Abstract
Background:
Disparities in healthcare access and delivery caused by transportation and health workforce difficulties negatively impact individuals living in rural areas. These challenges are especially prominent in older adults.
Design:
We systematically evaluated the feasibility, acceptability and effectiveness in providing telemedicine searching the English-language literature for studies (January 2012 to July 2018) in the following databases: Medline (PubMed); Cochrane Library (Wiley); Web of Science; CINAHL; EMBASE (Ovid); and PsycINFO (EBSCO).
Participants:
Older adults (mean age ≥65 and none were less than 60 years)
Interventions:
Interventions consisted of live, synchronous, two-way video-conferencing communication in non-hospital settings. All medical interventions were included.
Measurements:
Quality assessment using the Cochrane Collaboration’s Risk of Bias Tool was applied on all included articles, including a qualitative summary of all articles.
Results:
Of 6,616 citations, we reviewed the full text of 1,173 articles, excluding 1,047 that did not meet criteria. Of the 17 randomized controlled trials, the United States was the country with the most trials (6 [35%]) with cohort sizes ranging from 3–844 (median 35) participants. Risk of bias among included studies varied from low to high. Our qualitative analysis suggests that telemedicine can improve health outcomes in older adults and that it could be used in this population.
Conclusions:
Telemedicine is feasible and acceptable in delivering care to older adults. Research should focus on well-designed randomized trials to overcome the high degree of bias observed in our synthesis. Clinicians should consider using telemedicine in routine practice to overcome barriers of distance and access to care.
Keywords: telemedicine, older adult, rural, effectiveness
INTRODUCTION
Despite improvements in life expectancy and advances in medical therapies1, individuals residing in rural areas in the United States face increasing disparities in healthcare delivery2–4. Remote and distant communities demonstrate higher rates of the five leading causes of death in the US5, 6, attributed in part to the lack of resources2, 5 in the ambulatory setting7, limited access to specialists and specialized resources, fewer transportation options, and socioeconomic disparities8–12. Rural healthcare is especially problematic in vulnerable populations including persons with disabilities13, children14, and older adults11.
Information and communication technologies provide an opportunity to improve rural healthcare delivery in older adults, the fastest growing user group of technology15, particularly in an era of burgeoning rural broadband and cellular connectivity16. While telemedicine or telehealth encompasses many different modalities of using technology to deliver care, synchronous, two-way video-conferencing (referred and defined in this manuscript as telemedicine or TMed) is a promising strategy in delivering rural healthcare17–19 that may address the long-standing challenge of rural health service availability. As a result of the Telecommunications Act signed in 1996, infrastructure changes have helped support the feasibility and dissemination of TMed delivery, particularly for rural healthcare providers, patients, and communities19 in the United States. With the expansion of high-speed broadband access to over 96% of the population20, there is now improved capability for TMed in surmounting the major barriers faced by rural residents and narrowing the rural-urban divide in healthcare utilization17. TMed has now become increasingly adopted, particularly in capitated and shared risk health care financing systems21–23, and emerging legislation24, 25 promises to further widespread dissemination.
While a number of observational studies and single-site pilot studies suggest that TMed may have long-term cost-effectiveness26–30, may reduce hospital utilization26, 31–33 or emergency department visits34, 35, data in ambulatory settings have been less commonly evaluated. Older adults have less experience with emerging technologies and have considerable sensory, memory and other aging-related barriers to engaging in TMed36, 37. Older adults’ multiple co-morbidities may also require in-person rather than remote-based care. The purpose of this review is to conduct a systematic evaluation of the evidence regarding TMed interventions conducted in older adults in non-hospital settings. Although the intent of our review is to consider implications for rural health care, we evaluated both rural and urban studies extending past the domestic United States to assess the feasibility, acceptability, and effectiveness of TMed in this population.
METHODS
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines38. See Supplemental Appendix #1 for a checklist of each component.
Study Protocol
We reviewed all English-language studies published from the year of CMS’s TMed coverage determination (January 2012) to July 201836, 39–44 Database searches were conducted in June 2017, and repeated in February and July 2018. The final search update covered the full date range and records found in the previous searches were removed, based on the methods described by Bramer and Bain45. We present the aggregate results of all searches below.
With the assistance of two reference librarians (HBB, PJB), the search included subject headings and keywords to capture the concepts of telemedicine and older adults in English language articles. The search strategy was adjusted for the syntax appropriate to each database. The following electronic databases were searched: Medline (PubMed); Cochrane Library (Wiley); Web of Science; CINAHL; EMBASE (Ovid); and PsycINFO (EBSCO). See Supplemental Appendix 2 for our full search strategy. As our focus was on peer-reviewed publications, we deliberately omitted any grey literature including websites, conference proceedings, abstract submissions or clinical trial registries. Bibliographies of identified systematic reviews and all included manuscripts were reviewed manually by the lead author (JAB) for additional studies.
Selection Criteria
We used the Patients, Intervention, Controls, Outcomes (PICO) framework to refine our criteria. Inclusion criteria consisted of: English language studies; human studies; studies with a mean participant age of 65 years and corresponding one standard deviation or range required to exceed 60 years, as conducted in our previous work46; and ambulatory TMed care delivered either in-home, or in an assisted living or long-term care setting on the receiving end of the intervention (not acute or hospital settings). For inclusiveness, participants were eligible if they had any co-morbid physical and mental health conditions were included. Interventions were considered only if TMed was defined as live, real-time, synchronous, two-way video-conferencing on both the receiving and delivery end, as this is the most common type used within clinical settings and one that is most fully reimbursed.47 This is in contrast to other modalities of telehealth, including remote monitoring, e-consultations or store-and-forward, whose feasibility, acceptability and preliminary effectiveness have been reviewed elsewhere.48–50 Inclusion criteria also required a focus on patient care with a health care provider or trained staff (i.e., physician, associate provider [advanced practice registered nurse or physician’s assistant], physical/occupational therapist, psychologists, social workers or dietitians, etc.) on one end, and a patient on the receiving end. We also included peer-to-peer therapy for medical conditions, as it ultimately resulted in delivering patient care. We excluded any TMed (video-conferencing) related to remote medical education. Studies involving social media (i.e., Facebook or Twitter) were excluded. Initially, all study types (randomized controlled (RCT) trials, observational or qualitative studies, etc.) were included as the study team was concerned that the number of high-quality RCTs would be limited. Following full-text review and identification of a sufficient amount of eligible RCTs (N=17), our review protocol was modified to include only RCTs.
Data Extraction
Searches were combined using Endnote X8 (Thomson Reuters, New York). Two sets of reviewers extracted data from the full-text articles identified in each search. Each set of reviewers conducted a test review for quality assurance purposes by manually conducting a title/abstract review of 200 citations, for which concordance was required to exceed 80%. Discrepancies between reviewers were adjudicated by the senior author (JAB), an approach previously used46.
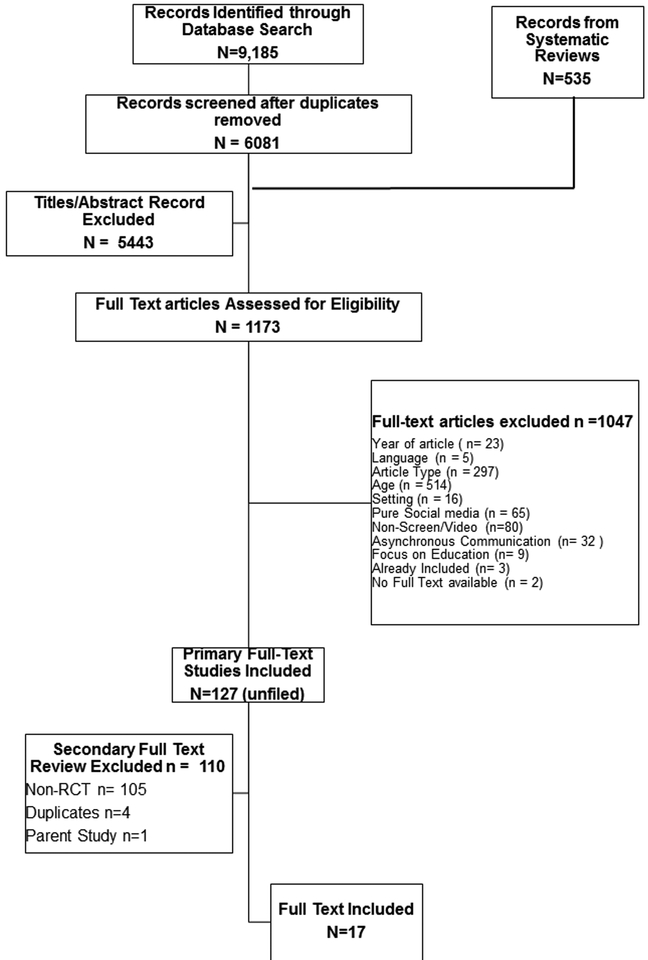
A total of 9,185 citations were identified using our full search criteria (see Figure 1). An additional 535 studies were identified from related systematic reviews during the search process. Pairs of reviewers manually reviewed citation titles and abstracts for inclusion criteria. Following initial title/abstract screening, discrepancies were reconciled before proceeding to full-text review. A second-level screening applied a hierarchical method of exclusion on the remaining full-text studies.
FIGURE 1:
Flow Diagram of Study Selection Process for the Systematic Review.
We reviewed n=36 systematic review bibliographies, which accounted for n=535 additional records of studies for review (accounted for in the flow diagram as ‘additional records identified through other sources.’). These articles were accounted for in the flow diagram.
Quality Review
The Cochrane Collaboration’s Risk of Bias Tool was used to evaluate bias for all included studies as conducted in our group’s previous work46. This tool focuses on the following: sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reports; and other sources of bias. Two reviewers (LMS, PRD) assessed each of the included studies, rating them as high, low or unclear risk of bias for each criterion. The senior author (JAB) adjudicated if any decisions differed.
Study-Level Outcomes
The primary outcomes were chosen a priori and intentionally left broad to ensure all potential effectiveness measures were captured. Our evaluation focused on effectiveness outcomes and acceptability of the intervention. All study data were extracted using a standardized data collection form, which included: publication year; country of origin; funding source; telemedicine modality (process, transmitting/receiving end, device used); study aim; number of study participants; mean age (and range); socioeconomic status (education, place of residence; function or frailty indicators; primary medical condition evaluated; sex-distribution; study setting; and description of the intervention and control groups. We qualitatively evaluated the study’s primary outcomes, video-contact time, and the estimate of effect and presented study limitations. Significant methodological heterogeneity precluded meta-analysis.
RESULTS
We present our PRISMA flow diagram in Figure #1. In total, our search strategy identified 9,720 total citations (Supplemental Appendix 2), of which 6,616 were reviewed after duplicates were removed. After initial title and abstract screening, 1,173 citations required full-text review. Non-RCT and asynchronous communications were the most common reasons for exclusion. The final count of included articles consisted of 17 studies, all of which were based on unique study populations.
Risk of Bias Assessment
Table 1 indicates the bias assessment according to the Cochrane Collaboration’s Risk of Bias Tool51 of all included studies according to the authors’ judgment. Subjective methodological quality of all included studies was considered low to intermediate based on the proportion of studies found to have a “high” risk of bias according to the Cochrane Tool. Methodological problems in the included studies consisted of non-blinded data collectors, outcome assessors, and treatment allocation. As expected, blinding of study participants and healthcare providers was not possible due to the nature of TMed interventions and hence we did not evaluate these components of the tool.
Table #1:
Methodological Quality of Telemedicine Randomized Controlled Studies (n=17) - Cochrane Risk-of-Bias Toola
| Reference | Year | Overall Risk of Biasb | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | |
|---|---|---|---|---|---|---|---|---|---|
| Data Collectors | Outcome Assessors | ||||||||
| Burns57 | 2017 | Low | High | High | Low | Unclear | High | High | Low |
| Burton84 | 2018 | Low | High | High | Low | Unclear | Low | Low | Low |
| Comin-Colet58 | 2016 | Low | High | High | High | Unclear | High | High | High |
| De Luca80 | 2015 | Low | Low | Unclear | Low | Unclear | High | High | Low |
| Dichmann Sorknaes52 | 2013 | Low | High | High | High | Unclear | High | High | High |
| Dy53 | 2013 | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Gandolfi85 | 2017 | Low | High | Unclear | High | High | High | High | Low |
| Homma59 | 2016 | Low | Low | Low | Unclear | Unclear | High | Low | Low |
| Hong86 | 2017 | Low | High | High | Unclear | Unclear | High | High | High |
| Hong69 | 2018 | High | High | High | High | High | High | High | Low |
| Ishani71 | 2016 | High | High | High | High | High | High | High | Low |
| Jelcic87 | 2014 | Low | Low | Low | High | High | High | High | Unclear |
| Orlandoni88 | 2016 | Low | High | High | Unclear | Unclear | High | High | High |
| cTakahash89 | 2012 | Low | High | High | High | High | High | High | High |
| dTrief54 | 2013 | Unclear | Unclear | Unclear | High | High | High | High | Unclear |
| Tsai70 | 2017 | High | High | High | High | High | High | High | High |
| Vahia55 | 2015 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| % Highe | --- | 3 (17.6) | 11 (64.7) | 10 (58.8) | 8 (47.1) | 6 (35.2) | 15 (88.2) | 13 (76.5) | 6 (35.2) |
Criteria for the author’s judgment of a summary assessment: “Yes” indicates a low risk of bias; “No” indicates a high risk of bias; “Unclear” indicates an uncertain risk of bias according to the Cochrane Collaboration tool. Blinding of Participants and Healthcare providers were not included in this evaluation.
A “Low” overall risk was assigned if all assessed domains were given a “Yes”, other than for Blinding. A “High” overall risk was assigned if there were one more domains given a “No.” Blinding of participants and healthcare providers was not taken into consideration when assessing a study’s overall risk of bias.
This paper is a secondary analysis of a previously conducted randomized controlled trial89
This paper is a secondary analysis of a previously conducted randomized controlled trial90
A percentage was calculated as the quotient of the number of “High” within a column and the total number of included citations.
Study Characteristics
The majority of the included RCTs were based in the United States (n=6), with Europe and South Korea both consisting of five and four studies, respectively (Supplemental Appendix 3). Only four studies focused in whole or in part on rural participants52–55. The majority of studies were funded by governmental or public agencies. Computers of all types (desktop, tablet, laptop) were used and included studies focused on effectiveness and participant perception of TMed usage. Study cohort number ranged from small pilot trials (n=3) to a larger, multi-site trial of 844 participants.
Participant Characteristics
Participants were older adults ranging from a mean age of 65.1 years to 86.45 years, although the ranges (when reported) consisted of adults aged 60 to >90 years (Table 3). Socioeconomic status was indicated in nine studies, and patient frailty or functional status was inconsistently reported using different indices. Most interventions focused on a spectrum of chronic disease entities including neurological disorders, depression, chronic obstructive pulmonary disease, diabetes, or high-risk older adults with different baseline characteristics. Studies varied in the sex-distribution of participants. Most interventions occurred in the participant’s home, with others delivered in nursing facilities or community centers.
Table 3:
Description of Study Population of Telemedicine Randomized Controlled Studies (n=17)
| Study | Arm | Age ± SD | Age Range | Sample Size | Sex Distribution | Socioeconomic Statusc | Baseline Functional or Frailty Statusd | Study Duration | Setting | Disorders or Conditions |
|---|---|---|---|---|---|---|---|---|---|---|
| Burns57 | Intervention | 64 ± 7.58 | 61–66 | 43 | 37M:6F | NR | NR | ~27 months | Local health facility | Head and neck cancer, post-treatment |
| Control | 65 ± 7.45 | 62–67 | 39 | 29M:10F | NR | NR | ||||
| Burton84 | Intervention | 71.33 | 66–80 | 3 | 0M:3F | 15±1.7 years of education | MMSE 27.3±1.5 | 8 weeks | Video Therapy Analysis Lab, university campus | Early-stage dementia, subjective cognitive impairment |
| Control | 72.33 | 68–77 | 3 | 1M:2F | 14.7±3.1 years of education | MMSE 24.3±6.4 | ||||
| Comin-Colet58 | Intervention | 74 ± 11 | NR | 81 | 46M:35F | NR | Fragility 19 (24%) | 6 months | Participant home | Congestive heart failure |
| Control | 75 ± 11 | NR | 97 | 59M:38F | NR | Fragility 25 (26%) | ||||
| De Luca80 | Intervention | 79.1±9.2 | NR | 32 | 11M:21F | 100% residing in nursing home | ADL 5.5 (2.0,6.0) IADL 3.0 (2.0,5.0) MMSE 24.1 (16.1,26.1) |
NR | Nursing home | Depression |
| Control | NR | 27 | 8M:19F | 100% residing in nursing home | ADL 1.0 (1.0,2.0) IADL 2.0 (2.0,3.0) MMSE 21.3 (17.9, 24.1) |
|||||
| Dichmann Sorknaes52 | Intervention | 71 ± 10 | NR | 132 | 53M:79F | 8 (6%) with 12–13 years of school | NR | 26 weeks | Participant home | Acutely-exacerbated COPD |
| Control | 72 ± 9 | NR | 134 | 51M:83 F | 4 (3%) with 12–13 years of school | NR | ||||
| Dy53 | Intervention | 83 |
65–93 | 11 | 7M:16F | 100% residing in nursing home | Anticipated ≥6 month residency | 6 months | Skilled nursing facility | Type II Diabetes Mellitus |
| Control | 12 | 100% residing in nursing home | Anticipated ≥6 month residency | |||||||
| Gandolfi85 | Intervention | 67.45 ± 7.18 | NR | 38 | 23M:15F | NR | MMSE 26.77±1.48 # Falls 0.58±1.44 |
7 weeks | Participant home | Parkinson’s Disease |
| Control | 69.84 ± 9.41 | NR | 38 | 28M:10F | NR | MMSE 28.64±6.96 # Falls 1.84±5.29 |
||||
| Homma59 | Intervention | 67.2 ± 1.5 | NR | 33 | 11M:22F | NR | NR | 3 months | District community center | Any lifestyle disease (i.e. HTN, dyslipidemia, diabetes, obesity) |
| Control | 65.1 ± 1.3 | NR | 35 | 13M:22F | NR | NR | ||||
| Hong86 | Intervention | 82.2 ± 5.6 | 69–93 | 11 | 5M:6F | NR | 8’ TUG 9.2±5.7s | 12 weeks | Residences in the community | Sarcopenia |
| Control | 81.5 ± 4.4 | 12 | 5M:7F | NR | 8’ TUG 10.9±4.8s | |||||
| Hong69 | Intervention | 78.1 ± 5.66 | 68–91 | 15 | 0M:15F | NR | 8’ TUG 9.55±4.03s | 12 weeks | Participant home | Fall Risk Assessment Scale score > 14 |
| Control | 81.54 ± 5.07 | 15 | 0M:15F | NR | 8’ TUG 8.27±2.27s | |||||
| Ishani71 | Intervention | 75.3 ± 8.1 | NR | 451 | 445M:6F | 115 (25.5%) ≥4 year degree | Good/excellent health 288 (63.9%) | 1 year | Participant home | Chronic Kidney Disease |
| Control | 74.3 ± 8.1 | 150 | 147M:3F | 34 (22.7%) ≥4 year degree | Good/excellent health 107 (71.3%) | |||||
| Jelcic87 | LSS-tele | 86±5.1 | NR | 7 | 2M:5F | 6±3.5 years of education | MMSE 23.7±2.8 | 3 months | Elderly Day care | Mild memory decline |
| LSS-direct | 82.7±6 | 10 | 3M:7F | 6.7±3.3 years of education | MMSE 24.9±2.5 | |||||
| Control | 82.3±5.9 | 10 | 1M:9F | 8.7±3.7 years of education | MMSE 24.8±2.7 | |||||
| Orlandoni88 | Intervention | 86.45 ± 7.03 | NR | 100 | 28M:72F | NR | Karnofsky index 42 ± 6.51 | 1 year | Participant home | Requires home enteral nutrition |
| Control | 84.36 ± 7.05 | 88 | 21M:67F | NR | Karnofsky index 42 ± 6.53 | |||||
| aTakahashi | Intervention | 80.3 ± 8.9 | NR | 102 | 50M:52F | NR | Grip strength 18.2±8.6 kg TUG 13.3±6.8 seconds Gait speed 0.70±0.38 m/s Barthel ADL Index 94.3±9.7 |
1 year | 4 sites within Mayo Clinic’s Employee/Community Health | High-risk elderly adultse |
| Control | 80.2 ± 7.6 | NR | 103 | 44M:59F | NR | Grip strength 18.8±9.4 kg TUG 15.8±15.4 seconds Gait speed 0.70±0.35m/s Barthel ADL Index 94.6±8.7 |
||||
| bTrief54 | Intervention | 70.79 ± 6.46 | NR | 844 | 308M:536F | 9.69±4.11 years of education | Charlson comorbidity index 2.88±2.00 | 5 years | NY-state residences | Type II Diabetes Mellitus |
| Control | 70.86 ± 6.78 | NR | 821 | 311M:510F | 9.85±4.13 years of education | Charlson comorbidity index 2.89±1.75 | ||||
| Tsai70 | Intervention | 73 ± 8 | NR | 19 | 12M:7F | NR | 6MWT: 363±66 | 8 weeks | Participant home | COPD |
| Control | 75 ± 9 | NR | 17 | 6M:11F | NR | 6MWT: 383±93 | ||||
| Vahia55 | Intervention | 70.1 ± 8.7 | NR | 11 | NR | 5.9±4.8 years of education | MMSE z-score (standard deviation, median) −0.73 (3.18,0) |
2 weeks | Residences in Imperial County, California | Suspected cognitive impairment |
| Control | 71.4 ± 10.6 | NR | 11 | NR | 5.0±3.7 years of education | MMSE z-score (standard deviation, median) −1.02 (3.03,−0.45) |
Values represented are mean ± standard deviations, counts (percent), or median (interquartile range)
Abbreviations: ADL – Activities of Daily Living; IADL – instrumental activities of daily living; COPD – Chronic obstructive pulmonary disease; LSS – lexical-semantic stimulation; MMSE – mini mental status examination; NR – not reported; NY – New York. TUG – timed up and go; 6MWT – 6-minute walk test
This paper is a secondary analysis of a randomized controlled trial89
This paper is a secondary analysis of a previously published randomized controlled trial90
socioeconomic status is defined as income, education, poverty, financial means, or Medicaid insurance status
each article either did not report frailty/functional status or defined it differently – please refer to the individual article for their precise definition
Intervention & Outcomes
Table 4 outlines the intervention description and control group of all included studies. All intervention-based groups used synchronous video-conferencing modalities. Control groups varied by studies predominantly consisting of standard, in-person, clinical care or usual health promotion care for the specific disease entity. Study duration varied from 2 weeks55 to 5 years54. One study56 did not report their study duration. Most primary outcome measures consisted of disease-specific outcome measures, including re-hospitalizations, non-fatal events, or clinical complications. Video contact time was ranged from monthly to three times per week. Only three studies commented on technical limitations of their video-delivery57–59, of which experienced considerable difficulty59.
Table 4:
Study Outcomes of Randomized Controlled Trials (n=17)
| Study | Intervention | Control | Primary Outcomes | Video Contact Time | Main Findings |
|---|---|---|---|---|---|
| Burns57 | Speech pathology care delivered by TMed | Standard, in-person speech pathology care | Cost, number, session length, efficiency; service | Telepractice sessions weekly; appointments as needed (1 hour each) | Significant reduction in number (p = 0.004) and duration (p = 0.024) of contact events required to manage cases by telepractice |
| Burton84 | Cognitive rehabilitation using TMed | Face-to-face care | Goal performance (Canadian Occupational Performance Measure) | Videoconferencing 1x/week | Lower rates of session completion among telehealth group may suggest lack of feasibility or acceptance. No statistical testing reported. |
| Comin-Colet58 | Telemonitoring with video-conferencing | Face-to-face encounters | Non-fatal heart failure events | NR | Significant decrease in non-fatal HF events (p<0.001) with lower readmission rates (p=0.007), among telehealth group |
| De Luca80 | Telemonitoring. Neurological / psychological video-counseling | Standard in-home nursing care | Psychological well-being; MMSE, ADL, IADL, GDS, BANSS, BPRS, EUROQoL | Video-counseling 1x per week | Significant differences only reported within telehealth group, T0 to T1: GDS (p<0.01), BPRS (p=.04), heart rate (p=.02), SAP (p<0.001), DAP (p=0.03) |
| Dichmann Sorknaes52 | Video consults one week post-discharge | Usual follow-up care | Total # of hospital readmissions | Teleconsulations daily for 1 week | No difference in # of hospital re-admissions (p=0.62) |
| Dy53 | Standard care with TMed | Standard home nursing care | Diabetes care; HbA1c point-of-care glucose, | Weekly or biweekly teleconsulations | SNF nurses reported TMed were a good use of their time; skills were effective for consult delivery . No statistical testing reported. |
| Gandolfi85 | Home-based Virtual Reality balance training | In-clinic sensory integration balance training | Gait and balance; Berg Balance Scale | Tele-rehab session 3x/week (50 minutes each) | Improved BBS scores for telerehab group (p = 0.04); significant Time × Group Interactions in Dynamic Gait Index for in-clinic (p = 0.04) |
| Homma59 | Lifestyle, health reports delivered by videophone | Printed document reports | Health status, body mass index, steps/day satisfaction;SBP/DBP, cholesterol | Monthly videophone sessions (15–20 minutes each) | Similar degrees of health status improvement & satisfaction levels (not significant) |
| Hong86 | Tele-exercise program with one-on-one remote instruction | Maintenance of usual lifestyle | Sarcopenia-related factors of health; total and AMM, chair sit-and-reach length, 2-min step, chair stand | Tele-exercise sessions 3x per week (20–40 min each) | Improved lower-limb muscle mass (p=0.017), AMM (p=0.032), total muscle mass (p=0.033), chair sit-and-reach length (p=0.019) |
| Hong69 | Exercise by TMed | Nutrition, exercise education, activity and nutrition monitoring | Fall-related risk factors | Tele-exercise sessions 3x/week (20–40 min each) | Greater improvement in chair stand test (p<0.001), Berg Balance Scale (p=0.02) |
| Ishani71 | Case management & care TMed | Usual kidney disease care | All-cause mortality, emergency department visits, nursing home admits | At least 1 video visit, with more as needed for acute care concerns | No significant difference between groups for any component of the primary outcome |
| Jelecic87 | Lexical tasks to enhance semantic verbal processing by Skype | Unstructured cognitive stimulation | Global cognitive performance; lexical-semantic; semantically-related or unrelated episodic verbal memory | One hour each morning | Improvements in global cognitive domain (p=0.001); non-inferior to in-person |
| Orlandoni88 | Nutritional assessment delivered by TMed | Standard home-visits with nutritional assessment | Incidence of metabolic and GI complications secondary to home enteral nutrition | At least 1 monthly video consultation (< 10 minutes on average) | Significantly lower incidence of metabolic and GI complications among video consultation group (both p<0.001); no significant difference in hospital admission rate |
| aTakahashi89 | Hospice care with TMed | Usual end-of-life care | # of hospital and emergency room visits | NR | No difference in hospitalizations, ER visits; mortality in telemonitoring higher compared to usual care (p=0.008) |
| bTrief54 | TMed for diabetic coaching (in Spanish if needed) | Usual diabetic care | Adherence to diabetes management; HbA1c, Diabetes Self-Care Activities scale | Tele-visits every 4–6 weeks | Self-reported adherence improved for intervention compared to control (p<0.001) |
| Tsai70 | Group-based telerehabilitation program | Usual care without exercise training | Endurance exercise capacity (ESWT) | Telerehab sessions 3x per week (1 hour each) | Improvement in ESWT (p<0.001) |
| Vahia55 | Neurocognitive testing using TMed | In-person neurocognitive testing | Various Neurocognitive tests | 1 test session per modality, administered 2 weeks apart | No differences in cognitive scores (p=0.280) |
Abbreviations: ADL - Activities of Daily Living; AMM – appendicular muscle mass; BANSS - Bedford Alzheimer Nursing Severity scale; BBS – berg balance scale; BPRS - Brief Psychiatric Rating Scale; DBP – diastolic blood pressure; DGI – Dynamic gait index; ER – emergency room; ESWT - endurance shuttle walk test; EUROQoL - standardized instrument as a measure of health outcomes and quality of life; GDS = Geriatric Depression Scale; HbA1c – hemoglobin A1c; HF – heart failure; HR - heart rate; IADL - Instrumental Activities of Daily Living Scale; IT – information technology; MMSE - Mini Mental State Examination; NR – not reported; PD – parkinson’s disease; QoL - quality of life; SBP – systolic blood pressure; SNF – skilled nursing facility; TMed - telemedicine
This paper is a upondary analysis of a Randomized Controlled Trial89
This paper is a secondary analysis of a previously published randomized controlled trial90
The main outcomes also varied between studies (Table 4). A number of studies (n=7) demonstrated similar outcomes compared to a corresponding control group; others demonstrated considerable acceptability, adherence and self-reported function. A number of studies (n=4) focused on fall, exercise or strength-based measures and demonstrated improvements. Three studies suggested that telemedicine could lead to improved cognitive function. All but one study demonstrated feasibility in their older adult population. However, improvements in utilization parameters were only observed in one study, while 5 studies demonstrated no differences. Each study had a number of major limitations, the main ones which are listed in the accompanying table (Supplemental Appendix 3).
DISCUSSION
We identified a number RCTs supporting TMed’s feasibility, acceptability and effectiveness across diverse health conditions, healthcare settings, and patient populations. Our data demonstrate that TMed can potentially be a useful modality of health service delivery. However, there were limitations with respect to the findings due to heterogeneity in study design, the plurality of underpowered studies in each arm, and other methodological limitations. This underscores the need for well-designed trials to minimize bias and provide definitive evidence of TMed use among ambulatory older adults.
Our review fills a gap as it focuses on trials conducted outside of the hospital setting. A number of included studies demonstrated equivalent outcomes highlighting the potential for telemedicine to address geographic barriers while delivering comparable health outcomes. Hospitals aim to achieve improved efficiency, prompting smaller systems in more remote areas to use telestroke and teleintensive care programs that are successful and sustainable60–62. Yet, there is less emphasis on ambulatory or skilled nursing facility care. Our results suggest that policymakers should promote further ambulatory coverage by eliminating barriers for both providers and patients, alike.
There is a critical need for high-quality studies investigating the impact of TMed interventions in older adults. The IDEATel study54, 63 integrated early TMed and remote monitoring with web-based informatics using a home-installed, low-bandwidth, TMed device. While their cohort exceeding 800 Medicare beneficiaries, the authors found that TMed was acceptable64, usable in lower socioeconomic65, ethnic66 and older adult populations67, and improved diabetes self-management68. Their data suggested a need for implementation strategies for future dissemination. The other three high methodologically high quality studies demonstrated sample size concerns69, 70 and a sample consisting predominantly of males71. Additional, adequately powered studies focusing on diverse populations are needed.
Our findings demonstrate that TMed interventions are feasible and acceptable among older adults and that similar outcomes are achievable compared to usual, in-person care. Few studies, though, focused specifically on rural adults and the results were mixed. While TMed may provide a unique opportunity to reach isolated, low-resource populations with limited access to in-person medical services, well-designed, high-quality studies are needed. It is unclear whether the considerable bias and misperception related to older adults’ use of technology72 play a role. Providers are often hesitant in recommending technologies in older adults due to potential physical, sensory, cognitive and visual-spatial abnormalities73–75. The population of older adults in the U.S. is rapidly growing76 with a workforce available to provide care for this demographic insufficient. TMed may help provide effective care, particularly in rural and underserved areas, and executing the Institute of Medicine’s recommendation to advance TMed resources77 is strongly supported by our observations.
Despite numerous limitations in study quality, our approach had a number of strengths supporting our conclusions. By using the PRISMA criteria, we reduced inherent bias and error that are present in conducting systematic reviews. Including research librarians increases the validity of our process. Our data substantiates that there are insufficient, well-designed RCTs in the use of TMed. The methodological inconsistencies in these trials provide an opportunity to focus on addressing these gaps in future work.
We acknowledge several limitations. First, many studies focused on specific diseases, and not multimorbid, frail older adults that often require a range of medical and social services78, impeding generalizability. The majority of studies did not highlight functional or socioeconomic status suggesting a need for future studies to report on these parameters. Second, laptops and computers which may have larger screens rather than tablets or smartphone technologies were used which are more affordable, widely available, but whose user interfaces may not necessarily be tailored to older adults - an important factor in usability79. Software and peripherals differ that may impact user experience and intervention effectiveness, which may increase the reach of future interventions. Data are needed to evaluate these devices, expanding upon traditional healthcare delivery to non-healthcare settings, beyond research or health centers. While our focus was on non-hospital based, only two RCTs were in nursing facilities53, 80. Observational studies exist81, 82; yet, the lack of rigorous studies in older adults have considerable implications as they are sicker, require increased medical assessment and acuity78, ultimately leading to increased utilization. Research to evaluate TMed interventions in such facilities are needed. Few studies described technological issues, particularly in areas with poor bandwidth, likely due to the urban-rural divide observed. Our findings are also prone to publication bias. Lastly, the heterogeneity of interventions and outcomes prevented us from conducting a formal meta-analysis, with some studies lacking formal statistical comparisons.
Our findings have a number of implications and provide a foundation for research priorities. The 2012 legislation covering TMed highlights an urgent need to develop novel, pragmatic interventions to evaluate TMed delivery, in both rural and non-rural populations. Currently, an Innovation Award is evaluating the impact of TMed on cost and reducible hospitalizations irrespective of locality in long-term care settings83. Understanding barriers and facilitators of effective TMed implementation strategies in systems as well as payment models to improve efficiency for both older adults and provider systems is helpful. We have an opportunity to integrate technology in older adults who traditionally are excluded from trials. Usability needs differ79 and future trials should adapt delivery systems to different chronological and physiological groups. While a number of RCTs using TMed in non-hospital settings exist, well-designed, powered trials will provide guidance in using this technology in older adults, particularly in rural areas.
Supplementary Material
Supplemental Appendix 1:
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist of evidence-based set of items for reporting in systematic reviews of randomized trials.
Supplemental Appendix 2
The search strategies of the main reference databases – MEDLINE (PubMed), Web of Science, Cochrane (Wiley), PsycInfo (Ebsco), CINAHL (EBSCO), Embase (Ovid) – that include results and ultimate number of references from each of these databases.
Supplemental Appendix 3
Evaluation of the extracted 17 studies that includes the country of origin, funding mechanisms and main study limitations.
Table 2:
Study Characteristics of Included Telemedicine Randomized Controlled Studies (n=17)
| Reference Year |
Telemedicine Model | Study Aim | # Participants | ||||
|---|---|---|---|---|---|---|---|
| Process | Transmitting End | Receiving End | Device | Active | Control | ||
| Burns57 2017 |
Expert to patient | Hospital-based speech pathologist | Patient with regional speech pathologist | Videoconferencing unit with Pan-Tilt-Zoom camera and handheld medical camera system | Evaluating speech pathology telepractice for swallowing of head/neck cancer patients | 43 | 39 |
| Burton84 2018 |
Expert to patient | Cognitive therapist | Patient | Video Therapy Analysis Lab with video set-up and peripherals | Comparability and feasibility of cognitive rehabilitation delivered by videoconferencing vs. in-person | 3 | 3 |
| Comin-Colet58 2016 |
Expert to patient | Nurse | Patient | Touchscreen computer, 3G access with videocall ability | Effectiveness of telemedicine check-ins & telemonitoring in improving CHF outcomes | 81 | 97 |
| De Luca80 2015 |
Expert to patient | Neurologist ± Psychologist | NH Resident | Videoconferencing-enabled PC and peripherals | Effectiveness of telehealth care model for managing NH residents | 32 | 27 |
| Dichmann Sorknaes52 2013 |
Expert to patient | Hospital-based nurses | Patient | Computer with web camera and microphone, and peripherals | Effectiveness of daily real-time video-consult vs. usual follow-up care in reducing readmission rates | 132 | 134 |
| Dy53 2013 |
Expert to expert | Endocrinologist | Nursing home nurse, dietician and patient | Laptop computer with secure videoconferencing and Skype freeware | Perception of telemedicine diabetes consultations by Skilled Nursing Facility Care Providers | 12 | 11 |
| Gandolfi85 2017 |
Expert to patient | Physio-therapist | Patient | Nintendo Wii console with web-camera & peripherals | Home virtual reality with in-clinic balance training in reducing instability in Parkinson’s patients | 38 | 38 |
| Homma59 2016 |
Expert to patient | Physician | Patient | Videophone (details not specified) | Effectiveness of counseling with telemonitoring vs. printed media in modifying lifestyle | 35 | 33 |
| Hong86 2017 |
Expert to patient | Exercise Instructor | Patient | PC with Internet connection; 15.6 inch touchscreen LCD, 2mp webcam, speaker, microphone | Development of a tele-exercise program on effectiveness of sarcopenia-related health factors | 11 | 12 |
| Hong69 2018 |
Expert to patient | Exercise instructor | Patient | Tablet with video-conferencing software | Effectiveness of a tele-exercise program on risk factors for falls | 15 | 15 |
| Ishani71 2016 |
Expert to patient | Interdisciplinary care team | Patient | Touch screen computer with peripherals | Feasibility and effectiveness of telehealth and case management for chronic kidney disease patients | 451 | 150 |
| Jelcic87 2014 |
Expert to patient | Therapist | Patient | Skype for Windows with network camera | Effect of domain-specific cognitive training delivered | 7c | 10 |
| 10 | |||||||
| Orlandoni88 2016 |
Expert to patient | Physician | Patient | Samsung Galaxy Tablet with videocall capabilities | Effectiveness of video consultation between home visits on outcomes of home enteral nutrition | 100 | 88 |
|
aTakahashi 2012 |
Expert to patient | Registered nurse | Patient | Intel Health Guide with videoconferencing capabilities and peripherals | Effectiveness of reducing ED visits and hospitalizations in older adults using telemonitoring | 102 | 103 |
|
bTrief54 2013 |
Expert to patient | Nurse case manager or dietician | Patient | Web-enabled computer with camera and peripherals | Adherence to diabetes care using telemedicine in Hispanic & African American patients | 844 | 821 |
| Tsai70 2017 |
Expert to patient | Physiotherapist based in tertiary hospital | Patient | Laptop computer with built-in camera (HP EliteBook 8560p) and peripherals | Effectiveness of videoconferencing tele-rehabilitation in improving physical fitness | 19 | 17 |
| Vahia55 2015 |
Expert to patient | UCSD Clinical evaluator | Patient | Tablet PC laptop, video camera, microphone and peripherals | Comparability of neuro-cognitive assessment via telepsychiatry vs. for older rural Latinos | 11 | 11 |
ACKNOWLEDGEMENTS
We would like to thank Patricia Erwin at Mayo Clinic Rochester for her assistance in the literature review.
Sponsor’s Role
The Sponsor had no role in the conduct, design or analysis of this study.
Funding Sources:
Dr. Batsis received funding from the National Institute on Aging of the National Institutes of Health under award number K23AG051681 and from the Friends of the Norris Cotton Cancer Center at Dartmouth and National Cancer Institute Cancer Center Support Grant 5P30 CA023108–37 Developmental Funds. Dr. Batsis has also received honoraria from the Royal College of Physicians of Ireland, Endocrine Society, and Dinse, Knapp, McAndrew LLC, legal firm. Support was also provided by the Department of Medicine, Geisel School of Medicine, Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention and the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- CMS
Centers for Medicare and Medicaid Services
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- TMed
Telemedicine
Footnotes
Conflicts of Interest
There are no Conflicts of Interest pertaining to this manuscript.
Work to be presented at the 2019 American Geriatrics Society Conference, Portland, Oregon
REFERENCES
- [1].Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356: 2388–2398. [DOI] [PubMed] [Google Scholar]
- [2].Ricketts TC. Workforce issues in rural areas: a focus on policy equity. Am J Public Health. 2005;95: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129: 611–620. [DOI] [PubMed] [Google Scholar]
- [4].Nawal Lutfiyya M, Bhat DK, Gandhi SR, Nguyen C, Weidenbacher-Hoper VL, Lipsky MS. A comparison of quality of care indicators in urban acute care hospitals and rural critical access hospitals in the United States. Int J Qual Health Care. 2007;19: 141–149. [DOI] [PubMed] [Google Scholar]
- [5].Warshaw R Health Disparities Affect Millions in Rural U.S. Communities In: News A, ed. AAMC News, Volume 2018, 2017. [Google Scholar]
- [6].Centers for Disease Control Vital Statistics. Volume 2013: United States Government, 2012. [Google Scholar]
- [7].Adler-Milstein J, Everson J, Lee SY. Sequencing of EHR adoption among US hospitals and the impact of meaningful use. J Am Med Inform Assoc. 2014;21: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DesRoches CM, Buerhaus P, Dittus RS, Donelan K. Primary care workforce shortages and career recommendations from practicing clinicians. Acad Med. 2015;90: 671–677. [DOI] [PubMed] [Google Scholar]
- [9].Gamm LD, Hutchison LL, Dabney BJ, Dorsey AM. Rural Health People 2010: A Companion Document to Health People 2010. College Station, Texas: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center, 2003. [Google Scholar]
- [10].Gamm L, Hutchison L, Bellamy G, Dabney BJ. Rural healthy people 2010: identifying rural health priorities and models for practice. J Rural Health. 2002;18: 9–14. [DOI] [PubMed] [Google Scholar]
- [11].Goins RT, Williams KA, Carter MW, Spencer M, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21: 206–213. [DOI] [PubMed] [Google Scholar]
- [12].MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10: 1531. [PMC free article] [PubMed] [Google Scholar]
- [13].Mainous AG 3rd, Kohrs FP. A comparison of health status between rural and urban adults. J Community Health. 1995;20: 423–431. [DOI] [PubMed] [Google Scholar]
- [14].AC S, RT S. Rural/Urban Differences in Barriers to and Burden of Care for Children With Special Health Care Needs. The Journal of Rural Health. 2007. [DOI] [PubMed] [Google Scholar]
- [15].Ao Aging. Internet Usage and Online Activities of Older Adults. Volume 2013: Administration on Aging, 2013. [Google Scholar]
- [16].Report of Task Force on Ensuring Access in Vulnerable Communities. American Hospital Assoication, 2016. [Google Scholar]
- [17].DS P Telecommunications in Rural America: Opportunities and Challenges for the Health Care System. Rockville, MD, 1992. [DOI] [PubMed] [Google Scholar]
- [18].S J, S P, M S. The Unique Application of Telemedicine to the Managed Healthcare System Am J Manag Care. 1996: 551–554. [Google Scholar]
- [19].Zollo SA, Kienzle MG, Henshaw Z, Crist LG, Wakefield DS. Tele-education in a telemedicine environment: implications for rural health care and academic medical centers. J Med Syst. 1999;23: 107–122. [DOI] [PubMed] [Google Scholar]
- [20].2018. Broadband Deployment Report. Volume 2019 Washington, DC: Federal Communications Commission, 2018. [Google Scholar]
- [21].Kvedar JC, Menn ER, Baradagunta S, Smulders-Meyer O, Gonzalez E. Teledermatology in a capitated delivery system using distributed information architecture: design and development. Telemed J. 1999;5: 357–366. [DOI] [PubMed] [Google Scholar]
- [22].Moses PL, McGowan JJ, Ricci MA. Efficacy of tele-endoscopy in a rural capitated market. Proc AMIA Annu Fall Symp. 1997: 398–402. [PMC free article] [PubMed] [Google Scholar]
- [23].Wells RS, Lemak CH. Beyond adoption to sustained use: telemedicine for rural communities. Telemed J. 1996;2: 285–293. [DOI] [PubMed] [Google Scholar]
- [24].Connect for Health Act of 2017. In: Congress t, ed. S1016, 2017. [Google Scholar]
- [25].Treat Paulsen E. and Reduce Obesity Act of 2017. HR1953, 2017. [Google Scholar]
- [26].Rosenberg CN, Peele P, Keyser D, McAnallen S, Holder D. Results from a patient-centered medical home pilot at UPMC Health Plan hold lessons for broader adoption of the model. Health Aff (Millwood). 2012;31: 2423–2431. [DOI] [PubMed] [Google Scholar]
- [27].Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare beneficiaries with chronic disease linked to savings. Health Aff (Millwood). 2011;30: 1689–1697. [DOI] [PubMed] [Google Scholar]
- [28].Dinesen B, Haesum LK, Soerensen N, et al. Using preventive home monitoring to reduce hospital admission rates and reduce costs: a case study of telehealth among chronic obstructive pulmonary disease patients. J Telemed Telecare. 2012;18: 221–225. [DOI] [PubMed] [Google Scholar]
- [29].Rojas SV, Gagnon MP. A systematic review of the key indicators for assessing telehomecare cost-effectiveness. Telemed J E Health. 2008;14: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cryer L, Shannon SB, Van Amsterdam M, Leff B. Costs for ‘hospital at home’ patients were 19 percent lower, with equal or better outcomes compared to similar inpatients. Health Aff (Millwood). 2012;31: 1237–1243. [DOI] [PubMed] [Google Scholar]
- [31].Darkins A, Ryan P, Kobb R, et al. Care Coordination/Home Telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14: 1118–1126. [DOI] [PubMed] [Google Scholar]
- [32].Dellifraine JL, Dansky KH. Home-based telehealth: a review and meta-analysis. J Telemed Telecare. 2008;14: 62–66. [DOI] [PubMed] [Google Scholar]
- [33].Grabowski DC, O’Malley AJ. Use of telemedicine can reduce hospitalizations of nursing home residents and generate savings for medicare. Health Aff (Millwood). 2014;33: 244–250. [DOI] [PubMed] [Google Scholar]
- [34].Hofmeyer J, Leider JP, Satorius J, Tanenbaum E, Basel D, Knudson A. Implementation of Telemedicine Consultation to Assess Unplanned Transfers in Rural Long-Term Care Facilities, 2012–2015: A Pilot Study. J Am Med Dir Assoc. 2016;17: 1006–1010. [DOI] [PubMed] [Google Scholar]
- [35].Stern A, Mitsakakis N, Paulden M, et al. Pressure ulcer multidisciplinary teams via telemedicine: a pragmatic cluster randomized stepped wedge trial in long term care. BMC Health Serv Res. 2014;14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van den Berg N, Schumann M, Kraft K, Hoffmann W. Telemedicine and telecare for older patients--a systematic review. Maturitas. 2012;73: 94–114. [DOI] [PubMed] [Google Scholar]
- [37].Forducey PG, Glueckauf RL, Bergquist TF, Maheu MM, Yutsis M. Telehealth for persons with severe functional disabilities and their caregivers: facilitating self-care management in the home setting. Psychol Serv. 2012;9: 144–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Expansion of Medicare Telehealth Services for CY 2012: Pub. 100–04. : U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services, 2011. [Google Scholar]
- [40].Expansion of Medicare Telehealth Services for CY 2012: Pub. 100–02. Baltimore, MD: U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services, 2011. [Google Scholar]
- [41].Barlow J, Singh D, Bayer S, Curry R. A systematic review of the benefits of home telecare for frail elderly people and those with long-term conditions. J Telemed Telecare. 2007;13: 172–179. [DOI] [PubMed] [Google Scholar]
- [42].Chi NC, Demiris G. A systematic review of telehealth tools and interventions to support family caregivers. J Telemed Telecare. 2015;21: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kampmeijer R, Pavlova M, Tambor M, Golinowska S, Groot W. The use of e-health and m-health tools in health promotion and primary prevention among older adults: a systematic literature review. BMC Health Serv Res. 2016;16 Suppl 5: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: A systematic literature review. Int J Med Inform. 2016;85: 17–26. [DOI] [PubMed] [Google Scholar]
- [45].Bramer W, Bain P. Updating search strategies for systematic reviews using EndNote. J Med Libr Assoc. 2017;105: 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Batsis JA, Gill LE, Masutani RK, et al. Weight Loss Interventions in Older Adults with Obesity: A Systematic Review of Randomized Controlled Trials Since 2005. J Am Geriatr Soc. 2017;65: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].CMS Telemedicine Services. Baltimore, MD: U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services, 2019. [Google Scholar]
- [48].Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Albahri OS, Albahri AS, Mohammed KI, et al. Systematic Review of Real-time Remote Health Monitoring System in Triage and Priority-Based Sensor Technology: Taxonomy, Open Challenges, Motivation and Recommendations. J Med Syst. 2018;42: 80. [DOI] [PubMed] [Google Scholar]
- [50].Kruse CS, Krowski N, Rodriguez B, Tran L, Vela J, Brooks M. Telehealth and patient satisfaction: a systematic review and narrative analysis. BMJ Open. 2017;7: e016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dichmann Sorknaes A The Effect of Tele-Consultation Between a Hospital-Based Nurse and a COPD Patient. Stud Health Technol Inform. 2016;225: 883–884. [PubMed] [Google Scholar]
- [53].Dy PM PC; Weinstock RS Use of telemedicine to improve glycemic management in a skilled nursing facility: a pilot study. Telemed J E Health. 2013;19: 643–645. [DOI] [PubMed] [Google Scholar]
- [54].Trief PM, Izquierdo R, Eimicke JP, et al. Adherence to diabetes self care for white, African-American and Hispanic American telemedicine participants: 5 year results from the IDEATel project. Ethn Health. 2013;18: 83–96. [DOI] [PubMed] [Google Scholar]
- [55].Vahia IVN B, Camacho A, Cardenas V, et al. Telepsychiatry for Neurocognitive Testing in Older Rural Latino Adults. Am J Geriatr Psychiatry. 2015;23: 666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim H, Jhoo JH, Jang JW. The effect of telemedicine on cognitive decline in patients with dementia. J Telemed Telecare. 2017;23: 149–154. [DOI] [PubMed] [Google Scholar]
- [57].Burns CL, Ward EC, Hill AJ, Kularatna S, Byrnes J, Kenny LM. Randomized controlled trial of a multisite speech pathology telepractice service providing swallowing and communication intervention to patients with head and neck cancer: Evaluation of service outcomes. Head Neck. 2017;39: 932–939. [DOI] [PubMed] [Google Scholar]
- [58].Comin-Colet J, Enjuanes C, Verdu-Rotellar J, et al. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: results of a randomized controlled trial. J Telemed Telecare, Volume 22, 2016, pp. 282–295. [DOI] [PubMed] [Google Scholar]
- [59].Homma SI H; Nakamura T; Fujimura K; Ito Y; Maeda Y; Kaneko I A comparative study on the effectiveness of one-way printed communication versus videophone interactive interviews on health promotion. J Telemed Telecare. 2016;22: 56–63. [DOI] [PubMed] [Google Scholar]
- [60].Gray LC, Fatehi F, Martin-Khan M, Peel NM, Smith AC. Telemedicine for Specialist Geriatric Care in Small Rural Hospitals: Preliminary Data. J Am Geriatr Soc. 2016;64: 1347–1351. [DOI] [PubMed] [Google Scholar]
- [61].Trombley MJ, Hassol A, Lloyd JT, et al. The Impact of Enhanced Critical Care Training and 24/7 (Tele-ICU) Support on Medicare Spending and Postdischarge Utilization Patterns. Health Serv Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zawada ET Jr., Kapaska D, Herr P, et al. Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system. S D Med. 2006;59: 391–393. [PubMed] [Google Scholar]
- [63].Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Izquierdo RE, Wang D, Huang D, Palmas W, Weinstock RS. Case Management with a Diabetes Team Using Home Telemedicine: Acceptance of Treatment Recommendations by Primary Care Providers in IDEATel. Telemed J E Health. 2015;21: 980–986. [DOI] [PubMed] [Google Scholar]
- [65].Shea S, Kothari D, Teresi JA, et al. Social impact analysis of the effects of a telemedicine intervention to improve diabetes outcomes in an ethnically diverse, medically underserved population: findings from the IDEATel Study. Am J Public Health. 2013;103: 1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Trief PM, Izquierdo R, Eimicke JP, et al. Adherence to diabetes self care for white, African-American and Hispanic American telemedicine participants: 5 year results from the IDEATel project. Ethn Health. 2013;18: 83–96. [DOI] [PubMed] [Google Scholar]
- [67].Lai AM, Kaufman DR, Starren J, Shea S. Evaluation of a remote training approach for teaching seniors to use a telehealth system. Int J Med Inform. 2009;78: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].West SP, Lagua C, Trief PM, Izquierdo R, Weinstock RS. Goal setting using telemedicine in rural underserved older adults with diabetes: experiences from the informatics for diabetes education and telemedicine project. Telemed J E Health. 2010;16: 405–416. [DOI] [PubMed] [Google Scholar]
- [69].Hong J, Kong HJ, Yoon HJ. Web-Based Telepresence Exercise Program for Community-Dwelling Elderly Women With a High Risk of Falling: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2018;6: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tsai LLYM RJ, Moddel C, Alison JA, McKenzie DK, McKeough ZJ. Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: The randomized controlled TeleR Study. Respirology. 2017;22: 699–707. [DOI] [PubMed] [Google Scholar]
- [71].Ishani A, Christopher J, Palmer D, et al. Telehealth by an Interprofessional Team in Patients With CKD: a Randomized Controlled Trial. Am J Kidney Dis, Volume 68, 2016, pp. 41–49. [DOI] [PubMed] [Google Scholar]
- [72].Batsis JA, Zagaria AB, Brooks E, et al. Obesity Stigma in Rural Older Adults: A qualitative study. [Submitted]. 2018. [DOI] [PMC free article] [PubMed]
- [73].Parker SJ, Jessel S, Richardson JE, Reid MC. Older adults are mobile too!Identifying the barriers and facilitators to older adults’ use of mHealth for pain management. BMC Geriatr. 2013;13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Westerman SJ, Davies DR. Acquisition and application of new technology skills: the influence of age. Occup Med (Lond). 2000;50: 478–482. [DOI] [PubMed] [Google Scholar]
- [75].Williams K, Pennathur P, Bossen A, Gloeckner A. Adapting Telemonitoring Technology Use for Older Adults: A Pilot Study. Res Gerontol Nurs. 2016;9: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].LA W, S C, D G, W H. US Census Bureau: 65+ in the United States: 2010. US Department of Health and Human Services: National Institutes of Health; Washington, DC, 2014. [Google Scholar]
- [77].Lustig T The Role of Telehealth in an Evolving Health Care Environment: Institute of Medicine, 2012. [PubMed]
- [78].Guiding principles for the care of older adults with multimorbidity: an approach for c. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60: E1–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sonderegger A, Schmutz S, Sauer J. The influence of age in usability testing. Appl Ergon. 2016;52: 291–300. [DOI] [PubMed] [Google Scholar]
- [80].De Luca RB A; De Cola MC; Trifiletti A; Tomasello P; Torrisi M; Reitano S; Leo A; Bramanti P; Calabro RS Tele-health-care in the elderly living in nursing home: the first Sicilian multimodal approach. Aging Clin Exp Res. 2016;28: 753–759. [DOI] [PubMed] [Google Scholar]
- [81].Farris G, Sircar M, Bortinger J, et al. Extension for Community Healthcare Outcomes-Care Transitions: Enhancing Geriatric Care Transitions Through a Multidisciplinary Videoconference. J Am Geriatr Soc. 2017;65: 598–602. [DOI] [PubMed] [Google Scholar]
- [82].Moore AB, Krupp JE, Dufour AB, et al. Improving Transitions to Postacute Care for Elderly Patients Using a Novel Video-Conferencing Program: ECHO-Care Transitions. Am J Med. 2017;130: 1199–1204. [DOI] [PubMed] [Google Scholar]
- [83].Initiative to Reduce Avoidable Hospitalizations Among Nursing Facility Residents: Phase Two. Volume 2019: Center for Medicare and Medicaid, 2019. [Google Scholar]
- [84].Burton RL, O’Connell ME. Telehealth Rehabilitation for Cognitive Impairment: Randomized Controlled Feasibility Trial. JMIR Res Protoc. 2018;7: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gandolfi M, Geroin C, Dimitrova E, et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson’s Disease: A Multicenter, Single-Blind, Randomized, Controlled Trial. Biomed Research International. 2017: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hong JK J; Kim SW; Kong HJ Effects of home-based tele-exercise on sarcopenia among community-dwelling elderly adults: Body composition and functional fitness. Exp Gerontol. 2017;87: 33–39. [DOI] [PubMed] [Google Scholar]
- [87].Jelcic N, Agostini M, Meneghello F, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: a pilot study. Clin Interv Aging. 2014;9: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Orlandoni P, Jukic Peladic N, Spazzafumo L, et al. Utility of video consultation to improve the outcomes of home enteral nutrition in a population of frail older patients. Geriatr Gerontol Int. 2016;16: 762–767. [DOI] [PubMed] [Google Scholar]
- [89].Takahashi PY, Pecina JL, Upatising B, et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172: 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Izquierdo R, Lagua CT, Meyer S, et al. Telemedicine intervention effects on waist circumference and body mass index in the IDEATel project. Diabetes Technol Ther. 2010;12: 213–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Appendix 1:
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist of evidence-based set of items for reporting in systematic reviews of randomized trials.
Supplemental Appendix 2
The search strategies of the main reference databases – MEDLINE (PubMed), Web of Science, Cochrane (Wiley), PsycInfo (Ebsco), CINAHL (EBSCO), Embase (Ovid) – that include results and ultimate number of references from each of these databases.
Supplemental Appendix 3
Evaluation of the extracted 17 studies that includes the country of origin, funding mechanisms and main study limitations.