Abstract
BACKGROUND:
Obesity is considered associated with an increase of resistin levels that plays a role in the regulation of energy and maintaining fasting blood glucose. Polymorphism of resistin is thought to be correlated with the levels of resistin and insulin resistance.
AIM:
This study aimed to examine the association of +299G > A and -420C > G resistin (RETN) gene with resistin level and insulin resistance in obese people of Indonesia.
METHODS:
We examined 142 healthy unrelated subjects consisting of 71 obese and 71 controls. Fasting blood glucose was measured by the enzymatic method while the resistin and insulin levels were measured by Elisa method. Insulin resistance was calculated by HOMA-IR index. Polymorphisms of RETN genes were examined by the Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) method, and the data was tested. The data were correlated with Kruskal Wallis continue logistic regression and simple linear regression
RESULTS:
In the obese group, there was an increased level of insulin (17.74 vs 11.27 mU/L) and insulin resistance (HOMA-IR 3.9 vs 1.46) compared to the control group. Polymorphism of +299G > A was associated with insulin resistance (GA and GA + AA genotype significantly different compare GG genotype with P < 0.001). Resistin level was negatively correlated with insulin level (P = 0.017).
CONCLUSION:
In this study, polymorphism of +299G > A was identified as a risk factor for insulin resistance, and there was a significant association of serum resistin level with insulin level in the population of Indonesia.
Keywords: Blood glucose, Insulin resistance, Obesity, Polymorphism, Resistin gene
Introduction
Obesity is a chronic inflammatory state characterised by elevated pro-inflammatory adipokines [1]. Adipose tissue in obese subjects is characterised by increased macrophages infiltration, which is thought to play a role in the pathogenesis of pro-inflammatory events [2]. Resistin is one of the pro-inflammatory adipokines and has been associated with obesity. The expression of resistin increases with adipose differentiation [3]. Resistin plays an important role in regulating energy, glucose and fat homeostasis by modulating hepatic insulin action with single nucleotide polymorphisms (SNPs) [4].
Two-thirds of plasma resistin variations are influenced by genetics [5]. Resistin gene is on the 19p3.2 chromosome, consists of 4 exons and 3 introns. This gene is polymorphic in SNPs of promoters, introns and 3’UTR. The SNP promoter -420C/G (rs1862513) is associated with obesity [6], insulin sensitivity and resistance, elevated blood sugar levels and lipid profile [7], [8]. However, results from some studies in various populations did not show any association of lipid profiles [5] and obesity [3]. The effect of SNP -420C > G on its expression was estimated due to its effect on the binding of a transcription factor on RETN promoter [8]. Research on the +299 RETN gene polymorphism showed +299G > A polymorphism was significantly associated with elevated plasma resistin [9]. Polymorphism of +299G > A RETN gene in a cohort study showed that A allele is associated with increased risk of Metabolic Syndrome [10], but in a study of Iranian patients with Gestational Diabetes Mellitus, this allele was not significantly different with controls [11].
This study aimed to correlate the polymorphisms of +299G > A (rs3745367) and -420C > G (rs1862513) RETN gene in obese Indonesian population with levels of resistin and insulin resistance.
Methods
This research was a case-control study involving 142 healthy participants 18-40 years old, consisting of 71 subjects with Body Mass Index (BMI) ≥ 25 kg/m2 as the obese group and 71 subjects with BMI 18.50 to 23 Kg/m2 as the control group. Samples were taken with consecutive sampling.
All subjects had normal blood glucose concentrations. Subjects who consumed anti-diabetes and anti-inflammatory drugs were excluded from this research. All subjects agreed to participate and signed an informed consent form. This study was by the Helsinki Declaration and had approval from the Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia, number: KE/FK/569/EC/2015.
Samples
Blood was drawn from subjects after fasting 8 hours, and the plasma was separated from buffy coat. Fasting blood glucose, insulin and resistin levels were measured from plasma. DNA was isolated from buffy coat for determination of RETN polymorphism. Blood glucose was determined by the enzymatic method with the Dyasis kit. Resistin and insulin levels were measured with Elisa kit (Elabscience catalogue number: E-EL-H1213). Determination of insulin resistance was calculated by the following formula:
HOMA-IR = {insulin (µU/mL) X glucose (mmol/L)}/22.5
HOMA-IR > 2.0 was grouped as glucose intolerance [12].
Isolation of DNA was done with Promega kit, and genotyping of resistin genes was examined with the Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) methods.
Genotyping of +299G > A RETN gene
DNA was amplified with forwarding primer 5’GAGAGGATCCAGGAGGTCG 3’ and reverse primer 5’GTGAGACCAAACGGTCCCT 3’. The PCR was done as follows:
Denaturation at 95°C for 5 min, pre-annealing at 59°C 1 min, elongation at 72°C for 2 min, then 35 cycles of 30 s at 95°C, 30 s at 59°C and 1 min 15 s at 72°C, and elongation at 72°C for 10 min. Product of PCR was 373 base pair (bp) then digested by AluI enzymes (MBI-Fermentas, United Kingdom) and separated by electrophoresis with 2% agarose gel and stained with ethidium bromide [13].
Genotyping of -420G > C RETN gene
DNA was amplified with forwarding primer 5’- TGTCATTCTCACCCAGAGACA-3’ and reversed primer 5’-TGGGCTCAGCTAACCAAATC-3’. The PCR method was as follows: denaturation at 95°C for 7 min, pre-annealing at 61°C for 1 min, elongation at 72°C for 2 min followed by 35 cycles of 30 s at 95°C, 30 s at 64°C and for 1 min 15 s at 72 °C and final elongation for 10 min at 72°C. The 534 bp PCR products were digested with BbsI enzyme at 37°C for 16 hours. The digestion products were separated by electrophoresis with 2% agarose gel. The G allele was visualised at 327 bp and C allele at 207 bp [14].
Statistical Analysis
All statistical analyses were performed using SPSS 17 software. Variables that were not normally distributed were presented as mean with Interquartile Range (IQR). The associations of +299 G > A and -420 C > G RETN with resistin level were analysed with the Kruskal Wallis test. The associations of +299G > A and -420C > G RETN with insulin resistance were analysed with logistic regression. Serum resistin level associated with BMI and insulin resistance parameters were analysed with simple linear regression, and the results were considered significantly different when p < 0.05.
Results
Characteristics of subjects
The BMI, insulin level and HOMA-IR in the obese group were higher and significantly different compared with the control group (p < 0.05) (Table 1).
Table 1.
Characteristic of subjects
| Variable | Obese (n = 71) | Control (n = 71) | P |
|---|---|---|---|
| Sex | |||
| Male | 30 (42.3%) | 25 (35.2%) | 0.389 |
| Female | 41 (57.7%) | 46 (64.8%) | |
| Age (Year) | 25.8 ± 5.9 | 25.4 ± 5.7 | 0.545 |
| BMI (kg/m2) | 31.9 ± 4.02 | 20.86 ± 1.66 | < 0.0001 |
| FBG (mg/dL) | 89.10 ± 1.23 | 88.55 ± 1.15 | 0.830 |
| Insulin (mU/L) | 17.74 ± 1.88 | 11.27 ± 1.88 | < 0.0001 |
| HOMA-IR | 3.90 ± 2.04 | 1.46 ± 1.26 | < 0.0001 |
| Resistin (pg/mL) | 310.1 (1.07-1645.89) | 313.35 (1.10-1835.99) | 0.375 |
BMI: Body Mass Index; FBG: Fasting Blood Glucose; HOMA-IR: Homeostatic Model Assessment-Insulin Resistance. Data were presented as the mean ± standard deviation for normally distributed data. Data were presented as median (minimum-maximum) for the data not normally distributed.
The polymorphism of +299G > A RETN gene, frequency of GG, GA and AA genotypes were not significantly different (p = 0.429) and G allele compared to A allele between obese and control groups were not significantly different (p = 0.719). The frequency of CC genotype compared with other types (CG and GG) of -420 C > G RETN gene were not significantly different (p = 0.583) and C allele compared to G allele in obese, and control groups were not significantly different (p = 0.905) (Figure 1).
Figure 1.
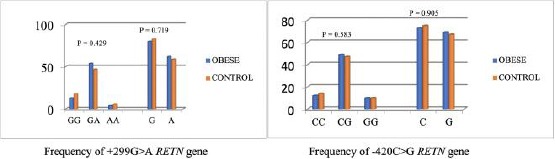
Frequency of +299G > A and -420C > G genotypes and allele RETN gene in obese and control groups
The association of resistin level with +299G > A and -420C > G RETN gene showed there were no significant differences in the obese and control groups (Table 2).
Table 2.
Level of Resistin in +299G>A and -420C>G RETN genotype in obese and control groups
| Resistin level (pg/mL) | P | |||
|---|---|---|---|---|
| +299G > A | GG | GA | AA | |
| Obese (n = 71) | 239.53 (390.47) | 337.52 (555.75) | 522.59 (1079.84) | 0.390 |
| Control (n = 71) | 438.51 (292.55) | 310.10 (562.05) | 165.52 (370.21) | 0.339 |
| -420C > G | CC | CG | GG | |
| Obese (n = 71) | 168.42 (708.32) | 310.10 (2508.71) | 310.45 (224.52) | 0.722 |
| Control (n = 71) | 376.27 (373.33) | 336.62 (498.96) | 82.89 (149.44) | 0.224 |
Data presented as median (IQR) because the data was not normally distributed.
The association between polymorphism of +299G > A and -420C > G RETN gene with insulin resistance showed GA genotypes compared with GG genotype were correlated with insulin resistance (p < 0.001), as well as GA+AA genotype, was correlated with insulin resistance (p < 0.001). Polymorphism of -420C > G RETN gene, however, was not correlated with insulin resistance (p > 0.05) (Table 3).
Table 3.
Association of +299G>A and -420C/G polymorphism of the RETN gene with insulin resistance
| +299 RETN gene (GG compared with GA, AA and GA+AA) | Coefficient | Odds Ratio | 95% CI for OR | P |
|---|---|---|---|---|
| GA | 1.68 | 5.375 | (2.20 - 13.13) | < 0.001 |
| AA | 2.13 | 8.437 | (0.95 - 74.85) | 0.055 |
| GA + AA | 1.72 | 5.566 | (2.31 - 13.44) | < 0.001 |
| -420 RETN gene (CC compared with CG, GG and CG+GG) | ||||
| CG | -0.43 | 0.649 | (0.20, 2.09) | 0.470 |
| GG | -0.86 | 0.424 | (0.10, 1.78) | 0.240 |
| CG + GG | -0.51 | 0.599 | (0.19, 1.89) | 0.383 |
Serum resistin levels were significantly associated with insulin level with simple linear regression and had negative coefficient (p = 0.017), which indicated there were increases of resistin levels that were correlated with decreases of insulin level (Tables 4).
Table 4.
Simple linear regression of the variables with resistin level
| Variable | Coefficient | 95% CI | P | R2 |
|---|---|---|---|---|
| BMI | 2.24 | (-8.84) – (13.32) | 0.721 | 0.001 |
| Blood glucose | 2.05 | (-2.24) – (6.33) | 0.342 | 0.007 |
| INSULIN | -6.33 | (-11.44) – (-1.16) | 0.017 | 0.040 |
| HOMA-IR | -19.13 | (-39.94) - (1.69) | 0.075 | 0.023 |
Discussion
The results of this study show there were significant differences in the levels of insulin and HOMA-IR between obese compared with the controls. The polymorphism of +299G > A RETN gene was correlated with insulin resistance but not for the -420C > G RETN gene. In the association of resistin level to insulin level and insulin resistance, we found serum resistin levels were significantly correlated with insulin level but for HOMA-IR index.
HOMA-IR index in the obese group was higher than the control group like findings in some studies [5,15]. Resistin level between obese and control groups in this study did not differ significantly. This is in line with a study by Savage et al., found no relationship between adiposity and resistin mRNA expression in freshly adipocytes subjects with ranging BMI from 22 – 59 kg/m2 and insulin resistance [16]. Resistin gene in humans is on chromosome 19p13.3 in an area that is not related to the vulnerability of obesity and insulin resistance [17].
One study by Kuminski [18] found expression of mRNA was not directly associated with expression of the protein, which, when modified both in the post-transcriptional and post-translational process can influence resistin levels. The relationship between the expression and secretion of resistin and other inflammatory markers, including IL-6, C- reactive protein (CRP) were reported in patients with severe inflammatory diseases. Mild inflammation due to obesity will interfere with the level of resistin [19]. The differences of this result with others are probably due to differences in obesity grouping [20]. Additionally, differences in the environmental factors may be affecting the HOMA-IR index. Polymorphism of +299G > A was associated with insulin resistance showing GA and GA+AA genotypes were risk factors for insulin resistance compared with GG genotype, but the polymorphism of -420C > G genotype was not a risk factor for insulin resistance.
This finding was different from studies in Korea [21] and Thailand which showed no association between polymorphisms of +299G > A and -420C > G RETN gene with the parameters of the metabolic syndrome [8]. A Lebanon population showed +299G > A polymorphism was not involved in the pathogenesis of obesity and diabetes mellitus [17]. Research in Japan and Egypt indicated polymorphism of -420C > G RETN gene was associated with obesity [22], [23]. The differences in some of these studies may be because there were differences in ethnicity that cause unequal frequency in each population.
Polymorphism of +299G > A and -420C > G RETN gene in this study was not associated with levels of resistin and is in line with research in China [24]. This result is in contrast with other studies showing this polymorphism is associated with increased levels of resistin in some East Asian populations, including Japanese, Korean, and Chinese, although data from the Caucasian population shows that this polymorphism is not associated with higher levels of resistin [1]. RETN polymorphism is correlated to increased BMI, blood pressure, fasting blood glucose, HOMA-IR, triglycerides, total cholesterol, resistin level, and decreased in HDL-cholesterol [5].
The results of research in non-obese type 2 diabetes mellitus patients showed the polymorphism of AA/AG genotypes of +299G > A RETN gene is considered as a risk factor for increased insulin resistance, based on fasting blood glucose and HOMA-IR index [13]. These results demonstrate ethnicity as a factor underlying the differences of SNPs in the influencing of human resistin expression [19]. In this study, polymorphism of +299G > A RETN gene was associated with insulin resistance. This result is similar to research in Iraq which showed that polymorphism RETN +299G > A gene plays a role in insulin resistance [25] and this polymorphism was found to increase the risk of diabetes mellitus in a population in Thailand [8]. Polymorphism of -420C > G RETN gene in this study was not associated with insulin resistance. This result is similar to a study in the Han population of China showing polymorphism of the RETN gene is not a risk factor for diabetes mellitus [26]. A study by Wen [27] in China reported the opposite results, finding this polymorphism increases the risk of diabetes mellitus.
Obesity is a polygenic effect that is influenced by a combination of several genes and environmental factors. Frequency of RETN gene varies with ethnicity and environment. Exposure to different environments such as dietary habits and genetic background other than differences in study design may cause different effects on obesity [5]. These differences probably cause the different results in studies concerning the influence of the polymorphism of the RETN gene in the pathogenesis of obesity along with the small number of samples that might contribute to differences in results. Additional research is needed to identify factors associated with inflammation due to obesity and the correlation of resistin levels with polymorphisms of genes should be further investigated.
In conclusion, polymorphism of +299G > A of RETN gene may be considered as a risk factor for insulin resistance but not the -420C > T of RETN gene. The level of resistin is correlated with the insulin level in Indonesia.
Acknowledgement
We would like to thank the Faculty of Medicine, Public Health and Nursing through Universitas Gadjah Mada which support this research through funding of the Community Fund with number UPPM/241/M/04/05.15, Indonesia.
Footnotes
Funding: This research was financially supported by the Faculty of Medicine, Public Health and Nursing through Universitas Gadjah Mada through the funding of the Community Fund with number UPPM/241/M/04/05.15, Indonesia
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Zhu ZL, Yang QM, Li C, Chen J, Xiang M, Chen MM, et al. Association between the resistin gene -420C>G polymorphism and obesity:an updated meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20(23):4922–4929. [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. https://doi.org/10.1172/JCI200319246 PMid:14679176 PMCid:PMC296995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers S, Zegers D, Van Camp JK, Boudin E, Nielsen TL, Brixen K. Resistin polymorphisms show associations with obesity, but not with bone parameters in men:results from the Odense Androgen Study. Mol Biol Reports. 2013;40(3):2467–2471. doi: 10.1007/s11033-012-2327-z. https://doi.org/10.1007/s11033-012-2327-z PMid:23203410. [DOI] [PubMed] [Google Scholar]
- 4.Osawa H, Yamada K, Onuma H, Murakami A, Ochi M, Kawata H, et al. The G/G genotype of a resistin single-nucleotide polymorphism at-420 increases type 2 diabetes mellitus susceptibility by inducing promoter activity through specific binding of Sp1/3. Am J Hum Genet. 2004;75(4):678–686. doi: 10.1086/424761. https://doi.org/10.1086/424761 PMid:15338456 PMCid:PMC1182055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzaghi C, Coco A, Salvemini L, Thompson R, Cosmo S, De Doria A, et al. Heritability of serum resistin and its genetic correlation with insulin resistance-related features in nondiabetic Caucasians. J Clin Endocrinol Metab. 2006;91(7):2792–5. doi: 10.1210/jc.2005-2715. https://doi.org/10.1210/jc.2005-2715 PMid:16670163. [DOI] [PubMed] [Google Scholar]
- 6.Hivert MF, Manning AK, Mc Ateer JB, Dupuis J, Fox CS, Cupples LA, et al. Association of variants in RETN with plasma levels and diabetes-related traits in the Framingham Offspring Study. Diabetes. 2009;58(3):750–6. doi: 10.2337/db08-1339. https://doi.org/10.2337/db08-1339 PMid:19074981 PMCid:PMC2646076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Chu WS, Hemphill C, Elbein SC. Human resistin gene:molecular scanning and evaluation of association with insulin sensitivity and type 2 diabetes in Caucasians. J Clin Endocr Metab. 2002;87(6):2520–4. doi: 10.1210/jcem.87.6.8528. https://doi.org/10.1210/jcem.87.6.8528 PMid:12050208. [DOI] [PubMed] [Google Scholar]
- 8.Suriyaprom K, Tungtrongchitr R, Namjuntra P. Association of resistin levels with resistin gene polymorphism and metabolic syndrome in Thais. J Med Biochem. 2015;34(2):170–178. doi: 10.2478/jomb-2014-0034. https://doi.org/10.2478/jomb-2014-0034 PMid:28356829 PMCid:PMC4922327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asano H, Izawa H, Nagata K, Nakatochi M, Kobayashi M, Hirashiki A, et al. Plasma resistin concentration determined by common variants in the resistin gene and associated with metabolic traits in an aged Japanese population. Diabetologia. 2010;53(2):234–46. doi: 10.1007/s00125-009-1517-2. https://doi.org/10.1007/s00125-009-1517-2 PMid:19727657. [DOI] [PubMed] [Google Scholar]
- 10.Tsukahara T, Nakashima E, Watarai A, Hamada Y, Naruse K, Kamiya H. Polymorphism in resistin promoter region at-420 determines the serum resistin levels and may be a risk marker of stroke in Japanese type 2 diabetic patients. Diabetes Res Clin Pr. 2009;84(2):179–186. doi: 10.1016/j.diabres.2008.10.021. https://doi.org/10.1016/j.diabres.2008.10.021 PMid:19269054. [DOI] [PubMed] [Google Scholar]
- 11.Takhshid MA, Zare Z. Resistin - 420 C/G polymorphism and serum resistin level in Iranian patients with gestational diabetes mellitus. J Diabetes & Met Disorders. 2015;14:37. doi: 10.1186/s40200-015-0165-y. https://doi.org/10.1186/s40200-015-0165-y PMid:25945322 PMCid:PMC4419403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalil O, Alnahal A, Ghonium M, Fawzy S, Ibrahem M, Raafat N. Does resistin gene polymorphisms +299 (G>A) participate in insulin resistance in Egyptian non-obese Type 2 Diabetes? Int J Genomic Med. 2014;2(1):1–7. [Google Scholar]
- 13.Wongwananuruk T, Rattanachaiyanont M, Leerasiri P, Indhavivadhana S, Techatraisak K, Angsuwathana S. The usefulness of homeostatic measurement assessment -insulin resistance (HOMA-IR) for detection of glucose intolerance in Thai women of reproductive age with polycystic ovary syndrome. Int J Endocrinol 2012. 2012 doi: 10.1155/2012/571035. 571035. https://doi.org/10.1155/2012/571035 PMid:22737168 PMCid:PMC3378956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunnari A, Ukkola O, Kesäniemi YA. Resistin polymorphisms are associated with cerebrovascular disease in Finnish Type 2 diabetic patients. Diabet Med. 2005;22(5):583–9. doi: 10.1111/j.1464-5491.2005.01480.x. https://doi.org/10.1111/j.1464-5491.2005.01480.x PMid:15842513. [DOI] [PubMed] [Google Scholar]
- 15.Lim SM, Choi DP, Rhee Y, Kim HC. Association between obesity indices and insulin resistance among healthy Korean adolescents:The JS High School Study. PloS One. 2015;10(5):e0125238. doi: 10.1371/journal.pone.0125238. https://doi.org/10.1371/journal.pone.0125238 PMid:25970186 PMCid:PMC4429969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage D, Sewter C, Klenk E, Segal D, Vidal-Puig A, Considine R, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor gamma action in humans. Diabetes. 2001;50(10):2199–2202. doi: 10.2337/diabetes.50.10.2199. https://doi.org/10.2337/diabetes.50.10.2199 PMid:11574398. [DOI] [PubMed] [Google Scholar]
- 17.Fakhoury R. The study of the association of the resistin gene RETN polymorphism with obesity and T2DM in Lebanese diabetic subjects. J Mol Biomark Diagn. 2013;4:3. [Google Scholar]
- 18.Kusminski CM, Ternan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond) 2005 Sep;109(3):243–56. doi: 10.1042/CS20050078. https://doi.org/10.1042/CS20050078 PMid:16104844. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Yang Z. Resistin's, obesity and insulin resistance:the continuing disconnect between rodents and humans. J Endocrinol Invest. 2016;39(6):607–615. doi: 10.1007/s40618-015-0408-2. https://doi.org/10.1007/s40618-015-0408-2 PMid:26662574. [DOI] [PubMed] [Google Scholar]
- 20.Piestrzeniewicz K, Łuczak K, Komorowski J, Maciejewski M, Wika JJ, Goch JH. Resistin increases with obesity and atherosclerotic risk factors in patients with myocardial infarction. Metabolism Clin and experiment. 2008;57:488–493. doi: 10.1016/j.metabol.2007.11.009. https://doi.org/10.1016/j.metabol.2007.11.009 PMid:18328349. [DOI] [PubMed] [Google Scholar]
- 21.Cho YM, Youn BS, Chung SS, Kim KW, Lee HK, Yu KY. Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia. 2004 Mar;47(3):559–65. doi: 10.1007/s00125-003-1319-x. https://doi.org/10.1007/s00125-003-1319-x PMid:14740159. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto Y, Morisaki H, Kokubo Y, Yamanaka I, Tomoike H, Okayama A, Yoshimasa Y, Morisaki T. Resistin gene variations are associated with the metabolic syndrome in Japanese men. Obes Res Clin Pract. 2009;3(2):65–74. doi: 10.1016/j.orcp.2008.11.003. https://doi.org/10.1016/j.orcp.2008.11.003 PMid:24345560. [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed Moustafa JS, Froguel P. From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol. 2013;9(7):402–13. doi: 10.1038/nrendo.2013.57. https://doi.org/10.1038/nrendo.2013.57 PMid:23529041. [DOI] [PubMed] [Google Scholar]
- 24.Chen BH, Song Y, Ding EL, Manson JE, Roberts CK, Rifai N. Association of resistin promoter polymorphisms with plasma resistin levels and type 2 diabetes in women and men. Int J Mol Epidemiol Genet. 2010;1(3):167–174. [PMC free article] [PubMed] [Google Scholar]
- 25.Al-hilali HA, Abduljaleel AK. The role of TNF and Resistin Gene +299 (G/A) polymorphism in the development of insulin resistance in non-obese Type 2 Diabetes Mellitus Iraqi patients. Int J Curr Microbiol App Sci. 2015;4(10):475–486. [Google Scholar]
- 26.Jiang B, Liu Y, Liu Y, Fang F, Wang X, Li B. Association of four insulin resistance genes with type 2 diabetes mellitus and hypertension in the Chinese Han population. Mol Biol Rep. 2014;41(2):925–33. doi: 10.1007/s11033-013-2937-0. https://doi.org/10.1007/s11033-013-2937-0 PMid:24414038 PMCid:PMC3929032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen Y, Lu P, Dai L. Association between resistin gene -420 C / G polymorphism and the risk of type 2 diabetes mellitus:a meta-analysis. Acta Diabetol. 2013;50(2):267–72. doi: 10.1007/s00592-010-0247-8. https://doi.org/10.1007/s00592-010-0247-8 PMid:21190046. [DOI] [PubMed] [Google Scholar]


