, implement and evaluate these systems. Gombe National Park (Tanzania) hosts the longest continual field research site for chimpanzee ecology and behavior in the world. High levels of disease-associated chimpanzee (Pan troglodytes schweinfurthii) mortalities (Williams et al. 2008), combined with growing interest in wildlife disease surveillance, spurred the creation of the Gombe Ecosystem Health Program in 2004 to collect standardized, observational data on syndromes of disease (Lonsdorf et al. 2006; Travis et al. 2008). This syndromic surveillance system parallels ongoing behavioral data collection, pathology (Terio et al. 2011), and fecal screening for enteric pathogens (Gillespie et al. 2010) and Simian Immunodeficiency Virus of chimpanzees (SIVcpz) (Keele et al. 2009).
Syndromic surveillance is the collection of pre-diagnosis health data that can signal a disease outbreak, triggering responses to reduce morbidity and mortality (Henning 2004). This type of surveillance targets groups of signs or symptoms that characterize a particular abnormality, although not a single disease process (Dόrea et al. 2011; Rodríguez-Prieto et al. 2014). For example, clinical symptoms of fever or respiratory illness have been used in syndromic surveillance for respiratory infections in humans (Schindeler et al. 2009; Babin 2010; van den Wijngaard et al. 2010; Hiller et al. 2013; Johnson et al. 2014). Although less specific than laboratory data, strong correlation between respiratory syndromic data and respiratory virus diagnosis has been demonstrated (Bourgeois et al. 2006; Hall et al. 2013). Syndromic surveillance is sensitive to changes in a population’s disease levels, providing early warning of disease outbreaks in human and animal populations (Henning 2004; Dόrea et al. 2011). It also has the advantage of being noninvasive; thus, when applied to wildlife, such as primates, it does not interfere with natural behavior and/or habituation.
Although syndromic surveillance is employed for domestic animal health, it is an incipient approach to disease detection in wildlife populations (Arenas et al. 2002; Warns-Petit et al. 2010; Oleaga et al. 2011). Consequently, there is a need for formal evaluations of system performance in wildlife (Stoto et al. 2006; Dorea et al. 2011; Rodríguez-Prieto et al. 2014). Formal evaluations involve a thorough analysis of data quality, an epidemiological profile of the population to characterize baseline and systematic variations in disease patterns (e.g., seasonal or day-of-the-week fluctuations), followed by development of an algorithm to detect abnormal variations (i.e., outbreaks) (Lescano et al. 2008). Detection algorithms are methods used to systematically monitor incoming data and generate a signal when disease levels exceed an established threshold (Stoto et al. 2006). The Gombe syndromic surveillance system was created with the goal of characterizing baseline disease levels to improve outbreak detection, but up until now, surveillance data had not being systematically analyzed and optimized for real-time outbreak detection.
Syndromic surveillance is best known for its application in large populations; thus, its application in a small chimpanzee population is unique. However, monitoring small populations is important since they are more susceptible to extinction risks from disease, making early detection critical to population viability. We systematically evaluated the Gombe syndromic surveillance system for the period of 2004–2012. Study objectives included (1) an assessment of data quality, (2) an epidemiological profile of baseline respiratory disease trends, (3) the development of algorithms for outbreak detection, and (4) a quantitative assessment of surveillance sensitivity. Here we report on Objectives 1–3; Objective 4 is reported by Wolf et al. (2019). We focused this initial work on respiratory syndrome given the recognized importance of respiratory outbreaks as causes of morbidity and mortality among great ape populations (Köndgen et al. 2008; Williams et al. 2008; Palacios et al. 2011).
Materials and Methods
Study Population
Gombe National Park, Tanzania, is home to a population of chimpanzees, which appears to have declined from as many as 120–150 in the 1960s (Pusey et al. 2007) to 96–100 at the beginning of 2013 [Jane Goodall Institute Research Center, Duke University, unpublished data]. Two of three chimpanzee communities, Mitumba and Kasekela, are habituated to humans, with individually identifiable chimpanzees that have been the focus of behavioral research since the mid-1990s and 1960s, respectively. These two communities were the focus of this study. All research was conducted with permission from the Tanzania Commission for Science and Technology, Tanzania Wildlife Research Institute, and Tanzania National Parks Association.
Syndromic Disease Surveillance
In 2004, a prospective health monitoring system was established to collect observational data on the presence or absence of clinical signs associated with specific disease syndromes (Lonsdorf et al. 2006; Travis et al. 2008). It is comprised of two standardized and one non-standardized data streams (Lonsdorf et al. 2006). The Daily Health Record (DHR) captures detailed, standardized health data on individuals, whether healthy or unhealthy, that are subjects of full-day behavioral data collection. DHR protocol requires at least 1 h of health-focused observation to gather respiratory, gastrointestinal, musculoskeletal, dermatologic, and general body condition data (Lonsdorf et al. 2006; Lonsdorf et al. 2018). If an individual in contact with the primary subject demonstrates abnormal health, an additional DHR is generated for that individual. The goal to date has been to capture DHR data on 60% of each community monthly. Weekly Health Record (WHR) data are collected on all chimpanzees, whether included in the DHR or not. WHR data are collected once a week, relying on recall to document presence or absence of abnormal clinical signs for all chimpanzees seen during the previous 7 days. This stream of data was designed to supplement the DHR, capturing less detailed health data on a greater number of chimpanzees. DHR and WHR data were collected by dedicated researchers having demonstrated familiarity with community members. Lastly, detailed, non-standardized monthly health reports are prepared by the resident veterinarian summarizing abnormalities observed within each community. All standardized data were entered into IMPACTtm (Internet-supported Management Program to Assist Conservation Technology), an online health surveillance database tool developed in partnership with Gorilla Doctors ™. In concert with syndromic surveillance is a pathology program, where all recovered carcasses undergo systematic necropsy and histopathology to determine cause of death and underlying pathologies (Terio et al. 2011). Data from this program were examined to identify mortalities associated with respiratory disease.
In this study, DHR and WHR data on syndromic respiratory disease were formally analyzed for the period of March 2004 to December 2012. This includes 5170 Kasekela DHRs from 84 individuals, 2846 Mitumba DHRs from 33 individuals, and 28,000 WHRs from 429 weeks in Kasekela and 10,721 WHRs from 437 weeks in Mitumba. Syndromic respiratory disease includes clinical signs of coughing, sneezing, and rhinorrhea. Veterinary reports and pathology data were used to validate findings. During this time, the Kasekela community size ranged 56–68 individuals, and Mitumba ranged 20–29 individuals [Jane Goodall Institute Research Center, Duke University, unpublished data].
Data Quality
Community coverage, data completeness, and consistency between DHR and WHR data sets were examined over the study period. Community coverage was assessed as the proportion of the community on which health data was collected each month. Monthly coverage was examined for seasonal changes in data quality, recognizing that it may be impacted by seasonal variations in animal behavior and observability as well as community size (Goodall 1986). Data completeness was assessed as the percentage of completed respiratory data fields (i.e., no missing data) in the DHR and WHR. To determine whether some clinical signs are more easily observed than others, we also examined DHR data for differences in completeness by clinical sign. For example, with limited visibility, a researcher may have difficulty confirming presence or absence of rhinorrhea, resulting in missing data.
Finally, consistency between DHR and WHR data was evaluated to assess the reliability of WHR data, which may be subject to recall bias. Percentage of agreement between the two records was calculated for all weeks when sick chimpanzees were reported using two approaches: Analysis 1: number of cases, and Analysis 2: identification of individuals reported sick. Since the WHR was designed to capture data on all chimpanzees seen, including those not in the DHR, WHR data was expected to have equal to or higher weekly counts of sick chimpanzees than DHR data. Thus, Analysis 1 was classified as consistent if WHR data had greater than or equal to the weekly number of sick chimpanzees reported in the corresponding DHR data. In Analysis 2, corresponding WHR and DHR data were classified as consistent if all sick chimpanzees identified in the DHR were also noted as sick in the WHR.
Respiratory Disease Trends
An epidemiological profile of baseline respiratory disease was performed as a descriptive analysis to facilitate selection of an optimal outbreak detection algorithm. We focused remaining analyses on DHR data based on WHR limitations revealed by data quality analyses. Cumulative number and prevalence of respiratory cases among observed chimpanzees each week were averaged over the study period. The justification for using both metrics was to account for weekly variations in the number of chimpanzees observed, which is not captured by the cumulative case count, but also recognizing that prevalence may appear abnormally high when low numbers of chimpanzees are observed. Weeks with previously recognized major respiratory outbreaks (Kasekela: n = 5 weeks; Mitumba: n = 8 weeks) were excluded to obtain baseline estimates; major outbreaks have been previously described as those in which 20% or more of a community is affected (Williams et al. 2008). Individuals observed with any respiratory abnormality were counted only once, even if observed more frequently within an observation week. As individuals are generally observed with approximately equal frequency when sick and healthy, we assumed the weekly sample prevalence to be representative of the community.
Weekly counts of respiratory cases were analyzed for seasonal and secular (over periods of years) trends using all data. Missing weeks (which consisted of < 3% and < 1% of Kasekela and Mitumba data, respectively) were excluded from analysis. To explore whether there are seasonal trends in rates of respiratory disease, we aggregated data by season. The rainy season in Gombe spans November-April (Lonsdorf et al., 2011); the remaining months constitute the dry season. We calculated prevalence ratios (PR) and exact 95% confidence intervals using weekly prevalence data (number of cases divided by number of observed chimpanzees) to determine whether there were differences in levels of respiratory disease in the dry vs wet season.
Next, cumulative weekly case counts were modeled by Poisson regression in Stata, version 15.1, as a simple descriptive analysis of whether there were secular trends in respiratory disease (i.e., trends over a period of years) and determine rate of change. Each community was modeled separately, with the weekly sum of respiratory cases as the dependent variable, time (expressed as chronological study weeks) as the fixed predictor variable, and the weekly sum of observed chimpanzees as the offset. Time trends were modeled in various ways. First, to test for an overall linear trend, we included week as a continuous variable in the model. Second, to allow a more flexible approach to estimating secular trends, we fit a Poisson model with restricted cubic splines (Harrell 2001). In the Kasekela data, we used five knots (at the 10th, 25th, 50th, 75th, and 90th percentiles) applied to the weekly time variable to allow for a flexible nonlinear relationship between time and weekly case frequency. However, the Mitumba data had fewer cases of respiratory disease and could not support as many knot locations. For the Mitumba data, we used restricted cubic splines with 4 knots (at the 10th, 25th, 50th, and 75th percentiles). We chose different knot locations for sensitivity analysis; however, interpretation was unchanged by these different locations. Bootstrap confidence intervals were used in all regression models to help adjust for autocorrelation. For graphical presentations, we calculated the expected number of cases we would observe from these models if the number of observed chimps was held constant at the average number observed at each location (5.2 chimps for Mitumba and 10 chimps for Kasekela). This allows more straightforward comparison of counts across weeks.
Algorithms for Respiratory Outbreak Detection
A simple set of detection algorithms was developed for respiratory outbreak detection. A weekly interval was used to minimize time to outbreak detection with future realtime application. Community thresholds were set so that approximately 95% of baseline weekly case data (count and prevalence) over March 2004-February 2005 fell below the threshold level. Use of a subset of surveillance data free of outbreaks, in this case, the first 12 study months, is typical for the generation of detection thresholds for a population (Lescano et al. 2008; Dόrea et al. 2014). Thus, in practice, when the number or prevalence of respiratory cases in the DHR exceeds these weekly thresholds, managers would be alerted to a possible outbreak.
We screened the remaining years of weekly DHR data (March 2005-December 2012) for clusters of respiratory cases by each algorithm for comparison. Here we use the term cluster to refer to the occurrence of respiratory cases exceeding our established weekly thresholds but unconfirmed as a transmission event (i.e., outbreak). For each detection, we examined the DHR, WHR, and veterinary reports to confirm that illness was observed in more than one individual, either within the same week or consecutive weeks of the detection. Although optimal for assessing surveillance specificity, diagnostic data were not available to confirm a detected respiratory cluster as an outbreak; thus, count and prevalence algorithms were evaluated on detection agreement. This approach is used for diagnostic test assessment when a gold standard test is unavailable for comparison and true infection status is unknown (Basta et al. 2013). Chamberlain’s percent positive agreement was used as the measure of reliability for this analysis (Szklo and Nieto 2007). It is an index of reliability for situations where disease incidence is very low or high. This was considered the best estimate of agreement since the number of weeks where clusters occurred was far lower than the number of weeks with none.
Results
Data Quality
Monthly community coverage by the DHR averaged 50% (± SD 4.9%) and 53% (± SD 4.8%) for the Kasekela and Mitumba communities, respectively, whereas the WHR covered an average of 87% (± SD 3.3%) and 95% (± SD 2.0%) of Kasekela and Mitumba, respectively. There were no differences in mean coverage between months in either community (Fig. 1), suggesting that seasonal changes in chimp behavior and distribution did not impact data collection. Data completeness averaged 96.7% (± SD 0.4%) and 97.2% (± SD 0.4%) of the DHR (documenting either absence or presence of respiratory signs) and 99.6% (± SD 0.4%) and 99.8% (± SD 0.1%) of the WHR in Kasekela and Mitumba, respectively. There were no differences by clinical sign in the amount of missing data, suggesting that clinical signs of respiratory disease (i.e., coughing, sneezing, and rhinorrhea) were equivalently observed within the forest environment. However, our data sets limited our ability to fully assess measurement bias in the case where unobserved abnormal signs were documented as absent.
Fig. 1.
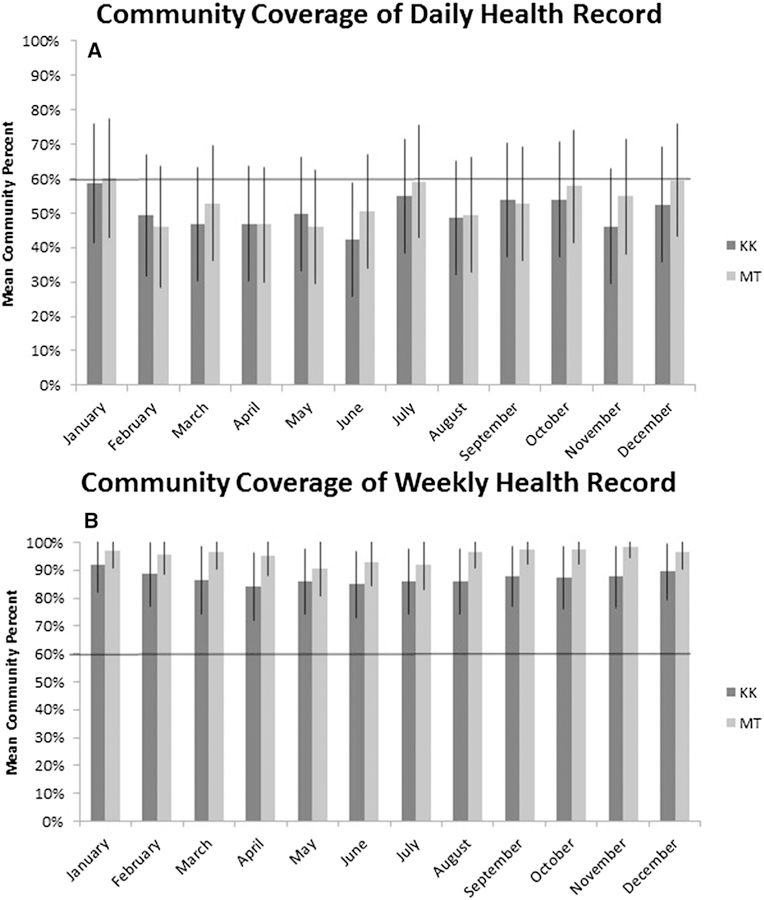
Mean community coverage across months. Panel A shows coverage of each community by the Daily Health Record for each month, whereas Panel B shows mean coverage by the Weekly Health Record for each month. The solid black horizontal lines indicate the projected goal of 60% coverage. KK Kasekela community; MT Mitumba community.
Data consistency between DHR and WHR records was analyzed under two scenarios to assess the reliability of WHR data. Of 416 surveillance weeks, 80 and 73 weeks contained reports of respiratory cases in either data set in Kasekela and Mitumba, respectively. Agreement on the weekly number of cases (Analysis 1) averaged 60% (± SD 5.4%) and 64.4% (± SD 5.6%) in Kasekela and Mitumba, respectively (Fig. 2). Consistency between the two records on the identities of sick chimpanzees (Analysis 2) averaged only 24% (± SD 6%) in Kasekela and 46.2% (± SD 6.9%) in Mitumba. Based on low consistency revealed by Analysis 2, we chose the DHR for epidemiological profiling and algorithm development to avoid potential impacts of recall bias associated with WHR data.
Fig. 2.
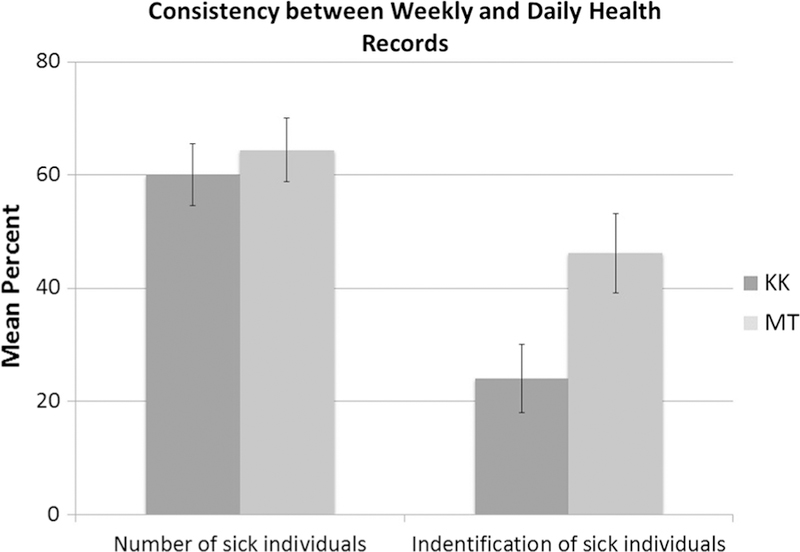
Percent agreement between Weekly Health Record (WHR) and Daily Health Record (DHR) data for each community. The first column set depicts the mean percent agreement on the number of individuals with respiratory disease. The second column set represents the mean percent agreement on the identities of respiratory cases reported in the two data sets. These estimates include only weeks where respiratory cases were reported, and takes into account that, by design, WHR data should contain at least the same number and identities of cases as DHR data. KK Kasekela community; MT Mitumba community.
Respiratory Disease Trends
The mean weekly number of chimpanzees on which data were collected in the DHR was 10 (SD ± 6) and 5 (SD ± 3) in Kasekela and Mitumba, respectively. Among these, the mean number of chimpanzees with any combination of respiratory signs in weeks outside of previously recognized major outbreaks was 0.12 (median 0; range 0–4) and 0.11 (median 0; range 0–2) in Kasekela and Mitumba, respectively. Considering that most weeks contained no cases of respiratory disease, the mean number of cases reported when respiratory illness was observed (n = 44 weeks in each community) was 1.2 (median 1; range 1–4) and 1.1 (median 1; range 1–2) in Kasekela and Mitumba, respectively (again, excluding weeks with recognized major outbreaks). Weekly respiratory disease prevalence was 1.3% (median 0%; range 0–50%) and 2.3% (median 0%; range 0–66.7%) in Kasekela and Mitumba, respectively, over all weeks of study. Similarly, case prevalence among observed chimpanzees estimated only for weeks when respiratory illness was observed was 13.9% (median 10.2%; range 450%) and 23.8% (median 20%; range 11.1–66.7%) in Kasekela and Mitumba, respectively.
When comparing respiratory disease prevalence between seasons, there was little evidence of a seasonal trend in Kasekela; although more respiratory cases were observed in the dry season in Kasekela, the difference was not significant (PR 1.4; 95% CI 0.9–2.2). However, in Mitumba the prevalence in the dry season was half the rate in the wet season (PR 0.5; 95% CI 0.3–0.9). Visual examination of respiratory case trends (Fig. 3) revealed that, in both communities, changes in case numbers across seasons appear to be largely a result of distinct clusters of respiratory disease in single years as opposed to regular seasonal fluctuation. These findings demonstrate that a detection algorithm utilizing a dynamic threshold to account for seasonal variation (e.g., temporal scan statistic) may be unnecessary.
Fig. 3.
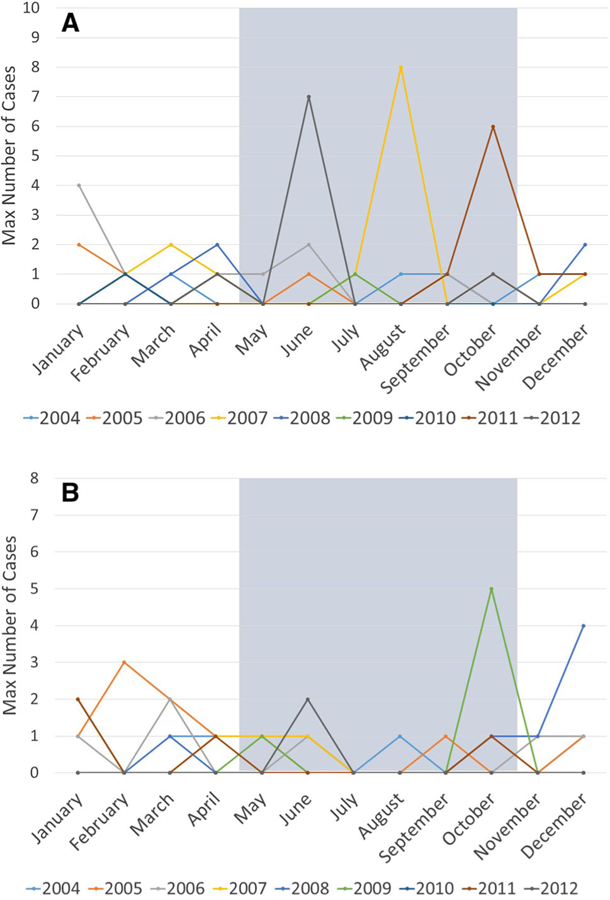
Monthly respiratory case trends of the habituated chimpanzee communities. Monthly numbers represent the maximum number of weekly respiratory cases across months of each study year, and each study year is displayed by a different color. Months that fall within the wet season in Gombe are shaded in gray. Panel A shows monthly case indices for Kasekela community and Panel B for Mitumba.
Descriptive analysis of secular trends indicated no evidence of a linear trend over time in Kasekela (P = 0.53); however, in Mitumba there was some evidence of a slight decrease in the rate of respiratory outbreaks over time (P < 0.01). Descriptive analyses in Kasekela using splines suggest little secular trend, as well. However, there was some decline in the expected number of cases between weeks 250 and 400 with a slight increase in expected cases at the very end of the study. Descriptive analyses in Mitumba using splines suggest a slight decrease in the number of respiratory events over time, with the earliest weeks in the study having the highest rates (Fig. 4).
Fig. 4.
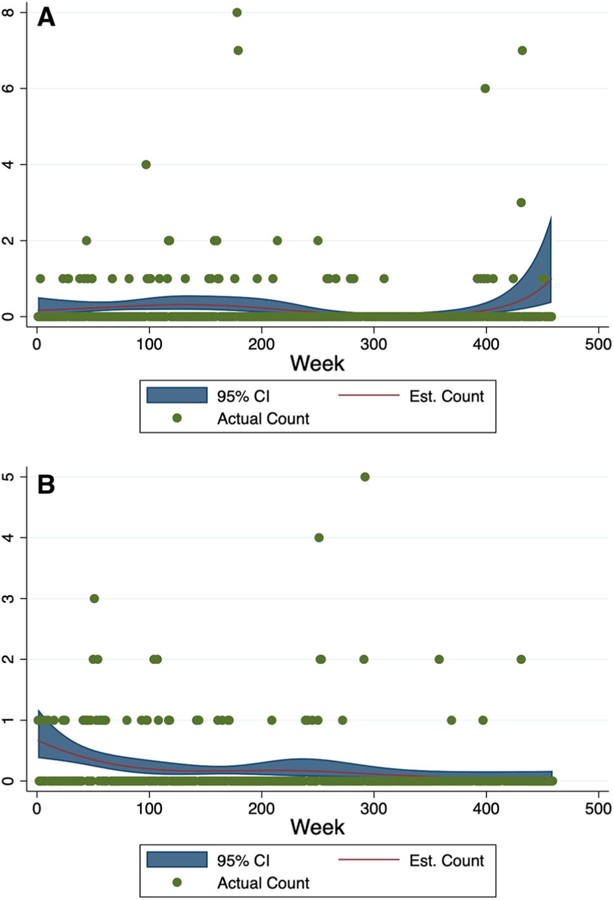
Weekly trends of respiratory cases in Kasekela and Mitumba from 2004 to 2012. The observed weekly counts of respiratory cases (green dots) are plotted across weeks of study, along with the expected number of cases (red line) and corresponding 95% confidence intervals (blue band) generated from Poisson models. We calculated the expected number of cases we would observe from these models if the number of observed chimps was held constant at the average number observed at each location (5.2 chimps for Mitumba and 10 chips for Kasekela). Kasekela is shown in Panel A and Mitumba in Panel B.
Algorithms for Respiratory Outbreak Detection
The thresholds representing the 95th percentile of the data (Fig. 5) were a count of ≤ 2 sick chimpanzees in either community and a prevalence of ≤ 16.7% and ≤ 33.3% in Kasekela and Mitumba, respectively (Table 1). Detections in 2 weeks by the prevalence threshold involved illness in only one chimpanzee and were considered false alarms. These occurred during weeks where few chimpanzees were observed, resulting in a prevalence of illness above threshold despite illness in only a single animal. In Table 1, summary results do not include these false alarms.
Fig. 5.
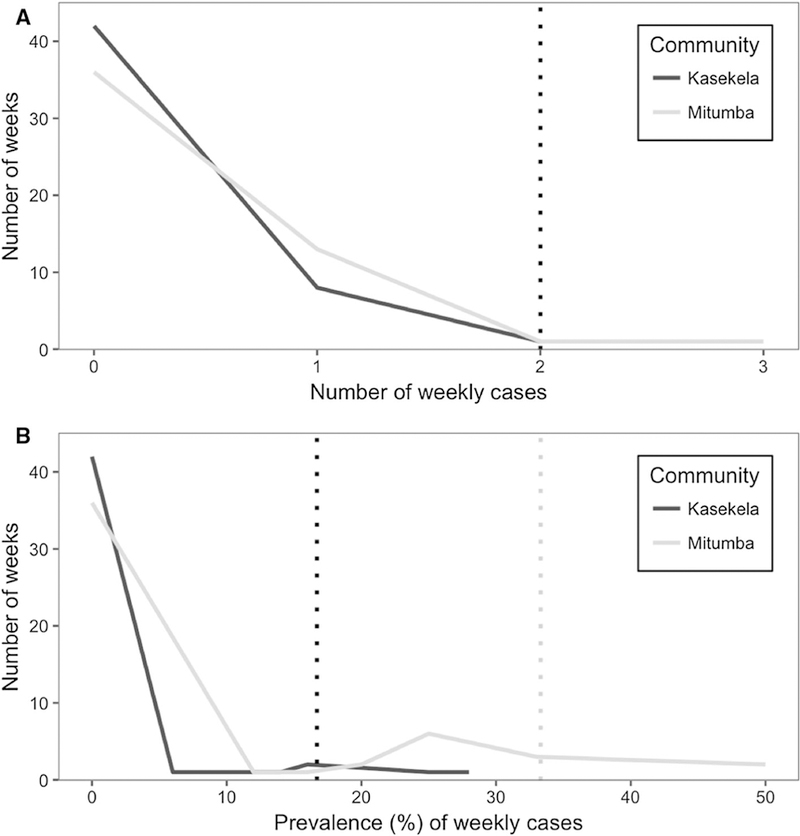
Frequency distribution of the baseline cumulative number and prevalence of weekly respiratory cases from the Daily Health Record in Year 1 of the study. Thresholds for outbreak detection in each community were set at the 95th percentile of the data, represented by the vertical dashed lines. Panel A represents the distribution of the weekly number of respiratory cases, where both communities had the same threshold (single vertical line). Panel B represents the distribution of the weekly prevalence of cases, where each community’s threshold is represented by individual vertical lines.
Table 1.
Baseline Frequencies of Respiratory Cases Observed in Year 1, Disease Detection Thresholds and Retrospective Respiratory Cluster Detection for Years 2005–2012. Prevalence Describes the Proportion of Chimpanzees with Respiratory Signs Among the Individuals Observed Within the Week. Community Thresholds Were Set so that Approximately 95% of Baseline Data Fell Within the Threshold Level. Thresholds Were Used to Detect Clusters of Respiratory Cases that Significantly Exceeded Year 1 Baseline Levels in Daily Health Record (DHR) Data from March 2005 to December 2012. For Each Detection, DHR and Weekly Health Record (WHR) Data Were Examined to Estimate the Cluster Size (i.e., Number of Cases) and Rule out False Alarms (i.e., a Signaled Event with < 2 Cases).
| Kasekela | Mitumba | |
|---|---|---|
| Year 1 baseline weekly number of cases, mean (range) | 0.2 (0–2) | 0.35 (0–3) |
| Count threshold | ≥ 2 | ≥ 2 |
| Number of weeks with detections | 12 | 9 |
| Number of respiratory clusters | 8 | 5 |
| Range of cluster sizes | 2–55 | 2–24 |
| Year 1 baseline weekly prevalence of cases, mean (range) | 2.7% (0–28.6%) | 8.2% (0–50%) |
| Prevalence threshold | ≥ 16.7% | ≥ 33.3% |
| Number of weeks with detections | 10 | 8 |
| Number of respiratory clusters | 5 | 4 |
| Range of cluster sizes | 6–55 | 3–24 |
Combined, the algorithms revealed 13 clusters of respiratory cases in the two communities from 2005 to 2012. Mean cluster size was 14 (range 2–55) and 8 (range 2–24) in Kasekela and Mitumba, respectively. Clusters of cases spanned 3 weeks on average (range 1–5). Five clusters involved illness observed in only two (n = 2) or three individuals (n = 3) over the course of one (n = 4) or four (n = 1) weeks. A single mortality was diagnosed with respiratory disease during the study (Streptococcus pneumoniae pleuropneumonia), but was not associated with any respiratory clusters. The percent positive agreement between the count and prevalence detection thresholds was 69.2% (± SD 1.7%). Disagreement occurred when few animals were sick in a single week. Most of these were a 1-week event, but one signal involved a cluster of respiratory illness in 7–9 animals over 5–10 weeks.
Veterinary reports were available for 12/13 clusters and examined for further validation of detected clusters. Respiratory disease was described in ≤ 2 cases in 11/12 clusters; however, in one situation where clusters were detected in both communities, a veterinary report described cases in only one community. Available veterinary reports consistently validated large clusters of respiratory disease when 20% of a community was affected.
Discussion
Here we present the first formal assessment of a syndromic surveillance system developed for noninvasive disease detection in a wildlife population, building upon past descriptive evaluations of efforts (Lonsdorf et al. 2006, 2011, 2018) and bridging a quantitative assessment of system performance (i.e., surveillance system sensitivity) (Wolf et al. 2019). Here, we used several metrics to assess data quality, revealing specific areas that necessitate refinement. Notably, community coverage of the DHR fell short of the projected goal of 60% each month in both communities. When established, this goal was considered a reasonable expectation of researcher efforts to find and collect data on community members. In general, an increase in community coverage would require either greater researcher effort to log data on more chimpanzees or a reduction in repeated sampling. Since some level of repeated sampling may be unavoidable, establishing a weekly goal exceeding a bottom-line estimate may better aid researchers in meeting the projected goal. Although WHR data provide greater community coverage, consistency between DHR and WHR data was lacking, suggesting that WHR data were not collected as intended or was subject to recall bias. This was a key finding in our early analyses, as it influenced our decision to focus remaining analyses on DHR data so as to avoid impacts associated with recall bias. Our results suggest that an optimal system would require a primary daily data collection system to ensure data accuracy, but a complimentary weekly approach could provide broader population coverage. In any case, a weekly approach should not be a sole data source for syndromic disease detection.
A set of disease detection algorithms that can be implemented for real-time outbreak detection were generated following an epidemiological profile of baseline disease. Preliminary comparison of the two detection algorithms revealed the weekly count threshold to be more sensitive, having identified four more clusters than the prevalence threshold. The prevalence approach was also more vulnerable to false alarms, based on two signals that occurred when only a single animal was sick. While both algorithms detected rapid increases in respiratory cases, the weekly count threshold appears better at identifying low-level clusters that were distributed widely over time (detecting one event where 7–9 animals became ill over 510 weeks). The weekly count threshold is also advantageous for prospective outbreak detection in real time given its simplicity, enabling early detection (within the observation week) by population managers when the predefined threshold number of cases is observed. A quantitative analysis of the sensitivity of both algorithms is described by Wolf et al. (2019).
The ultimate goal of an efficient syndromic surveillance system is the early detection of infectious disease outbreaks with few false alarms. The evaluation of the current system was limited by a lack of pathogen data from the study site, precluding confirmation of detected respiratory disease clusters as infectious disease outbreaks. With a low detection threshold (as established in this study), false alarms would be expected. However, considering the low baseline level of respiratory disease in this population, a low, sensitive detection threshold, even if producing occasional false alarms, is favorable for triggering a rapid mitigation response. The integration of syndromic surveillance with a noninvasive diagnostic response plan (Leendertz et al. 2006; Gillespie et al. 2008; Kondgen et al. 2010) would be a powerful approach to early outbreak detection and pathogen identification for disease mitigation.
We emphasize through this work that the design and implementation of syndromic surveillance systems should also include a formal analysis of system performance. Such evaluations should be integrated into the surveillance process and undertaken by a team that includes managers, observers, and epidemiologists or biostaticians. While this analysis focused only on respiratory data, the methods can be applied to other disease syndromes. We did not report here on the standardization of observer training protocols or inter-observer reliability assessment, which are important in reducing measurement error and/or bias. When syndromic surveillance can be integrated into ongoing observational studies, large amounts of health data can be accrued with comparably little effort and expense, but the system itself should be monitored to ensure optimal performance.
Acknowledgements
We thank the staff of the Jane Goodall Institute’s Gombe Stream Research Centre for collecting daily health observations, as well as to the Gombe Ecosystem Health Project staff who manage and organize the data as it originates from the field. Thanks also to Andres Perez for insight and direction on time series analysis and Richard Maclehose for the constructive feedback on other aspects of the biostatistical analyses. Alex Krupnick, Emma Finestone, Emma Lantz, Emily Seidl, Anna Sjodin, and Edward Wilkerson provided key data management support. We also thank the Tanzania Commission for Science and Technology (COSTECH), Tanzania Wildlife Research Institute (TAWIRI), and Tanzania National Parks Association (TANAPA) for their continued support and approval of this research. Funding support comes from the Zoetis/Morris Animal Foundation Veterinary Research Fellowship [D10ZO-902] and the University of Minnesota Doctoral Dissertation Fellowship, the National Institute of Health (R01 AI058715, R01 AI 120810 and R00 HD057992), National Science Foundation (LTREB-1052693), Arcus Foundation, USFWS Great Ape Conservation Fund. Monetary support and invaluable time and effort were provided by staff and volunteers at Lincoln Park Zoo’s Davee Center for Epidemiology and Endocrinology and Lester E. Fisher for the Study and Conservation of Apes.
References
- Arenas AJ, Gόmez F, Salas R, Carrasco P, Borge C, Maldonado A, O’Brien DJ, Martinez-Moreno FJ (2002) An evaluation of the application of infrared thermal imaging to the tele-diagnosis of sarcoptic mange in the Spanish ibex (Capra pyrenaica). Veterinary Parasitology 109:111–117 [DOI] [PubMed] [Google Scholar]
- Babin SM (2010) Using syndromic surveillance systems to detect pneumonic plague. Epidemiology and Infection 138:1–8. 10.1017/S0950268809990689 [DOI] [PubMed] [Google Scholar]
- Basta NE, Stuart JM, Nascimento MC, Manigart O, Trotter C, Chandramohan D, Sow SO, Berthe A, Bedru A, Yenenesh K, Daugla DM, Gamougam K, Hodgson A, Forgor AA, Omotara A, Gadzama GB, Watkins ER, Rebbetts LS, Diallo K, Noel S, Halloran ME, Maiden MCJ, Greenwood B (2013) Methods for identifying Neisseria meningitidis carriers: a multi-center study in the African meningitis belt. PLoS ONE 8:6–11. 10.1371/journal.pone.0078336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois FT, Olson KL, Brownstein JS, McAdam AJ, Mandl KD (2006) Validation of syndromic surveillance for respiratory infections. Annals of Emergency Medicine 47:265.e1–265.e1. 10.1016/j.annemergmed.2005.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvignac-Spencer S, Leendertz S, Gillespie TR, Leendertz FH (2012) Wild great apes as sentinels and sources of infectious disease. Clinical Microbiology and Infection 18:521–527. 10.1111/j.1469-0691.2012.03816.x [DOI] [PubMed] [Google Scholar]
- Dόrea FC, Lindberg A, McEwen BJ, Revie CW, Sanchez J (2014) Syndromic surveillance using laboratory test requests: a practical guide informed by experience with two systems. Preventive Veterinary Medicine 116:313–324. 10.1016/j.pre-vetmed.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Dόrea FC, Sanchez J, Revie CW (2011) Veterinary syndromic surveillance: current initiatives and potential for development. Preventive Veterinary Medicine 101:1–17 [DOI] [PubMed] [Google Scholar]
- Fruth B, Benishay J, Bila-Isia I, Coxe S, Dupain J, Furuichi T, Hart J, Hart T, Hashimoto C, Hohmann G, Hurley M, Ilambu O, Mulavwa M, Ndunda M, Omasombo V, Reinartz G, Scherlis J, Steel L, Thompson J (2008) Pan paniscus. In: IUCN Red List Threat. Species. Version 2012.2 www.iucnredlist.org. Accessed 1 May 2013
- Gillespie TR, Lonsdorf EV, Canfield EP, Meyer DJ, Nadler Y, Raphael J, Pusey AE, Pond J, Pauley J, Mlengeya T, Travis DA (2010) Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. American Journal of Physical Anthropology 143:534–544. 10.1002/ajpa.21348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie TR, Nunn CL, Leendertz FH (2008) Integrative approaches to the study of primate infectious disease: implications for biodiversity conservation and global health. American Journal of Physical Anthropology 47:53–69. 10.1002/ajpa.20949 [DOI] [PubMed] [Google Scholar]
- Goodall J (1986) The Chimpanzees of Gombe: Patterns of Behavior, Cambridge, Massachusetts: Belknap Press of Harvard University Press [Google Scholar]
- Hall G, Krahn T, Van Dijk A, Evans G, Moore K, Maier A, Majury A (2013) Emergency department surveillance as a proxy for the prediction of circulating respiratory viral disease in Eastern Ontario. Canadian Journal of Infectious Diseases and Medical Microbiology 24:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE (2001) Regression Modelling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis, New York, NY: Springer [Google Scholar]
- Henning K (2004) Overview of syndromic surveillance: What is syndromic surveillance? Morbidity and Mortality Weekly Report 53(Suppl):5–11 [Google Scholar]
- Hiller KM, Stoneking L, Min A, Rhodes SM (2013) Syndromic surveillance for influenza in the emergency department-A systematic review. PLoS ONE 8:e73832 10.1371/journal.pone.0073832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Alianell A, Radcliffe R (2014) Seasonal patterns in syndromic surveillance emergency department data due to respiratory Illnesses. Online Journal of Public Health Informatics 6:e66 10.5210/ojphi.v6i1.5164 [DOI] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH (2009) Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460:515–519. 10.1038/nature08200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647 10.1038/nature09575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köndgen S, Kühl H, N’Goran PK, Walsh PD, Schenk S, Ernst N, Biek R, Formenty P, Matz-Rensing K, Schweiger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin WI, Pauli G, Boesch C, Leendertz FH (2008) Pandemic human viruses cause decline of endangered great apes. Current Biology 18:260–264. 10.1016/j.cub.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Kondgen S, Schenk S, Pauli G, Hoesch C, Leendertz F (2010) Noninvasive monitoring of respiratory viruses in wild chimpanzees. Ecohealth 7:332–341 [DOI] [PubMed] [Google Scholar]
- Leendertz FH, Pauli G, Maetz-Rensing K, Boardman W, Nunn C, Ellerbrok H, Jensen SA, Junglen S, Christophe B (2006) Pathogens as drivers of population declines: the importance of systematic monitoring in great apes and other threatened mammals. Biological Conservation 131:325–337. 10.1016/j.biocon.2006.05.002 [DOI] [Google Scholar]
- Lescano AG, Larasati R, Sedyaningsih ER, Bounlu K, Araujo-Castillo RV, Munayco-Escate CV, Soto G, Mundaca CC, Blazes DL (2008) Statistical analyses in disease surveillance systems. BMC Proceedings 2:S7 10.1186/1753-6561-2-s3-s7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf E, Travis D, Pusey A, Goodall J (2006) Using retrospective health data from the Gombe chimpanzee study to inform future monitoring efforts. American Journal of Primatology 908:897–908. 10.1002/ajp [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Gillespie TR, Wolf TM, Lipende I, Raphael J, Bakuza J, Murray CM, Wilson ML, Kamenya S, Mjungu D, Collins DA, Gilby IC, Stanton MA, Terio KA, Barbian HJ, Li Y, Ramirez M, Krupnick A, Seidl E, Goodall J, Hahn BH, Pusey AE, Travis DA (2018) Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park Tanzania. American Journal of Primatology 80(1):e22562 10.1002/ajp.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Murray CM, Lonsdorf EV, Travis DA, Gilby IC, Chosy J, Goodall J, Pusey AE (2011) A retrospective analysis of factors correlated to chimpanzee (Pan troglodytes schwein-furthii) respiratory health at Gombe National Park, Tanzania. Ecohealth 8:26–35. 10.1007/s10393-011-0683-0 [DOI] [PubMed] [Google Scholar]
- Oates J, Tutin C, Humle T, Wilson M, Baillie J, Balmforth Z, Blom A, Boesch C, Cox D, Davenport T, Dunn A, Dupain J, Duvall C, Ellis C, Farmer K, Gatti S, Greengrass E, Hart J, Herbinger I, Hicks C, Hunt K, Kamenya S, Maisels F, Mitani J, Moore J, Morgan B, Morgan D, Nakamura M, Nixon S, Plumptre A, Reynolds V, Stokes E, Walsh P (2008) Pan troglodytes. In: IUCN Red List Threat. Species. Version 2012.2 www.iucnredlist.org
- Oleaga A, Casais R, Balseiro A, Espí A, Llaneza L, Hartasánchez A, Gortázar C (2011) New techniques for an old disease: Sarcoptic mange in the Iberian wolf. Veterinary Parasitology 181:255–266. 10.1016/j.vetpar.2011.04.036 [DOI] [PubMed] [Google Scholar]
- Palacios G, Lowenstine L, Cranfield MR, Gilardi KVK, Spelman L, Lukasik-Braum M, Kinani J-F, Nyirakaragire Mudakikwa Antoine, Elisabeth Bussetti AV, Savji N, Hutchison S, Egholm M, Ian L (2011) Human metapneumovirus infection in wild mountain gorillas, Rwanda. Emerging Infectious Diseases 17:711713 10.3201/eid1704100883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J (2007) The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conserv Biol 21:623–634. 10.1111/j.1523-1739.2007.00704.x [DOI] [PubMed] [Google Scholar]
- Robbins M, Williamson L (2008) Gorilla beringei. In: IUCN Red List Threat. Species Version 2012.2 www.iucnredlist.org. Accessed 1 May 2012
- Rodriguez-Prieto V, Vicente-Rubiano M, Sánchez-Matamoros A, Rubio-Guerri C, Melero M, Martínez-Lόpez B, Martínez-Avilés M, Hoinville L, Vergne T, Comin A, Schauer B, Dόrea F, Pfeiffer DU, Sánchez-Vizcaíno JM (2014) Systematic review of surveillance systems and methods for early detection of exotic, new and re-emerging diseases in animal populations. Epidemiology and Infection 10.1017/s095026881400212x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindeler SK, Muscatello DJ, Ferson MJ, Rogers KD, Grant P, Churches T (2009) Evaluation of alternative respiratory syndromes for specific syndromic surveillance of influenza and respiratory syncytial virus: a time series analysis. BMC Infectious Diseases 9:1–9. 10.1186/1471-2334-9-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoto MA, Fricker RD, Jain A, Diamond A, Davies-Cole JO, Glymph C, Kidane G, Lum G, Jones L, Dehan K, Yuan C (2006) Evaluating statistical methods for syndromic surveillance. In: Statistical Methods in Counterterrorism: Game Theory, Modeling, Syndromic Surveillance, and Biometric Authentication, Wilson A, Wilson G, Olwell D (editors), New York, NY: Sprnger, pp 141–172 [Google Scholar]
- Szklo M, Nieto F (2007) Epidemiology: Beyond the Basics, 2nd ed., Sudbury, MA: Jones and Bartlett Publishers, LLC [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, Mlengeya T, Kirchhoff CA, Gilagiza B, Michael L, Kamenya S, Estes JD, Brandon F, Rudicell RS, Liu W, Patton S, Collins A, Hahn BH, Travis DA, Lonsdorf EV (2011) Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from Gombe National Park, Tanzania, 20042010. Journal of Zoo and Wildlife Medicine 42:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis DA, Lonsdorf EV, Mlengeya T, Raphael J (2008) A science based approach to managing disease risks for ape conservation. American Journal of Primatology 70:745–750. 10.1002/ajp.20566 [DOI] [PubMed] [Google Scholar]
- van den Wijngaard CC, van Asten L, van Pelt W, Doornbos G, Nagelkerke NJD, Donker GA, van der Hoek W, Koopmans MPG (2010) Syndromic surveillance for local outbreaks of lower-respiratory infections: Would It Work? (syndromic outbreak detection) PLoS ONE 5:e10406 10.1371/journal.pone.0010406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P, Tutin C, Oates J, Baillie J, Maisels F, Stokes E, Gatti S, Bergl R, Sunderland-Groves J, Dunn A (2008) Gorilla gorilla. In: IUCN Red List Threat. Species. Version 2012.2 www.iucnredlist.org. Accessed 1 May 2012
- Warns-Petit E, Morignat E, Artois M, Calavas D, Warns-petit E (2010) Unsupervised clustering of wildlife necropsy data for syndromic surveillance. BMC Veterinary Research 6:56 10.1186/1746-6148-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Lonsdorf E, Wilson M, Schumacher-Stankey J, Goodall J, Pusey A (2008) Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. American Journal of Primatology 70:766–777. 10.1002/ajp.20573 [DOI] [PubMed] [Google Scholar]
- Wolf TM, Wang WA, Lonsdorf EV, Gillespie T, Pusey A, Gilby IC, Travis DA, Singer RS, Wolf T (2019) Optimizing syndromic health surveillance in free ranging great apes: the case of Gombe National Park. Journal of Applied Ecology 56(3):509–518. 10.1111/1365-2664.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse MEJ, Gowtage-Sequeria S (2005) Host range and emerging and reemerging pathogens. Emerging Infectious Diseases 11:1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]


