Abstract
BACKGROUND:
People co-evolved with members of the microbiota and developed, used and adapted many complex immune mechanisms, which are used for monitoring and control of the microbiota. The gut microbiota in cooperation with humans became its essential part, so-called “hidden organ” with many important and indispensable functions. Quantitative and/or qualitative deficiency of the gut microbiota (dysbiosis) probably is a basis of many disorders, including obesity.
AIM:
To present an overview of the possible association between gut microbiota and obesity.
METHODS:
Meta-analysis of available scientific and published data including PubMed, Web of Science, Scopus and Cochrane Library.
RESULTS:
In the intestinal microbiota at obese people is detected a specific increase in the proportion between class Firmicutes and Bacteroidetes despite the non-obese people. Also, it is detected a decrease in this proportion if the person lost weight. These facts may be secondary to obesity. The colonisation of germ-free mice with microbiota from ordinarily feed or obese mice, without changes in the feed style leads to increase body fat to more than 50%.
CONCLUSION:
The human gut microbiota directly affects the food digestion, absorption and metabolism. The gut microbiota of obese people has a higher capacity for receiving energy from the food than the microbiota at slim people. The gut microbiota affects appetite control and energy balance. Lifestyle and food regimen affect the diversity of the gut microbiota and the presence of dysbiosis.
Keywords: gut, microbiota, association, obesity
Introduction
Malnutrition is a wide term, which embraces obesity and undernutrition and is featuring with an imbalance between energy input and output. In 2014 in the World, more than 600 million were obese [1]. Obesity is a condition with excess body fat and increased body weight. There are some disorders, which are associated with obesity; such are atherosclerosis, diabetes, non-alcoholic fatty liver disease and some types of cancer, which are leading causes of death in the USA [2].
Some studies on GFM (germ-free mice) indicate that GFM are resistant to high-calorie foods and fattening besides feeding with high-calorie foods [3]. Colonisation the GFM with microbiota derived from “obese donor” results with an increase of the full body fat and body weight, backwards if GFM is colonised with microbiota derived from “slim donor”, they won’t get fat beside feeding with high-calorie foods [4]. GFM, which received faecal transplantation from “obese donor”, will increase their body weight [5]. All of these studies impose an opinion that the human gut microbiota may have a role in the development of obesity and obesity-associated disorders.
Results
Composition and taxonomic distribution of gut microbiota
Human Microbiome Project included 554 individuals, selected by many criteria. The average age of them was 26, and the average Body Mass Index (BMI) was 24 kg/m2. Samples were collected from 18 regions of the body and gut sample just from faeces [6]. Compositions of the microbiota were determined by 16S rRNA genetic sequencing performing metagenomics profiles of all sequenced genomes. All knowledge about gut microbiota was published in 2012 in two studies [7], [8]. The conclusion was that the healthy human gut microbiota consists of 70-100 known bacterial species [7]. However, 16S rRNA gene sequencing techniques guessed that the number of bacteria in the human gut microbiota might exceed 1000 species because of consisting some very rare, thin and non-cultivable or non-classified categories of bacteria [9].
Oesophagus, stomach and small intestine
There are no data for permanent microbiota in the proximal part of the oesophagus. Studies have detected that in proximal oesophagus, the diversity of microbes is low. Studies for the distal part of the oesophagus say that there is reduced diversity and a dominant species of it is Streptococcus. Also, it is known that the reduced diversity of microbes in the oesophagus is good to sustain the wellbeing of the oesophagus [9]. The low pH of stomach limits many types of microbes, which get there. Attendance or absence of Helicobacter pylori affects the composition of the stomach microbiota. When H. pylori are there, it is a dominant species. Otherwise, Streptococcus is dominant [10]. H. pylori can be commensal or pathogen species of the stomach [4]. Besides this, stomach microbiota consists of other species as Prevotella, Veillonella and Rothia.
It is very hard to derive data about the microbiota in the small intestine because of insufficiency of healthy volunteers. Based on some routine microbiological examinations and molecular studies, it is known that Streptococcus is a dominant species in duodenum and jejunum. Generally, it is accepted that the diversity and complexity of bacterial communities increases in proximal-distal direction (from the duodenum to ileum).
Large intestine and faeces
The composition of microbiota in the large intestine is very similar to those in the distal part of the small intestine, actually ileum. It is much easier to examine the composition of the large intestine microbiota than other parts of the gut. Five divisions of bacteria are present; such are Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria and Proteobacteria. Divisions Firmicutes and Bacteroidetes are more numerous than the others. They take 90% of the number of all bacteria in the large intestine [9]. Less than 0.1% of human gut microbiota are some pathogens as Campylobacter jejuni, Salmonella enterica and Vibrio cholerae (Figure 1).
Figure 1.
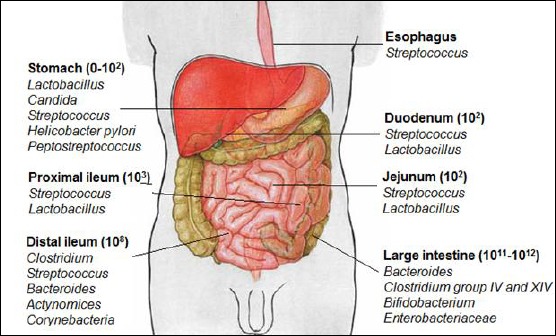
Composition and number of major gut microbial species
Factors that influence the human gut microbiota
The composition and count of gut microbiota are dynamic and variable, even under the subtle influences of some environmental factors [11]. Some of these factors are:
Genetics - Studies have revealed that host genetics has a small but statistically significant effect on the microbiota’s composition and count. Studies on mice with variation in the MHC (Major Histocompatibility Complex) genes have shown that the host’s genetics influences the microbiota. These mice had a higher susceptibility to autoimmune disease because of influences of the gut microbiota.
Age - There are some acknowledgements that the humans are exposed to microbes even during the intrauterine period, and there were identified bacterial DNA in healthy placentas, amniotic liquid and meconium of term newborns. The delivery mode (vaginal or cesarean section), the feeding style (breast milk or baby milk) are the main factors which determine the microbiota at newborns. During the first three years of life, the microbiota of children is getting more numerous and more complex.
Diet - Food, which people eat, obtain nutrients for the host, but also the gut microbiota. This fact is remarkable at children who are weaning from baby milk and are starting to eat some different food, which is getting a low number of Bifidobacterium species in their intestines. Domination of gut bacteria which are metabolising vegetative polysaccharides (Roseburia, Eubacterium rectangle and Ruminococcus bromii) is marked in veggies. People who eat meat have bile-tolerant microbes; such are Alistipess, Bilophila and Bacteroides.
Drugs - As it is well known, all the medications affect the gut microbiota. The most studied affections are connected with the use of antibiotics. For example, during the treatment with Ciprofloxacin, which does not influence anaerobic bacteria, reduction of 1/3 of anaerobic gut bacteria may be detected. This effect is a result of the elimination of some basic bacterial species, which are necessary for the survival of the others.
Figure 2.

Main factors affecting the gut microbiota composition highlighting the great influence of diet and lifestyle on the composition
Lifestyle - Many everyday activities can affect the gut microbiota. For example, the microbiota on the skin and in the intestine are similar between family members, especially if they have a pet, which is some vector transferring microbes between family members. Also, travelling in different parts of the World derives variation in the gut microbiota, very similar to those of the domestic people.
Circadian rhythms - Period of the day, ambient of lights and temperature and food availability affects the human physiological processes and the human gut microbiota. Some oscillations in the microbiota in people, who work in shifts or work hard, can because of some metabolic disorders.
Discussion
Dysbiosis
Dysbiosis is a condition, determined by quantitative and/or qualitative (functional) reduction of the composition of microbiota [12]. Dysbiosis has two main characteristics:
- Reduced diversity of microbial community against healthy individuals;
- Some degree of the inflammatory state, despite the primary process of the disease/condition.
Many daily activities, such as feeding style, hygiene habits, physical activity, medications, host genetics, living region, etc., probably cause dysbiosis.
Obesity
Malnutrition encompasses obesity and undernutrition are characterised by an imbalance between energy input and output. In 2014, assessments were about more than 600 million obese people, and more than one billion suffering from undernutrition [1]. The epidemic of obesity has spread in more than 1/3 of the elderly population, and the average costs for obesity treatment complications are 1429 dollars higher than costs for slim people [2]. Data from National Health and Nutrition Examination Surveys point than 64% from elderly Americans (>20 years old) was with BMI (Body Mass Index) higher than 25 between the years 1999-2000 [13].
Obesity is a condition of the excess of body fat and scarcely ever is a condition of higher body mass, because the body mass can be increased at people with higher muscle volume or women who are pregnant. Obesity-associated diseases, like atherosclerosis, diabetes, non-alcoholic liver disease and some kinds of cancer are the leading causes of death in the USA and the World [2]. Also, the distribution of body fat influences morbidity. For example, intra-abdominal and abdominal fatty tissue is more implicated in the initiation of obesity complications than the subcutaneous fatty tissue on the thigh and arm.
Appetite control and energy balance
Body weight is controlled by endocrine and neural mechanisms, which influence the energy input and output. This complex regulatory system is essential because even small balance disorders lead to big bodyweight disturbances. By the weight loss, the appetite is getting up, and consumption of energy decreases, but this mechanism does not work very often.
The appetite is controlled by many factors, which are integrated into the central nervous system (CNS), rather the hypothalamus. Signals, which arrive, to the hypothalamus include afferent neural, hormone and metabolic signals. Neural signals are derived by the Vagus nerve, which is produced by the distension of the digestive system walls. Hormone signals include leptin, insulin, cortisol, ghrelin, peptide YY (PYY), cholecystokinin and some peptides from the digestive system. Also, a lot of physiological and culturally factors play a role in appetite control (Figure 3).
Figure 3.
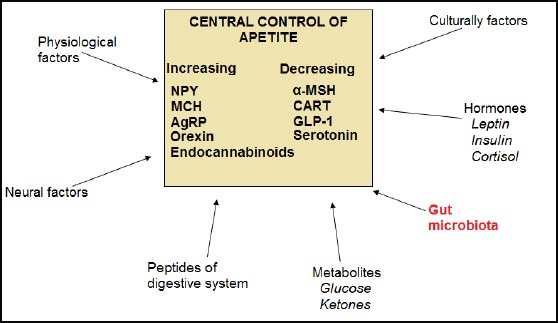
Factors that regulate appetite through effects on CNS. (NPY-neuropeptide Y; MCHmelanin-concentrating hormone; AgRP-agouti-related transcript; MSH-melanocyte stimulating hormone; CART-cocaine and amphetamine-related transcript; GLP-1-glucagon-like peptide; CCK-cholecystokinin; PYY-peptide YY)
Gut microbiota and appetite control
Gut microbiota is included in the regulation of food intake by the influence to the hormones, which regulate the metabolic function and some brain regions responsible for the food intake, also defined as gut-brain axis [1].
The brain constantly comes up to different neural and hormone signals, which monitor the energy level in the body. Most of these signals are evolved in the gut and are part of the gut-brain axis. The axis is affected by the feeding style, genetics, anatomy of the gut and the gut microbiota. The food intake influences the releasement of some hormones and makes sense of fullness and appetite decrease. Distension of the stomach evolves Vagus nerve afferent signal to the brain (rhomb encephalon) and release of hormones from the stomach mucosa, such as CCK, GLP-1, PYY and leptin. All of these neural and hormone factors influence the gut-brain axis affecting the hypothalamic arcuate nucleus (Figure 4).
Figure 4.
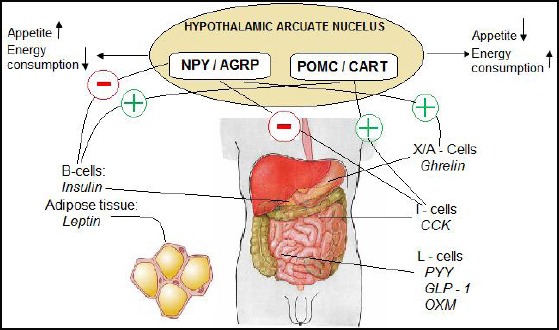
Central hormonal control of appetite and energy expenditure. (NPY-neuropeptide Y; AgRP-agouti-related peptide; POMC-proopiomelanocortin; CART-cocaine and amphetamine-related peptide; CCK-cholecystokinin; GLP-1-glucagon-like peptide; OXM-oxyntomodulin)
The hypothalamus plays the central role in the energy homeostasis by influencing appetite and energy consumption. Two different groups of neurons are responsible for the interpretation of the peripheral signals. Appetite suppressing neurons (anorexigenic), proopiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART) are located in the lateral part of the hypothalamic arcuate nucleus (Fig. 4) which expresses α-melanocyte stimulating hormone. Melanocortin and α-melanocyte stimulation hormone induce a negative energy balance. These peptides are synthesised because of the increase in fatty tissue. In the medial part of the hypothalamic arcuate nucleus are located the orexigenic neurons which express the neuropeptide Y (NPY) and agouti-related protein (AGRP). NPY and AGRP are the main central neurotransmitters, which stimulate appetite and decrease energy consumption.
The gut microbiota composition and dysbiosis influence on the hormone orchestration and indirectly affect the appetite. Breton et al., [20] studied how the intestinal infusion of Escherichia coli proteins in mice affects the increase of plasmatic levels of GLP-1 and PYY. They found that the changes in the gut microbiota (dysbiosis) induced changes in the plasmatic levels of GLP-1 and PYY, which are hormones that affect the hypothalamic arcuate nucleus and the appetite.
Queipo-Ortuño et al., [21] studied the association between some genus from the gut microbiota and the plasmatic levels of ghrelin and leptin. They noticed that there is a significant positive correlation between the number of Bifidobacterium and Lactobacillus and a significant negative correlation between the genus Clostridium, Bacteroides and Prevotella with the plasmatic levels of leptin. Also, they noticed that the plasmatic levels of ghrelin are negatively correlated with the genus Bifidobacterium, Lactobacillus and B. coccoides and positively correlated with Bacteroides and Prevotella (Table 2).
Table 1.
Taxonomic review of the members of gut microbiota into divisions, classes, orders, families and species
| Division | Class | Order | Family | Species |
|---|---|---|---|---|
| Firmicutes | Clostridia | Clostridiaels | Clostridiaceae | Clostridium |
| Ruminococcaceae | Ruminococcus | |||
| Faecalibacterium | ||||
| Eubacteriaceae | Eubacterium | |||
| Bacilli | Lactobacilliales | Lactobacillaceae | Lactobacillus | |
| Streptococcaceae | Streptococcus | |||
| Enterococcus | ||||
| Erysiphelotrichia | Erysiphelotrichales | Erysiphelotrichaceae | Turicibacter | |
| Catenibacterium | ||||
| Coprobacillus | ||||
| Allobaculum | ||||
| Negativicutes | Selemonadales | Selenomonadaceae | Megamonas | |
| Veillonellales | Veillonellaceae | Dialister | ||
| Megasphaera | ||||
| Veillonella | ||||
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella |
| Bacteroidaceae | Bacteroides | |||
| Actinobacteria | Coriobacteria | Coriobacteriales | Coriobacteriaceae | Collinsella |
| Atopobiaceae | Olsenella | |||
| Egerthellales | Eggerthellaceae | Slackia | ||
| Eggerthella | ||||
| Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | |
| Fusobacteria | Fusobacteria | Fusobacteriales | Fusobacteriaceae | Fusobacterium |
| Proteobacteria | Gammaproteo- bacteria | Enterobacteriales | Enterobacteriaceae | Escherichia |
| Shigella | ||||
| Aeromonadales | Succinivibrionaceae | Succinivibrio | ||
| Anaerobiospirillum | ||||
Table 2.
Relations between plasmatic levels of leptin and ghrelin with gut microbiota composition
| ↑Leptin | ↑Lactobacillus and Bifidobacterium |
| ↓Prevotella and Clostridium | |
| ↑Ghrelin | ↑Prevotella and Bacteroides |
| ↓Lactobacillus and Bifidobacterium | |
All these acknowledgements indicate that the differences in feeding style and physical activity influence the gut microbiota composition, affecting its diversity. Generally, the food restriction and increase of physical activity have a potentially negative influence on the number and composition of the intestinal bacteria, which are health promoters.
Generally, there is a trend of eating high-calorie foods, which affect the gut microbiota. In a way, the gut microbiota is described as an environmental factor, which activity results with an increased depot of adipose tissue and obesity [1].
In the past fifteen years, many scientists were exploring the association of the gut microbiota and some diseases, and the possibility for treatment affecting the gut microbiota. Conclusions from these studies resulted in inconsistent findings of the association between gut microbiota and obesity. The association was studied mainly on animal models. GFM (germ-free mice) are resistant to high-calorie food and high food intake, which by science are the main promoters of obesity. Additionally, colonisation of GFM with “obese gut microbiota” increased the fatty tissue and body weight, in contrast to colonisation with “slim gut microbiota” [14].
Contact between microbes and the intestinal epithelial cells determine which signals are good and which are bad. Enhanced pilling up to energy by the “obese gut microbiota” is the most common explanation for obesity. Reduced number of the members of the division Bacteroidetes and proportionally increased number of the members of the division Firmicutes, which are associated with a bigger capacity to supply energy from food, such as degradation and fermentation to complex carbohydrates [15], characterise “obese gut microbiota”.
The “obese gut microbiota” has a bigger capacity to derive energy from the food, and to stimulate gene reprogramming in the large intestine, to change the secretion of polypeptide hormones and other bioactive molecules from enteroendocrine cells and too weak the defence and immunologic homeostasis in the intestine. The gut microbiota also communicates with the adipose tissue, the liver and the brain [16], (Figure 5).
Figure 5.
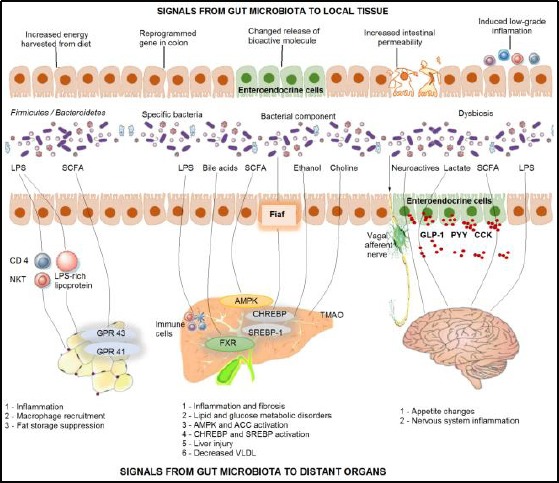
Local and distant effects of gut microbiota in the pathogenesis of obesity [16]. (LPS-lipopolysaccharide; SCFAsshort-chain fatty acids; Fiaf-fasting-induced adiposity factor; NKT-natural killer cell; GPR 43/41-G-protein-related receptor; AMPKAMP-activated protein kinase; CHREBP-carbohydrate related element-binding protein; SREBP-1-sterol regulating element-binding protein; FXR-farnesoid X receptor; TMAO-trimethylamine N-oxide; GLP-1-glucagon-like peptide; PYY-peptide YY; CCK-cholecystokinin)
Axis gut microbiota-adipose tissue: The gut microbiota is included in the regulation of adipose tissue through different mechanisms. LPS (lipopolysaccharide) incites immune response with inflammation and immune cells penetration in the adipose tissue. SCFAs (short-chain fatty acids) are included in insulin-related fat accumulation in the adipose tissue through activation of GPR43 and GPR41, which inhibit the lipolysis and enhance the differentiation of the adipose cells.
Axis gut microbiota-liver: Dysbiotic microbiota induces increased penetration at the intestinal mucosa for some pathogens and parts or metabolites from intestinal bacteria, such as LPS and ethanol. In the liver, LPS induces inflammation through stimulation the immune cells. Some metabolites as the bile acids, SCFAs and trimethylamine-N-oxide (TMAO) play a role in the pathogenesis of the non-alcoholic fatty liver disease (NAFLD).
Axis gut microbiota-brain: Afferent neurons from the digestive system and intestinal hormones are key signal molecules included in the communication between gut and brain and the metabolism of the host. Bioactive molecules which are included in the process are LPS, intestinal peptides, SCFAs, lactates etc. [16], (Figure 5).
Fiaf (Fasting-induced adipose factor) is a circulating inhibitor to the lipoprotein lipase, which is normally suppressed by the gut microbiota in the intestinal epithelium, and play a central role in the metabolism of triglycerides through inhibition the lipoprotein lipase production in the adipose tissue [3]. From this, it can be concluded that GFM are protected from obesity because of increased levels of Fiaf and increased activity of AMPK (AMP-activated protein kinase).
Polypeptide hormones and other bioactive molecules released from enteroendocrine intestinal cells are included in the food intake regulation. Different TLRs (Toll-like receptors) expressed on enteroendocrine cells recognise different PAMPs (Pathogen-associated molecular patterns) and influence the polypeptide hormones release. For example, LPS from Gram-negative bacteria is recognised by TLR4 and induce secretion of CCK (satiety hormone) [16], which decrease the appetite and increases energy consumption [1].
Injured intestinal barrier and penetration of bacteria or bacterial products through the barrier is an important mechanism to the pathogenesis of obesity. Some bacterial products play a role in the regulation of the intestinal barrier, associated with SCFAs. Also, obesity is related to mild chronic inflammation, and some antigens from the gut may be inducers of this activity. Dysbiosis affects the innate and adaptive immunity through some microbe cell components and metabolic signals.
Besides the local influence, the gut microbiota influences distant organs, mainly the adipose tissue. Obesity is characterised by an increase of the volume of adipose tissue, and the gut microbiota enhances the mechanisms of metabolic disorders in the axis gut microbiota-adipose tissue. For example, LPS is identified as an inducing factor to the insulin resistance of the adipose tissue, which is transported to the adipose cells together with other lipoproteins through translocases. LPS-reach lipoproteins are absorbed by the bigger adipose cells, which has high metabolic activity. SCFAs synthesised by the gut microbiota participate in the insulin-mediated accumulation of lipids in the adipose cells, through activation of the SCFA-receptors (GPR43 and GPR41) which inhibits the lipolysis and induces differentiation of adipose cells. Mice with GPR43 deficiency are obese besides the normal feeding, while the mice, which has a high expression of GPR43, are lean, besides feeding with high-calorie foods. GPR43 activation by SCFAs suppresses insulin signals to the adipose cells, which enhances the metabolism of lipids and carbohydrates in the other tissues. Suspect that GPR43 is a sensor for excessively receiving energy from the foods and keep the metabolic homeostasis [18]. A conclusion may be that GPR43 activation causes insulin resistance in the adipose tissue, but increased sensitivity in the muscles and the liver.
The liver is constantly exposed to signals by the digestive system, gut microbes and their particles. Alterations in the gut microbiota composition are firmly connected with risk for disorders in the liver associated with obesity, such as non-alcoholic fatty liver disease (NAFLD) [4]. The NAFLD severity is associated with the degree of dysbiotic gut microbiota, especially with the number of Bacteroidetes related to NAFLD and Ruminococcus related to liver fibrosis [19].
Gut microbiota has a deep influence on the metabolism of bile acids through deconjugation, dehydrogenation and dihydroxylation of primary bile acids. Alteration in the gut microbiota gets changes in the bile-acid pool, which influences the nuclear antagonist of bile-acid X receptor (FXR) included in the regulation of bile acids, as the regulation of lipid and carbohydrate metabolism and may induce metabolic dysfunction, obesity and insulin resistance.
Also, Fiaf is included in the mechanisms which relate the gut microbiota and NAFLD, where the dysbiotic gut microbiota inhibits secretion of Fiaf from the intestinal cells and activates LPL, CHREBP and SREBP-1 and resulted in accumulation of triglycerides in the liver [3]. Ethanol and other bacterial products included in the NAFLD progression may be related to a high amount of alcohol producing bacteria from the Proteobacteria genus.
Gut microbiota has impinged upon distant organs and CNS, which recipes the abundance of neural and hormone signals from the digestive system. Bacteria and their metabolites may affect directly through vagus nerve stimulation or indirectly through immune-neuroendocrine mechanisms. Vagus nerve activation particularly is depended from the secretion of signal molecules as PYY, GLP- 1 and CCK. SCFAs is not just a source of energy, but also plays a role as a chemical transmitter [18].
Conclusion
Gut microbiota directly affects the metabolism of nutrients and vitamins, essential for the host organism. It is noted that the gut microbiota of obese people has a bigger capacity for getting energy from the food than the gut microbiota of lean people and the division Firmicutes is more effective in that. The gut microbiota is included in the control of appetite through hormones, which influence the metabolism and some parts of the brain responsible for the eating behaviour. Feeding style and physical activity affect the gut microbiota composition and its diversity - lower diversity relates to obesity. “Obese gut microbiota” has a lower number of members of the division Bacteroidetes and a high number of member of the division Firmicutes. Diet has maybe the biggest influence on the gut microbes, which is known through observation of the composition of microbiota after permanently taking some food. Undoubtedly, it may be concluded that the gut microbiota has a big influence in the pathogenesis of obesity and other obesity-related disorders.
Acknowledgements
The paper was awarded the Best Poster Presentation at the 2nd Students Congress of General Medicine, May 9-10, 2019, Shtip, the Republic of Macedonia by the Scientific Foundation SPIROSKI, Skopje, Republic of Macedonia.
Footnotes
Funding: This research was financially supported by the Scientific Foundation SPIROSKI, Skopje, Republic of Macedonia
Competing Interests: The authors have declared that no competing interests exist
References
- 1.De Clercq NC, Groen AK, Romijn JA, Nieuwdorp M. Gut Microbiota in Obesity and Undernutrition. The Advances in Nutrition journal. 2016;15(7(6)):1080–1089. doi: 10.3945/an.116.012914. https://doi.org/10.3945/an.116.012914 PMid:28140325 PMCid:PMC5105041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parekh PJ, Arusi E, Vinik AI, Johnson DA. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. The Frontiers in endocrinology journal. 2014;5:47. doi: 10.3389/fendo.2014.00047. https://doi.org/10.3389/fendo.2014.00047 PMid: 24778627 PMCid:PMC3984999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):979–84. doi: 10.1073/pnas.0605374104. https://doi.org/10.1073/pnas.0605374104 PMid:17210919 PMCid:PMC1764762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. https://doi.org/10.1038/natur.e05414 PMid:17183312. [DOI] [PubMed] [Google Scholar]
- 5.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semekovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. https://doi.org/10.1126/science.1241214 PMid:24009397 PMCid:PMC3829625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aagard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, Patel S, Cutting M, Madden T, Hamilton H, Harris E. The Human Microbiome project strategy for comprehensive sampling of the human microbiome and why it matters. The FASEB Journal. 2013;27(3):1012–22. doi: 10.1096/fj.12-220806. https://doi.org/10.1096/fj.12-220806 PMid:23165986 PMCid:PMC3574278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttenhower C et collaborators. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. https://doi.org/10.1038/natur.e11234 PMid:22699609 PMCid:PMC3564958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Methe BA et collaborators. A framework for human microbiome research. Nature. 2012;486(7402):215–21. doi: 10.1038/nature11209. https://doi.org/10.1038/natur.e11209 PMid:22699610 PMCid:PMC3377744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. The Gastroenterology journal. 2014;146(6):1449–58. doi: 10.1053/j.gastro.2014.01.052. https://doi.org/10.1053/j.gastro.2014.01.052 PMid:24486050 PMCid:PMC4181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyr?n P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. The PloS One Journal. 2008;3(7):e2836. doi: 10.1371/journal.pone.0002836. https://doi.org/10.1371/journal.pone.0002836 PMid:18665274 PMCid:PMC2475661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. The Human Microibiome. 20th edition. McGraw-Hill Education; 2018. Harrison's principles of Internal medicine; pp. 3379–3390. [Google Scholar]
- 12.Passos MDCF, Moraes-Filho JP. Intestinal microbiota in digestive diseases. The Arquivos de gastroenterologia. 2017;54(3):255–262. doi: 10.1590/S0004-2803.201700000-31. https://doi.org/10.1590/s0004-2803.201700000-31 PMid:28723981. [DOI] [PubMed] [Google Scholar]
- 13.Fauci AS, Braunwald E, Kasper DL, Stephen LH, Longo DL, Jameson JL, Loscalzo J. Harrison's principles of internal medicine 17th edition:Biology of obesity. McGrawHill Medical. 2008:462–468. [Google Scholar]
- 14.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. https://doi.org/10.1038/nature07540 PMid:19043404 PMCid:PMC2677729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology:human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. doi: 10.1038/4441022a. https://doi.org/10.1038/4441022a PMid:17183309. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. Insights into the role of gut microbiota in obesity:pathogenesis, mechanisms and therapeutic perspectives. The Proetin & Cell journal. 2018;9(5):397–403. doi: 10.1007/s13238-018-0546-3. https://doi.org/10.1007/s13238-018-0546-3 PMid:29725936 PMCid:PMC5960470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via short-chain fatty acid receptor GPR43. The Nature communications journal. 2013;4:1829. doi: 10.1038/ncomms2852. https://doi.org/10.1038/ncomms2852 PMid:23652017 PMCid:PMC3674247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nohr MK, Pedersen MH, Gille A, Egerol KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. The Endocrinology journal. 2013;154(10):3552–64. doi: 10.1210/en.2013-1142. https://doi.org/10.1210/en.2013-1142 PMid:23885020. [DOI] [PubMed] [Google Scholar]
- 19.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Huanault G, Oberti F, Cal?s P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. The Hepatology journal. 2016;63(3):764–75. doi: 10.1002/hep.28356. https://doi.org/10.1002/hep.28356 PMid:26600078 PMCid:PMC4975935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemont J, Goichon A, Gu?rin C, Peltier J, Pestel-Caron M, Chan P, Vaudry D, do Rego JC, Li?nard F, P?nicaud L, Fioarmonti X, Ebenezer IS, H?kfelt T, D?chelotte P, Fetissov SO. Gut Commensal E.coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. The Cell Metabolism journal. 2016;23(2):324–34. doi: 10.1016/j.cmet.2015.10.017. https://doi.org/10.1016/j.cmet.2015.10.017 PMid:26621107. [DOI] [PubMed] [Google Scholar]
- 21.Queipo-Ortun˜o MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. The PloS one journal. 2013;8(5):e65465. doi: 10.1371/journal.pone.0065465. https://doi.org/10.1371/journal.pone.0065465 PMid:23724144 PMCid:PMC3665787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaser MJ. Hypothesis:the changing relationship of Helicobacter pylori and humans:implications for health and disease. The journal of infectious diseases. 1999;179(6):1523–30. doi: 10.1086/314785. https://doi.org/10.1086/314785 PMid:10228075. [DOI] [PubMed] [Google Scholar]
- 23.Cho I, Yamanishi S, Cox L, Meth? BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. doi: 10.1038/nature11400. https://doi.org/10.1038/natur.e11400 PMid:22914093 PMCid:PMC3553221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neish AS. Microbes in gastrointestinal health and disease. The Gastroenterology journal. 2009;136(1):65–80. doi: 10.1053/j.gastro.2008.10.080. https://doi.org/10.1053/j.gastro.2008.10.080 PMid:19026645 PMCid:PMC2892787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vulevic J, Juris A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of nutrition. 2013;143(3):324–31. doi: 10.3945/jn.112.166132. https://doi.org/10.3945/jn.112.166132 PMid:23303873. [DOI] [PubMed] [Google Scholar]


