Abstract
AIM:
The study aimed to investigate the association between advanced maternal age (AMA) and the risk of adverse maternal, perinatal and neonatal outcomes about parity in singleton pregnancies.
METHODS:
We retrospectively analysed 950 women who gave birth in the Department of Obstetrics and Perinatology of the University Hospital in Kraków for six months (between 1st January and 30th June 2018). The patients were divided into 3 groups according to their age (30-34 years old, 35-39 years old and over 40 years old). Each of these groups was subsequently subdivided into 2 groups depending on parity (primiparae and multiparae). Maternal, perinatal and neonatal outcomes were compared between the groups and the subgroups.
RESULTS:
Comparison of the three age groups revealed that advanced maternal age might constitute a predisposing factor for preterm birth, caesarean section and large for gestational age (LGA). From these parameters, statistical significance was reached in case of greater risk of LGA (OR = 2.17), caesarean section (OR = 2.03) and elective C-section (OR = 1.84) in women over 40 years old when compared to the patients aged 30-34. Furthermore, AMA increases the risk of postpartum haemorrhage (OR = 6.43). Additionally, there is a negative correlation between maternal age and gestational age at delivery (R = -0.106, p < 0.05).
CONCLUSIONS:
Advanced maternal age can undoubtedly be associated with several adverse perinatal outcomes. At the same time, the risk of perinatal complications begins to increase after the age of 35 but becomes significant in women aged ≥ 40.
Keywords: advanced maternal age, pregnancy outcomes, perinatology, pregnancy complications
Introduction
Delayed childbearing has become increasingly common in the past decades. Recent years have seen significant growth in mean maternal age at first childbirth as well as in number of pregnancies at advanced maternal age (AMA). In Poland, the percentage of live births to women aged 35 and over increased almost twice - from 9.1% in 2005 to 16.3% in 2016. At the same time, the rates of deliveries among patients over 40 rose by nearly 50% - from 1.8% in 2005 to 2.6% in 2016 [1]. Similar trends have been observed worldwide, in both high- and low-income countries [1], [2], [3], [4].
Various reasons for delayed childbearing can be identified [5]. Among them, the most significant one seems to be a progress in assisted reproductive technologies (ART) (e.g. in vitro fertilisation, oocyte donation), which enables patients in their 40s, 50s or even 60s to become pregnant [6], [7].
Furthermore, recent enormous changes in work and society have been reflected in women’s desire to develop their careers, obtain financial security and built a stable relationship with their partner before becoming mothers. Higher educational level among females led to a better knowledge and awareness of different types of contraception and, together with greater access to birth control methods, constitutes another factor responsible for an increase in maternal age [8], [9].
Historically, advanced maternal age was defined as age ≥ 35 years. However, in many contemporary studies, the cut-off for AMA has been changed to the age of 40 [5], [10], [11]. Access to ARTs and tendency towards postponing childbearing led to the creation of new definitions - very advanced maternal age (VAMA) and extremely advanced maternal age (EAMA) describing women delivering at age 45-49 and ≥ 50, respectively [12].
Delayed childbearing is believed to be associated with an increased rate of obstetrical and perinatal complications as well as adverse pregnancy outcomes. When compared to younger patients, women in advanced maternal age are reported to be at greater risk of congenital disorders, placenta previa, ectopic pregnancy, spontaneous abortion, stillbirth, preterm birth, induction of labour, caesarean delivery and small for gestational age (SGA). Also, the prevalence of chronic medical conditions (e.g. diabetes mellitus, hypertension) and other diseases with a possible influence on a course of pregnancy (such as cancer) are higher among older patients [5]. Multiple studies suggest that the incidence rate of perinatal complications only begins to increase after the age of 35, but the most significant growth can be observed after the age of 40 [9], [11].
Therefore, the objective of the study was to investigate the association between advanced maternal age (35-39 and ≥ 40 separately) and the risk of adverse maternal, perinatal and neonatal outcomes about parity in singleton pregnancies.
Methods
The retrospective study enrolled 950 women at age ≥ 30 years in singleton pregnancy who gave birth in the Department of Obstetrics and Perinatology of the University Hospital in Kraków during six months (between 1st January 2018 and 30th June 2018). The patients were divided into 3 main groups according to their age: 30-34 years (group 1), 35-39 years (group 2), ≥ 40 years (group 3). Each of these groups was subsequently subdivided into 2 subgroups depending on parity (primiparae and multiparae). The groups and the subgroups were compared about maternal, perinatal and neonatal outcomes.
Data were obtained from the hospital electronic medical records and included demographic features, maternal medical conditions, pregnancy complications, delivery mode (including indications for caesarean section) as well as perinatal and neonatal outcomes. The demographic information consisted of maternal age at delivery, BMI, gravidity and parity, history of caesarean sections and other surgical procedures. Pregnancy complications included pregnancy-induced hypertension (PIH) (defined as a blood pressure >140/90 mmHg measured on 2 separate occasions, > 6 hours apart, without proteinuria, developed after 20 weeks of gestation), preeclampsia (defined as PIH accompanied by proteinuria of >300 mg in a daily urine sample), anaemia (Hb <11 g/dl), hypothyroidism (TSH >2.5 mIU/l), oligohydramnios (defined as AFI ≤ 5 cm), polyhydramnios (defined as AFI >20 cm), preterm rupture of membranes (PROM) (defined as rupture of membranes > 1 hour before the onset of labour) and placenta praevia.
Perinatal and neonatal outcomes included: gestational age at delivery, preterm birth (PTB) (defined as delivery at < 37 weeks of gestation), birth asphyxia, stillbirth, lack of progress of labour, postpartum haemorrhage (PPH) (defined as loss of > 500 ml or > 1000 ml of blood within the first 24 hours after vaginal delivery and caesarean section, respectively), incidence of episiotomy and perineal tears, a 5-minute Apgar score (7-10 was considered normal, 4-6 - intermediate, 0-3 - low), incidence of congenital anomalies, macrosomia (defined as birth weight > 4500 g), small for gestational age (SGA) (defined as a weight <10th percentile for the gestational age), large for gestational age (LGA) (defined as a weight >90th percentile for the gestational age).
All the patients had either a vaginal delivery (VD) (non-induced or induced) or caesarean section (CS) (emergency or elective).
Statistical analysis was performed using STATISTICA 13.1 (StatSoft®, Poland) statistical analysis software. The patients’ characteristics were presented as medians with interquartile ranges (IQR) or means with standard deviations (SD) for continuous variables and numbers of cases and percentages for categorical data. Comparison of qualitative values was assessed with the Chi-squared test and exact Fisher’s test; quantitative variables were compared with the use of the Mann-Whitney U test, p-value < 0.05 was considered statistically significant. Odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Results
Out of 1500 women who delivered at the Department of Obstetrics and Perinatology of the University Hospital in Kraków during the study period, 950 were ≥30 years old and in singleton pregnancy. General characteristics of the patients, perinatal complications and pregnancy outcomes, about parity, are presented in Table 1, 2 and 3.
Table 1.
General characteristics, perinatal complications and pregnancy outcomes among the whole study population (*percentage of all the vaginal deliveries)
| Median (IQR) or n(%) | Maternal age | |||
|---|---|---|---|---|
| 30-34 (n = 594) | 35-39 (n = 298) | ≥40 (n = 58) | ||
| Demographic features | ||||
| Pre-pregnancy BMI | 21.97 (4.53) | 21.61 (4.19) | 24.22 (3.12) | |
| Previous caesarean delivery | 166 (28) | 114 (38.3) | 19 32.8 | |
| Gravidity | ||||
| 1st | 238 (40) | 54 (18.1) | 9 (15.5) | |
| 2nd | 233 (39) | 119 (40) | 14 (24.1) | |
| 3rd | 78 (13) | 77 (25.8) | 20 (34.5) | |
| 4th | 33 (6) | 27 (9.1) | 9 (15.5) | |
| ≥5th | 12 (2) | 21 (7) | 6 (10.4) | |
| Parity | ||||
| 1st | 266 (44.8) | 75 (25.2) | 14 (24.1) | |
| 2nd | 273 (46) | 150 (50.3) | 22 (38) | |
| 3rd | 37 (6.2) | 59 (19.8) | 18 (31) | |
| 4th | 12 (2) | 11 (3.7) | 3 (5.2) | |
| ≥5th | 6 (1) | 3 (1) | 1 (1.7) | |
| Pregnancy complications | ||||
| Pregnancy-induced hypertension (PIH) | 52 (8.8) | 19 (6.4) | 8 (13.8) | |
| Hypothyroidism | 229 (38.6) | 106 (35.6) | 18 (31) | |
| Anaemia | 61 (10.3) | 23 (7.7) | 4 (6.9) | |
| Placenta praevia | 8 (1.3) | 3 (1) | 2 (3.4) | |
| PROM | 24 (4) | 11 (3.7) | 4 (6.9) | |
| Oligohydramnios | 13 (2.2) | 5 (1.7) | 2 (3.4) | |
| Polyhydramnios | 5 (0.8) | 4 (1.3) | 0 (0) | |
| Mode of delivery | ||||
| Caesarean section | 361 (60.8) | 193 (64.8) | 45 (77.6) | |
| Emergency C-section | 113 (19) | 62 (20.8) | 12 (20.7) | |
| Elective C-section | 248 (41.8) | 131 (44) | 33 (56.9) | |
| Non-induced vaginal delivery | 172 (29) | 87 (29.2) | 11 (19) | |
| Induced vaginal delivery | 61 (10.2) | 18 (6) | 2 (3.4) | |
| Perinatal outcome | ||||
| Gestational age at delivery | 39 (1+5) | 38+6 (1+2) | 38+4 (1+3) | |
| Preterm birth | 65 (10.9) | 40 (13.4) | 9 (15.5) | |
| Birth asphyxia | 48 (8.1) | 22 (7.4) | 1 (1.7) | |
| Lack of progress of labour | 27 (4.5) | 4 (1.3) | 1 (1.7) | |
| Postpartum haemorrhage | 5 (0.8) | 1 (0.3) | 3 (5.2) | |
| Episiotomy | 104 (44.6*) | 39 (37.1*) | 3 (23.1*) | |
| Perineal tears | 66 (28.3*) | 23 (21.9*) | 5 (38.5*) | |
| Neonatal outcome | ||||
| Birth weight [g] | 3320 (640) | 3350 (698) | 3275 (510) | |
| Macrosomy | 4 (6.7) | 2 (0.7) | 0 (0) | |
| SGA | 43 (7.2) | 16 (5.4) | 3 (5.2) | |
| LGA | 52 (8.8) | 32 (10.7) | 10 (17.2) | |
| APGAR score: | 0-3 | 2 (0.3) | 1 (0.3) | 0 (0) |
| 4-6 | 4 (6.7) | 1 (0.3) | 2 (3.4) | |
| 7-10 | 588 (99) | 296 (99.3) | 56 (96.6) | |
| Stillbirth | 2 (0.3) | 0 (0) | 0 (0) | |
| Congenital anomalies | 37 (6.2) | 20 (6.7) | 3 (5.2) | |
Table 2.
General characteristics, perinatal complications and pregnancy outcomes among primiparas
| Median (IQR) or n(%) | Maternal age | |||
|---|---|---|---|---|
| 30-34 (n = 266) | 35-39 (n = 75) | ≥40 (n = 14) | ||
| Demographic features | ||||
| Pre-pregnancy BMI | 20.92 (4.30) | 20.90 (1.57) | 21.56 (0) | |
| Previous caesarean delivery | - | - | - | |
| Gravidity | ||||
| 1st | 238 (89.5) | 54 (72) | 9 (64.3) | |
| 2nd | 19 (7.1) | 14 (18.7) | 2 (14.3) | |
| 3rd | 7 (2.6) | 3 (4) | 0 (0) | |
| 4th | 2 (0.8) | 3 (4) | 1 (7.1) | |
| ≥5th | 0 (0) | 1 (1.3) | 2 (14.3) | |
| Parity | ||||
| 1st | 266 (100) | 75 (100) | 14 (100) | |
| 2nd | - | - | - | |
| 3rd | - | - | - | |
| 4th | - | - | - | |
| ≥5th | - | - | - | |
| Pregnancy complications | ||||
| Pregnancy-induced hypertension (PIH) | 24 (9) | 5 (6.7) | 3 (21.4) | |
| Hypothyroidism | 125 (47) | 21 (28) | 3 (21.4) | |
| Anaemia | 23 (8.6) | 6 (8) | 0 (0) | |
| Placenta praevia | 1 (0.4) | 0 (0) | 1 (7.1) | |
| PROM | 12 (4.5) | 4 (5.3) | 0 (0) | |
| Oligohydramnios | 7 (2.6) | 2 (2.7) | 1 (7.1) | |
| Polyhydramnios | 0 (0) | 1 (1.3) | 0 (0) | |
| Mode of delivery | ||||
| Caesarean section | 157 (59) | 50 (66.7) | 13 (92.9) | |
| Emergency C-section | 72 (27) | 22 (29.3) | 4 (28.6) | |
| Elective C-section | 85 (32) | 28 (37.3) | 9 (64.3) | |
| Non-induced vaginal delivery | 75 (28.2) | 18 (24) | 1 (7.1) | |
| Induced vaginal delivery | 34 (12.8) | 7 (9.3) | 0 (0) | |
| Perinatal outcome | ||||
| Gestational age at delivery | 39+2 (1+5) | 39 (1+6) | 38+5 (1+5) | |
| Preterm birth | 29 (10.9) | 9 (12) | 1 (7.1) | |
| Birth asphyxia | 33 (12.4) | 9 (12) | 0 (0) | |
| Lack of progress of labour | 24 (9) | 3 (4) | 1 (7.1) | |
| Postpartum haemorrhage | 3 (1.1) | 0 (0) | 2 (14.3) | |
| Episiotomy | 75 (68.8*) | 18 (72*) | 1 (100*) | |
| Perineal tears | 21 (19.3*) | 2 (8*) | 0 (0*) | |
| Neonatal outcome | ||||
| Birth weight | 3260 (610) | 3330 (600) | 3265 (258) | |
| Macrosomy | 1 (0.4) | 1 (1.3) | 0 (0) | |
| SGA | 19 (7.1) | 3 (4) | 2 (14.3) | |
| LGA | 26 (9.8) | 9 (12) | 1 (7.1) | |
| APGAR score: | 0-3 | 2 (0.8) | 0 (0) | 0 (0) |
| 4-6 | 2 (0.8) | 0 (0) | 0 (0) | |
| 7-10 | 262 (98.5) | 75 (100) | 14 (100) | |
| Stillbirth | 2 (0.8) | 0 (0) | 0 (0) | |
| Congenital anomalies | 19 (7.1) | 5 (6.7) | 2 (14.3) | |
Table 3.
General characteristics, perinatal complications and pregnancy outcomes among multiparas
| Median (IQR) or n(%) | Maternal age | |||
|---|---|---|---|---|
| 30-34 (n = 328) | 35-39 (n = 223) | ≥40 (n = 44) | ||
| Demographic features | ||||
| Pre-pregnancy BMI | 22.68 (4.45) | 22.58 (4.18) | 24.57 (2.62) | |
| Previous caesarean delivery | 166 (50.6) | 114 (51.1) | 19 (43.2) | |
| Gravidity | ||||
| 1st | - | - | - | |
| 2nd | 214 (65.2) | 105 (47.1) | 12 (27.3) | |
| 3rd | 71 (21.6) | 74 (33.2) | 20 (45.4) | |
| 4th | 31 (9.5) | 24 (10.7) | 8 (18.2) | |
| ≥5th | 12 (3.7) | 20 (9) | 4 (9.1) | |
| Parity | ||||
| 1st | - | - | - | |
| 2nd | 273 (83.2) | 150 (67.3) | 22 (50) | |
| 3rd | 37 (11.3) | 59 (26.5) | 18 (40.9) | |
| 4th | 12 (3.7) | 11 (4.9) | 3 (6.8) | |
| ≥5th | 6 (1.8) | 3 (1.3) | 1 (2.3) | |
| Pregnancy complications | ||||
| Pregnancy-induced hypertension (PIH) | 22 (6.7) | 14 (6.3) | 5 (11.4) | |
| Hypothyroidism | 84 (25.6) | 85 (38.1) | 15 (34.1) | |
| Anaemia | 36 (11) | 17 (7.6) | 4 (9.1) | |
| Placenta praevia | 6 (1.8) | 3 (1.3) | 1 (2.3) | |
| PROM | 12 (3.7) | 7 (3.1) | 4 (9.1) | |
| Oligohydramnios | 6 (1.8) | 3 (1.3) | 1 (2.3) | |
| Polyhydramnios | 5 (1.5) | 3 (1.3) | 0 (0) | |
| Mode of delivery | ||||
| Caesarean section | 204 (62.2) | 143 (64.1) | 32 (72.7) | |
| Emergency C-section | 41 (12.5) | 40 (17.9) | 8 (18.2) | |
| Elective C-section | 163 (49.7) | 103 (46.2) | 24 (54.5) | |
| Non-induced vaginal delivery | 97 (29.6) | 69 (31) | 10 (22.7) | |
| Induced vaginal delivery | 27 (8.2) | 11 (4.9) | 2 (4.6) | |
| Perinatal outcome | ||||
| Gestational age at delivery | 39 (1+3) | 38+6 (1+2) | 38+3 (1+2) | |
| Preterm birth | 36 (11) | 31 (13.9) | 8 (18.2) | |
| Birth asphyxia | 13 (4) | 13 (5.8) | 1 (2.3) | |
| Lack of progress of labour | 3 (0.9) | 1 (0.4) | 0 (0) | |
| Postpartum haemorrhage | 1 (0.3) | 1 (0.4) | 1 (2.3) | |
| Episiotomy | 29 (23.4*) | 21 (26.3*) | 2 (16.7*) | |
| Perineal tears | 35 (28.2*) | 21 (26.3*) | 5 (41.7*) | |
| Neonatal outcome | ||||
| Birth weight | 3380 (655) | 3350 (730) | 3360 (608) | |
| Macrosomy | 3 (0.9) | 1 (0.4) | 0 (0) | |
| SGA | 18 (5.5) | 13 (5.8) | 1 (2.3) | |
| LGA | 23 (7) | 23 (10.3) | 9 (20.5) | |
| APGAR score: | 0-3 | 0 (0) | 1 (0.4) | 0 (0) |
| 4-6 | 2 (0.6) | 1 (0.4) | 2 (4.6) | |
| 7-10 | 326 (99.4) | 221 (99.1) | 42 (95.4) | |
| Stillbirth | 0 (0) | 0 (0) | 0 (0) | |
| Congenital anomalies | 14 (4.3) | 15 (6.7) | 1 (2.3) | |
Comparison of the women aged 35-39 and ≥ 40 with the control group (30-34) (Table 4) revealed that advanced maternal age was associated with a lower gestational age at delivery (p = 0.0025, 38+6 vs 39 and p = 0.0003, 38+4 vs 39, respectively), lower incidence of vaginal delivery and elevated rate of caesarean section - both elective and emergency. The results met statistical significance criteria only for the group aged ≥ 40 (p = 0.0238, OR = 2.23 for CS and p = 0.0262, OR = 1.84 for elective CS).
Table 4.
Comparison of all the women aged 35-39 and ≥ 40 with the control group (30-34)
| Outcome | 35-39 | ≥40 | |||
|---|---|---|---|---|---|
| p-value | OR (95% CI) | p-value | OR (95% CI) | ||
| Pregnancy complications | |||||
| Pregnancy-induced hypertension (PIH) | 0.2158 | 0.71 (0.41-1.22) | 0.2055 | 1.67 (0.75-3.71) | |
| Hypothyroidism | 0.3857 | 0.88 (0.66-1.17) | 0.2599 | 0.72 (0.40-1.29) | |
| Anaemia | 0.2185 | 0.73 (0.44-1.20) | 0.5560 | 0.65 (0.23-1.86) | |
| Placenta praevia | 0.9104 | 0.74 (0.19-2.81) | 0.4944 | 2.62 (0.54-12.64) | |
| PROM | 0.8000 | 0.91 (0.44-1.88) | 0.4935 | 1.76 (0.59-5.26) | |
| Oligohydramnios | 0.6091 | 0.76 (0.27-2.15) | 0.8792 | 1.60 (0.35-7.27) | |
| Polyhydramnios | 0.7261 | 1.60 (0.43-6.00) | - | - | |
| Mode of delivery | |||||
| Caesarean section | 0.3356 | 1.19 (0.89-1.59) | 0.0238 | 2.23 (1.18-4.22) | |
| Emergency C-section | 0.5273 | 1.12 (0.79-1.58) | 0.7583 | 1.11 (0.57-2.16) | |
| Elective C-section | 0.5290 | 1.09 (0.82-1.44) | 0.0262 | 1.84 (1.07-3.17) | |
| Non-induced vaginal delivery | 0.9410 | 1.01 (0.74-1.37) | 0.1060 | 0.57 (0.29-1.26) | |
| Induced vaginal delivery | 0.0360 | 0.56 (0.32-0.97) | 0.1483 | 0.31 (0.07-1.30) | |
| Perinatal outcome | |||||
| Gestational age at delivery | 0.0025 | - | 0.0003 | - | |
| Preterm birth | 0.2783 | 1.26 (0.83-1.92) | 0.2949 | 1.49 (0.70-3.17) | |
| Birth asphyxia | 0.7145 | 0.91 (0.54-1.54) | 0.1358 | 0.20 (0.03-1.48) | |
| Lack of progress of labour | 0.0232 | 0.29 (0.10-0.84) | 0.5014 | 0.37 (0.05-2.77) | |
| Postpartum haemorrhage | 0.6613 | 0.40 (0.05-3.44) | 0.0254 | 6.43 (1.50-27.63) | |
| Episiotomy | 0.2344 | 0.73 (0.46-1.17) | 0.1573 | 0.37 (0.10-1.38) | |
| Perineal tears | 0.2420 | 0.71 (0.41-1.22) | 0.5297 | 1.58 (0.50-5.01) | |
| Neonatal outcome | |||||
| Birth weight [g] | 0.9030 | - | 0.9305 | - | |
| Macrosomy | - | - | - | - | |
| SGA | 0.2892 | 0.73 (0.40-1.32) | 0.7505 | 0.70 (0.21-2.33) | |
| LGA | 0.3386 | 1.25 (0.79-1.99) | 0.0355 | 2.17 (1.04-4.54) | |
| APGAR score: | 0-3 | 0.5417 | 1.00 (0.09-11.07) | - | - |
| 4-6 | 0.8713 | 0.50 (0.06-4.49) | 0.1639 | 5.27 (0.94-29.41) | |
| 7-10 | 0.8966 | 1.51 (0.30-7.53) | 0.3246 | 0.29 (0.06-1.47) | |
| Stillbirth | - | - | - | - | |
| Congenital anomalies | 0.7811 | 1.08 (0.62-1.90) | 0.9733 | 0.82 (0.24-2.75) | |
At the same time, AMA seems to reduce the risk of lack of progress of labour (p = 0.0232, OR = 0.29 for the age group 35-39) and, in case of vaginal birth, it increases the probability of no need for episiotomy and lack of perineal tear (p = 0.0167, OR = 1.87 for the age group 35-39). Analysis of the cohorts ≥ 40 and 30-34 revealed that large for gestational age (LGA) occurred statistically more often in advanced maternal age (p = 0.0355, OR = 2.17). Furthermore, delivery among the women aged ≥ 40 was more frequently followed by postpartum haemorrhage than childbirth among younger patients (p = 0.0254, OR = 6.43).
Examination of primiparous women revealed that advanced maternal age in this cohort was associated with a lower gestational age at delivery and a higher incidence of caesarean section (p = 0.0107, OR = 9.03 for CS and p = 0.0187, OR = 3.83 for elective CS among the group aged ≥ 40). In addition, AMA increased risk of postpartum haemorrhage among primiparas (p = 0.0213, OR = 14.61 for the patients ≥ 40).
Considering only multiparas, children of older women were more often large for gestational age (LGA) than those born to younger mothers (p = 0.0029, OR = 3.41 for ≥ 40 cohorts). At the same time, labour was more frequently complicated by postpartum haemorrhage. Advanced maternal age in multiparas was also associated with a lower gestational age at delivery (p = 0.0028, 38+3 vs 39 for the patients ≥ 40). Table 5 and 6 show detailed results of a comparison of the age groups about parity.
Table 5.
Comparison of the women 35-39 and ≥ 40 with the control group among primiparas (**one-tailed Mann-Whitney U test)
| Outcome | 35-39 | ≥40 | |||
|---|---|---|---|---|---|
| p-value | OR (95% CI) | p-value | OR (95% CI) | ||
| Pregnancy complications | |||||
| Pregnancy-induced hypertension (PIH) | 0.5189 | 0.72 (0.26-1.96) | 0.1416 | 2.75 (0.72-10.54) | |
| Hypothyroidism | 0.0033 | 0.44 (0.25-0.77) | 0.0960 | 0.31 (0.08-1.14) | |
| Anaemia | 0.8595 | 0.92 (0.36-2.35) | - | - | |
| Placenta praevia | - | - | 0.0977 | 20.38 (1.21-344.39) | |
| PROM | 0.9906 | 1.19 (0.37-3.80) | - | - | |
| Oligohydramnios | 0.6958 | 1.01 (0.21-4.97) | 0.3401 | 2.85 (0.33-24.91) | |
| Polyhydramnios | - | - | - | - | |
| Mode of delivery | |||||
| Caesarean section | 0.5697 | 1.39 (0.81-2.38) | 0.0107 | 9.03 (1.16-70.05) | |
| Emergency C-section | 0.6982 | 1.12 (0.64-1.97) | 1.0000 | 1.08 (0.33-3.55) | |
| Elective C-section | 0.3821 | 1.27 (0.74-2.17) | 0.0187 | 3.83 (1.25-11.78) | |
| Non-induced vaginal delivery | 0.4712 | 0.80 (0.44-1.45) | 0.1219 | 0.20 (0.03-1.56) | |
| Induced vaginal delivery | 0.4180 | 0.70 (0.30-1.65) | - | - | |
| Perinatal outcome | |||||
| Gestational age at delivery | 0.0700 | - | 0.0498** | - | |
| Preterm birth | 0.7899 | 1.11 (0.50-2.46) | 1.0000 | 0.63 (0.08-5.00) | |
| Birth asphyxia | 0.9248 | 0.96 (0.44-2.11) | - | - | |
| Lack of progress of labour | 0.2378 | 0.42 (0.12-1.44) | 1.0000 | 0.78 (0.10-6.22) | |
| Postpartum haemorrhage | - | - | 0.0213 | 14.61 (2.23-95.79) | |
| Episiotomy | 0.9427 | 1.17 (0.45-3.06) | - | - | |
| Perineal tears | 0.2449 | 0.36 (0.08-1.65) | - | - | |
| Neonatal outcome | |||||
| Birth weight [g] | 0.5890 | - | 0.6750 | - | |
| Macrosomy | 0.9180 | 3.58 (0.22-57.93) | - | - | |
| SGA | 0.4762 | 0.54 (0.16-1.88) | 0.2829 | 2.17 (0.45-10.41) | |
| LGA | 0.5754 | 1.26 (0.56-2.82) | 1.0000 | 0.71 (0.09-5.65) | |
| APGAR score: | 0-3 | - | - | - | - |
| 4-6 | - | - | - | - | |
| 7-10 | - | - | - | - | |
| Stillbirth | - | - | - | - | |
| Congenital anomalies | 0.8869 | 0.93 (0.36-2.58) | 0.2829 | 2.17 (0.45-10.41) | |
Table 6.
Comparison of the women 35-39 and ≥ 40 with the control group among multiparas
| Outcome | 35-39 | ≥40 | |||
|---|---|---|---|---|---|
| p-value | OR (95% CI) | p-value | OR (95% CI) | ||
| Pregnancy complications | |||||
| Pregnancy-induced hypertension (PIH) | 0.8414 | 0.93 (0.47-1.86) | 0.2643 | 1.78 (0.64-4.97) | |
| Hypothyroidism | 0.0018 | 1.79 (1.24-2.58) | 0.2320 | 1.50 (0.77-2.93) | |
| Anaemia | 0.1902 | 0.67 (0.37-1.23) | 0.9046 | 0.81 (0.27-2.40) | |
| Placenta praevia | 0.9223 | 0.73 (0.18-2.95) | 0.6984 | 1.25 (0.15-10.63) | |
| PROM | 0.7431 | 0.85 (0.33-2.19) | 0.2033 | 2.63 (0.81-8.55) | |
| Oligohydramnios | 0.9223 | 0.73 (0.18-2.95) | 0.6984 | 1.25 (0.15-10.63) | |
| Polyhydramnios | 0.8491 | 0.88 (0.21-3.72) | - | - | |
| Mode of delivery | |||||
| Caesarean section | 0.5699 | 1.09 (0.77-1.55) | 0.2862 | 1.62 (0.80-3.26) | |
| Emergency C-section | 0.0769 | 1.53 (0.95-2.46) | 0.2960 | 1.56 (0.68-3.59) | |
| Elective C-section | 0.4188 | 0.87 (0.62-1.22) | 0.5457 | 1.21 (0.64-2.28) | |
| Non-induced vaginal delivery | 0.7311 | 1.07 (0.74-1.55) | 0.3462 | 0.70 (0.33-1.47) | |
| Induced vaginal delivery | 0.1336 | 0.58 (0.28-1.19) | 0.5775 | 0.53 (0.12-2.31) | |
| Perinatal outcome | |||||
| Gestational age at delivery | 0.0880 | - | 0.0028 | - | |
| Preterm birth | 0.3023 | 1.31 (0.78-2.19) | 0.1651 | 1.80 (0.78-4.17) | |
| Birth asphyxia | 0.3160 | 1.50 (0.68-3.30) | 0.8954 | 0.56 (0.07-4.39) | |
| Lack of progress of labour | 0.9033 | 0.49 (0.05-4.74) | - | - | |
| Postpartum haemorrhage | 0.6552 | 1.47 (0.09-23.63) | 0.5630 | 7.60 (0.47-123.74) | |
| Episiotomy | 0.8988 | 1.17 (0.61-2.24) | 0.7337 | 0.66 (0.14-3.19) | |
| Perineal tears | 0.5006 | 0.91 (0.48-1.71) | 0.7591 | 1.25 (0.37-4.17) | |
| Neonatal outcome | |||||
| Birth weight [g] | 0.3420 | - | 0.5050 | - | |
| Macrosomy | 0.9033 | 0.49 (0.05-4.74) | - | - | |
| SGA | 0.8643 | 1.07 (0.51-2.23) | 0.5858 | 0.40 (0.05-3.07) | |
| LGA | 0.1691 | 1.53 (0.84-2.80) | 0.0029 | 3.41 (1.46-7.95) | |
| APGAR score: | 0-3 | - | - | - | - |
| 4-6 | 0.7360 | 0.73 (0.07-8.10) | 0.1099 | 7.76 (1.06-56.55) | |
| 7-10 | 0.9033 | 0.68 (0.10-4.86) | 0.1099 | 0.13 (0.02-0.95) | |
| Stillbirth | - | - | - | - | |
| Congenital anomalies | 0.2047 | 1.62 (0.77-3.43) | 0.8229 | 0.52 (0.07-4.05) | |
Further evaluation of the whole study population, as well as primiparous and multiparous women, separately revealed a correlation between maternal age and gestational age at delivery. Nonetheless, the findings met statistical significance criteria only when analysing all the patients irrespective of parity and in the case of multiparas (Figure 1).
Figure 1.
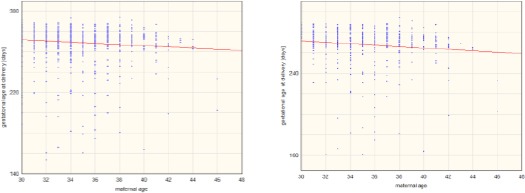
Correlation between maternal age and gestational age at delivery among the whole study population (p<0.05, r= -0.106) (left); Correlation between maternal age and gestational age at delivery among multiparas (p<0.05, r= -0.107) (right)
Discussion
In our study, 77.6% of women over 40 years delivered via caesarean section in comparison to 64.8% women aged 35-39 and 60.8% at age 30-34. Caesarean section rate in the group over 40 was significantly higher than in the control group (women aged 30-34) (p = 0.0238, OR 2.23). Moreover, an elective caesarean section among women aged ≥ 40 was performed almost twice as often as among younger patients (p = 0.0262, OR = 1.84).
Furthermore, when analysing only primiparas, the incidence of cesarean section in the group ≥ 40 was nine times higher than in the control group (p = 0.0107, OR = 9.03). Out of that, elective cesarean section was proposed almost four times more often to the older women (p = 0.0187, OR = 3.38).
Similar to our findings, different studies also report that advanced maternal age predisposes to caesarean delivery. A. Dietl et al. indicated that C-section rate was higher in the study group (> 40 years old) comparing to the control group (< 30 years old) (42.7% vs 24.7%) [6]. Furthermore, according to the study, the percentage of caesarean deliveries was increasing with a growing maternal age at childbirth: 24.7% (< 30 years old), 26.8% (30-34), 34.8% (35-39), up to 42.7% (> 40 years old, p < 0.001). In the group of nulliparous women aged > 40, the percentage of caesarean section was reaching up to 59.1%, out of which 30.7% were the elective ones (p < 0.001) [6]. Similar results have been presented in other studies [1], [13].
Advanced maternal age is known as an independent risk factor for delivery via cesarean section. Several hypotheses for the increased need for cesarean sections among women at advanced maternal age were proposed, including atherosclerotic changes in uterine arteries [14], lower contraction potential and decreased oxytocin receptor level [15] as well as generally longer labour duration [16]. There is a persistent negative relationship between the age of pregnant women and the function of the uterus [17].
Other reasons for such a high proportion of cesarean sections among older patients might be an increased occurrence of medical conditions (both pre-existing and gestational), induction of labour, fetal malposition and maternal request for cesarean section [18].
As mentioned above, the rate of CCs among AMA women was significantly increased, thus the percentage of vaginal delivery was lower (39.2% for 30-34, 35.2% for 35-39 and 22.4% for ≥ 40 in total, both induced and non-induced). We obtained a result of a decreased episiotomy rate and an increased perineal tears rate in the whole group of women aged ≥40. Furthermore, the highest rate of episiotomy was observed among 35-39 primiparous women (72%) and the gap between episiotomy and perineal tear percentages was the biggest for ≥ 40 multiparas (16.7% vs 41.7%). Simultaneously, in the group of primiparas, the perineal tears rate was quite low (8%). Considering general indications for episiotomy, risk factors for perineal tears (such as primiparity or fetal weight) and the outcomes of the group aged 35-39, we suppose that episiotomy was accurately performed as prevention of unintended laceration of peritoneum [19].
In the whole group of women aged ≥ 40 as well as in the cohort of primiparas ≥ 40 years old, the incidence of postpartum haemorrhage (PPH) was nearly 7 and 14 times higher than in the relevant control groups, respectively. The literature confirms these results, listing the age of 40 and obesity as risk factors for postpartum bleeding [20]. Therefore, we strongly believe that appropriate prophylaxis of PPH, as well as strict control of anaemia parameters, should be performed in this group of the patients [24].
Our analysis revealed that the incidence of large for gestational age (LGA) was significantly higher in the whole group of women aged ≥ 40 when compared to the control group (p = 0.0355, OR = 2.17). Similar results were observed among multiparas aged ≥ 40 (p = 0.029, OR = 3.41). Other studies also report the LGA rate to be increased among mothers giving birth at an advanced age [6]. M.S. Schimmel at al. reported the twice more frequent occurrence of LGA among AMA mothers (OR = 1.64 p = 0.0001) and, what is more, the weights of newborns were simultaneously increasing with the growth of maternal age [21]. For primiparas, such a correlation has not been observed, similar to our study. Although LGA may result from maternal diabetes and multiparity, the bigger tendency for LGA among AMA women prevailed after calibrating for the two latter variables in the multivariable analysis.
In our study, the higher maternal age, the lower gestational age at delivery was: control group delivered at 39 (1+5) weeks of gestation, the group aged 35-39 at 38+6 (1+2) weeks of gestation and women aged ≥ 40 at 38+4 (1+3) gestational weeks. Both relationships met statistical significance criteria (p = 0.0025 and p = 0.0003, respectively). Our results confirm the conclusions from other studies [12].
Moreover, we observed a negative correlation between maternal age and gestational age at delivery. The correlation was statistically significant for the whole study population as well as for multiparous women (p = 0.003, r = -0.0949 and p = 0.004, r = -0.1168, respectively).
As for preterm delivery (PTB), several researchers reported that its incidence was dramatically increasing with maternal age (9.4 vs 19.1, p < 0.001) [22]. Nonetheless, our study revealed no statistically significant difference between the preterm birth rate among younger women and AMA patients.
Finally, in general population of pregnant women, the normal ratio of pregnancy-induced hypertension (PIH) ranges from 6-10 % and is one of the main causes of perinatal morbidity and mortality of both women and the newborns [23]. In our study the rates for the women aged 30-39 fitted in that range (8.8% for 30-34 and 6.1% for 35-39 considering whole study population), but in the group aged ≥40 it was much higher – 13.8% for the whole population of the age ≥ 40, 11.4% for the multiparas and even 21.4% for primiparas. Even though these results weren’t statistically significant, they might confirm the immunological hypothesis of PIH hazard decreasing with each subsequent pregnancy with the same partner [Dudenhausen]. Dietl et al. investigated similar outcomes (AMA women more likely evolving PIH). However, the percentages in his study were remarkably lower (2.0% for women aged > 40 and 0.9% for 30-34) comparing to our study [6].
In conclusion, in addition to observing the increased frequency of pregnancy-induced hypertension, lower gestational age at delivery, increased cesarean section rate and higher incidence of LGA were observed among advanced maternal age patients. What is more, we showed that these adverse effects were proceeding with age. Most studies compare only two groups of mothers, above 40 years old and younger. Our research is uncommon since we have divided the advanced maternal age group into two subgroups (≥ 40 and 35-39) and compared them with the control group aged 30-34. Furthermore, each group was further subdivided into primiparas and multiparas for a better evaluation of results. Based on the study, delayed child-bearing seems to be associated with an increased rate of obstetrical and perinatal complications. Care providers need to be aware of these complications and adapt obstetrical supervision for better pregnancy outcomes.
Acknowledgements
The paper was awarded the Best Oral Presentation at the 42. International Medical Congress, May 27-29, 2019, Ohrid, the Republic of Macedonia by the Scientific Foundation SPIROSKI, Skopje, Republic of Macedonia.
Footnotes
Funding: This research was financially supported by the Scientific Foundation SPIROSKI, Skopje, Republic of Macedonia
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Ben-David A, Glasser S, Schiff E, Zahav AS, Boyko V, Lerner-Geva L. Pregnancy and Birth Outcomes Among Primiparae at Very Advanced Maternal Age:At What Price? Maternal and Child Health Journal. 2016;20:833–842. doi: 10.1007/s10995-015-1914-8. https://doi.org/10.1007/s10995-015-1914-8 PMid:26686195. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa K, Urayama KY, Tanigaki S, et al. Association between very advanced maternal age and adverse pregnancy outcomes:A cross sectional Japanese study. BMC Pregnancy and Childbirth. 2017;17 doi: 10.1186/s12884-017-1540-0. https://doi.org/10.1186/s12884-017-1540-0 PMid:29017467 PMCid:PMC5635576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida NKO, Almeida RMVR, Pedreira CE. Adverse perinatal outcomes for advanced maternal age:A cross-sectional study of Brazilian births. Jornal de Pediatria. 2015;91:493–498. doi: 10.1016/j.jped.2014.12.002. https://doi.org/10.1016/j.jped.2014.12.002 PMid:26054772. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Tanbo T, Åbyholm T, Henriksen T. The impact of advanced maternal age and parity on obstetric and perinatal outcomes in singleton gestations. Archives of gynecology and obstetrics. 2011;284(1):31–7. doi: 10.1007/s00404-010-1587-x. https://doi.org/10.1007/s00404-010-1587-x PMid:20632182 PMCid:PMC3112324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JA, Tough S, Wilson RD, Audibert F, Blight C, Brock S JA, Cartier L, Desilets VA, Gagnon A, Langlois S, Murphy-Kaulbeck L. Delayed child-bearing. Journal of obstetrics and gynaecology Canada. 2012;34(1):80–93. doi: 10.1016/S1701-2163(16)35138-6. https://doi.org/10.1016/S1701-2163(16)35138-6. [DOI] [PubMed] [Google Scholar]
- 6.Dietl A, Cupisti S, Beckmann MW, Schwab M, Zollner U. Pregnancy and obstetrical outcomes in women over 40 years of age. Geburtshilfe und Frauenheilkunde. 2015;75(08):827–32. doi: 10.1055/s-0035-1546109. https://doi.org/10.1055/s-0035-1546109 PMid:26366002 PMCid:PMC4554509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciancimino L, Lagana AS, Chiofalo B, Granese R, Grasso R, Triolo O. Would it be too late? A retrospective case-control analysis to evaluate maternal-fetal outcomes in advanced maternal age. Archives of gynecology and obstetrics. 2014;290(6):1109–14. doi: 10.1007/s00404-014-3367-5. https://doi.org/10.1007/s00404-014-3367-5 PMid:25027820. [DOI] [PubMed] [Google Scholar]
- 8.Kalayci H, Ozdemir H, Alkas D, Cok T, Tarim E. Is primiparity a risk factor for advanced maternal age pregnancies? The Journal Of Maternal-fetal & Neonatal Medicine. 2017;30(11):1283–7. doi: 10.1080/14767058.2016.1211633. https://doi.org/10.1080/14767058.2016.1211633 PMid:27406982. [DOI] [PubMed] [Google Scholar]
- 9.Wielgos A, Szymusik I, Bartnik P, Kacperczyk J, Kosinska-Kaczynska K, Pietrzak B. Pregnancy beyond the age of 40-the influence of parity on perinatal outcome. Neuroendocrinology Letters. 2015;36(4):101–7. [PubMed] [Google Scholar]
- 10.Chan BC, Lao TT. Effect of parity and advanced maternal age on obstetric outcome. International Journal of Gynecology & Obstetrics. 2008;102(3):237–41. doi: 10.1016/j.ijgo.2008.05.004. https://doi.org/10.1016/j.ijgo.2008.05.004 PMid:18606410. [DOI] [PubMed] [Google Scholar]
- 11.Kenny LC, Lavender T, McNamee R, O'Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome:evidence from a large contemporary cohort. PloS one. 2013;8(2):e56583. doi: 10.1371/journal.pone.0056583. https://doi.org/10.1371/journal.pone.0056583 PMid:23437176 PMCid:PMC3577849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osmundson SS, Gould JB, Butwick AJ, Yeaton-Massey A, El-Sayed YY. Labor outcome at extremely advanced maternal age. American journal of obstetrics and gynecology. 2016;214(3):362–e1. doi: 10.1016/j.ajog.2015.09.103. https://doi.org/10.1016/j.ajog.2015.09.103 PMid:26454124. [DOI] [PubMed] [Google Scholar]
- 13.Karlström A, Lindgren H, Hildingsson I. Maternal and infant outcome after caesarean section without recorded medical indication:findings from a Swedish case-control study. BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120(4):479–86. doi: 10.1111/1471-0528.12129. https://doi.org/10.1111/1471-0528.12129 PMid:23316937. [DOI] [PubMed] [Google Scholar]
- 14.Crawford BS, Davis J, Harrigill K. Uterine artery atherosclerotic disease:histologic features and clinical correlation. Obstetrics & Gynecology. 1997;90(2):210–5. doi: 10.1016/S0029-7844(97)00225-1. https://doi.org/10.1016/S0029-7844(97)00225-1. [DOI] [PubMed] [Google Scholar]
- 15.Bayrampour H, Heaman M. Advanced maternal age and the risk of cesarean birth:a systematic review. Birth. 2010;37(3):219–26. doi: 10.1111/j.1523-536X.2010.00409.x. https://doi.org/10.1111/j.1523-536X.2010.00409.x PMid:20887538. [DOI] [PubMed] [Google Scholar]
- 16.Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega-Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstetrics & Gynecology. 2004;104(5):943–51. doi: 10.1097/01.AOG.0000142713.53197.91. https://doi.org/10.1097/01.AOG.0000142713.53197.91 PMid:15516383. [DOI] [PubMed] [Google Scholar]
- 17.Main DM, Main EK, Moore II DH. The relationship between maternal age and uterine dysfunction:a continuous effect throughout reproductive life. American journal of obstetrics and gynecology. 2000;182(6):1312–20. doi: 10.1067/mob.2000.106249. https://doi.org/10.1067/mob.2000.106249 PMid:10871444. [DOI] [PubMed] [Google Scholar]
- 18.Callaway LK, Lust K, McIntyre HD. Pregnancy outcomes in women of very advanced maternal age. Australian and New Zealand journal of obstetrics and gynaecology. 2005;45(1):12–6. doi: 10.1111/j.1479-828X.2005.00333.x. https://doi.org/10.1111/j.1479-828X.2005.00333.x PMid:15730358. [DOI] [PubMed] [Google Scholar]
- 19.Corrêa MD, Junior, Passini R., Júnior Selective episiotomy:indications, techinique, and association with severe perineal lacerations. Revista Brasileira de Ginecologia e Obstetrícia. 2016;38(6):301–7. doi: 10.1055/s-0036-1584942. https://doi.org/10.1055/s-0036-1584942 PMid:27399925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weeks A. The prevention and treatment of postpartum haemorrhage:what do we know, and where do we go to next? BJOG: An International Journal of Obstetrics & Gynaecology. 2015;122(2):202–10. doi: 10.1111/1471-0528.13098. https://doi.org/10.1111/1471-0528.13098 PMid:25289730. [DOI] [PubMed] [Google Scholar]
- 21.Schimmel MS, Bromiker R, Hammerman C, Chertman L, Ioscovich A, Granovsky-Grisaru S, Samueloff A, Elstein D. The effects of maternal age and parity on maternal and neonatal outcome. Archives of gynecology and obstetrics. 2015;291(4):793–8. doi: 10.1007/s00404-014-3469-0. https://doi.org/10.1007/s00404-014-3469-0 PMid:25227657. [DOI] [PubMed] [Google Scholar]
- 22.Yaniv SS, Levy A, Wiznitzer A, Holcberg G, Mazor M, Sheiner E. A significant linear association exists between advanced maternal age and adverse perinatal outcome. Archives of gynecology and obstetrics. 2011;283(4):755–9. doi: 10.1007/s00404-010-1459-4. https://doi.org/10.1007/s00404-010-1459-4 PMid:20376672. [DOI] [PubMed] [Google Scholar]
- 23.Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-Induced hypertension. Hormones (Athens) 2015;14:211–223. doi: 10.14310/horm.2002.1582. https://doi.org/10.14310/horm.2002.1582 PMid: 26158653. [DOI] [PubMed] [Google Scholar]
- 24.Prevention and Management of Postpartum Haemorrhage: BJOG: An International. Journal of Obstetrics & Gynaecology. 2017;124(5):e106–e149. doi: 10.1111/1471-0528.14178. https://doi.org/10.1111/1471-0528.14178 PMid:27981719. [DOI] [PubMed] [Google Scholar]


