Abstract
BACKGROUND:
Prostate hyperplasia and prostate cancer are two of the most common pathological condition of the prostate to be found on male. Both of these diseases share common pathogenesis involving inflammation of prostatic tissues. Chronic inflammation will induce the release of cytokines, followed by cells injury and tissues damage. One of the cytokines that play a role in prostate pathology is IL-6. The inflammation will also induce the releases of anti-inflammatory cytokines such as TGFβ-1.
AIM:
This study aims to analyse the expression of IL-6 and TGFβ-1, in prostate hyperplasia and prostate cancer.
MATERIAL AND METHODS:
This is an observational study, using paraffin-embedded tissue samples of prostate hyperplasia and prostate cancer. Samples were obtained from the laboratory of Pathological Anatomy, Faculty of Medicine, Andalas University, Padang, Indonesia. Immunohistochemistry was performed to detect the cytokine expression, and a semiqunatitaves measurement according to Immunoreactive score (IRS) was performed for evaluation. For the TGFβ-1, the stromal expression was also analysed by measurement of the stromal stained area. The correlation of cytokine expression to Gleason index score was also analysed in prostate cancer.
RESULTS:
The result showed that this study found that TGFβ-1 was detected both in the stromal component as well as epithelial. With the stromal being the dominant site of expression. The stromal TGFβ-1 expression was of significantly higher in prostate hyperplasia compares to prostate cancer (p < 0.05), while the epithelial expression of TGFβ-1 was not found to be significantly different. IL-6 was mostly expressed intracytoplasmic in epithelia. The IL-6 expression was significantly higher in prostate cancer compared to hyperplasia. However, there was no significant correlation to found between IL-6 expression to the Gleason Score among prostate cancers.
CONCLUSION:
This study concluded that there were differences in expression of both TGFβ-1 and IL-6 between prostate hyperplasia and prostate cancer tissue by immunohistochemistry.
Keywords: Prostate Hyperplasia, Prostate Cancer, IL-6, TGFβ-1, Gleason Index
Introduction
The benign prostate hyperplasia and prostate cancer are the most common pathological condition to be found on the male’s urothelial system. An epidemiological study showed that the incidence of this disease was increasing annually, worldwide [1]. Prostate hyperplasia is the most common benign neoplasm on elderly male. Histopathological study on autopsy reports showed that the prevalence of prostate hyperplasia was found up to 50% on males between 50-60 years of age, and it increases to over 80% in the 70 years group of ages [1], [2], [3], [4], [5]. In the other hand, prostate cancer is the most common non-skin cancer to be found on male, worldwide. This disease serves as the second most killer cancer among males in the United States and Europe [2], [6].
Prostate hyperplasia and prostate cancer share a similar pathogenesis, in which both are related to hormones and inflammation [7], [8], [9]. Despite having different predilection, both diseases are known as a chronic disease that has an early initiation, followed by a slow progression course, until it shows clinical symptom [2], [7]. In the recent five years, it has been revealed that chronic inflammation of prostate tissue is one of the risk factors for both prostate hyperplasia and prostate cancer [2], [3], [7], [10], [11], [12]. Although the pathogenesis is still unclear, nowadays there are many studies showed the relations of chronic inflammation to both prostate hyperplasia and prostate cancer [3], [4], [9], [13]. Recently the treatment of BPH and prostate cancer also started to incorporate the prevention and treatment of chronic inflammation as an integrated treatment [10], [14], [15], [16].
Chronic inflammation will induce the release of cytokine and other inflammatory factors, both from inflammatory cells, as well as the hypoxic prostate epithelium [1], [17]. These cytokines will interact with stromal cells and cause further tissue damage [2], [17]. The inflammation will induce infiltrations of T cells, B cells and macrophages [8], [18]. These inflammatory cells will induce the release of the proinflammatory IL-2, IL-8 and IL-6 from epithelial as well as stromal cells [19]. In return, the increasing of proinflammatory cytokines will induce the production and secretion of an anti-inflammatory cytokine such as TGF β and FGF. The complex interaction of those cytokines will eventually affect the function of prostate gland [18], [19], [20], [21], [22]. TGF-β is known as a controlling factor of tumour progressiveness. This cytokine has a biphasic role in carcinogenesis. On the early phase of cancer, it serves as a tumour suppressor agent by inhibiting cell proliferation [23], [24]. However, on the later stage, it functions as a tumour promoter, in which, it will induce the cellular changes related to tumour cells invasion [23], [24], [25].
IL-6 is a cytokine that involves in the malignancies process and could serve as a factor that inhibits the apoptosis of tumour cells as well as inducing angiogenesis [24], [26], [27], [28]. Pace et al. (2011) showed that the IL-6 was found to be significantly higher on a patient with prostate cancer compared to prostate hyperplasia [27]. The present study aims to analyse the differential expression of cytokines that involves in the pathogenesis of BPH and prostate cancer, especially TGFβ-1 and IL-6 in prostate tissues.
Materials and Methods
Experimental Design
The present study used formalin fixed paraffin embedded tissues from prostate lesion obtained from a surgical procedure. Forty samples of previously diagnosed as histologically, (29), as well as 40 samples of prostate cancers, were used in this study. The tissues were obtained from the laboratory of pathological anatomy, Faculty of Medicine, Andalas University, Padang, Indonesia.
Immunohistochemistry
Immunohistochemistry was used to investigate the expression of cytokines using the avidin-biotin complex method. Following primary antibody were used; Rabbit polyclonal anti-human TGFβ-1, Bioss, 0086R with a dilution of 1:200, and Rabbit polyclonal anti-human IL-6. Bioss, 07-82R, with dilution 1:100. The goat anti-rabbit Igg, Vector laboratories, of dilution 1:200 was used as secondary antibody and the 3-3’ diaminobenzidine (DAB), Dojindo Laboratories, was applied as the chromogen. The immunohistochemistry was performed as suggested in the primary antibody datasheet accordingly. The cellular expression of the cytokines was performed by a semi-quantitative system according to Immunoreactive score (IRS). Both the proportion and intensity of cellular staining were measured. The final IRS score is the multiplication of proportion score to intensity score, which ranges between 0 to 12. In this present study, the IRS score of 0 to 4 was considered as “low IRS score”, and the score above 4 is treated as “high IRS score”. The stromal staining of TGFβ-1were also analysed by measuring the proportion of stained area using the Image J software, (Image J 1.49v software, National Institute of Health, Bethesda, MD, USA). For the samples of prostate cancer, the correlation of cytokine expression to the Gleason score was also analysed. The prostate cancers samples were grouped either as “high Gleason score” (score 8-10) or “low Gleason score” (score 7 or below).
Statistical analysis
Chi-square was used for statistical analysis. And the Shapiro-Wilk test was performed as a test of normality.
Results
Before data analysis, the distribution of data was assessed for normality test. Since each of the groups contains less than 50 samples, the normality test was performed with the Shapiro-Wilk test, as shown in Table 1.
Table 1.
Normality Test for Independent Variables
| Variables | Group | Shapiro-Wilk | ||
|---|---|---|---|---|
| Statistic | df | Sig. | ||
| TGFβ-1 | Prostate hyperplasia | 0.852 | 40 | 0.000 |
| Prostate cancer | 0.743 | 40 | 0.000 | |
| IL-6 | Prostate hyperplasia | 0.955 | 40 | 0.109 |
| Prostate cancer | 0.914 | 40 | 0.005 | |
Table 1 shows the distribution of variables. None of the variables shows a normal distribution (p < 0.05). Therefore, the test was continued by transforming the data, and a repeating normality test was performed, as shown in Table 2.
Table 2.
Post-Transformation Normality Test
| Variables (Log) | Group | Shapiro-Wilk | ||
|---|---|---|---|---|
| Statistic | df | Sig. | ||
| LOG TGFβ-1 | Prostate hyperplasia | 0.899 | 23 | 0.024 |
| Prostate cancer | 0.946 | 24 | 0.223 | |
| LOG IL-6 | Prostate hyperplasia | 0.799 | 23 | 0.000 |
| Prostate cancer | 0.868 | 24 | 0.005 | |
Post-transformation normality test showed that only TGFβ-1 on prostate cancer data shows normal distribution. Therefore, to investigate the correlation between independent and dependent variables, the statistical analysis will be done using the non-parametric Mann Whitney test. The value of each variable will be presented by the average value and standard of deviation.
The Epithelial Expression of TGFβ-1 on Prostate Lesions
Immunohistochemistry shows that TGFβ-1 was expressed both in the epithelial component as well as the stromal component (Figure 1). However, the majority of the staining with strong signal intensities were to be found in the stromal area. The epithelial expression of TGFβ-1 can be found in prostatic epithelial both in prostate hyperplasia as well as prostate cancer (Figure 1A and B). Most of the staining of the TGFβ-1 in prostate hyperplasia showing of low expression in IRS score (97.5%). Prostate cancer also mostly showed low TGFβ-1 epithelial expression (87.5%) (Table 3). The average IRS values of TGFβ-1 epithelial expression is slightly lower in prostate Hyperplasia (IRS score 0.7) compared to the prostate cancer group (IRS score 1.3). However, the differences are statistically non-significant. Interestingly there are 5 samples of prostate cancers that showed high epithelial TGFβ-1 expression as can be seen in Table 3 and Figure 1B.
Figure 1.

The Expression of TGFβ-1 by Immunohistochemistry on Human Prostate Tissue. Prostate Hyperplasia (A) and Prostate Cancer (B). TGFβ-1 are mostly Expressed on Stromal Component Prostate Hyperplasia (A); The Stromal Staining is Greatly Reduced in Prostate Cancer, However Cancer Epithelial can Show TGFβ-1 Staining in Some Cases (B) Immunoperoxidase of Rabbit Anti Human TGFβ-1. Scale Bar 100 µm
Table 3.
The Epithelial Expression of TGFβ-1 on Prostate Hyperplasia and Prostate Cancer Tissue
| Group | n | Epithelial TGFβ-1 (IRS Score) | ||
|---|---|---|---|---|
| Low (%) | High (%) | p | ||
| Prostate hyperplasia | 40 | 39 (97.5%) | 1 (2.5%) | 0.201 |
| Prostate carsinoma | 40 | 35 (87.5%) | 5 (12.5%) | |
Table 3 shows the expression of epithelial TGFβ-1 on prostate hyperplasia and prostate cancer. Both groups show mostly low IRS scores. The carcinoma groups contain slightly higher IRS score. However, there are no significant differences to be found between groups (p = 0.21).
The Stromal Expression of TGFβ-1 on Prostate Lesions
The immunohistochemistry staining of TGFβ-1 showed a distinct pattern between hyperplasia and prostatic cancer. Most of the prostate hyperplasia showed a high stromal staining intensity surrounding the gland (Figure 1A and 2A). In the other hand, prostate cancer shows low expression of TGFβ-1 in the stromal area (Figure 1B and 2C). The measurement of stromal staining was done by Image J software by selecting the brown staining area, converting the image into a black and white image and measured the stained area.
Figure 2.

The Expression of TGFβ-1 by Immunohistochemistry on Human Prostate Tissue. Prostate Hyperplasia (A) and Prostate Cancer (B); The Stomal Area is Measured by Image J Software by Isolate the Brown Stained Area and Measuring the Proportion of Stained Area. There is a Higher Proportion of Stained Area in Prostate Hyperplasia (B) Compared to Prostate Cancer (D). Immunoperoxidase of Rabbit Anti Human TGFβ-1. Scale Bar 100 µm
The average stromal area of TGFβ-1 in prostate hyperplasia is 15.3% and is significantly higher compares to that of the prostate cancer 4.5%. The stromal expression of TGFβ-1 can be seen in Table 4.
Table 4.
The Stromal Expression of TGF-β1 on Prostate Hyperplasia and Prostate Cancer Tissue
| Group | n | TGFβ-1 (% Area) | ||
|---|---|---|---|---|
| Average Value | St-Deviation | p | ||
| Prostate hyperplasia | 40 | 15.32 | 8.74 | 0.00 |
| Prostate carcinoma | 40 | 4.47 | 4.84 | |
Table 4 shows the expression of stromal TGFβ-1 on prostate hyperplasia and prostate cancer. There is a significantly higher expression of TGFβ-1 on hyperplasia prostate, compared to prostate cancers (p < 0.05).
The Expression of IL-6 on Prostate Lesions and its Correlation to the Gleason Score in Prostate Cancer
Immunohistochemistry shows that IL- 6 was expressed in the epithelial component both in hyperplasia prostate and prostate cancer. The staining can also be detected in the stromal area but a faintly low intensity (Figure 3). The majority (62.5%) of prostate hyperplasia showed low expression of IL-6 in IRS score, in contrast, the majority (85%) of samples in carcinoma group showed a stronger expression, as can be seen in Table 5.
Figure 3.
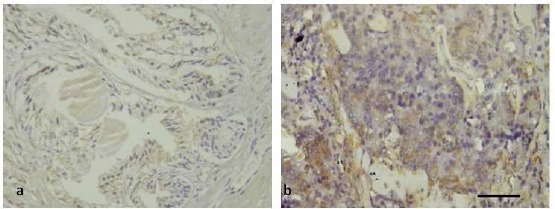
The Expression of IL-6 by Immunohistochemistry on Human Prostate Tissue. Prostate Hyperplasia (A) and Prostate Cancer (B). IL-6 is Expressed on Low Intensity on Prostate Hyperplasia (A). The Expression is Higher in Prostate Cancer Cells (B) Immunoperoxidase of Rabbit Anty Human IL-6. Scale Bar; 100µm
Table 5.
The Expression of IL-6 on Prostate Hyperplasia and Prostate Cancer Tissue
| Group | n | IL-6 (IRS Score) | ||
|---|---|---|---|---|
| Low | High | p | ||
| Prostate hyperplasia | 40 | 25 (62.5%) | 15 (37.5%) | 0.001 |
| Prostate carsinoma | 40 | 6 (15%) | 34 (85%) | |
Table 5 shows the expression of IL-6 on prostate hyperplasia and prostate cancer. There is a significantly higher expression of IL-6 on prostate cancers compared to hyperplasia prostate (p < 0.05).
When the prostate cancer was grouped into “high” and “low” Gleason score index group, both groups showed high IL-6 IRS score, there is no significant correlation between IL-6 expression to the Gleason index score in prostate cancer, as can be seen in Table 6.
Table 6.
The Correlation of Gleason Score to the Expression of IL-6 on Prostate Carcinoma
| Gleason Score | n | IL-6 IRS score (%) | ||
|---|---|---|---|---|
| Low | High | p | ||
| Low | 40 | 1 (7.1%) | 13 (92.9%) | 0.399 |
| High | 40 | 5 (19.2%) | 21 (80.8%) | |
Discussion
The Expression of TGFβ-1 on Prostate Lesions: Prostate Hyperplasia and Prostate Cancer
Chronic inflammation plays a significant role in the initiation and progression to the wide spectrum of pathogenesis in prostate lesions. The inflammation will attract the infiltrations of B cells, T Cells, as well as macrophages. These immune cells will increase the secretion of proinflammatory cytokine IL-2, IL-6 and IL-8 both on the epithelial tissue or from storms. In response to the increases of proinflammatory cytokines, the epithelial and stromal cells will produce anti-inflammatory cytokines such as TGFβ-1 and FGF, which in turn will affect the normal function of prostate glands.
In this present study, we found that TGFβ-1 was highly expressed in hyperplasia prostate tissue in the extracellular matrix surrounding the glands. The expression of TGFβ-1 was significantly different compared to prostate cancer. This result is suggesting that TGFβ-1 play an important role in prostate hyperplasia pathogenesis. It has been known that TGFβ-1, acts as a modulator of other protein such as bFGF 2, synthesis of extracellular matrix and angiogenesis via VEGF. This interaction plays roles in the pathogenesis of hyperplasia prostate.
TGFβ-1 is a potent mitotic factor for fibroblast as well as other mesenchymal cells [22], [30]. It also regulates the synthesis of the extracellular matrix and induces the secretion of fibrogenic bFGF-2. TGFβ-1 expression act as chemoattractant towards fibroblast, which played roles in the early process of fibrosis on early phase and was instrumental in the process of fibrosis [31]. TGFβ-1 stimulates fibroblast transformation into myofibroblast and smooth muscle cells through induction of extracellular matrixes. Other studies showed the role of TGFβ-1 in fibrosis. Zuhirman (2014) shows the increases of TGFβ-1 in chronic ureteral obstruction [32]. Untergasser et al., (2005) showed the increases of TGFβ-1 stimulates the collagen synthesis by stromal cells on prostate hyperplasia, as well as the transformation of fibroblast into myofibroblast [6].
In Contrast to prostatic hyperplasia, our present study shows that there are decreases in stromal TGFβ-1 expression in prostate cancer. Interestingly we also found the increases of cytoplasmic expression of TGFβ-1 in some samples. There were variations of the stromal TGFβ-1 expression level among prostate cancer in our study, and the average values of stained area proportion were of 4%. Most prostate cancers in the present study show negative cytoplasmic staining of TGFβ-1, 19 samples showed positive staining but only on a low level (IRS score 1). TGFβ-1 was known to have a biphasic role in carcinogenesis and has been well known as a factor of tumour progression in the early phase TGFβ-1 act as a tumour suppressor by inhibiting cell proliferation, and induces cells differentiation as well as apoptosis. However, in the late stage, TGFβ-1 loses its tumour suppressing-function and act as a tumour promoter. In the present study, most of our samples showed a low expression of TGFβ-1 in cancer cells, suggesting a decreased synthesis or secretion of this protein in prostate cancer. The low expression might have roles in the pathogenesis of prostate cancers. Loss of TGFβ-1 function with low expression was also reported in some cancers such as breast, ovarium, oesophagus, and head and neck cancer.
However, in the late stages of carcinogenesis, TGFβ-1 can play an opposite function as a tumour growth promoter. It is suggested that some tumour is actively secreted TGFβ-1 in the late stages of carcinogenesis. The TGFβ-1 in these tumour plays a complex role related to angiogenesis, immune cells suppression, cellular transformation related to invasiveness and metastatic ability, as well as mediating interactions of tumour cells and extracellular matrix. Interestingly in our present study, we found 5 samples with higher expression of TGFβ-1 (IRS score 4). The high TGFβ-1 expression on some cancer cells raises the question of whether these differences related to the biological behaviour of tumour cells, the tendencies to metastasise or other clinical outcomes. Further study is required to answers these questions in prostate cancers.
The Expression of IL-6 on Prostate Lesions; Prostate Hyperplasia and Prostate Cancer
Chronic inflammation plays a significant role in the initiation and progression of the prostate lesion. Inflammation was believed to have a strong correlation to prostatitis, prostatic hyperplasia and prostate cancer. Inflammation will invite T cells, B cells and macrophages to the prostate glandular structures and stroma. After the initiation process, the dendritic cells will be activated and maintained the T cells responses within the prostate gland; this will cause a chronic and progressive pathological process that will eventually facilitate the progression of prostate hyperplasia or prostate cancer.
Immune cells infiltration will increase the secretion of a pro-inflammatory cytokine such as IL-2, IL-6, and IL-8. The activation of various cytokines will disrupt the balance of cell proliferation a d apoptosis. IL-6 is produced by various type of cells, including macrophages endothelial, and lymphocytes. IL-6 expression and can be detected both intracellular within cells cytoplasm as well on extracellular matrix. Higher expression of IL-6 was detected on a prostate cancer group with strong intensities. In the other hand, most of the prostate hyperplasia showed weak expression of IL-6.
Our present study showed a similar result with some other study. Engelhard et al. (2014) found that the expression of IL-6 in prostate cancer is significantly higher compared to prostate hyperplasia [20]. We also observed a variation of IL-6 expression among prostate cancer; 7 of the samples exhibit a high IL-6 expression of IRS score 9 or above. The strong expression in some of prostate cancer samples raises the question of whether this finding has any relation to the biological behaviour of cancer cells. Duscharla et al. (2017) reported that a high serum level of IL-6 is related to the bone metastasis of prostate cancer [33].
IL-6 secreted by immune cells infiltrate will be captured by IL-6-R, this will activate JAK, STAT3 and MAPK pathway, these, in turn, will induce cell proliferation through androgen receptor inductionangiogenesis and facilitates metastasis. IL-6 are also known to induce intraprostatic testosterone through activation of steroidogenic enzymes. Iliopoulos et al. (2009) suggested the correlation between inflammation, IL-6 activation, STAT1, PI3K, and NFkB in the pathogenesis of prostate cancer. Another study also confirmed that the increase of IL-6 was correlated to the prognosis and showed a negative relation to the survival rate. Based on the above results, the present study supports the theory that IL-6 plays a role significant role in the pathogenesis and progression of prostate cancer (34).
However, in the present study, the expression of IL-6 was not correlated to the Gleason score significantly. Our study employed immunohistochemistry to detect the expression of IL-6 in prostate tissues. The nature of IL-6 as a soluble cytokine could sometimes be difficult to be measured quantitatively by immunohistochemistry. Further study is required to investigate the relation of IL-6 to the Gleason score quantitatively, using a different and more sensitives method.
In summary, the present study reveals the differential expression of TGFβ-1 and IL-6 between prostate hyperplasia and prostate cancer. The TGFβ-1 were highly expressed in the stromal component of prostate hyperplasia, compared to prostate cancer. In the other hand, the IL-6 showed a higher expression in prostate cancer cells compared to prostate hyperplasia. Further study is required to investigate the function of each cytokine in the pathogenesis of prostate lesion.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Abedelmalek S, Wong DP, Chtourou H, Souissi N, Tabka Z. Racial Variation of Interleukin-6 in Soccer Players:The Effect of Short-Term Maximal Exercise. International Journal of Medicine and Pharmaceutical Sciences (IJMPS) 2014;4(1):37–48. https://doi.org/10.1080/09291016.2014.904574. [Google Scholar]
- 2.Elkahwaji JE. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res Rep Urol. 2012;5:1–10. doi: 10.2147/RRU.S23386. https://doi.org/10.2147/RRU.S23386 PMid:24400229 PMCid:PMC3826944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schröder F, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer:The role of inflammation. Eur Urol. 2011;60(1):106–17. doi: 10.1016/j.eururo.2011.03.055. https://doi.org/10.1016/j.eururo.2011.03.055 PMid:21497433. [DOI] [PubMed] [Google Scholar]
- 4.Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, Salonia A, Sciarra A, Montorsi F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH) BJU international. 2013;112(4):432–41. doi: 10.1111/bju.12118. https://doi.org/10.1111/bju.12118 PMid:23650937. [DOI] [PubMed] [Google Scholar]
- 5.Bardan R, Dumache R, Dema A, Cumpanas A, Bucuras V. The role of prostatic inflammation biomarkers in the diagnosis of prostate diseases. Clinical biochemistry. 2014;47(10-11):909–15. doi: 10.1016/j.clinbiochem.2014.02.008. https://doi.org/10.1016/j.clinbiochem.2014.02.008 PMid:24560954. [DOI] [PubMed] [Google Scholar]
- 6.Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia:age-related tissue-remodeling. Experimental gerontology. 2005;40(3):121–8. doi: 10.1016/j.exger.2004.12.008. https://doi.org/10.1016/j.exger.2004.12.008 PMid:15763388. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Hu MB, Bai PD, Zhu WH, Liu SH, Hou JY, Xiong ZQ, Ding Q, Jiang HW. Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. BioMed research international 2015. 2015 doi: 10.1155/2015/249741. https://doi.org/10.1155/2015/249741 PMid:25722971 PMCid:PMC4334627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciarra A, Mariotti G, Salciccia S, Gomez AA, Monti S, Toscano V, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108(3-5):254–60. doi: 10.1016/j.jsbmb.2007.09.013. https://doi.org/10.1016/j.jsbmb.2007.09.013 PMid:17935971. [DOI] [PubMed] [Google Scholar]
- 9.Sfanos KS, De Marzo AM. Prostate cancer and inflammation:the evidence. Histopathology. 2012;60(1):199–215. doi: 10.1111/j.1365-2559.2011.04033.x. https://doi.org/10.1111/j.1365-2559.2011.04033.x PMid:22212087 PMCid:PMC4029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaren ID, Jerde TJ, Bushman W. Role of interleukins, IGF and stem cells in BPH. Differentiation. 2011;82(4-5):237–43. doi: 10.1016/j.diff.2011.06.001. https://doi.org/10.1016/j.diff.2011.06.001 PMid:21⇉72 PMCid:PMC3∡82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid AR, Umbas R, Mochtar CA. Recent role of inflammation in prostate diseases:chemoprevention development opportunity. Acta Med Indones. 2011;43(1):59–65. [PubMed] [Google Scholar]
- 12.Bergamini S, Bellei E, Bonetti LR, Monari E, Cuoghi A, Borelli F, Sighinolfi MC, Bianchi G, Ozben T, Tomasi A. Inflammation:an important parameter in the search of prostate cancer biomarkers. Proteome science. 2014;12(1):32. doi: 10.1186/1477-5956-12-32. https://doi.org/10.1186/1477-5956-12-32 PMid:24944525 PMCid:PMC4061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thapa D, Ghosh R. Chronic inflammatory mediators enhance prostate cancer development and progression. Biochemical pharmacology. 2015;94(2):53–62. doi: 10.1016/j.bcp.2014.12.023. https://doi.org/10.1016/j.bcp.2014.12.023 PMid:25593038. [DOI] [PubMed] [Google Scholar]
- 14.Chughtai B, Lee R, Te A, Kaplan S. Inflammation and benign prostatic hyperplasia:clinical implications. Current urology reports. 2011;12(4):274–7. doi: 10.1007/s11934-011-0191-3. https://doi.org/10.1007/s11934-011-0191-3 PMid:21519898. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari A. Elocalcitol, a vitamin D3 analog for the potential treatment of benign prostatic hyperplasia, overactive bladder and male infertility. IDrugs: the investigational drugs journal. 2009;12(6):381–93. [PubMed] [Google Scholar]
- 16.Adorini L, Penna G, Fibbi B, Maggi M. Vitamin D receptor agonists target static, dynamic, and inflammatory components of benign prostatic hyperplasia. Annals of the New York Academy of Sciences. 2010;1193(1):146–52. doi: 10.1111/j.1749-6632.2009.05299.x. https://doi.org/10.1111/j.1749-6632.2009.05299.x PMid:20398021. [DOI] [PubMed] [Google Scholar]
- 17.Manchanda PK, Kibler AJ, Zhang M, Ravi J, Bid HK. Vitamin D receptor as a therapeutic target for benign prostatic hyperplasia. Indian J Urol. 2012;28(4):377–81. doi: 10.4103/0970-1591.105745. https://doi.org/10.4103/0970-1591.105745 PMid:23450267 PMCid:PMC3579114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porcaro AB, Novella G, Molinari A, Terrin A, Minja A, De Marco V, Martignoni G, Brunelli M, Cerruto MA, Curti P, Cavalleri S. Prostate volume index and chronic inflammation of the prostate type IV with respect to the risk of prostate cancer. Urologia internationalis. 2015;94(3):270–85. doi: 10.1159/000362176. https://doi.org/10.1159/000362176 PMid:25170543. [DOI] [PubMed] [Google Scholar]
- 19.Kashyap M, Pore S, Wang Z, Gingrich J, Yoshimura N, Tyagi P. Inflammasomes are important mediators of prostatic inflammation associated with BPH. Journal of inflammation. 2015;12(1):37. doi: 10.1186/s12950-015-0082-3. https://doi.org/10.1186/s12950-015-0082-3 PMid:25991911 PMCid:PMC4436794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt PF, Seklehne S, Brustrmann H, et al. Immmuno Histochemical Espression of Interleukin-2 Receptor and Interleukin -6 in Patients with Prostate Cancer and Benigh Prostatic Hiperplasia:Association with asymptomatic Inflamatory Prostatitis NIH Category IV. Scan J Urol. 2014;49(2):120–126. doi: 10.3109/21681805.2014.971427. https://doi.org/10.3109/21681805.2014.971427 PMid:25363611. [DOI] [PubMed] [Google Scholar]
- 21.Basanta D, Strand DW, Lukner RB, et al. The Role of Transforming Growth Factor-βMediated Tumor-Stroma Interactons in Prostate Cancer Progression:An Integrative Approach. Cancer Res. 2009;69(17):7111–7120. doi: 10.1158/0008-5472.CAN-08-3957. https://doi.org/10.1158/0008-5472.CAN-08-3957 PMid:19706777 PMCid:PMC2748342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funahashi Y, O'Malley KJ, Kawamorita N, et al. Upregulation of Androgen-Responsive Gen and Transforming Growth Factor β1 Cascade Genes in a Rat Model of Non-Bacterial Prostatic Inflamation. Prostate. 2014;74(4):337–345. doi: 10.1002/pros.22668. https://doi.org/10.1002/pros.22668 PMid:24446128 PMCid:PMC3898594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucia MS, Lambret JR. Growth Factor in Benign Prostatic Hyperplasia:Basic Science Implications. Curren Prostate Reports. 2007;5:78–84. doi: 10.1007/s11934-008-0048-6. https://doi.org/10.1007/s11918-007-0011-x. [DOI] [PubMed] [Google Scholar]
- 24.Cao Z, Kyprianou N. Mechanism Navigating theTGF-βPathway in Prostat Cancer. Asian Journal of Urology. 2015;2:11–1. doi: 10.1016/j.ajur.2015.04.011. https://doi.org/10.1016/j.ajur.2015.04.011 PMid:29051866 PMCid:PMC5645057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kattan MW, Shariat SF, Andrews B, et al. The addition of Interleukin 6 Soluble Receptor and Transformng Growth Factor Beta, improves a Preoperative Nomogram for Predicting Biochemical Progression in Patients with Clinically Located Prostate Cancer. Journal of Clinical Oncology. 2003;21(9):3573–3579. doi: 10.1200/JCO.2003.12.037. https://doi.org/10.1200/JCO.2003.12.037 PMid:12913106. [DOI] [PubMed] [Google Scholar]
- 26.Smith PC, Hobisch A, Lin DL, et al. Interleukin 6 and Prostate Cancer Progression. Cytokine Growth Factor. Reviews. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. https://doi.org/10.1016/S1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 27.Pace G, Di Massimo C, De Amicis D, Vicentini C, Ciancarelli MGT. Inflammation and endothelial activation in benign prostatic hyperplasia and prostate cancer. Int Braz J Urol. 2011;37(5):617–22. doi: 10.1590/s1677-55382011000500008. https://doi.org/10.1590/S1677-55382011000500008 PMid:22099274. [DOI] [PubMed] [Google Scholar]
- 28.Cung Z, Steiner H Bartsch, et al. Interleukin-6 Regulation of Prostate Cancer Cell Growth. Journal of Cellular Biochemistry. 2005;95:497–505. doi: 10.1002/jcb.20477. https://doi.org/10.1002/jcb.20477 PMid:15838876. [DOI] [PubMed] [Google Scholar]
- 29.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Current opinion in urology. 2013;23(1):5–10. doi: 10.1097/MOU.0b013e32835abd4a. https://doi.org/10.1097/MOU.0b013e32835abd4a PMid:23159991. [DOI] [PubMed] [Google Scholar]
- 30.Steiner MS, Zhou ZZ, Tonb DC, et al. Expression of Transforming Growth Factor β1 in Prostat Cancer Endocrinology. 1994;135(5):2240–2246. doi: 10.1210/endo.135.5.7956947. https://doi.org/10.1210/endo.135.5.7956947 PMid:7956947. [DOI] [PubMed] [Google Scholar]
- 31.Flavel RA, Sanjabi S, Wrzesinski SH, et al. The Polarization of Immune Cells in the Tumor Environment by TGF-β. Nature Reviews Immunology. 2010;10:554–567. doi: 10.1038/nri2808. https://doi.org/10.1038/nri2808 PMid:20616810 PMCid:PMC3885992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuhirman . Disertasi: Fakultas Kedokteran Universitas Andalas Padang; 2014. Hubungan Transforming Growth Factor β1, Tumor Necrosis Factor α, Matrix Metallo Proteinase-1 dan Fibroblast Growth Factor 2 dengan Fibrosis Ureter pada penderita batu ureter; pp. 133–139. [Google Scholar]
- 33.Duscharla D, Reddy KRK, Dasari C, et al. Interleukin-6 Induced Over expression of valosin-Containing Protein (VCP) / p97 is Associated with Androgen-Independent Prostate Cancer (AIPC) Progession Wiley Cellular Physiology. 2018:1–17. doi: 10.1002/jcp.26639. https://doi.org/10.1002/jcp.26639 PMid:29693262. [DOI] [PubMed] [Google Scholar]
- 34.Illiopoulos D, Hirsch H.A, Struhl K. An epigenetic switch involving NF-kB, Lin28, let-7 microRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. https://doi.org/10.1016/j.cell.2009.10.014 PMid:19878981 PMCid:PMC2783826. [DOI] [PMC free article] [PubMed] [Google Scholar]


