Abstract
Background.
Among patients with a core biopsy diagnosis of ductal carcinoma in situ (DCIS), approximately 10% have microinvasion (DCISM), which, like DCIS, is subject to upstaging by surgical excision, but for which the rates of T and N upstaging are unknown, as is the role of sentinel lymph node biopsy (SLNB), since current studies of SLNB for DCISM are based on the final pathologic report, not the core needle biopsy. In this study, we identified the rates of T and N upstaging following surgical excision in patients with a suspected vs. definite core needle biopsy diagnosis of DCISM.
Methods.
Overall, 369 consecutive patients (2007–2017) with a core biopsy diagnosis of suspected versus definite DCISM and surgical excision were stratified by extent of DCISM on core biopsy: suspicious focus, single focus, multiple foci/single biopsy, and multiple foci/multiple biopsies. Within strata, we identified clinicopathologic features associated with T and N upstaging.
Results.
Across core biopsy strata, there were no clear differences in imaging characteristics or median invasive tumor size (0.2 cm). Among 105 patients with a core biopsy of suspicious versus 264 with a core biopsy of definite for DCISM, 28% and 37%, respectively, were upstaged to at least pT1a, but only 1% and 6%, respectively, to pN1.
Conclusions.
Although 28% of patients with suspected DCISM on core biopsy were surgically upstaged to invasive cancer, the frequency of pN1 SLN metastasis (1%) was comparable with that of DCIS, and was insufficient to recommend SLNB at initial surgery. SLNB remains reasonable for patients with definite DCISM on core biopsy.
INTRODUCTION
Following core needle biopsy, the current standard of care for the diagnosis of breast cancer prior to surgery, approximately 20% of patients with a preoperative diagnosis of ductal carcinoma in situ (DCIS) will be upstaged to invasive cancer on final pathology, a rate insufficient to justify sentinel lymph node biopsy (SLNB)—a procedure with some morbidity—at the initial operation. DCIS with microinvasion (DCISM), defined as DCIS with one or more foci of stromal invasion, none larger than 1 mm, is the subject of an extensive literature comprising a meta-analysis of 24 previous series (1999–2012)1 and 6 more recent reports (2012–2016),2–7 demonstrating SLN metastases in 2–20% of patients and suggesting a role for SLNB. However, with a core biopsy diagnosis of DCISM, the surgeon’s conundrum is threefold: (1) the diagnosis of DCISM in the above reports is based on the final surgical pathology, not the core biopsy; (2) a core biopsy diagnosis may be ‘suspicious’ but not ‘definite’ for DCISM; (3) the extent of T and N upstaging and the role of SLNB following a core biopsy diagnosis of DCISM, suspected or definite, is unclear. We aim to address these issues in a large series of patients with a core biopsy diagnosis of suspected or definite DCISM followed by surgical excision, making detailed clinicopathologic correlations between core biopsy and final pathology, and reporting both the rate and extent of T and N upstaging.
METHODS
Consecutive patients with a core needle biopsy of DCISM who had surgical excision between 14 March 2007 and 15 November 2017 at Memorial Sloan Kettering Cancer Center (MSKCC) were identified through query of our prospective Breast Pathology and Breast Service databases, under an Institutional Review Board-approved Waiver of Authorization. All patients with prior DCIS or invasive breast cancer were excluded. All initial diagnoses were based on core needle biopsy. Current American Joint Committee on Cancer (AJCC)8 staging criteria were followed in defining DCISM as no invasive focus >1 mm, and in categorizing node status as negative (pN0), isolated tumor cells (pN0i+), micrometastasis (0.2–2 mm, pN1mi), and macrometastasis (>2 mm, pN1). All diagnoses of DCISM, including biopsies performed outside MSKCC, were based on unblinded review by two or more MSKCC breast pathologists at the time of diagnosis, characterizing the DCISM lesion as a suspicious focus, single definite focus, multiple definite foci in a single biopsy, or multiple definite foci in multiple biopsies. Foci suspicious for DCISM typically comprised very small cell clusters that could not be fully characterized by epithelial and myoepithelial stains (Fig. 1). All imaging was reviewed by MSKCC breast radiologists. The size of core biopsy under stereotactic and magnetic resonance imaging (MRI) guidance was 9 gauge, and 14 gauge under sonographic guidance. All surgical specimens were processed uniformly per College of American Pathologists (CAP) guidelines and MSKCC pathology protocols.
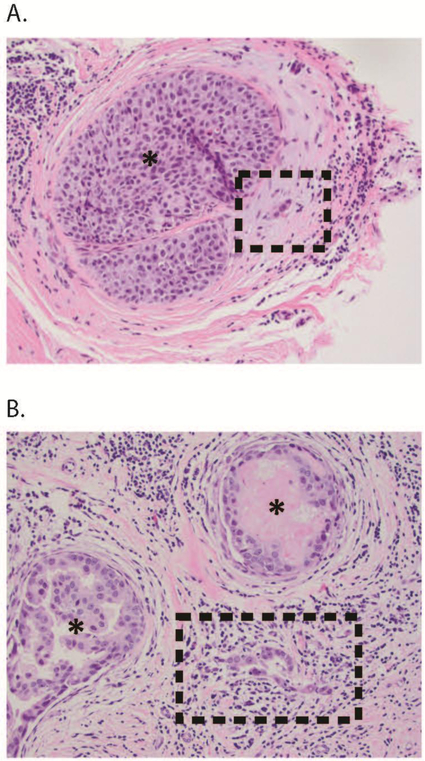
FIG. 1.
(a) Suspected DCISM: here, a cluster of three cells is in the stroma adjacent to a duct containing DCIS (*). Although suspicious, this focus is not definitely diagnostic of microinvasion, and could represent prominent endothelial cells in a small periductal vessel. Work-up with appropriate myoepithelial and/or epithelial markers may help to resolve the differential diagnosis in similar cases. (b) Definite DCISM: here, the microinvasion comprises a cluster of 15–20 neoplastic cells devoid of myoepithelium, spanning <1 mm in the lymphocyte-rich stroma between two ducts (*) harboring DCIS. DCISM ductal carcinoma in situ with microinvasion, DCIS ductal carcinoma in situ
We identified demographic, clinical, radiographic, and pathologic variables by retrospective chart review. Lesion size comprised the greatest extent of disease seen on mammography, ultrasound, and/or MRI. Whenever possible, DCIS subtype was defined as the dominant morphology. Final pathologic diagnosis was the composite of the core biopsy and surgical excision. SLNB followed our service protocol as previously reported,9 defining blue, hot, and/or clinically suspicious nodes as SLN. All axillary lymph node dissections (ALNDs) were performed only after a positive SLNB. Tumor markers were performed if possible: estrogen receptor (ER) for DCIS; and ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) when sufficient invasive tumor was present.
Patient and disease characteristics were summarized using the median for continuous variables and frequency for categorical variables. Between-group comparisons used the Kruskal–Wallis test for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. A p-value <0.05 was considered significant. All statistical analyses used STATA/SE version 12.1 (StataCorp LLC, College Station, TX, USA).
RESULTS
Overall, 369 patients had a core biopsy diagnosis of DCISM—105 (28%) suspected and 264 (72%) definite—and a majority had a single focus (Table 1). Although patients with definite DCISM were somewhat younger (p = 0.04), the median age of the entire cohort (55 years) did not differ by extent of DCISM. Most patients were diagnosed by mammography/stereotactic biopsy, and no group was more likely to present with a palpable mass. With increasing extent of DCISM, multiple imaging studies were more frequent (p = 0.005) and there were non-significant trends toward more frequent mass/asymmetry and greater extent of calcifications.
TABLE 1.
Clinical presentation, imaging, and biopsy characteristics by extent of the DCISM lesion
| DCISM suspected [n = 105] | DCISM definite 1 focus [n =175] | DCISM definite >1 focus 1 biopsy [n = 67] | DCISM definite >1 focus >1 biopsy [n = 22] | p-Value | DCISM definite total [n = 264] | p-Value | |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| Age, years [median (IQR)] | 57 (48–65) | 53 (46–61) | 54 [47–62] | 55 (48–61) | 0.20 | 53 (47–61) | 0.04 |
| Palpable mass | 3 (3) | 17 (10) | 3 (5) | 2 (9) | 0.13 | 22 (8) | 0.06 |
| Imaging characteristics | |||||||
| Diagnosed by: | 0.005 | 0.004 | |||||
| Mammogram | 93 (89) | 137 (78) | 52 (78) | 12 (54) | 201 (76) | ||
| Ultrasound | 2 (29) | 6 (3) | 2 (3) | 0 (0) | 8 (3) | ||
| MRI | 4 (48) | 11 (6) | 4 (6) | 1 (5) | 16 (6) | ||
| Multiple | 6 (67) | 21 (11) | 9 (12) | 9 (41) | 39 (15) | ||
| Mass or asymmetry on imaging | 18 (17) | 31 (18) | 19 (28) | 5 (23) | 0.25 | 55 (21) | 0.42 |
| Extent of calcificationsa | 0.13 | 0.67 | |||||
| Small cluster(s) or <1 cm | 23 (23) | 44 (28) | 17 (30) | 2 (10) | 63 (27) | ||
| 1 to <5 cm | 47 (49) | 74 (47) | 28 (49) | 9 (43) | 111 (47) | ||
| 5 to <10 cm | 19 (20) | 22 (14) | 10 (18) | 7 (33) | 39 (17) | ||
| ≥10 cm or diffuse | 2 (2) | 7 (5) | 1 (2) | 3 (14) | 11 (5) | ||
| Calcifications present but no size provided | 6 (6) | 9 (6) | 1 (2) | 0 (0) | 10 (4) | ||
| Biopsy guidance | <0.001 | 0.02 | |||||
| Stereotactic | 95 (91) | 136 (78) | 53 (79) | 14 (64) | 203 (77) | ||
| Ultrasound | 7 (7) | 29 (17) | 9 (13) | 0 (0.0) | 38 (14) | ||
| MRI | 3 (3) | 9 (5) | 5 (8) | 1 (5) | 15 (6) | ||
| Multiple biopsy types | 0 (0.0) | 1 (1) | 0 (0.0) | 7 (32) | 8 (3) | ||
| Pathologic characteristics on core needle biopsy | |||||||
| Necrosisb | 93 (93) | 149 (96) | 58 (92) | 17 (90) | 0.61 | 224 (94) | 0.70 |
| Nuclear gradeb,c | 0.008 | 0.19 | |||||
| Low | 3 (3) | 7 (4) | 1 (25) | 0 (0) | 8 (3) | ||
| Low to intermediate | 5 (5) | 7 (4) | 4 (6) | 0 (0) | 11 (4) | ||
| Intermediate | 32 (31) | 36 (21) | 11 (17) | 3 (14) | 50 (19) | ||
| Intermediate to high | 17 (16) | 43 (25) | 7 (11) | 4 (18) | 54 (21) | ||
| High | 47 (45) | 80 (46) | 43 (65) | 15 (6) | 138 (52) | ||
| Lymphocytic reaction | 5 (5) | 7 (4) | 5 (8) | 1 (5) | 0.74 | 5 (5) | 0.95 |
| Presence of calcifications | 90 (94) | 149 (97) | 50 (94) | 21 (100) | 0.48 | 220 (97) | 0.27 |
| Architectureb | 0.31 | 0.60 | |||||
| Solid | 32 (31) | 57 (33) | 22 (33) | 4 (18) | 83 (32) | ||
| Cribriform | 8 (8) | 9 (5) | 3 (5) | 0 (0) | 12 (5) | ||
| Micropapillary | 0 (0) | 2 (1) | 1 (2) | 0 (0) | 3(1) | ||
| Papillary | 0 (0) | 0 (0.0) | 2 (3) | 0 (0) | 2 (18) | ||
| Tubular | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (0.4) | ||
| Mixed | 63 (61) | 104 (60) | 38 (58) | 18 (82) | 160 (61) | ||
| Treatment | |||||||
| Surgical management | 0.002 | 0.72 | |||||
| Lumpectomy | 57 (54) | 97 (55) | 38 (57) | 3 (14) | 138 (52) | ||
| Mastectomy | 48 (46) | 78 (45) | 29 (43) | 19 (86) | 126 (48) | ||
| Axillary evaluation | <0.001 | <0.001 | |||||
| No axillary evaluation | 24 (23) | 12 (7) | 3 (5) | 0 (0) | 15 (6) | ||
| SLNB only | 81 (77) | 154 (88) | 58 (87) | 18 (82) | 230 (87) | ||
| SLNBandALND | 0 (0) | 9 (5) | 6 (9) | 4 (18) | 19 (7) |
Data are expressed as n (%) unless otherwise specified
Percentages are rounded
DCISM ductal carcinoma in situ with microinvasion, IQR interquartile range, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, MRI magnetic resonance imaging
Data on the extent of calcifications were missing in 9 patients (2.4%), while the 29 remaining patients had no calcifications
Data on necrosis were missing in 31 patients (8%; on nuclear grade in 4 patients (1%) and on architecture in 5 patients (1%)
There were no significant differences in pathologic subtype by extent of DCISM, and most patients had intermediate-or high-grade disease. Mastectomy was more frequent for patients with the most extensive DCISM (86%, p = 0.002), but, across the other strata, the proportions of mastectomy and lumpectomy were approximately equal. Axillary staging was more frequent for definite than suspected DCISM (94% vs. 77%, p < 0.001); more frequent with younger age, mass/asymmetry on imaging, and higher nuclear grade; and more frequent for mastectomy (173/174, 99%) than for lumpectomy (158/195, 81%). Among 39 patients who did not have axillary staging, 24 (62%) had suspected DCISM and 14 (38%) had definite DCISM. Nineteen patients had ALND—16 concurrent and 3 as second procedures.
Seven patients had no residual DCIS or invasive disease in their surgical specimens, three of whom had atypical ductal hyperplasia. Surgical specimens containing only DCIS were more frequent with suspected DCISM than with definite DCISM (51% vs. 29%). Among 105 patients with suspected DCISM on core biopsy, 51 (49%) had invasive cancer on final pathology—22 (21%) confirming DCISM, and 29 (28%) upstaged to T1 disease (Table 2). Among patients with definite DCISM on core biopsy, 98 (35%,p < 0.001) were upstaged to T1 disease and one patient was upstaged to T2 disease. The median invasive tumor size of 0.2 cm (interquartile range 0.1–0.4) did not vary by extent of DCISM.
TABLE 2.
Final pathologic characteristics stratified by extent of the DCISM lesion
| DCISM suspected [n = 105] | DCISM definite 1 focus [n = 175] | DCISM definite >1 focus 1 biopsy [n = 67] | DCISM definite >1 focus >1 biopsy [n = 22] | p- Value | DCISM definite total [n = 264] | p-Value | |
|---|---|---|---|---|---|---|---|
| PT | <0.001 | <0.001 | |||||
| Tis | 54 (51) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | ||
| T1mi | 22 (21) | 113 (65) | 36 (54) | 15 (68) | 164 (62) | ||
| Tl | 29 (28) | 60 (34) | 31 (46) | 7 (32) | 98 (37) | ||
| T2–4 | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 2 (1) | ||
| Tumor size, cm [median (IQR)] | 0.2 (0.1–0.6) | 0.14 (0.1–0.4) | 0.2 (0.1–0.4) | 0.1 (0.1–0.4) | 0.71 | 0.17 (0.1–0.4) | 0.55 |
| pNa | 0.16 | 0.07 | |||||
| pN0 | 78/81 (96) | 147/163 (90) | 59/67 (88) | 18/22 (82) | 224/252 (89) | ||
| pN0(i+) | 2 (3) | 2 (1) | 0 (0) | 0 (0) | 2 (1) | ||
| pN1mi | 0 (0) | 4 (3) | 1 (2) | 2 (9) | 7 (3) | ||
| ≥pN1+ | 1 (1) | 10 (6) | 4 (6) | 2 (9) | 16 (6) | ||
| Extensive/multicentric DCIS | 17 (16) | 36 (21) | 11 (16) | 4 (18) | 0.79 | 51 (19) | 0.48 |
| Lymphovascular invasion | 5 (5) | 19 (11) | 4 (6) | 3 (14) | 0.33 | 26 (10) | 0.28 |
| Multicentric/multifocal invasive disease | 22 (21) | 61 (35) | 27 (40) | 16 (73) | <0.001 | 104 (39) | 0.001 |
| Estrogen receptor | 0.13 | 0.08 | |||||
| Positive | 69 (65) | 126 (72) | 51 (76) | 12 (55) | 189 (72) | ||
| Negative | 28 (27) | 43 (25) | 16 (23) | 9 (41) | 68 (26) | ||
| Not performed | 8 (8) | 6 (3) | 0 (0) | 1 (5) | 7 (3) | ||
| Progesterone receptor positive | 0.002 | 0.004 | |||||
| Positive | 35 (33) | 81 (46) | 36 (54) | 6 (27) | 123 (47) | ||
| Negative | 33 (31) | 54 (31) | 25 (37) | 11 (50) | 90 (34) | ||
| Not performed | 37 (35) | 40 (23) | 6 (9) | 5 (23) | 51 (19) | ||
| HER2-positive | <0.001 | <0.001 | |||||
| Positive | 12 (11) | 42 (24) | 24 (34) | 8 (36) | 73 (28) | ||
| Negative | 34 (32) | 69 (39) | 31 (46) | 8 (36) | 108 (41) | ||
| Not performed | 59 (56) | 64 (37) | 13(19) | 6 (27) | 83 (31) |
Data are expressed as n (%) unless otherwise specified
Percentages are rounded
DCISM ductal carcinoma in situ with microinvasion, IQR interquartile range, DCIS ductal carcinoma in situ, HER2 human epidermal growth factor receptor 2
Denominator = patients with axillary node assessment
Among patients with suspected DCISM, no clinicopathologic features were significantly predictive of upstaging to definite invasion. Among the 81 patients with suspected DCISM who had SLNB, 2 had pN0i+ disease and only 1 (1%) had pN1 disease. Among the 264 patients with definite DCISM, 2 (1%) had pN0i+ disease, 7 (3%) had pN1mi disease, and 16 (6%) had pN1 or greater disease (Table 2). The category of pN1 or greater included 1 patient with pN2 disease and 1 patient with pN3 disease. Of the 19 patients who underwent ALND after SLNB, 5 had additional positive nodes. There was no association between the extent of the DCISM lesion and nodal burden: 2 patients with a single focus of DCISM had three and six macrometastases, respectively, 1 patient with multiple foci of DCISM had 11 macrometastases, and 2 patients with multiple foci/multiple sites of DCISM had one micrometastasis and one macrometastasis, respectively. On final pathology, there was a non-significant trend toward more lymphovascular invasion and a significant trend toward more multifocal/multicentric invasive disease(p < 0.001) with increasing extent of DCISM. ER was performed more frequently than PR and HER2, reflecting a study population with minimal invasive disease, with no differences between strata in ER status.
Among patients with suspected or definite DCISM who had axillary assessment, pN1 disease was more frequent for patients treated by mastectomy than those treated by lumpectomy (7.5% vs. 2.5%) [Table 3]. On univariate analysis (Table 4), patients with pN1 or greater disease were significantly younger (p = 0.006) and had a greater extent of calcifications (p = 0.001). In a multivariate analysis incorporating these characteristics and adjusting for procedure type, there was a significantly increased risk of nodal metastasis associated with definite DCISM (odds ratio 7.85, 95% confidence interval 1.01–60.7, p = 0.048).
TABLE 3.
Proportion of patients with pN1 diseasea by operation performed
| Operation | Suspected DCISM | Definite DCISM | Total |
|---|---|---|---|
| Lumpectomy | 1/33 (3) | 3/124 (2) | 4/157 (2.5) |
| Mastectomy | 0/48 (0) | 13/125 (10) | 13/173 (7.5) |
| Total | 1/81 (1) | 16/249 (6) | 17/330 (5) |
Data are expressed as n/N (%)
DCISM ductal carcinoma in situ with microinvasion
Includes two patients with pN2–3 disease, and excludes pN0i+ and pN1mi
TABLE 4.
Clinical, imaging, and pathologic characteristics associated with ≥pN1 disease (univariate)
| pNO [n = 309] | ≥pN1a [n = 24] |
p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years [median (IQR)] | 54 (47–63) | 49 (41–56) | 0.006 |
| Palpable mass | 22 (6) | 2 (9) | 0.61 |
| Imaging characteristics | |||
| Diagnostic imaging | 0.69 | ||
| Mammogram | 279 (80) | 17 (77) | |
| Ultrasound | 10 (3) | 0 (0) | |
| MRI | 19 (6) | 1 (5) | |
| Multiple | 40 (12) | 4 (18) | |
| Mass or asymmetry on imaging | 65 (19) | 7 (32) | 0.13 |
| Extent of calcifications | 0.001 | ||
| Small cluster(s) or <1 cm | 84 (27) | 4 (21) | |
| 1 to <5 cm | 154 (49) | 5 (26) | |
| 5 to <10cm | 51 (16) | 5 (26) | |
| ≥10 cm or diffuse | 9 (3) | 4 (21) | |
| Calcifications present but no size provided | 15 (5) | 1 (5) | |
| Biopsy imaging | 0.86 | ||
| Stereotactic | 282 (81) | 18 (82) | |
| Ultrasound | 42 (12) | 2 (9) | |
| MRI | 17 (5) | 1 (5) | |
| Multiple biopsy types | 7 (2) | 1 (5) | |
| Pathologic characteristics on core needle biopsy | |||
| Lymphocytic reaction | 18 (5) | 0 (0) | 0.27 |
| Necrosis | 298 (86) | 20 (100) | 0.24 |
| Presence of calcifications | 292 (84) | 18 (100) | 0.35 |
| Nuclear grade | 0.71 | ||
| Low | 11 (3) | 0 (0) | |
| Low to intermediate | 15 (4) | 1 (5) | |
| Intermediate | 78 (23) | 4 (18) | |
| Intermediate to high | 69 (20) | 3 (14) | |
| High | 171 (50) | 14 (64) | |
| Architecture | 0.36 | ||
| Solid | 109 (32) | 7 (32) | |
| Cribriform | 20 (6) | 0 (0) | |
| Micropapillary | 2 (1) | 1 (5) | |
| Papillary | 2 (1) | 0 (0) | |
| Tubular | 1 (0.3) | 0 (0) | |
| Mixed | 209 (64) | 14 (64) | |
| Hormone receptor status | |||
| Estrogen receptor-positive | 211 (69) | 17 (71) | 0.94 |
| Progesterone receptor-positive | 134 (44) | 12 (50) | 0.58 |
| HER2-positive | 71 (23) | 12 (50) | 0.23 |
Data are expressed as n (%) unless otherwise specified
Percentages are rounded
IQR interquartile range, MRI magnetic resonance imaging, HER2 human epidermal growth factor receptor
Comprises pN1mi plus pN1, and includes pN2 and pN3 (one patient each)
DISCUSSION
With widespread screening, DCIS now comprises 24% of all new breast cancer diagnoses in the US,10 with suspected or definite DCISM comprising approximately 10% of these.11 Management of the breast and regional nodes in DCIS is well-defined: breast conservation vs. mastectomy, dependent on the extent of disease, and SLNB primarily for patients who require mastectomy.12 Long-term breast cancer-specific survival exceeds 95%,13 and the rate of clinically significant node metastasis (pN1) is approximately 1%, insufficient to recommend routine axillary node staging. Like DCIS, the long-term prognosis of DCISM is excellent. In a Surveillance, Epidemiology, and End Results (SEER) study11 comparing DCIS(n = 87,695) with DCISM (n = 8863), 10-year cancer-specific mortality was 1.5% versus 4.0%, respectively. In two DCISM studies of our own from earlier eras, Matsen et al.5 reported 414 patients (1997–2010) with an overall survival at 5 years of 98%, and Lyons et al.14 reported 112 patients (1996–2004) with only five locoregional events and no distant events at 5 years. Unlike DCIS, management of the axilla in DCISM is more controversial.
To date, the literature on SLNB for DCISM is problematic. In a systematic review of 24 studies from 1999 to 2012 (most with fewer than 50 patients),1 the SLN contained isolated tumor cells (pN0i+) in 2.9% of patients, micrometastases (pN1mi) in 4.0% of patients, and macrometastases (pN1) in 3.2% of patients. In the seven studies that did not specify the size of nodal metastasis, 12.6% of patients were SLN-positive. In the 10 studies that specified the size of SLN metastasis, combined with the results of the six larger and more recent studies,2–7 SLNB was performed in 86% of 1443 patients; 3.4% were pN0i+, 4.9% were pN1mi, and 2.5% were pN1. Limitations of these studies include small size, retrospective design, variations in surgical technique, and non-standardized pathologic processing. The most significant limitation is that the diagnosis of DCISM was based on the final pathologic diagnosis, not on the results of the core biopsy, which is the basis for the surgeon’s decision to perform SLNB at the first operation. Further difficulty arises from those cases in which the diagnosis of DCIS is ‘suspected’ rather than ‘definite’. Is SLNB required for a core biopsy diagnosis of DCISM, and do the results differ based on the certainty of the diagnosis?
Namm et al.15 presciently addressed this issue in a report that we believe is the first and only study prior to our own. Among 103 women (2000–2014) with a core biopsy diagnosis of DCISM, 72 (70%) had suspected DCISM (32% of these were upstaged to pT1 invasive cancer), and 72% had SLNB. Our proportion of suspected DCISM was much smaller (28%), but our rate of upstaging to pT1 invasive cancer was comparable (28%), as was our rate of axillary staging (77%). Among their 52 patients with suspected DCISM and SLNB, 3 (6%) were SLN-positive [2 (4%) with pN1mi disease and 1 (2%) with pN2 disease]. Our results were quite similar. Among 81 patients with suspected DCISM and SLNB, 3 were SLN-positive [2 (3%) with pN0i+ disease and 1 (1%) with pN1 disease]. A 1% rate of clinically significant nodal metastasis is comparable with that of ‘pure’ DCIS, and we fully agree with their conclusion that SLNB is not required for patients with a core biopsy diagnosis of suspected DCISM.
Among the 31 patients with definite DCISM in the study by Namm et al., 30 (97%) had SLNB and 3 (10%) were positive [2 with pN1mi disease (6.5%) and 1 with pN1 disease (3%)]. Among our 264 patients with definite DCISM, 245 (93%) had SLNB and 25 (10%) were positive [2 (1%) with pN0i+ disease, 7 (3%) with pN1mi disease, and 16 (6.5%) with pN1 disease]. We cannot explain the difference in the rates of clinically significant (pN1) nodal metastasis (3% vs. 6%), but, based on our results, must conclude that SLNB is reasonable for most patients with a core biopsy diagnosis of definite DCISM.
Neither the study by Namm et al.15 nor our own identified useful predictors of final T and N pathology status following a core biopsy diagnosis of suspected or definite DCISM beyond what wouldbe dictated by common sense. Among those patients with suspected DCISM in the study by Namm et al., upstaging to invasive cancer was associated with larger lesion size and a smaller size (14 gauge) biopsy needle, while, among our patients, upstaging to pN1 disease was associated with definitive microinvasion, greater extent of calcifications, and younger age. A smaller size biopsy needle probably accounts for the larger proportion of patients in their series categorized as suspected DCISM (70% vs. 28%) and suggests that more extensive sampling by larger core biopsy devices may resolve diagnostic uncertainty by categorizing fewer patients as suspected DCISM and more as definite DCISM. The similarity of results following a core biopsy diagnosis of suspected DCISM suggests that our pathologists’ diagnostic criteria for suspicion are similar to those reported by Namm et al.15
The decision to perform SLNB for DCISM—suspected or definite—is also a function of the extent of the breast surgery. Among our 17 patients with pN1 disease, 13 (76%) had mastectomy, and among our 173 patients treated by mastectomy, 7.5% were pN1 (Table 3). These results support a policy of SLNB for all patients with definite DCISM treated by mastectomy, as is currently recommended for DCIS requiring mastectomy,12 but demonstrate a very low yield of pN1 disease (1%) for suspected DCISM whether treated by lumpectomy or mastectomy.
The strengths of this study are the prospective data collection, large sample size, and treatment by a team of breast-specific radiologists, surgeons, and pathologists following standardized criteria and treatment algorithms. Weaknesses include a lack of axillary staging for 23% of patients with suspected DCISM, and the absence of long-term follow-up, adding some uncertainty to the prognostic significance of SLN metastases in DCISM. Of note, we do address this issue in a separate study by Matsen et al.5 asking whether the extent of DCISM was related to SLN status. Among 414 patients with definite DCISM from an earlier time period (1997–2010), 5-year overall survival was 98% in SLN-negative patients and 100% in SLN-positive patients, and recurrence-free survival was 96%, with all local, regional, and distant events in the SLN-negative cohort.
CONCLUSIONS
We conclude that SLNB is not indicated for patients with a core biopsy diagnosis of suspected DCISM. Although 28% of patients were upstaged to T1 invasive cancer, clinically significant SLN metastases (pN1) were found in only 1%, similar to that of DCIS in general. We also conclude that SLNB is indicated for patients with definite DCISM on core biopsy, among whom 6% were upstaged to pN1 disease. Among patients with either suspected or definite DCISM, we did not find any patient, imaging, or other core biopsy characteristics to reliably select patients for SLNB at the initial operation. We welcome confirmatory studies from other institutions.
SYNOPSIS.
We identified pathologic T and N upstaging rates in patients having surgical excision based on suspected versus definite core needle biopsy diagnosis of ductal carcinoma in situ with microinvasion, and concluded that for these patients, clinically significant sentinel lymph node (SLN) metastasis frequency supports SLN biopsy at initial surgery.
FUNDING
The preparation of this manuscript was supported in part by NIH/NCI Cancer Center Support Grant No. P30CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICTS OF INTEREST
Dr. Monica Morrow has received speaking honoraria from Roche and Genomic Health. Meghan R. Flanagan, Michelle Stempel, Edi Brogi, and Hiram S. Cody III have no potential conflicts of interest to disclose.
DISCLOSURES
Portions of the data from this study were presented in poster format at the 2018 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, USA, 1–5 June 2018.
All authors have read and approved the manuscript, and the findings of this study have not been published elsewhere.
REFERENCES
- 1.Gojon H, Fawunmi D, Valachis A. Sentinel lymph node biopsy in patients with microinvasive breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2014;40(1):5–11. [DOI] [PubMed] [Google Scholar]
- 2.Ko BS, Lim WS, Kim HJ, Yu JH, Lee JW, Kwan SB, et al. Risk factor for axillary lymph node metastases in microinvasive breast cancer. Ann Surg Oncol. 2012;19(1):212–6. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor NS, Shamonki J, Sim MS, Chung CT, Giuliano AE. Impact of multifocality and lymph node metastasis on the prognosis and management of microinvasive breast cancer. Ann Surg Oncol. 2013;20(8):2576–81. [DOI] [PubMed] [Google Scholar]
- 4.Margalit DN, Sreedhara M, Chen YH, Catalano PJ, Nguyen PL, Golshan M, et al. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast-conserving therapy or mastectomy. Ann Surg Oncol. 2013;20(3):811–8. [DOI] [PubMed] [Google Scholar]
- 5.Matsen CB, Hirsch A, Eaton A, Stempel M, Heerdt A, Van Zee KJ, et al. Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol. 2014;21(10):3330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna MG, Jaffer S, Bleiweiss IJ, Nayak A. Re-evaluating the role of sentinel lymph node biopsy in microinvasive breast carcinoma. Mod Pathol. 2014;27(11):1489–98. [DOI] [PubMed] [Google Scholar]
- 7.Orzalesi L, Casella D, Criscenti V, Gjondedaj U, Bianchi S, Vezzosi V, et al. Microinvasive breast cancer: pathological parameters, cancer subtypes distribution, and correlation with axillary lymph nodes invasion. Results of a large single-institution series. Breast Cancer. 2016;23(4):640–8. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol. 2018;25(7):1783–5. [DOI] [PubMed] [Google Scholar]
- 9.Cody HS 3rd, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol. 1999;8(2):85–91. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Zhu W, Du F, Luo Y, Xu B. The Demographic Features, Clinicopathological Characteristics and Cancer-specific Outcomes for Patients with Microinvasive Breast Cancer: A SEER Database Analysis. Sci Rep. 2017;7:42045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32(13):1365–83. [DOI] [PubMed] [Google Scholar]
- 13.Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyons JM 3rd, Stempel M, Van Zee KJ, Cody HS 3rd. Axillary node staging for microinvasive breast cancer: is it justified? Ann Surg Oncol. 2012;19(11):3416–21. [DOI] [PubMed] [Google Scholar]
- 15.Namm JP, Mueller J, Kocherginsky M, Kulkarni S. The utility of sentinel lymph node biopsy in patients with ductal carcinoma in situ suspicious for microinvasion on core biopsy. Ann Surg Oncol. 2015;22(1):59–65. [DOI] [PubMed] [Google Scholar]