Abstract
OBJECTIVES:
To examine the association between self-reported vision impairment (VI), hearing impairment (HI), and dual sensory impairment (DSI) stratified by dementia status on hospital admissions, hospice use, and healthcare costs.
DESIGN:
Retrospective analysis.
SETTING:
Medicare Current Beneficiary Survey from 1999 to 2006.
PARTICIPANTS:
Rotating panel of community-dwelling Medicare beneficiaries aged 65 years and older (N=24,009).
MEASUREMENTS:
Vision and hearing impairment were ascertained by self-report. Dementia status was determined by self-report or diagnosis codes in claims data. Primary outcomes included any inpatient admission over a two-year period, hospice use over a two-year period, annual Medicare Fee-for-Service costs, and total healthcare costs (which included information from Medicare claims data and other self-reported payments).
RESULTS:
Self-reported DSI was present in 30.2% (n=263/871) of participants with dementia and 17.8% (n=4,112/23,138) without dementia. In multivariable logistic regression models, HI, VI, or DSI were generally associated with increased odds of hospitalization and hospice use regardless of dementia status. In a generalized linear model adjusted for demographics, annual total healthcare costs were greater for those with DSI and dementia compared to DSI without dementia ($28,875 vs. $3,340, respectively). Presence of any sensory impairment was generally associated with higher healthcare costs. In a model adjusted for demographics, Medicaid status, and chronic medical conditions, DSI compared with no sensory impairment was associated with a small but statistically significant difference in total healthcare spending in those without dementia ($1,151 vs. $1,056, p<0.001) but not in those with dementia ($11,303 vs. $10,466, p=0.395).
CONCLUSION:
Older adults with sensory and cognitive impairments constitute a particularly prevalent and vulnerable population who are at increased risk of hospitalization and contribute to higher healthcare spending.
Keywords: sensory impairment, hearing impairment, vision impairment, dementia, healthcare cost
Introduction
Multiple chronic conditions (MCC) affect two in three adults over the age of 65 in the United States, and the number of co-existing conditions is associated with higher rates of disability, death, healthcare utilization, and healthcare cost.1,2 Given the rising prevalence of MCC and the growing emphasis on improving value in U.S. health care, it is important to identify specific conditions for which co-occurrence may lead to excessive healthcare utilization and cost.3 Sensory and cognitive impairments are two such conditions that commonly co-exist in older adults. One in ten adults aged 65 and older has Alzheimer’s disease, and around one in nine adults aged 80 years and older has hearing and vision impairment combined, termed dual sensory impairment (DSI).4,5 Hearing impairment (HI) and vision impairment (VI) are independently associated with adverse health outcomes, including hospitalization, cognitive impairment, poor physical functioning, reduced quality of life, and increased mortality.6–9 Compared to either HI or VI alone, DSI leads to higher rates of functional dependence and hospitalization.10,11 Sensory and cognitive impairments are also independently associated with increased healthcare cost.5,12–15 The estimated direct medical cost for hearing impairment alone ranges from $3.3 to $12.8 billion per year, and the total monetary cost of dementia in 2010 was estimated to be between $157 billion and $215 billion.13,14
Despite the high prevalence of sensory and cognitive impairments in older adults, there are several key gaps in knowledge about the effects of this combination on outcomes such as healthcare utilization and cost.16 This combination may be a potent driver of healthcare utilization and cost due to high rates of functional impairment, increased skilled nursing utilization, and increased risk for steeper cognitive decline.7,17,18 These impairments may also adversely affect a person’s capacity to self-manage chronic conditions and access preventive care, which could lead to higher utilization of acute services and spiraling cost. One barrier to characterizing this relationship is the lack of population data that contains utilization and cost information as well as reliable assessments of dementia diagnosis and sensory impairments. The Medicare Current Beneficiary Survey (MCBS), which collects data on the costs, use, and healthcare status of a nationally representative cohort of the Medicare population, serves as an ideal tool to address these issues. The objective of the current study was to examine how self-reported sensory impairments in the MCBS relate to healthcare utilization and cost in community-dwelling older adults with and without dementia. As our primary measure of healthcare utilization, we assessed inpatient admissions because hospitalization contributes to increased functional decline, loss of independence, institutionalization, and a large percentage of adjusted Medicare expenditures.19,20 Hospice use was also considered given the rising prevalence of Medicare beneficiaries with dementia who utilize hospice services and its potential to reduce inpatient admissions.5
Methods
Study Population
We examined a total of 24,009 community dwelling Medicare beneficiaries aged 65 and older enrolled in the MCBS between 1999 to 2006. The MCBS is a continuous survey of a nationally representative sample of Medicare Beneficiaries, based on a rotating panel whereby participants enter and leave the survey annually.21 New participants remain in the study for four years. Interviews are conducted up to three times per year. The MCBS is linked to the Centers for Medicare and Medicaid Services (CMS) enrollment file and further connected to Medicare claims files that provide information on healthcare utilizations and costs.
We defined periods of two-year units between the index years of 1999 to 2006 with each subject included only once in the analysis, and we used the first two-year period that contained sufficient baseline information to ascertain our primary variables of interest. We used two-year periods to provide a representative sample of each subject’s average utilization tendencies, recognizing that cost and utilization fluctuates due to acute illnesses and adverse health events. Inclusion criteria included: age-entitled beneficiaries, community dwelling at the start of the study period, and available data on all independent variables, dependent variables, and covariates of interest. We included proxy responses when available which accounted for roughly 10% of survey responses. This study was approved by the institutional review board (IRB) of Duke University Medical Center.
Independent Variables
Hearing impairment was defined based on two self-reported questions: 1) Which statement best describes your hearing (with a hearing aid, if you use one)? (no trouble, a little trouble, or a lot of trouble) and 2) Do you use a hearing aid (yes, no, or deaf)? If participants reported “a little trouble” or “a lot of trouble” or if they used hearing aids or indicated deafness, then the participants were classified as hearing impaired.
Vision impairment was defined based on one self-reported question: How much trouble do you have with your vision? (no trouble, little trouble, or a lot of trouble). Subjects who reported “little trouble” or “a lot of trouble” were classified as visually impaired. We did not define vision impairment based on reported use of glasses or contacts because 83% of subjects reported using glasses, suggesting that many respondents referred to reading glasses.
Dementia was defined by either answering yes to the question, “Have you ever been told you have a diagnosis of Alzheimer’s disease or dementia?” or the presence of any ICD-9 dementia diagnosis codes from either inpatient or outpatient Medicare claim files (Supplementary Table 1). We used both approaches to maximize case finding as has been done in previous studies using MCBS data.22–24
Dependent Variables
Healthcare utilization outcomes were any inpatient admission and any hospice use during the two-year study units as verified in the Medicare claims files. Healthcare costs were defined by the annual per subject Medicare Fee for Service (FFS) costs and total healthcare costs, in 2006 dollars. Total cost includes payments from Medicare, Medicaid, Medicare HMO, private HMO, Veterans Affairs, employer sponsored payments, individual purchased insurance, unknown private insurance, out-of-pocket payments, uncollected liability, and other sources. While all Medicare costs were derived from the Medicare claim files, non-Medicare costs are based on self-reported data and adjustments using MCBS algorithms for estimation.
Covariates
Covariates included age, gender, race, annual income less than $10,000, and Medicaid insurance coverage eligibility. A variable indicating whether the participant lived alone was also included because several studies have demonstrated a relationship between living alone and healthcare utilization, albeit in different directions.25,26 Chronic health conditions were divided into two groups: Group A conditions were those with lower short-term risk of mortality, including hypertension, stroke, diabetes, rheumatoid and other arthritis, and osteoporosis; and Group B conditions were those with relatively higher short-term risk of mortality, including coronary heart disease, myocardial infarction, cancer, or lung disease. We grouped conditions in this manner to evaluate if healthcare cost varied in hierarchical models based on the pattern of chronic medical conditions.
Statistical Analyses
Differences in demographic and clinical characteristics among those with and without dementia were assessed using chi-square tests for categorical variables and t-test or Wilcoxon tests for continuous variables. In a stratified analysis of participants with and without dementia, multivariable logistic regressions were used to measure the relationship between all combinations of vision and hearing impairment with any inpatient admission or hospice use. We stratified our analysis into participants with and without dementia as we felt that dementia may act as an effect modifier. The analyses were adjusted in a series of four hierarchical models: 1) adjusted for demographics only (age, gender, race, income, and lives alone); 2) adjusted for demographics and Medicaid eligibility; 3) adjusted for demographics, Medicaid eligibility, and sum of Group A chronic conditions; 4) adjusted for demographics, Medicaid eligibility, sum of Group A chronic conditions, and sum of Group B chronic conditions. For healthcare cost data, a generalized linear model with log-link and gamma distribution measured the relationship between all combinations of vision and hearing impairments with annual total healthcare costs and Medicare FFS costs in participants with and without dementia.27,28, All statistical analyses were performed using SAS statistical software version 9.0 (SAS Institute, Inc., Cary, NC). All hypothesis tests were two-sided with a significance level of p<0.05.
Results
Baseline characteristics
The study population consisted of 24,009 older community-dwelling adults (Table 1). Among this sample, 871 participants (3.6%) had dementia, 5,854 participants (24.4%) had HI alone, 3,488 participants (14.5%) had VI alone, and 4,375 participants (18.2%) had DSI. Participants with dementia were more likely to be older, female, African American, and have an annual income less than $10,000 compared to participants without dementia. They also had a higher total score of pre-existing comorbidities (3.2 vs. 2.7) and were more likely to have DSI (30.2% vs. 17.8%, respectively).
Table 1:
Baseline characteristics of community-dwelling older adults aged 65 years and older enrolled in the 1999–2006 Medicare Current Beneficiary Survey stratified by dementia status.
| Dementia (N=871) | No Dementia (N=23,138) | P-value | |
|---|---|---|---|
| Age (years) (SD) | 81.9 (7.0) | 76.4 (7.0) | <0.001 |
| Female gender (%) | 63.7 | 57.9 | <0.001 |
| Black (%) | 18.7 | 12.7 | <0.001 |
| Annual income less than $10,000 (%) | 32.6 | 20.9 | <0.001 |
| Lives alone (%) | 27.7 | 35.9 | <0.001 |
| Medicaid insurance coverage eligible (%) | 24.8 | 11.2 | <0.001 |
| Sensory impairment | |||
| No vision or hearing impairment | 261 (30.0%) | 10,031 (43.3%) | <0.001 |
| Vision impairment, no hearing impairment | 137 (15.7%) | 3,351 (14.5%) | |
| Hearing impairment, no vision impairment | 210 (24.1%) | 5,644 (24.4%) | |
| Dual sensory impairment | 263 (30.2%) | 4,112 (17.8%) | |
| Comorbidity score, up to 11 points (SD) | 3.2 (1.8) | 2.7 (2.7) | <0.001 |
| Coronary heart disease (%) | 18.4 | 12.9 | <0.001 |
| Hypertension (%) | 65.7 | 63.4 | .18 |
| Heart disease (CHF, valve disease, arrhythmias) (%) | 26.0 | 23.4 | .08 |
| Previous myocardial infarction (%) | 20.3 | 14.4 | <0.001 |
| Stroke (%) | 30.3 | 11.3 | <0.001 |
| Cancer (%) | 18.3 | 20.0 | .21 |
| Diabetes mellitus (%) | 22.6 | 19.7 | .03 |
| Rheumatoid arthritis (%) | 14.5 | 10.9 | <0.001 |
| Other arthritis (e.g. osteoarthritis) (%) | 63.0 | 61.6 | .40 |
| Osteoporosis (%) | 25.8 | 20.6 | <0.001 |
| Lung disease (emphysema, COPD, asthma) (%) | 16.1 | 14.4 | .17 |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; SD, standard deviation
Hospital admission
The results of multivariable logistic regression analyses between sensory impairments and any inpatient hospitalization over two years are shown in Table 2. Among participants with dementia, higher odds of hospital admission were seen in participants with VI only (OR=2.03, 95% CI=1.32, 3.11) and DSI (OR=1.58, 95% CI=1.11, 2.25) but not HI only (OR=1.15, 95% CI=0.79, 1.68) in the model adjusted for demographics only. Most associations were attenuated in the fully adjusted model, but higher odds of hospitalization remained statistically significant for the VI only group (OR=1.82, 95% CI=1.17, 2.82). Among those without dementia, higher odds of hospitalization were seen across all three sensory impairment groups in most models except for the fully adjusted model where statistical significance was seen only with the DSI group (OR=1.13, 95% CI=1.04, 1.23).
Table 2:
Association between sensory impairment and inpatient admission and hospice use in older adults stratified by dementia status.
| Inpatient Admission | Hospice Use | |||||||
|---|---|---|---|---|---|---|---|---|
| Dementia (N=871) | No Dementia (N=23,138) | Dementia (N=871) | No Dementia (N=23,138) | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Model 1 | ||||||||
| VI Only | 2.03 | 1.32, 3.11* | 1.22 | 1.12, 1.33* | 1.15 | 0.50, 2.66 | 1.04 | 0.74, 1.46 |
| HI Only | 1.15 | 0.79, 1.68 | 1.15 | 1.07, 1.24* | 1.41 | 0.66, 2.99 | 0.92 | 0.69, 1.22 |
| DSI | 1.58 | 1.11, 2.25* | 1.41 | 1.31, 1.53* | 1.94 | 1.00, 3.77* | 1.46 | 1.11, 1.91* |
| Model 2 | ||||||||
| VI Only | 2.00 | 1.30, 3.07* | 1.20 | 1.11, 1.31* | 1.11 | 0.48, 2.58 | 1.03 | 0.73, 1.45 |
| HI Only | 1.14 | 0.78, 1.67 | 1.15 | 1.07, 1.24* | 1.40 | 0.66, 2.98 | 0.92 | 0.69, 1.22 |
| DSI | 1.56 | 1.10, 2.23* | 1.41 | 1.30, 1.52* | 1.90 | 0.98, 3.71 | 1.45 | 1.11, 1.90* |
| Model 3 | ||||||||
| VI Only | 1.89 | 1.23, 2.92* | 1.14 | 1.04, 1.24* | 1.19 | 0.51, 2.77 | 1.04 | 0.74, 1.46 |
| HI Only | 1.10 | 0.75, 1.61 | 1.10 | 1.03, 1.19* | 1.48 | 0.69, 3.17 | 0.92 | 0.69, 1.22 |
| DSI | 1.46 | 1.02, 2.09* | 1.27 | 1.17, 1.38* | 2.09 | 1.05, 4.13* | 1.46 | 1.11, 1.91* |
| Model 4 | ||||||||
| VI Only | 1.82 | 1.17, 2.82* | 1.07 | 0.98, 1.17 | 1.18 | 0.50, 2.76 | 1.00 | 0.71, 1.41 |
| HI Only | 1.03 | 0.70, 1.52 | 1.04 | 0.96, 1.12 | 1.39 | 0.65, 3.01 | 0.87 | 0.65, 1.16 |
| DSI | 1.39 | 0.96, 2.01 | 1.13 | 1.04, 1.23* | 2.11 | 1.05, 4.21* | 1.36 | 1.03, 1.78* |
Indicates p < 0.05 compared to those without sensory impairments. Model 1 was adjusted for age, gender, race, income, and living situation. Model 2 added adjustment for Medicaid eligibility. Model 3 added adjustment for Group A chronic conditions (hypertension, stroke, diabetes, rheumatoid and other arthritis, and osteoporosis). Model 4 added adjustment for Group B chronic conditions (coronary heart disease, myocardial infarction, cancer, or lung disease). Abbreviations: CI, confidence interval; DSI, dual-sensory impairment; HI, hearing impairment; OR, odds ratio; VI, vision impairment.
Hospice use
Table 2 summarizes the association between sensory impairment and hospice use. Among participants with dementia, those with DSI had higher odds of hospice use in most models (OR=2.11, 95% CI=1.05, 4.21 for Model 4). Similarly, DSI but not VI or HI was associated with hospice use in people without dementia.
Total annual healthcare cost
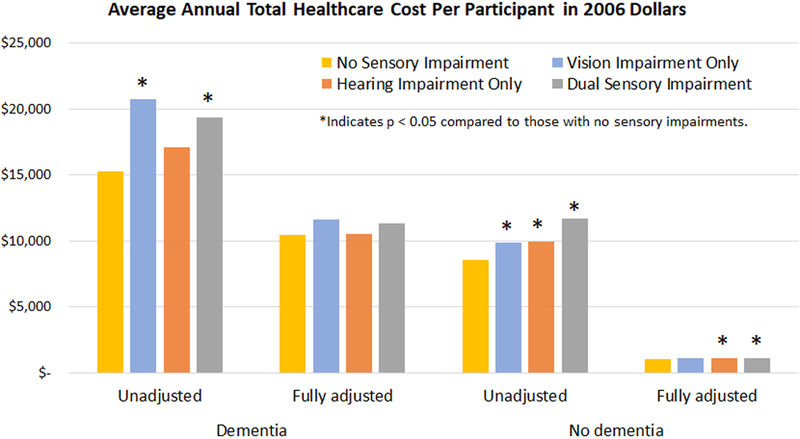
Total annual healthcare costs per participant were consistently higher among those with dementia compared with those without dementia across all models (Figure 1, Table 3). For example, those with DSI and dementia spent $11,303 per year on average compared with $1,151 among those with DSI and without dementia in the fully adjusted model. Generally, regardless of dementia status, VI and DSI were associated with higher annual total costs.
Figure 1:
Relationship between sensory impairment and average annual total healthcare cost per individual in 2006 dollars stratified by dementia status.
Table 3:
Relationship between sensory impairment and average annual total healthcare cost per individual in 2006 dollars stratified by dementia status.
| Dementia | No Dementia | |||
|---|---|---|---|---|
| Average Cost (95% CI) | P value | Average Cost (95% CI) | P value | |
| Unadjusted | ||||
| No VI or HI | $15,273 (13,609–17,140) | REF | $8,531 (8,349–8,718) | REF |
| VI only | $20,771 (17,105–25,201) | 0.002 | $9,896 (9,555–10,408) | <0.001 |
| HI only | $17,106 (14,357–20,313) | 0.207 | $9,982 (9,640–10,408) | <0.001 |
| DSI | $19,397 (16,495–22,757) | 0.004 | $11,688 (11,261–12,200) | <0.001 |
| Model 1 | ||||
| No VI or HI | $22,737 (9,991–51,741) | REF | $2,589 (2,180–3,075) | REF |
| VI only | $29,557 (10,690–36,151) | 0.008 | $2,952 (2,823– 3,081) | <0.001 |
| HI only | $26,602 (22,282–31,831) | 0.092 | $2,900 (2,797–3,004) | <0.001 |
| DSI | $28,875 (24,328–34,105) | 0.005 | $3,340 (3,211–3,470) | <0.001 |
| Model 2 | ||||
| No VI or HI | $17,961 (7,909–40,792) | REF | $2,167 (1,821–2,577) | REF |
| VI only | $21,554 (17,602–26,224) | 0.074 | $2,427 (2,340–2,535) | <0.001 |
| HI only | $20,117 (16,704–23,889) | 0.228 | $2,427 (2,340–2,535) | <0.001 |
| DSI | $21,733 (18,321–25,685) | 0.029 | $2,773 (2,665–2,903) | <0.001 |
| Model 3 | ||||
| No VI or HI | $13,396 (5,944–30,187) | REF | $1,237 (1,044–1,466) | REF |
| VI only | $15,137 (12,458–18,486) | 0.230 | $1,336 (1,287–1,398) | <0.001 |
| HI only | $14,199 (11,788–16,878) | 0.551 | $1,349 (1,312–1,398) | <0.001 |
| DSI | $14,735 (12,458–17,414) | 0.273 | $1,460 (1,398–1,510) | <0.001 |
| Model 4 | ||||
| No VI or HI | $10,466 (4,600–23,812) | REF | $1,056 (896–1,245) | REF |
| VI only | $11,617 (9,524–14,233) | 0.282 | $1,099 (1,056–1,141) | 0.063 |
| HI only | $10,570 (8,896–12,664) | 0.890 | $1,120 (1,077–1,151) | 0.002 |
| DSI | $11,303 (9,524–13,291) | 0.395 | $1,151 (1,109–1,204) | <0.001 |
Model 1 was adjusted for age, gender, race, income, and living situation. Model 2 added adjustment for Medicaid eligibility. Model 3 added adjustment for Group A chronic conditions (hypertension, stroke, diabetes, rheumatoid and other arthritis, and osteoporosis). Model 4 added adjustment for Group B chronic conditions (coronary heart disease, myocardial infarction, cancer, or lung disease). Abbreviations: CI, confidence interval; DSI, dual sensory impairment; HI, hearing impairment; REF, reference; VI, vision impairment.
Among participants with dementia, no statistically significant differences in spending were seen in the fully adjusted model among the different combinations of sensory impairment. Among those without dementia, small but statistically significant differences in total spending were generally seen in participants with all combinations of sensory impairment compared to those without any sensory impairment. In the fully adjusted model, participants with neither sensory impairment nor dementia spent an average of $1,056 per year compared to $1,099 among those with VI alone (p=0.063), $1,120 among those with HI alone (p=0.002), and $1,151 among those with DSI (p<0.001).
Annual Medicare Fee for Service cost
Results for average annual Medicare FFS cost per participant in 2006 dollars are displayed in Table 4. Those with dementia had higher annual Medicare FFS cost compared with those without dementia in all models. In the fully adjusted model, no statistically significant differences in cost were seen with any combination of sensory impairment in those with dementia. Among those without dementia, DSI was associated with the largest differences in spending compared to those without sensory impairments. This relationship was statistically significant in all models, except for the fully adjusted model (average spending of $193 in those with DSI compared to $184 in those without sensory impairments, p=0.17).
Table 4:
Relationship between sensory impairment and average annual Medicare Fee for Service cost per individual in 2006 dollars stratified by dementia status.
| Dementia | No Dementia | |||
|---|---|---|---|---|
| Average Cost (95% CI) | P value | Average Cost (95% CI) | P value | |
| Unadjusted | ||||
| No VI or HI | $8,867 (7,360–10,683) | REF | $4,518 (4,360–4,682) | REF |
| VI only | $13,655 (9,931–18,798) | 0.007 | $5,331 (4,970–5,738) | <0.001 |
| HI only | $10,197 (7,714–13,478) | 0.318 | $5,150 (4,879–5,467) | <0.001 |
| DSI | $12,236 (9,399–15,872) | 0.017 | $6,416 (6,009–6,867) | <0.001 |
| Model 1 | ||||
| No VI or HI | $9,421 (2,411–36,812) | REF | $684 (515–909) | REF |
| VI only | $13,849 (10,080–19,124) | 0.019 | $773 (719–835) | <0.001 |
| HI only | $11,117 (8,290–14,791) | 0.272 | $725 (678–767) | 0.080 |
| DSI | $12,341 (9,420–16,110) | 0.050 | $862 (808–924) | <0.001 |
| Model 2 | ||||
| No VI or HI | $5,585 (1,418–21,996) | REF | $455 (341–606) | REF |
| VI only | $7,205 (5,194–9,941) | 0.127 | $497 (463–532) | 0.014 |
| HI only | $6,032 (4,524–8,098) | 0.585 | $482 (454–514) | 0.059 |
| DSI | $6,590 (5,027–8,657) | 0.224 | $570 (533–609) | <0.001 |
| Model 3 | ||||
| No VI or HI | $4,055 (1,039–15,831) | REF | $250 (188–333) | REF |
| VI only | $4,907 (3,528–6,772) | 0.259 | $266 (248–285) | 0.089 |
| HI only | $4,136 (3,123–5,556) | 0.879 | $259 (244–275) | 0.270 |
| DSI | $4,380 (3,325–5,759) | 0.592 | $286 (267–306) | <0.001 |
| Model 4 | ||||
| No VI or HI | $3,338 (851–13,097) | REF | $184 (139–244) | REF |
| VI only | $3,872 (2,804–5,374) | 0.364 | $186 (173–199) | 0.799 |
| HI only | $3,238 (2,436–4,339) | 0.840 | $181 (170–192) | 0.567 |
| DSI | $3,505 (2,637–4,606) | 0.737 | $193 (180–206) | 0.173 |
Model 1 was adjusted for age, gender, race, income, and living situation. Model 2 added adjustment for Medicaid eligibility. Model 3 added adjustment for Group A chronic conditions (hypertension, stroke, diabetes, rheumatoid and other arthritis, and osteoporosis). Model 4 added adjustment for Group B chronic conditions (coronary heart disease, myocardial infarction, cancer, or lung disease). Abbreviations: CI, confidence interval; DSI, dual sensory impairment; HI, hearing impairment; REF, reference; VI, vision impairment
Discussion
In a representative sample of Medicare beneficiaries, we found that the presence of self-reported sensory impairments was generally associated with increased healthcare utilization, including inpatient admissions, and total annual healthcare cost. Sensory impairments were common in this population, with 18% of those without dementia and 30% of those with dementia reporting combined vision and hearing impairments. Prior studies demonstrate an increased risk of hospital admissions among those with sensory or cognitive impairments.6,8,20,29 Our findings among those with DSI are also in line with previous work showing that individuals with DSI experience more hospitalization days compared to those with normal vision or hearing.10 We extend these findings by showing that DSI is a risk factor for hospitalization regardless of dementia status.
Hospice enrollment was higher among those with DSI regardless of dementia status. Given that patients with dementia enrolled in hospice are more likely to die in their location of choice, our results highlight an encouraging trend in achieving goal concordant care at the end of life for people with multiple impairments.30 In those without dementia, higher hospice use among participants with DSI may reflect the higher degree of disease burden associated with DSI. Alternative possibilities that merit further exploration are whether sensory impairment influences one’s preferences for life-extending care or if the effect is modified by race and gender.31,32
Related to healthcare cost, participants with dementia had higher average costs compared to those without dementia regardless of coexisting sensory impairment, consistent with previous research.5,14,33,34 Drivers of healthcare cost among participants with dementia include increased functional impairments, greater need for home healthcare, higher prescription drug expenditures, multiple chronic conditions, and hospital admissions.23,34–36 Generally, those with DSI had the highest annual total costs regardless of dementia status. In those without dementia, we found a small but statistically significant difference in total healthcare spending among participants with HI and DSI compared to those without sensory impairments. As a comparison, one report using the Medical Expenditure Panel Survey showed that those with self-reported hearing loss had $392 in excess medical expenditures compared to those without hearing loss, corresponding to approximately $3.10 billion in excess medical expenditures given a prevalence of 7.91 million individuals with hearing loss in the U.S.37 We did not find a statistically significant relationship between sensory impairments and healthcare spending among those with dementia. One possible explanation is that of a ceiling effect. Given the high baseline level of spending, sensory impairments may be less likely to impact cost in this setting. Additionally, the relatively small number of participants with dementia may have diminished our power to detect meaningful associations.
Our findings related to healthcare utilization and cost may have several explanations. People with sensory and cognitive impairment are at high risk for falls, depression, social isolation, reduced health literacy, and impaired physical functioning.7,11,38 Social isolation is an underrecognized risk factor that may contribute to worse health outcomes through decreased medication adherence, reduced self-esteem, and poorer coping strategies.39 The threshold for hospitalization is likely lower in this population both from a physiologic perspective and social perspective (e.g., higher likelihood that emergency department physicians are concerned about safety of discharge).40 This is supported by an increased risk of hospitalization for ambulatory care sensitive conditions among patients with dementia.35,41 Sensory impairment in individuals with dementia is also associated with higher rates of neuropsychiatric symptoms which contribute to hospital admissions.42
Given the increased risk of dementia and cognitive decline in those with sensory impairments, the development of effective screening tools for cognitive impairment in this population will be crucial to identifying those at highest risk.16 Interventions to address medical risk factors that lead to loss of vision and hearing in addition to rehabilitative or restorative treatments such as hearing aids or cataract surgery may help to control excess healthcare costs. While the Over-The-Counter Hearing Aid Act of 2017 is the first step towards ensuring access to affordable hearing aids, legislation that expands access to hearing rehabilitative services such as communication counseling is equally important. Future studies can help to determine if increasing coverage for restorative sensory care services will result in decreased overall healthcare cost.43 Additionally, focusing on sensory health within the context of pre-existing dementia care programs may help to further promote functional independence and decrease healthcare spending.44 Another important consideration in this population is that hospitalization often represents a sentinel event that precipitates subsequent decline and institutionalization due to hospitalization associated disability and delirium.45 System and physician-led interventions such as promoting early mobility, reducing polypharmacy, and providing hearing aids and glasses in the hospital may be opportunities to prevent this chain of adverse outcomes.
Our study has several limitations that may affect the results. First, sensory impairments were assessed by self-report in the MCBS. Correlation between self-report and objective measures varies based on the cohort and has not been formally assessed within the MCBS.46,47 However, subjective measures remain important because they capture the perceived quality of hearing and vision. Similarly, dementia was classified by both survey report and Medicare claims data as used in previous studies involving the MCBS.23,24 Case definitions of dementia in database analyses may result in biased estimates.48,49 Second, data on the severity of sensory impairments, dementia, and co-morbidities were not available in this dataset. In prior work, greater severity of hearing impairment was associated with higher hospitalization risk.8 While greater severity of dementia is generally associated with higher healthcare cost, its effect on hospitalization risk is inconsistent across studies.35,40,50 Third, the dementia group represented a small percentage of the entire population (n=871, 3.6%), which likely impacts the precision of our estimates. The relatively low prevalence of dementia compared to previous reports may be due to selection bias if participants with more severe dementia did not accurately report the level of their impairment or were excluded because they did not answer certain survey items. However, the potential for this selection bias might have been reduced by including proxy responses when available. Fourth, although we adjusted for relevant demographic and health variables, there remains the possibility of unmeasured confounding. Fifth, because we categorized participants with sensory impairments into mutually exclusive groups, our findings likely underestimate the total costs associated with vision and hearing impairment alone.
Overall, our study highlights a prevalent and vulnerable population of community-dwelling older adults. Individuals with sensory impairments are at increased risk of hospitalization regardless of dementia status. Among individuals without dementia, sensory impairments were associated with higher total healthcare spending. While we did not identify an added effect of comorbid sensory impairment on healthcare cost in those with dementia, this may have been limited by a ceiling effect on healthcare spending and the relatively small sample size. Targeted interventions aimed at preventing and treating comorbid sensory and cognitive impairments may help to reduce healthcare utilization and cost.
Supplementary Material
Supplementary Table S1: List of ICD-9 codes used to identify cases of dementia in this study.
Acknowledgments
Sponsor’s Role: This work is supported by the Duke Claude D. Pepper Older American Independence Center (P30AG028716), the Physical Resilience Indicators and Mechanisms in the Elderly (PRIME) Collaborative (UH2AG056925), the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002553), and the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13–410) at the Durham VA Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Conflict of Interest: None of the authors have conflicts of interest including financial interests, activities, relationships, and affiliations.
References:
- 1.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. [DOI] [PubMed] [Google Scholar]
- 2.Steiner CA, Barrett ML, Weiss AJ, et al. Trends and Projections in Hospital Stays for Adults With Multiple Chronic Conditions, 2003–2014: Statistical Brief #183 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. [PubMed] [Google Scholar]
- 3.Burwell SM. Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899. [DOI] [PubMed] [Google Scholar]
- 4.Swenor BK, Ramulu PY, Willis JR, et al. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 2013;173(4):312–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2018 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2018;14(3):367–429. [Google Scholar]
- 6.Bal S, Kurichi JE, Kwong PL, et al. Presence of Vision Impairment and Risk of Hospitalization among Elderly Medicare Beneficiaries. Ophthalmic Epidemiol. 2017;24(6):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52(12):1996–2002. [DOI] [PubMed] [Google Scholar]
- 8.Genther DJ, Betz J, Pratt S, et al. Association Between Hearing Impairment and Risk of Hospitalization in Older Adults. J Am Geriatr Soc. 2015;63(6):1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genther DJ, Frick KD, Chen D, et al. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):2322–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huddle MG, Deal JA, Swenor B, et al. Association Between Dual Sensory Impairment, Hospitalization, and Burden of Disease. J Am Geriatr Soc. 2016;64(8):1735–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson JGS, Guthrie DM. Older Adults With a Combination of Vision and Hearing Impairment Experience Higher Rates of Cognitive Impairment, Functional Dependence, and Worse Outcomes Across a Set of Quality Indicators. J Aging Health. 2017:898264317723407. [DOI] [PubMed] [Google Scholar]
- 12.Prager AJ, Liebmann JM, Cioffi GA, et al. Self-reported Function, Health Resource Use, and Total Health Care Costs Among Medicare Beneficiaries With Glaucoma. JAMA Ophthalmol. 2016;134(4):357–365. [DOI] [PubMed] [Google Scholar]
- 13.Huddle MG, Goman AM, Kernizan FC, et al. The Economic Impact of Adult Hearing Loss: A Systematic Review. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1040–1048. [DOI] [PubMed] [Google Scholar]
- 14.Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koberlein J, Beifus K, Schaffert C, et al. The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3(11):e003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitson HE, Cronin-Golomb A, Cruickshanks KJ, et al. American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: Sensory Impairment and Cognitive Decline in Older Adults. J Am Geriatr Soc. 2018;66(11):2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PL, Cohen HJ, Fillenbaum GG, et al. Association of Co-Existing Impairments in Cognition and Self-Rated Vision and Hearing With Health Outcomes in Older Adults. Gerontol Geriatr Med. 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitson HE, Cousins SW, Burchett BM, et al. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55(6):885–891. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann J, Michalowsky B, Kaczynski A, et al. The Impact of Hospitalization on Readmission, Institutionalization, and Mortality of People with Dementia: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2018;64(3):735–749. [DOI] [PubMed] [Google Scholar]
- 20.Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–194. [DOI] [PubMed] [Google Scholar]
- 21.Adler GS. A profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev. 1994;15(4):153–163. [PMC free article] [PubMed] [Google Scholar]
- 22.Lin PJ, Kaufer DI, Maciejewski ML, et al. An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimers Dement. 2010;6(4):334–341. [DOI] [PubMed] [Google Scholar]
- 23.Hill J, Fillit H, Thomas SK, et al. Functional impairment, healthcare costs and the prevalence of institutionalisation in patients with Alzheimer’s disease and other dementias. Pharmacoeconomics. 2006;24(3):265–280. [DOI] [PubMed] [Google Scholar]
- 24.Gruber-Baldini AL, Stuart B, Zuckerman IH, et al. Treatment of dementia in community-dwelling and institutionalized medicare beneficiaries. J Am Geriatr Soc. 2007;55(10):1508–1516. [DOI] [PubMed] [Google Scholar]
- 25.Ennis SK, Larson EB, Grothaus L, et al. Association of living alone and hospitalization among community-dwelling elders with and without dementia. J Gen Intern Med. 2014;29(11):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pimouguet C, Rizzuto D, Lagergren M, et al. Living alone and unplanned hospitalizations among older adults: a population-based longitudinal study. Eur J Public Health. 2017;27(2):251–256. [DOI] [PubMed] [Google Scholar]
- 27.Gregori D, Petrinco M, Bo S, et al. Regression models for analyzing costs and their determinants in health care: an introductory review. Int J Qual Health Care. 2011;23(3):331–341. [DOI] [PubMed] [Google Scholar]
- 28.Deb P, Norton EC. Modeling Health Care Expenditures and Use. Annu Rev Public Health. 2018;39:489–505. [DOI] [PubMed] [Google Scholar]
- 29.Phelan EA, Borson S, Grothaus L, et al. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shega JW, Hougham GW, Stocking CB, et al. Patients dying with dementia: experience at the end of life and impact of hospice care. J Pain Symptom Manage. 2008;35(5):499–507. [DOI] [PubMed] [Google Scholar]
- 31.Kwak J, Haley WE, Chiriboga DA. Racial differences in hospice use and in-hospital death among Medicare and Medicaid dual-eligible nursing home residents. Gerontologist. 2008;48(1):32–41. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KS, Kuchibhatla M, Tulsky JA. Racial differences in self-reported exposure to information about hospice care. J Palliat Med. 2009;12(10):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z, Zhang K, Lin PJ, et al. A longitudinal analysis of the lifetime cost of dementia. Health Serv Res. 2012;47(4):1660–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deb A, Sambamoorthi U, Thornton JD, et al. Direct medical expenditures associated with Alzheimer’s and related dementias (ADRD) in a nationally representative sample of older adults - an excess cost approach. Aging Ment Health. 2018;22(5):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu CW, Cosentino S, Ornstein K, et al. Use and cost of hospitalization in dementia: longitudinal results from a community-based study. Int J Geriatr Psychiatry. 2015;30(8):833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin PJ, Biddle AK, Ganguly R, et al. The concentration and persistence of health care expenditures and prescription drug expenditures in Medicare beneficiaries with Alzheimer disease and related dementias. Med Care. 2009;47(11):1174–1179. [DOI] [PubMed] [Google Scholar]
- 37.Foley DM, Frick KD, Lin FR. Association between hearing loss and healthcare expenditures in older adults. J Am Geriatr Soc. 2014;62(6):1188–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs JM, Hammerman-Rozenberg R, Maaravi Y, et al. The impact of visual impairment on health, function and mortality. Aging Clin Exp Res. 2005;17(4):281–286. [DOI] [PubMed] [Google Scholar]
- 39.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health. 2004;94(5):823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph JL, Zanin NM, Jones RN, et al. Hospitalization in community-dwelling persons with Alzheimer’s disease: frequency and causes. J Am Geriatr Soc. 2010;58(8):1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin PJ, Zhong Y, Fillit HM, et al. Hospitalizations for ambulatory care sensitive conditions and unplanned readmissions among Medicare beneficiaries with Alzheimer’s disease. Alzheimers Dement. 2017;13(10):1174–1178. [DOI] [PubMed] [Google Scholar]
- 42.Russ TC, Parra MA, Lim AE, et al. Prediction of general hospital admission in people with dementia: cohort study. Br J Psychiatry. 2015;206(2):153–159. [DOI] [PubMed] [Google Scholar]
- 43.Reed NS, Lin FR, Willink A. Hearing Care Access?: Focus on Clinical Services, Not Devices. JAMA. 2018;320(16):1641–1642. [DOI] [PubMed] [Google Scholar]
- 44.Jennings LA, Laffan AM, Schlissel AC, et al. Health Care Utilization and Cost Outcomes of a Comprehensive Dementia Care Program for Medicare Beneficiaries. JAMA Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. [DOI] [PubMed] [Google Scholar]
- 46.Sindhusake D, Mitchell P, Smith W, et al. Validation of self-reported hearing loss. The Blue Mountains Hearing Study. Int J Epidemiol. 2001;30(6):1371–1378. [DOI] [PubMed] [Google Scholar]
- 47.Klein BE, Klein R, Lee KE, et al. Associations of performance-based and self-reported measures of visual function. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1999;6(1):49–60. [DOI] [PubMed] [Google Scholar]
- 48.Taylor DH Jr., Ostbye T, Langa KM, et al. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu CW, Ornstein KA, Cosentino S, et al. Misidentification of Dementia in Medicare Claims and Related Costs. J Am Geriatr Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaller S, Mauskopf J, Kriza C, et al. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015;30(2):111–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: List of ICD-9 codes used to identify cases of dementia in this study.