Abstract
Aims:
To assess the effects of walnuts on cardiometabolic outcomes in obese people and to explore the underlying mechanisms using novel methods including metabolomic, lipidomic, glycomic and microbiome analysis, integrated with lipid particle fractionation, appetite-regulating hormones and haemodynamic measurements.
Materials and Methods:
A total of 10 obese individuals were enrolled in this crossover, randomized, double-blind, placebo-controlled clinical trial. The participants had two 5-day inpatient stays, during which they consumed a smoothie containing 48 g walnuts or a macronutrient-matched placebo smoothie without nuts, with a 1-month washout period between the two visits.
Results:
Walnut consumption improved aspects of the lipid profile; it reduced fasting small and dense LDL particles (P < 0.02) and increased postprandial large HDL particles (P < 0.01). Lipoprotein insulin resistance score, glucose and the insulin area under the curve (AUC) decreased significantly after walnut consumption (P < 0.01, P < 0.02 and P < 0.04, respectively). Consuming walnuts significantly increased 10 N-glycans, with eight of them carrying a fucose core. Lipidomic analysis showed a robust reduction in harmful ceramides, hexosylceramides and sphingomyelins, which have been shown to mediate effects on cardiometabolic risk. The peptide YY AUC significantly increased after walnut consumption (P < 0.03). No major significant changes in haemodynamic or metabolomic analysis or in microbiome host health-promoting bacteria such as Faecalibacterium were found.
Conclusions:
These data provide a more comprehensive mechanistic perspective of the effect of dietary walnut consumption on cardiometabolic variables. Lipidomic and lipid nuclear magnetic resonance spectroscopy analysis showed an early but significant reduction in ceramides and other atherogenic lipids with walnut consumption, which may explain the longer-term benefits of walnuts or other nuts on insulin resistance, cardiovascular risk and mortality.
Keywords: cardiovascular risk, ceramides, glycomics, lipidomics, Mediterranean diet, metabolomics, microbiota, nutrigenomics, walnuts
1 |. INTRODUCTION
Large interventional and observational studies have repeatedly demonstrated a link between increased walnut consumption and reductions in cardiovascular disease (CVD) risk and mortality.1,2 Clinical trials on the Mediterranean diet and other dietary patterns rich in walnuts have shown evidence of cardiometabolic benefits.3 While the majority of nuts contain high concentrations of monounsaturated fatty acids, walnuts (Juglans regia) are particularly rich in polyunsaturated fatty acids, primarily alpha-linolenic acid (ALA), an omega-3 fatty acid with anti-atherogenic effects.4 Walnuts are also rich in fibre and polyphenols that are potentially cardioprotective.3 The US Food and Drug Administration issued a qualified health claim for walnuts affirming that, in the context of a balanced diet, the consumption of approximately 42.5 g walnut per day reduces CVD risk5 and the American Diabetes Association also currently recommend walnut consumption.6 The beneficial effects of walnuts on CVD risk have been primarily attributed to altered lipid profile,7 glycaemic metabolism8 and vascular physiology9; however, prior studies do not fully explain the mechanisms underlying the beneficial effects of dietary walnuts on cardiometabolic health. A more comprehensive scientific approach which could provide a better understanding of these effects involves targeted analysis of several previously hypothesized pathways and the untargeted agnostic analysis using omics technologies, including changes in the relative abundance of metabolites (metabolomics), lipids (lipidomics), N-glycans (glycomics), and host-microbial communities (microbiome).10
We performed analysis of metabolomics, lipidomics, glycomics and the microbiome, assessed lipid fractionation and measured appetite-regulating hormones to explore mechanisms underlying the effects of dietary walnut consumption on metabolic and cardiovascular parameters in obese people. The present study is the first cross-over, randomized, double-blind, placebo-controlled short-term, inpatient feeding study using this integrated approach to evaluate the full spectrum of mechanisms underlying the cardiovascular and metabolic effects of walnut consumption in obese individuals.
2 |. MATERIALS AND METHODS
Ten individuals with obesity, as defined by body mass index ≥30 kg/m2, were enrolled in a randomized (1:1), double-blind, placebo-controlled, cross-over, 5-day inpatient study of either 48 g of walnuts (approximately the recommended daily dose)5 or “placebo” consumption, which was approved by the Beth Israel Deaconess Medical Center (BIDMC) institutional review board (Figure S1). All participants provided written informed consent. The study design has been described previously.11 Briefly, participants were admitted at the Clinical Research Centre of the BIDMC for 5 days during each phase (walnut or placebo). Participants consumed walnuts, or placebo, in which safflower oil and walnut flavouring replaced walnuts, in the form of a smoothie for breakfast during the five inpatient days, with the same macronutrient composition, allowing double-blinding, as previously described9,12 (Table S1). During both inpatient visits, participants followed an isocaloric diet to minimize variability. Baseline measurements were performed on day 1 (resting metabolic rate, body composition, haemodynamic and central blood pressure measures and blood draws after an overnight fast). The same measurements were repeated on day 5 and along with serial blood draws, at 0, 30, 60, 120 and 180 minutes after smoothie consumption. Fecal collection methods are described in File S1. Participants also had an ad libitum or weighed buffet meal to assess caloric consumption and food preferences (File S1). The participants were asked not to consume nuts during the 1-month washout period before they came back to receive the opposite smoothie (patients who received the walnut smoothie on the first visit received the placebo smoothie on the second visit and vice versa).
2.1 |. Body composition and energy expenditure measurements
Body composition was measured using a dual-energy X-ray absorptiometry scanner (Hologic 4500; Hologic, Waltham, Massachusetts) and resting metabolic rate was measured using indirect calorimetry (Vmax Spectra; Yorba Linda, CA, USA, Sensor Medics). Methods for cardiovascular and haemodynamic measurements and microbiome analysis are described in File S1.
2.2 |. Biochemical measurements
Blood samples drawn through venipuncture were processed for plasma or serum and stored at −80 ○C until assayed in duplicate. Measurements were acquired using the following techniques: commercially available ELISA kits, radioimmunoassays, automated immunoassay analyser (Immulite 1000; Siemens Healthcare Diagnostic) or a CLIA-certified external laboratory (the latter for basic cholesterol panel). Lipoprotein subclass profiles were measured using a 400-MHz proton nuclear magnetic resonance (NMR) spectrometer13 (File S1).
2.3 |. Omics measurements
Metabolomics and lipidomics were performed at the Whitehead Institute for Biomedical Research at the Massachusetts Institute of Technology, using liquid chromatography–mass spectrometry, as previously described.14 Glycomics were performed at the National Centre for Functional Glycomics at the BIDMC, as previously described15 (File S1).
2.4 |. Plasma fatty acids, total antioxidant capacity, total phenolic content and fecal short-chain fatty acid analysis
The short-chain fatty acid (SCFA) analysis was performed using gas chromatography as previously described.16 The analysis of total antioxidant capacity, total phenolic content and the quantification of plasma fatty acids using gas chromatography were performed at Hospital Clínic de Barcelona (File S1).
2.5 |. Statistical analysis
The Statistical Package for Social Sciences, v.19 was used for statistical analysis. Results are presented as means ± SE. Variables were checked for normality with the Kolmogorov–Smirnov test. Variables not normally distributed were log-transformed. A general linear mixed model was used to assess the treatment effect on anthropometric, clinical and laboratory variables with the variables of treatment, sequence and visit included as fixed effects, participant-within-sequence included as a random effect, and baseline values included as a covariate when available. The sample size has been calculated on a previously published functional MRI outcome11; however, the power to detect changes in other study variables was similar to that of our previous study,9,12 and thus, we hypothesized that changes would be detected in cardiometabolic outcomes, which were expanded in the present analysis. P values <0.05 were considered statistically significant.
Multivariate statistical analysis of the metabolomics, lipidomics and glycomic data was carried out using the significance analysis for microarrays algorithm in the TM4 MeV (version 4.9.0) data analysis software, and the partial least squares-discriminant analysis (PLS-DA) algorithm in the XLSTAT statistical software (version 2013.4.03). The trial was registered at ClinicalTrials.gov: (https://clinicaltrials.gov/ct2/show/NCT02673281).
3 |. RESULTS
3.1 |. Participant characteristics
Basic characteristics of the participants (six men and four women, mean age 50.7 ± 2.3 years) are shown in Table 1.
TABLE 1.
Demographic, anthropometric, body composition and energy expenditure measurements after 5 days of walnut or placebo
| Placebo |
Walnut |
||||
|---|---|---|---|---|---|
| Variables | Day 1 | Day 5 | Day 1 | Day 5 | P |
| Demografic | 10 | ||||
| n | 10 | 50.7 ± 2.3 | |||
| Age, years | 50.7 ± 2.3 | 6 (60) | |||
| Men, n (%) | 6 (60) | 4 (40) | |||
| Women, n (%) | 4 (40) | 5 (50) | |||
| African American, n (%) | 5 (50) | 4 (40) | |||
| White, n (%) | 4 (40) | 1 (10) | |||
| Hispanic, n (%) | 1 (10) | ||||
| History of disease | 3 (30) | ||||
| Hypertension, n (%) | 3 (30) | 1 (10) | |||
| Hyperlipidaemia, n (%) | 1 (10) | ||||
| Anthropometry | |||||
| Body mass index, kg/m2 | 36.8 ± 2.5 | 36.6 ± 2.5 | 37.1 ± 2.5 | 36.8 ± 2.4 | 0.35 |
| Body weight, kg | 107 ± 5.8 | 106 ± 5.6 | 108 ± 5.8 | 107 ± 5.52 | 0.35 |
| Waist circumference iliac, cm | 122 ± 7.1 | 121 ± 5.5 | 121 ± 5.7 | 124 ± 6.01 | 0.95 |
| Hip, cm | 118 ± 5.2 | 121 ± 5.5 | 123 ± 5.5 | 122 ± 5.61 | 0.16 |
| Waist/Hip | 0.97 ± 0.02 | 0.99 ± 0.02 | 0.97 ± 0.02 | 0.97 ± 0.03 | 0.57 |
| Fat body mass, kg | NDa | 42.9 ± 5.5 | NDa | 42.6 ± 5.3 | 0.73 |
| Fat (% of body mass) | NDa | 38.8 ± 3.4 | NDa | 38.4 ± 3.1 | 0.31 |
| Lean body mass, kg | NDa | 61.8 ± 2.3 | NDa | 62.5 ± 2.3 | 0.33 |
| VAT mass, g | NDa | 651 ± 95.5 | NDa | 665 ± 93.3 | 0.72 |
| RQ | NDa | 0.85 ± 0.01 | NDa | 0.86 ± 0.019 | 0.47 |
| REE, kcal/d | NDa | 1733 ± 87.1 | NDa | 1775 ± 78.1 | 0.51 |
| Fecal fat | |||||
| Total fecal fat, g/24 h | 1.76 ± 0.45 | 2.18 ± 0.65 | 2.71 ± 1.32 | 2.14 ± 0.72 | 0.52 |
| Fecal wet weight, g | 96.2 ± 21.3 | 163 ± 32.1 | 68.5 ± 13.6 | 164 ± 25.1 | 0.71 |
| % fat excreted | 1.9 ± 0.45 | 1.43 ± 0.48 | 2.49 ± 0.92 | 1.33 ± 0.47 | 0.62 |
Abbreviations: ND, no data; REE, resting energy expenditure; RQ, respiratory quotient; VAT, visceral adipose tissue.
Some assessments were done at select visits. Data shown as means ± SEM. P values are from a general linear mixed-model analysis of day 5 values of the walnut and placebo phases. The variables of treatment, visit and sequence were included in the model as fixed effects, and participant-within-sequence was included as a random effect, baseline values from the first visits in each arm were included as covariates when available. There was no significant difference between the baseline values of both the phases using a Mann–Whitney U-test. VAT = a random effect. Baseline values from the first visits in each RQ.
3.2 |. Effects of walnut consumption on food preferences
Participants tended to consume fewer kilocalories and total fats (not significant) in the ad libitum meal, while they ate significantly less total protein (P < 0.02), with a higher percentage of kilocalories derived from carbohydrates (P < 0.01) in the walnut phase (Table S2). Notably, no change in respiratory quotient was observed between the groups (Table 1).
3.3 |. Effects of walnut consumption on energy expenditure, body composition, fecal fat and SCFAs
No changes in resting energy expenditure or body composition were observed after 5 days of walnut or placebo smoothie consumption (Table 1). Total fecal fat was unchanged between the groups (Table S3). Fecal SCFAs showed a significant reduction of isobutyrate (P < 0.01; Table S3) and isovalerate (P < 0.02; Table S3) in the walnut group, without changes in acetate, propionate, and butyrate, compared to placebo (Table S3).
3.4 |. Effects of walnut consumption on gut hormones and metabolic variables
No significant changes in fasting measures of cardiometabolic markers were observed (Table S4). Glucose and insulin area under the curves (AUCs) were reduced with walnut consumption (P < 0.02 and P < 0.04, respectively), while the peptide YY (PYY) AUC was increased (P < 0.03; Table 2 and Figure S2); however only the PYY post-smoothie incremental AUC (AUC) change remained significant (Table S5).
TABLE 2.
Post-smoothie area under the curve of metabolic biomarkers and appetite-regulating hormones after 5 days of treatment with walnut or placebo
| Variablesa | Placebo Day 5 | Walnut Day 5 | P |
|---|---|---|---|
| Glucose, mg/dL*min | 18 496 ± 476 | 17 820 ± 402 | 0.02 |
| Insulin, μIU/mL*min | 4822 ± 640 | 4678 ± 799 | 0.04 |
| PYY, pg/mL*min | 19 892 ± 1147 | 22 314 ± 1340 | 0.03 |
| Leptin, pg/mL*min | 7940 ± 1936 | 7482 ± 1681 | 0.07 |
| Adiponectin, μg/mL*min | 4208 ± 368 | 4413 ± 217 | 0.31 |
| Ghrelin, pg/mL*min | 91 248 ± 8330 | 92 241 ± 7071 | 0.71 |
| Oxyntomodulin, pg/mL*min | 88 583 ± 9524 | 90 212 ± 10 275 | 0.84 |
| GLP-1, pg/mL*min | 7489 ± 1038 | 7368 ± 756 | 0.97 |
| GIP, pg/mL*min | 37 622 ± 2325 | 35 050 ± 4109 | 0.61 |
| FGF-21, pg/mL*min | 13 081 ± 2651 | 12 039 ± 2429 | 0.31 |
| C-peptide, ng/mL*min | 1002 ± 152 | 946 ± 125 | 0.63 |
| GH, ng/mL*min | 74 ± 25 | 40 ± 6.7 | 0.32 |
| IGF-1, ng/mL*min | 23 971 ± 4025 | 24 706 ± 3408 | 0.68 |
| IGFBP3, ng/mL*min | 1522 ± 223 | 1695 ± 301 | 0.68 |
| Cortisol, μg/dL*min | 2189 ± 196 | 1840 ± 99 | 0.18 |
Abbreviations: AUC, area under the curve; FGF-21, fibroblast growth factor 21; GH, growth hormone; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor 1; IGFBP3, insulin-like growth factor-binding protein 3; PYY, peptide YY.
AUC varibles are expressed as concentration*time (0–30–60–120–180 minutes). Data shown as means ± SEM. P values are from general linear mixed-model analysis of day-5 values of the walnut and placebo phases. Variables of treatment, visit and sequence were included in the model as fixed effects and participant-within-sequence was included as a random effect.
3.5 |. Effects of walnut consumption on lipid fractionation and basic cholesterol panel
Walnut consumption, using the NMR fasting plasma lipoprotein particle measurements, increased medium HDL particles (P < 0.01; Table 3) and small VLDL particles (P < 0.001; Table 3), and decreased atherogenic small LDL particles (P < 0.02; Table 3). No significant changes in fasting basic cholesterol panel measures, such as total cholesterol, clusterin, HDL, tryglycerides, LDL or oxidized LDL were observed (Table 3).
TABLE 3.
Fasting and area under the curve plasma lipids and lipoprotein particle profiles by nuclear magnetic resonance measurements after 5 days of treatment with walnut or placebo
| Placebo |
Walnut |
||||
|---|---|---|---|---|---|
| Variables | Day 1 | Day 5 | Day 1 | Day 5 | P |
| Fasting measurements | |||||
| Basic cholesterol panel | |||||
| Total cholesterol, mg/dL | 171 ± 12 | 169 ± 11 | 171 ± 12 | 168 ± 10 | 0.79 |
| Triglycerides, mg/dL | 105 ± 19 | 131 ± 13 | 105 ± 19 | 113 ± 13. | 0.57 |
| HDL cholesterol, mg/dL | 49 ± 1.9 | 43 ± 1.6 | 49 ± 1.9 | 44 ± 2.1 | 0.67 |
| Clusterin, μg/mL | 99 ± 9.01 | 97.6 ± 4.01 | 99 ± 8.9 | 103 ± 8.7 | 0.24 |
| VLDL cholesterol, mg/dL | 21 ± 3.8 | 26 ± 3.8 | 21 ± 3.8 | 22 ± 2.7 | 0.71 |
| LDL cholesterol, mg/dL | 100 ± 11 | 99 ± 10 | 100 ± 12 | 98 ± 9 | 0.70 |
| Oxidized LDL, ng/mL | 230 ± 28 | 256 ± 28 | 243 ± 23 | 237 ± 27 | 0.27 |
| NMR lipoprotein particles | |||||
| Large VLDL, nmol/L | 2.63 ± 0.37 | 3.04 ± 0.5 | 2.81 ± 0.29 | 2.35 ± 0.28 | 0.16 |
| Medium VLDL, nmol/L | 4.6 ± 1.2 | 6.9 ± 0.91 | 5.1 ± 1.5 | 8.4 ± 1.8 | 0.65 |
| Small VLDL, nmol/L | 40 ± 4.7 | 41 ± 3.8 | 36 ± 5 | 45 ± 3.7 | <0.001 |
| Large LDL, nmol/L | 278 ± 52 | 290 ± 52 | 231 ± 61 | 343 ± 83 | 0.80 |
| IDL, nmol/L | 250 ± 28 | 209 ± 53 | 292 ± 41 | 233 ± 39 | 0.64 |
| Small LDL, nmol/L | 339 ± 32 | 377 ± 53 | 384 ± 45 | 283 ± 33 | 0.02 |
| Large HDL, μmol/L | 3.34 ± 0.27 | 2.77 ± 0.36 | 3.5 ± 0.55 | 3.06 ± 0.2 | 0.45 |
| Medium HDL, μmol/L | 3.11 ± 0.8 | 3.47 ± 0.42 | 4.28 ± 0.86 | 5.8 ± 0.82 | 0.04 |
| Small HDL, μmol/L | 17.9 ± 0.89 | 17.5 ± 1.05 | 17.78 ± 0.94 | 17.5 ± 1.05 | 0.27 |
| Post-smoothie AUC measurementsa | |||||
| Large VLDL particles, nmol/L | NDb | 469 ± 71 | NDb | 392 ± 39 | 0.06 |
| Medium VLDL particles, nmol/L | NDb | 1180 ± 165 | NDb | 1829 ± 385 | 0.04 |
| Small VLDL particles, nmol/L | NDb | 6730 ± 742 | NDb | 6875 ± 539 | 0.82 |
| IDL particles, nmol/L | NDb | 46 591 ± 5619 | NDb | 42 603 ± 7062 | 0.21 |
| Large LDL particles, nmol/L | NDb | 42 448 ± 8284 | NDb | 41 176 ± 7456 | 0.37 |
| Small LDL particles (total), nmol/L | NDb | 71 889 ± 4822 | NDb | 71 694 ± 6220 | 0.96 |
| Large HDL particles, μmol/L | NDb | 526 ± 53 | NDb | 605 ± 36 | 0.01 |
| Medium HDL particles, μmol/L | NDb | 936 ± 115 | NDb | 946 ± 132 | 0.92 |
| Small HDL particles, μmol/L | NDb | 2974 ± 135 | NDb | 2985 ± 121 | 0.88 |
| Lipoprotein insulin resistance score | NDb | 9619 ± 787 | NDb | 8359 ± 500 | 0.01 |
Note: Data shown as means ± SEM. P values are from a general linear mixed-model analysis of day-5 values of the walnut and placebo phases. The variables of treatment, visit and sequence were included in the model as fixed effects and participant-within-sequence was included as a random effect. Baseline values from the first visits in each arm were included as covariates when available. Baseline values were not available for AUC analysis and screening values for basic cholesterol panel were used as baseline values for both phases. There was no significant difference between the baseline values of both the phases using a Mann–Whitney U-test.
Abbreviations: AUC, area under the curve; ND, no data; NMR, nuclear magnetic resonance.
AUC varibles are expressed as concentration*time (0–30–60–120–180 minutes).
Some assessments were done at select visits.
The post-smoothie VLDL particle AUC showed a tendency towards decreased triglyceride-enriched large VLDL particle AUC (P < 0.06; Table 3), balanced by an increase of the fewer triglyceride-rich, medium VLDL particle AUC (P < 0.04, Table 3). A significant decrease in the lipoprotein insulin resistance score AUC, an NMR lipid analysis-based and validated method to assess insulin resistance,17 was observed with walnuts (P < 0.01; Table 3). The large HDL particle AUC significantly increased in the walnut diet (P < 0.01; Table 3 and Figure S2). None of the aforementioned lipids showed a significant change between the two groups according to the incremental AUC (Table S6), indicating a contribution of baseline changes to the observed AUC changes.
3.6 |. Effects of walnut consumption on lipidomics and metabolomics
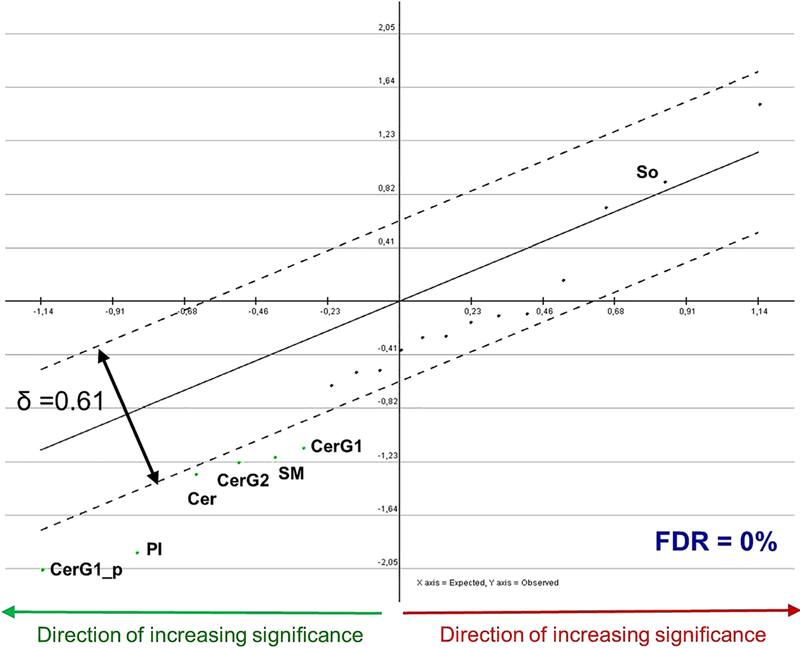
The multivariate statistical analysis indicated a collective significant decrease in the total abundance of the 19 monitored lipid classes in the walnut diet compared to the placebo diet (~ 4%) and identified five lipid classes which were significantly reduced in the walnut group (six profiles in total as CerG1 was considered with both its positive and negative ion mode measurements). These, in order of decreasing significance, are: hexosylceramides (CerG1_p) positive reading, phosphatidylinositol), ceramides, hexosylceramides ceramides G2 (CerG2), sphingomyelins, and hexosylceramides (CerG1) negative reading, while sphingosine concentration showed an increasing trend in the walnut group. No change in dihydroceramides concentration was observed (Figure 1). Multivariate statistical analysis of the metabolomic data showed no significance difference in the abundance of any of the 71 monitored metabolites.
FIGURE 1.
Paired significance analysis for microarrays curve of standardized lipids between the walnut and placebo. The analysis uses standardized values of the lipid measurements (ie, the mean value of a lipid concentration in all samples is subtracted from the measurement in this lipid in a particular sample and the residual is divided by the standard deviation of this lipid class measurement among all samples). The x-axis represents the expected score of the function that depends on a lipid class quantity. The y-axis represents the observed score of the function that depends on a lipid class quantity. If the absolute value of (observed – expected) score for a lipid class is larger than δ, then this lipid class is positively (if the residual is positive) or negatively (if the residual is negative) significantly changed in the walnut compared to the placebo samples. Each dot of the graph corresponds to a lipid class used in the analysis. Green dots below the lower dotted line correspond to the negatively significant lipid classes in the walnut compared to the placebo diet samples, while those above the lower dotted line correspond to the negatively non significant lipid classes in the walnut compared to the placebo diet samples. The dark-red dots, which are all between the two dotted lines, correspond to the positively non-significant lipid classes in the walnut compared to the placebo diet samples. The more distant a dot is from the origin of the axes, the more negatively significant the corresponding lipid class is. The threshold of significance (δ) is the smallest corresponding to a zero (0). FDR, false discovery rate–median, FDR-median (%). CerG1_p, hexosylceramides G1 positive reading; PI, phosphatidylinositol; Cer, ceramides; CerG2, hexosylceramides G2; SM, sphingomyelins; CerG1, hexosylceramides G1 negative reading; so, sphingosine
3.7 |. Effects of walnut consumption on fasting plasma fatty acids, total antioxidant capacity and polyphenol content
Walnut consumption increased the proportion of ALAs in plasma (P < 0.02), while the placebo smoothie consumption, rich in safflower oil, led to a significant increase in the proportion of plasma oleic acid (P < 0.03; Table S7). No differences in total antioxidant capacity or polyphenol content between the two groups were observed (Table S7).
3.8 |. Effects of walnut consumption on serum protein N-Glycans
A total of 58 different N-glycans structures, ranging from 1579 m/z to 4587 m/z were reported (Table S8). Using a paired significance analysis for microarrays, the relative abundance of 10 N-glycans was identified as significantly increased in the walnut diet false discovery rate (median = 0%; Figures S3 and S4B). These 10 N-glycans are complex N-glycans, with eight of them carrying a core fucose (fucose attached to the first N-acetylglucosamine residue). Four of these N-glycans are sialylated, carrying up to three sialic acids (N-acetylneuraminic acid).
3.9 |. Effects of walnut consumption on haemodynamic and cardiovascular measures
No changes were observed in 24-hour central blood pressure and haemodynamic measurements (Table S9), using Mobil-O-Graph (Table S10), or in the acute flow mediated dilation or hyperaemic response 3 hours after walnut smoothie consumption (Table S11).
4 |. DISCUSSION
We observed significant changes in insulin and glucose AUC after walnut consumption accompanied by a beneficial effect of walnuts on some lipid classes. We also applied, for the first time, an integrated approach, including multiomics and microbiome analyses, to broadly investigate using an untargeted approach the mechanisms underlying the positive cardiometabolic effect of dietary walnut consumption in people with obesity. Our results extend previous findings on the beneficial effects of walnut consumption on lipids7 and glucose.8 Additionally, in the present study, we showed for the first time that short-term walnut consumption significantly decreased atherogenic small and dense LDL particle levels and reduced harmful lipid classes such as ceramides and sphingomyelins, suggesting decreased lipotoxicity, which may lead to the previously demonstrated improvements in cardiometabolic health.2,8
Ceramides are metabolites of sphingolipid, can contribute to metabolic-related obesity disorders interfering with insulin signalling, leading to insulin resistance,18 and can be produced via different pathways (Figure 2). Insulin resistance in adipose tissue leads to an increase in circulating free fatty acids which, in turn, leads to the de novo production of ceramides.19
FIGURE 2.
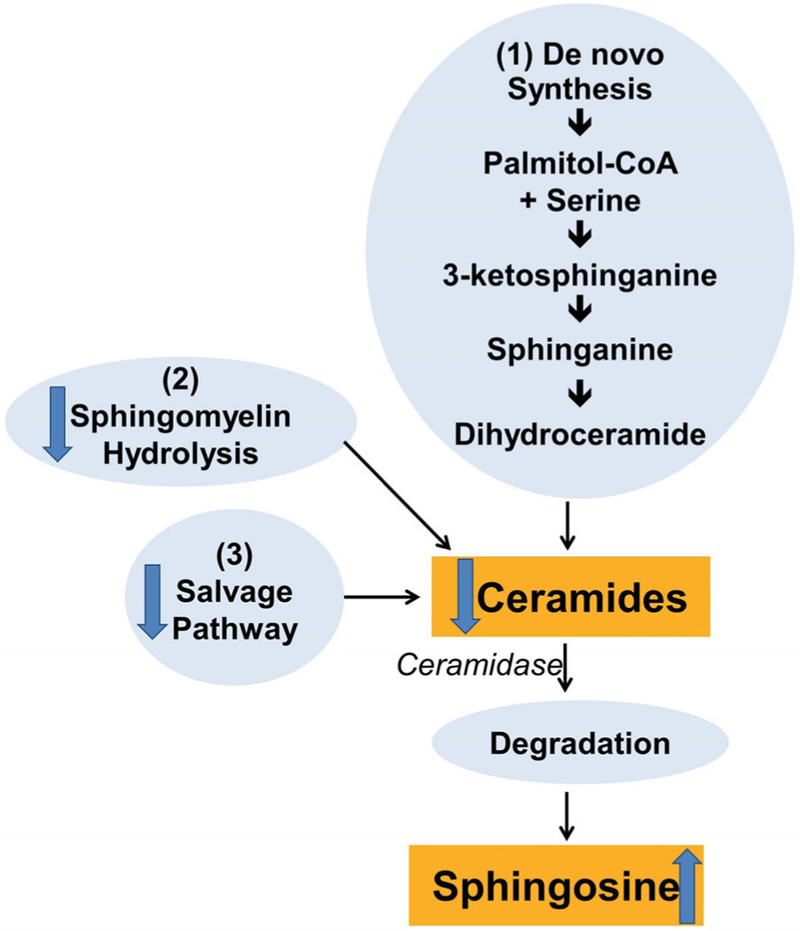
Pathways of ceramides synthesis. The schematic diagram depicts the pathways of ceramide metabolism. Cellular ceramide can be produced via several pathways: (1) by a de novo biosynthesis from precursor palmitate which is metabolized to dihydroceramides prior to formation of ceramides; (2) through the sphyngomyelin hydrolysis pathway; (3) through the “salvage pathway” consisting in the breakdown of more complex sphingolipids; ceramides are degraded by ceramidase leading to the production of sphingosine. The arrows indicate the increasing/decreasing (arrow up/arrow down) response to walnut consumption
To determine which ceramide synthetic pathway was regulated after walnut consumption, we analysed the serum concentrations of dihydroceramide (markers of de novo synthesis), sphingomyelins (markers of sphingomyelin hydrolysis), hexosylceramides (markers of the salvage pathway) and sphingosine (marker of ceramides degradation; Figure 2). Walnut consumption decreased total ceramides, hexosylceramides and sphingomyelins, and increased sphingosines without changes in dihydroceramide concentration. The general decrease in levels of different ceramide classes may indicate either a reduction in ceramide production and/or an increase of ceramide degradation. Particularly, the reduction of hexosylceramides and sphingomyelins may represent a decrease in the activation of the salvage pathway and shingomielin hydrolysis pathway, respectively, while an increase in sphingosines may suggest that walnut consumption could have decreased ceramide concentrations through increasing ceramidase activity, with subsequent increases of sphingosine, which is the breakdown product of ceramides.
Walnuts are rich in ALA content, and we observed the expected significant increase in plasma ALAs with walnut consumption. ALAs could also be a mediator of the observed reduction in ceramides. ALA consumption alters adipokine concentrations, particularly adiponectin,20,21 which can also reduce ceramide concentrations through the conversion of ceramides to sphingosine.22 Our group has previously shown, using a similar protocol, that 4-day walnut consumption increased fasting concentration of adiponectin,9 confirming the results of Lozano et al.20 Since ALAs and linoleic acids are the main lipid components of the walnut and the placebo smoothies, respectively, our data could be considered as reflecting the results of the effect of two different dietary lipid classes on plasma metabolites and lipids; however, this will need to be studied in more detail in future targeted studies that would vary only with respect to these lipids, given that the differences between the walnut and placebo smoothies used in the present study also included differences in fibre and other phytonutrients that are present in walnuts, but not in safflower oil. It remains to also be studied whether other nuts may have similar effects, as expected.
In the present study, although we did not see changes in the basic cholesterol panel, we did observe a significant modulation of NMR-analysed lipid particles, particularly LDL, HDL and VLDL particles after 5 days. Our results showed a significant reduction in small LDL particle levels. LDL cholesterol is positively associated with CVD mortality, and small LDL particles are more atherogenic than large LDL particles23 and their atherogenicity is increased by oxidation.24 In endothelial cells, endogenously produced ceramides are involved in the transcytosis of oxidized LDL across the endothelial cell barrier. Moreover, ceramides facilitated the subendothelial retention of these oxidized LDL, additionally stimulating the development of atherosclerosis.25 In the present study, we did not observe a significant reduction of oxidized LDL despite a significant reduction of small LDL particles, which are highly susceptible to oxidation.
We have shown a significant increase in large and medium HDL particles after consuming walnuts, and previous studies showed that circulating concentrations of large HDL particles are inversely related to CVD, while the opposite effect is associated with small HDL concentrations.26 In addition, we observed a significant reduction of triglyceride-rich lipoprotein VLDL AUC with walnut consumption, and this lipid class is strongly associated with CVD risk.27 Significant changes in the AUCs of glucose, insulin and certain NMR lipids were not replicated in the respective incremental AUCs (Tables S6 and S7). The different results for these two metrics are reasonably attributable to the distinct methods involved in their calculation. AUCs reflect changes in baseline values, whereas incremental AUCs evaluate only changes above and beyond any baseline changes. While the incremental AUC, which is usually used to evaluate acute glucose/lipid responses to meals relative to baseline values, eliminates results that drop under the baseline, the AUC does not. In summary, considering these differences, we propose that the significant effects observed in AUCs may be attributable to overall changes in the short-term 5-day effect of walnut consumption and less to specific only post-smoothie mixed-meal changes.
As a post-translational modification, glycans are commonly found attached to proteins (glycoproteins) and lipids (glycolipids) on the external surface of cells and also on circulating protein. They have numerous biological roles, such as immune functions and cell adhesion/migration. N-glycosylation is the major type of glycosylation found on circulating proteins. Variations or alterations of the N-glycans of serum proteins have been observed under physiological and pathological conditions. In the present study, we observed a significant increase of 10 N-glycans in the walnut group compared to the placebo group, all of which are complex N-glycans. Eight of them are core fucosylated and four of these are sialylated, carrying up to three sialic acids. With the exception of three (2418, 2968 and 3777 m/z) of these 10 N-glycans, all of the N-glycans identified in the present study are among the N-glycans found to decorate the constant Fc region of human immunoglobulin G (IgG), one of the most abundant glycoproteins in human serum.28 Changes in IgG N-glycans, especially changes in sialylation and core fucosylation, are known to impact the effector function of immunoglobulins,29 including antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity.30,31 Enhanced sialylation of IgG N-glycans has been shown to have anti-inflammatory effects, whereas increased core fucosylation was observed to decrease antibody-dependent cellular cytotoxicity response.30 While it remains to be confirmed and tested with further analyses, including of IgG N-glycans, our data suggest that the N-glycosylation and the effector functions of IgG may have been affected by the walnut diet.
No effects of walnut consumption on vascular and haemodynamic variables were observed, potentially because of the short timeframe, limited number of participants and the low rate of cardiovascular comorbidities in our population. Some limited, early changes in the microbiome are observed (see File S1 for details of this exploratory analysis).
The present study has some important strengths and limitations. The study is strengthened by its use of a previously validated and tested placebo/walnut smoothie delivery system, which allowed double-blinding.9,12 Another strength is the inpatient setting and the confirmative increase of plasma ALA proportion in the walnut arm, which ensured patient compliance. This may indicate that one of the primary differences between the walnut and placebo groups is ALA versus linoleic acid, and this is certainly a major difference; however, other phytonutrients also differ between the walnut and placebo groups from the other components of walnut versus safflower oil. Future studies will need to test directly whether this is attributable to only ALA versus linoleic acid differences and/or may reflect walnut-specific changes. The short-term 5-day effect of walnut consumption and the 180-minute-long duration of mixed-meal tests used in the present study, although extremely useful as a starting point, could also be considered as limitations of the study. Longer studies with more weeks of walnut consumption and/or mixed-meal tests of longer duration, for example, 300-minute-long tests are needed to extend these observations and may provide stronger and more significant changes in study outcomes including plasma lipids. Despite the small number of participants, the a priori power calculation in this study led to statistically significant results. The cross-over design reduced potential baseline participant differences and/or uncontrolled confounders. In this study we examined many variables, which were treated as discrete hypotheses.
To date, multiomic studies in humans have only been correlative, comparing sphingolipid concentrations in serum or tissues with one or more diseases/conditions (eg, insulin resistance, hepatic steatosis).32 Preclinical studies in rodents have been able to use interventional methods (eg, ceramide synthesis inhibition) to ascertain the ceramide role in metabolic disorders.33 In humans, ceramide plasma concentrations correlate with hypertension, myocardial infarction and stroke34 and they are independent predictors of plaque instability and/or future mortality, also exceeding the conventional predictive value of LDL cholesterol.35 These data show a possible clinical use of ceramides, and our results suggest that dietary walnuts may represent an effective nutritional modulator of ceramide concentrations that, in turn, could improve cardiometabolic health in the obese. Finally, no major differences among mass spectrometry-detectable metabolites were found between the walnut and placebo phases. This was to be expected, as short-term walnut consumption is not thought to substantially affect the primary metabolism, but rather the secondary metabolism and lipid biosynthesis and degradation pathways. Increased PYY concentration after walnut consumption may explain the improved sense of satiety, which has been reported with walnuts.12
In conclusion, in the present study, we explored the effect of walnut consumption on lipids and insulin resistance using a multiple approach analysis to provide a more in-depth and comprehensive analysis of walnuts on metabolic and cardiovascular variables in obesity. Data from lipidomics and NMR spectroscopy measurements demonstrated a significant reduction in harmful ceramides and some atherogenic lipids, also in the postprandial phase. These findings may emphasize the relevance of the postprandial state in the understanding of the possible cardioprotective mechanisms associated with dietary omega-3 fatty acids. While lipidomic and metabolomic analysis is quite well established, the understanding of glycomics results need to be further investigated with future studies. Considering the exploratory nature of the present study, future, larger studies are warranted to confirm findings of the present study on the walnut-mediated mechanisms that improve cardiometabolic health.
Supplementary Material
ACKNOWLEDGMENTS
The project was supported by Harvard Clinical and Translational Science Center Grant UL1 RR025758 from the National Centre for Research Resources and by NIH DK081913. The California Walnut Commission provided walnuts and supported the study through an investigator-initiated study grant. The California Walnut Commission approved funding the study, but had no role in study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Funding information
The California Walnut Commission; Harvard Clinical and Translational Science Centre Grant UL1 RR025758; National Centre for Research Resources and National Institute of Health (NIH) DK081913; National Centre for Functional Glycomics NIH grant P41GM103694
Footnotes
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13773.
CONFLICT OF INTEREST
C.S.M. has served as a consultant to the California Walnut Commission. All other authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev 2001;59: 103–111. [DOI] [PubMed] [Google Scholar]
- 2.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 3.Ros E Health benefits of nut consumption. Nutrients 2010;2: 652–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sala-Vila A, Cofan M, Nunez I, Gilabert R, Junyent M, Ros E. Carotid and femoral plaque burden is inversely associated with the alpha-linolenic acid proportion of serum phospholipids in Spanish subjects with primary dyslipidemia. Atherosclerosis 2011;214:209–214. [DOI] [PubMed] [Google Scholar]
- 5.Tarantino L Qualified health claims: letter of enforcement discretion — walnuts and coronary heart disease (docket no. 02P–0292) 2004:
- 6.Standards of Medical Care in Diabetes-2017. Summary of revisions. Diabetes Care 2017;40:S4–S5. [DOI] [PubMed] [Google Scholar]
- 7.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr 2009;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salas-Salvado J, Bullo M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronis KN, Vamvini MT, Chamberland JP, et al. Short-term walnut consumption increases circulating total adiponectin and apolipoprotein A concentrations, but does not affect markers of inflammation or vascular injury in obese humans with the metabolic syndrome: data from a double-blinded, randomized, placebo-controlled study. Metabolism 2012;61:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson JF, Allayee H, Gerszten RE, et al. Nutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a scientific statement from the American Heart Association. Circ Cardiovasc Genet 2016;9:291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farr OM, Tuccinardi D, Upadhyay J, Oussaada SM, Mantzoros CS. Walnut consumption increases activation of the insula to highly desirable food cues: a randomized, double-blind, placebo-controlled, cross-over fMRI study. Diabetes Obes Metab 2018;20:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan AM, Sweeney LL, Liu X, Mantzoros CS. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity (Silver Spring) 2010;18: 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab 2002;48:171–180. [PubMed] [Google Scholar]
- 14.Smulan LJ, Ding W, Freinkman E, Gujja S, Edwards YJK, Walker AK. Cholesterol-independent SREBP-1 maturation is linked to ARF1 inactivation. Cell Rep 2016;16:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehoux S, Mi R, Aryal RP, et al. Identification of distinct glycoforms of IgA1 in plasma from patients with immunoglobulin A (IgA) nephropathy and healthy individuals. Mol Cell Proteomics 2014;13: 3097–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 2014;4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada PHN, Demler OV, Dugani SB, et al. Lipoprotein insulin resistance score and risk of incident diabetes during extended follow-up of 20 years: the Women’s Health Study. J Clin Lipidol 2017;11:1257–1267 e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–594. [DOI] [PubMed] [Google Scholar]
- 19.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res 2009;50 (Suppl:S91–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozano A, Perez-Martinez P, Marin C, et al. An acute intake of a walnut-enriched meal improves postprandial adiponectin response in healthy young adults. Nutr Res 2013;33:1012–1018. [DOI] [PubMed] [Google Scholar]
- 21.Sekine S, Sasanuki S, Murano Y, Aoyama T, Takeuchi H. Alpha-linolenic acid-rich flaxseed oil ingestion increases plasma adiponectin level in rats. Int J Vitam Nutr Res 2008;78:223–229. [DOI] [PubMed] [Google Scholar]
- 22.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamarche B, St-Pierre AC, Ruel IL, Cantin B, Dagenais GR, Despres JP. A prospective, population-based study of low density lipoprotein particle size as a risk factor for ischemic heart disease in men. Can J Cardiol 2001;17:859–865. [PubMed] [Google Scholar]
- 24.Holvoet P, Mertens A, Verhamme P, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2001;21:844–848. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Yang X, Xing S, et al. Endogenous ceramide contributes to the transcytosis of oxLDL across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid Med Cell Longev 2014; 2014:823071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med 2009;150:84–93. [DOI] [PubMed] [Google Scholar]
- 27.Sacks FM, Alaupovic P, Moye LA, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation 2000;102:1886–1892. [DOI] [PubMed] [Google Scholar]
- 28.Xue J, Zhu LP, Wei Q. IgG-fc N-glycosylation at Asn297 and IgA O-glycosylation in the hinge region in health and disease. Glycoconj J 2013;30:735–745. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell 2018;9:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A 2017;114:3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karsten CM, Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology 2012;217: 1067–1079. [DOI] [PubMed] [Google Scholar]
- 32.Adams JM 2nd, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004;53:25–31. [DOI] [PubMed] [Google Scholar]
- 33.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179. [DOI] [PubMed] [Google Scholar]
- 34.Chaurasia B, Summers SA. Ceramides - Lipotoxic inducers of metabolic disorders: (trends in endocrinology and metabolism 26, 538–550; 2015). Trends Endocrinol Metab 2018;29:66–67. [DOI] [PubMed] [Google Scholar]
- 35.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.