Abstract
BACKGROUND:
The first two years of life constitute a critical period of rapid change. The events during this phase prepare the child for subsequent developmental competency.
AIM:
To determine the potential risk factors that affect an infant’s cognitive development in the first two years of life in a sample of Egyptian infants
SUBJECTS AND METHODS:
A cross-sectional comparative study included 655 male and female infants. Their age ranged from 3 – 24 months. Bayley Scales of Infant and Toddler Development (Bayley III) were used for cognitive assessment. Perinatal and nutritional data were recorded. Levels of serum Zinc, Copper, Iron, vitamin B12 and complete blood count (CBC) were assessed in a subsample of 193 infants.
RESULTS:
Infants having below the average cognitive composite score (CCS) represented 38.47% of the whole sample. The risk of having a low average (CCS) was determined by multiple factors. Poor maternal education and low family income were the most significant social risk factors (OR = 2.19, p = 0.0003; OR = 1.64, p = 0.002 respectively). Prematurity and complicated labor represented significant perinatal risks (OR = 1.22, p = 0.005; OR = 2.39, p =0.001respectively). Bottle feeding versus breastfeeding in the first six months of life was the most significant nutritional predictor of low average (CCS) (OR = 1.79, p = 0.001). Infants with low average (CCS) had significantly lower levels of serum zinc and vitamin B12 than those with average scores.
CONCLUSION:
Multiple factors appear to interact affecting the early cognitive development of Egyptian infants. Prematurity, complicated labour, poor maternal education, low family income and micronutrient deficiency are the main risk factors. Studying these factors is of great value in directing governmental intervention efforts.
Keywords: Cognitive development, Infants, Maternal education, Micronutrient deficiency, (Bayley-III) scales
Introduction
The first 2 years of life is a critical period of rapid growth and brain development. During this period, nutrition and environmental factors play important roles in growth and cognitive development of children [1]. Early cognitive development is related to the development of memory and social skills, language acquisition, logical reasoning, planning, and problem-solving [2]. Child cognitive development is influenced by genetic and environmental factors which interact in complex ways to determine how the brain develops and functions [3]. The child has a genetically determined potential for cognitive development. However environmental factors, such as prenatal and postnatal maternal and infant wellbeing [4], [5], nutritional factors as adequate breastfeeding and complementary feeding [6], socioeconomic conditions and the parents’ ability to create a good and stimulating home environment may also have a positive influence on the child’s cognitive development [7]. Factors such as malnutrition, micronutrient deficiency, poverty-related health problems, home environment, parenting practices, and living in poor neighbourhoods with high levels of crime and unemployment are all factors that may impact brain development in children and therefore influence the possibility of education [8]. The effects of under-nutrition may begin before the child is born [9]. Undernourished pregnant women are more likely to give birth to underweight babies who are generally more at risk. Disruption of normal development can result in dysregulation of neural systems during vulnerable periods of brain development, leading to pronounced neurocognitive deficits, delays in the development of IQ, language, social-emotional functioning, poor academic achievement and poor productivity in adulthood [10]. Neurocognitive assessment is crucial for early detection of developmental disorders, especially in the first years of life, subsequently enabling optimising the design of intervention strategies that respond to individual needs [11]. In developing countries, diminished data represents a big challenge for choosing the most appropriate cost-effective intervention procedure. Research in the area of early child development is highly needed to detect modifiable risk factors and direct intervention efforts.
The objective of this study is to inspect potential risk factors that affect an infant’s cognitive development in the first two years of life in a sample of Egyptian infants.
Material and Methods
Study design: A cross-sectional comparative study of Egyptian infants in the first two years of life. They were classified according to their performance on the cognitive domain of Bayley Scales of Infant and Toddler Development (Bayley-III) into two categories: infants having below the average composite score and infants having average and above average score. Potential risk factors that might impair the cognitive performance of these infants were studied.
Inclusion criteria
Infants were enrolled if they were over 1 month and not more than 24 months of age, belonging to the middle socioeconomic class and their caregivers consented to participate in the study.
Exclusion criteria
Infants were excluded if they demonstrated any obvious congenital anomalies, features of genetic diseases, or had a history of any metabolic or physical problems which may affect their cognitive development.
Study setting
Infants were recruited from Developmental and Behavioral paediatrics Clinic at the National Research Centre, and Pediatric Nutrition Clinic of Ain Shams University in the period from September 2016 to September 2018.
Sample Size Calculations
We are planning a study of independent cases and controls with 2 control(s) per case. Prior data indicate that the probability of exposure among controls is 0.26. If the true odds ratio for disease in exposed subjects relative to unexposed subjects is 1.8, we will need to study 190 patients and 380 controls to be able to reject the null hypothesis that this odds ratio equals 1 with probability (power) 0.85. The Type I error probability associated with this test of this null hypothesis is 0.05. We will use a continuity-corrected chi-squared statistic or Fisher’s exact test to evaluate this null hypothesis.
Two hundred fifty-two infants with below average cognitive composite score were recruited as cases and 403 infants with average and above average cognitive composite score as controls [12].
Methods
Socio-demographic assessment: Special questionnaire was designed for this study, which included questions about maternal age, maternal and paternal education and occupation, marital status, family income and order of childbirth [13].
Assessment of maternal and prenatal history: including parity, history of chronic diseases as hypertension, diabetes or hypothyroidism, diseases acquired during pregnancy as gestational diabetes or preeclampsia, gestational age of the infant, mode of delivery, postnatal problems as cyanosis, jaundice or convulsions and whether the infant was admitted to NICU or not.
Thorough physical examination and anthropometric measurements of weight and height: All measurements were made according to techniques described in the Anthropometric Standardization Reference Manual [14]. Weight-for-age z scores (WAZ), height-for-age z-scores (HAZ) and Body mass index- for age z-score for all children were calculated based on the WHO growth standards [15] with the help of Anthro-Program of PC.
Infant Feeding Practices in the first six months of life: was assessed to identify infants who were predominately breastfed, artificially-fed (who were consuming other milk including infant formula, fresh, tinned, and powdered milk from cows or other animals or mixed fed (artificial plus breast milk). The time of introduction of complementary feeding was recorded whether before or after the sixth month of age.
Cognitive ability assessment: Using the Bayley Scales of Infant and Toddler Development (Bayley-III). These scales were developed by Nancy Bayley (16) to assess the development of infants and toddlers between the age range of 1 month to 42 months. Bayley-III consists of 5 subscales, i.e. Cognitive Scale, Language Scale (Receptive Communication and Expressive Communication), Motor Scale (Fine Motor and Gross Motor), Social-Emotional Scale and Adaptive Behavior Scale. In this study, only the cognitive domain is being measured. The Cognitive Scale includes items that assess sensorimotor development, exploration and manipulation, object relatedness, concept formation, memory, and other aspects of cognitive processing. The test is administered according to the infant’s age-specific start point. Each correct response is given a score of 1, and the total raw score is then converted into its composite score.
Biochemical assessment: Serum Fe, Zn and Cu concentrations were measured using an atomic absorption flame emission spectrophotometer [17], [18]. Serum vitamin B12 was assessed using the Bayer Centaur chemiluminescence method. Complete blood count was performed by an automated cell counter. Haemoglobin concentration < 11 g/dl, was used as a cutoff point for the diagnosis of anaemia [19]. It was considered that iron deficiency existed when serum Iron < 45 ug/dL [20]. Other nutritional deficiencies, were assessed according to the following cutoff values; vitamin B12 < 203 pg/mL, (21) Zinc < 65 ug/dL [22] and Copper < 63.7 ug/dL [23].
Ethical Considerations
The study complies with the International Ethical Guidelines for Biomedical Research Involving Human Subjects [24]. The Research and Ethical Committee of NRC cleared the study protocol. The number of ethical approvals was 11020.
Informed Consent
It was obtained from the parents enrolled in the study Confidentiality: Mothers and children were identified by a serial number, and the information at the individual level was kept strictly confidential.
Results
In this study, 655 male and female infants were recruited. Forty-four per cent were males. Their age ranged from 3 to 24 months, with a mean of 14.7 ± 6 months. Anthropometric measurements revealed that the majority of infants were of normal weight for their age (91%), normal height (88%) and normal weight for height (91%)(Table 1). The mean cognitive composite score was 80.32 ± 12.48. The infants were classified according to their cognitive composite scores into 2 categories: Below average group whose score was less than 85, and average and above average group whose score was 85 or above (Table 1).
Table 1.
Characteristics of the studied sample
| Variable | Number (%) N = 655 |
|---|---|
| Sex | |
| Male | 290 (44.3%) |
| Female | 365 (55.7%) |
| Mean age in months | 14.7 ± 6.0 |
| Infants < 6 months | 231 (35.3%) |
| Infants 6 - < 12 months | 191 (29.1%) |
| Infants 12- 24 months | 233 (35.6%) |
| WAZ | |
| (mean ± SD) | -0.2048 ± 1.19 |
| Normal | 596 (91.0%) |
| Underweight | 46 (7.0%) |
| Overweight | 13 (2.1%) |
| HAZ | |
| (mean ± SD) | -0.4985 ± 1.49 |
| Normal | 578(88.2%) |
| Stunted | 77 (11.7%) |
| WHZ | |
| (mean ± SD) | 0.09 ± 1.17 |
| Normal | 597 (91.1%) |
| Wasted | 24 (3.7%) |
| Overweight& obese | 34 (5.2%) |
| Cognitive composite score | |
| (mean ± SD) | 80.32 ± 12.482 |
| Average& above average | 403 (61.52%) |
| Below average | 252 (38.47%) |
WAZ: weight for age z-score; HAZ: Height for age z-score; WHZ: Weight for height Z-score.
It was found that the risk of having below average cognitive composite score was significantly associated with the father’s income. In infants belonging to lower-middle-income families, the risk was 1.64 times higher than infants belonging to upper-middle-income families (OR = 1.64), and the P value was < 0.01). Being a low educated mother carried a highly significant risk (P < 0.001) of having a below average infant 2.01 times more than a highly educated mother (OR = 2.01). Other social variables as maternal age, maternal occupation and child order of birth looked to not influence cognitive development of this sample (Table 2).
Table 2.
Social risk Factors for below average cognitive composite score
| Infants having below average score (n = 252) | Infants having average &above average score (n = 403) | OR (95%CI) | P-value | |
|---|---|---|---|---|
| Child Order | ||||
| (≥ 3) n = 443 | 172 (68.3%) | 271 (67.2%) | 1.05 | 0.78 |
| (< 3) n = 212 | 80 (31.7%) | 132 (32.8%) | (0.75-1.49) | |
| Mother’s age | ||||
| (≤ 25 years) n = 241 | 96 (38.1%) | 145 (36.0%) | 1.09 | 0.58 |
| (> 25 years) n = 414 | 156 (61.9%) | 258 (64.0%) | (0.78-1.54) | |
| Father’sincome | ||||
| (lowermiddle) *n = 312 | 139 (55.2%) | 173 (42.9%) | 1.64 | 0.002 |
| (Upper Middle) **n = 343 | 113 (44.8%) | 230 (57.1%) | (1.18-2.27) | |
| Mother’s education | ||||
| (illiterate or read and write) n = 161 | 84 (33.3%) | 77 (19.1%) | 2.19 | 0.0003 |
| (High education) ***n = 494 | 168 (66.7%) | 326 (80.9%) | (1.38-3.48) | |
| Mother’s occupation | ||||
| (Housewife) n = 503 | 196 (77.7%) | 307 (76.2%) | 1.09 | 0.63 |
| (Working mother) = 152 | 56 (23.9%) | 96 (18.8%) | (0.74-1.62) |
Low- Middle Income: Father is Unemployed, Day by day worker, Farmer & Laborer;
Upper-Middle- Income: Father is Employee, Professional & Employer or dealer;
High Education= High School and University.
As regards perinatal medical circumstances presented in Table 3, preterm infants were more at risk of having below average cognitive composite score OR = 1.22, p-value = 0.005. Infants born by cesarean section were 1.26 times at risk of having below average cognitive composite score than vaginally born infant although this data didn’t reach statistically significant ratio p = 0.15. Complicated labour had a highly significant effect on cognitive composite score outcome as infants who experienced delivery problems were twice at risk of having below average cognitive composite score OR = 2.39, p-value 0.0001. As regards chronic maternal illness before and during pregnancy didn’t appear to have a significant effect on the infant cognitive composite score outcome in the current study.
Table 3.
Association of perinatal medical factors with below average cognitive composite score
| Infants having below average score (n = 252) | Infants having average &above average score (n = 403) | OR (95%CI) | P-value | |
|---|---|---|---|---|
| History of maternal chronic disease before pregnancy | ||||
| Yes (n = 124) | 45 (17.9%) | 79 (19.6%) | 0.89 (0.58-1.36) | 0.57 |
| No (n = 531) | 207 (82.1%) | 324 (80.4%) | ||
| Maternal diseases acquired during pregnancy | ||||
| Yes (n = 89) | 35 (13.9%) | 54 (13.4%) | 1.04 (0.64-1.69) | 0.858 |
| No (n = 566) | 217 (86.1%) | 349 (86.6%) | ||
| Gestational age | ||||
| Preterm < 37 weeks (n = 49) | 28 (11.1%) | 21 (5.2%) | 1.22 (0.65-2.28) | 0.005 |
| Full-term ≥ 37 weeks (n = 606) | 224 (88.9%) | 382 (94.8%) | ||
| Type of labor | ||||
| CS (n = 372) | 152 (60.3%) | 220 (54.6%) | 1.26 (0.91-1.76) | 0.15 |
| Normal (n = 283) | 100 (39.7%) | 183 (45.4%) | ||
| Complicated labor | ||||
| Yes (n = 86) | 49 (19.4%) | 37 (9.2%) | 2.39 (1.47-3.88) | 0.001 |
| No (n = 569) | 203 (80.6%) | 366 (90.8%) | ||
As shown in Table 4, The risk of having below average cognitive composite score in bottle-fed infants was near twice times higher than breastfed infants (p < 0.001, OR = 1.79). At the same occasion, bottle-fed infants had the probability of getting below average cognitive composite score 1.70 times greater than mixed fed infants with a significant P-value < 0.05.
Table 4.
Infant feeding practices as risk factors for below average cognitive composite score
| Feeding practices | Infants having below average score (n = 252) | Infants having average &above average score (n = 403) | Or (95% ci) | P |
|---|---|---|---|---|
| Type of feeding | ||||
| Bottle feeding vs Breastfeeding | ||||
| Bottle fed (240) | 113 (44.8%) | 127 (31.5%) | 1.79 (1.25-2.56) | 0.001** |
| Breast fed (322) | 107 (42.5%) | 215 (53.3%) | ||
| Bottle feeding vs Mixed feeding | ||||
| Bottle fed (240) | 113 (44.8%) | 127 (31.5%) | 1.7 (1.00-2.00) | 0.03* |
| Mixed fed (93) | 32 (12.7%) | 61 (15.1%) | ||
| Mixed feeding vs Breastfeeding | ||||
| Mixed fed (93) | 32 (12.7%) | 61 (15.1%) | 1.05 (0.63-1.76) | 0.833 |
| Breast fed (322) | 107 (42.5%) | 215 (53.3%) | ||
| Time of introduction of complementary food | ||||
| Before the age of six months (332) | 132 (52.1%) | 200 (49.6%) | 1.12 (0.78-1.62) | 0.53 |
| After the age of six months (323) | 120 (47.6%) | 203 (50.3%) | ||
Time of introduction of complementary food could not be considered as a predictor of the cognitive composite score. Starting CF before six months seemed to carry a risk of getting below the average cognitive composite score (OR = 1.12). However, the P value was not significant (P > 0.05).
A subsample of 193 infants was investigated for some micronutrient’s serum levels (Iron, copper, zinc and vitamin B12) and anaemia is owing to their association with cognitive development. It was found that none of the examined infants has subnormal levels of serum iron or serum copper, while 17 infants had subnormal zinc level, 9 had subnormal vitamin B12 level, and 89 infants were anaemic.
As shown in Table 5, there are significantly lower levels of serum zinc and serum vitamin B12 in infants with below average cognitive composite score if compared with their peers with average and above average scores.
Table 5.
Studied biochemical parameters as risk factors for below average cognitive composite score
| Biochemical parameter | N1 | Mean ± SD in infants having below average score | Mean ± SD in infants having average & above average score | Cutoff values indicating a deficiency | T | P |
|---|---|---|---|---|---|---|
| Cu (µg/dl) | 193 | 116.7 ± 41.2 | 130.0 ± 44.6 | < 63.7 µg/dl | 1.969 | 0.056 |
| Zn (µg/dl) | 193 | 83.5 ± 31.8 | 101.7 ± 47.9 | < 65 µg/dl | 4.396 | 0.015* |
| Vitamin B12 (pg/ml) | 193 | 981.6 ± 422.0 | 1109.4 ± 433.3 | < 203 pg/ml | 3.392 | 0.028* |
| Fe (µg/dl) | 193 | 163.0 ± 47.4 | 157.4 ± 54.2 | < 45 µg/dl | 0.701 | 0.484 |
| Hemoglobin (gm/dl) | 193 | 10.6 ± 1.2 | 10.8 ± 1.4 | < 11 gm/dl | 1.123 | 0.766 |
Biochemical parameters were done for a subsample of 193 children;
significant at p < 0.05.
Figures 1a, 1b, and 1c show that as being anaemic; having subnormal serum level of serum zinc, or subnormal serum level of vitamin B12 appeared to carry a non-significant risk for cognitive development in the studied sample (P was > 0.05 for each factor). It has been found that 33.7% of infants who were anaemic had a below average cognitive composite score on Bayley III scales (OR = 1.262), P > 0.05); 47.1% of infants having subnormal serum level of zinc had a below average cognitive composite score (OR = 2.24 (0.74-6.79), P > 0.05). Also, about 44.4% of infants having subnormal serum level of vitamin B12 had a below average cognitive composite score (OR = 1.91), P > 0.05).
Figure 1.
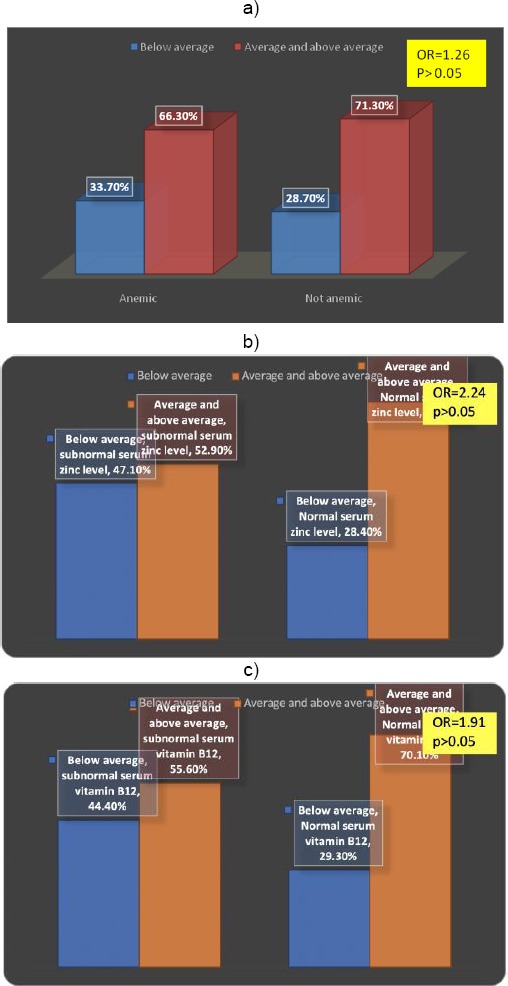
a) Anemia as a risk factor for below average cognitive composite score; b) Subnormal serum zinc as a risk factor for below average cognitive composite score; c) Subnormal serum vitamin B12 as a risk factor for below the average cognitive composite score
In this study, logistic regression analysis (Table 6) revealed the multifactorial interaction between infant feeding variables, socio-economic variables, maternal and perinatal health variables in predicting the cognitive developmental outcome. Complicated labour, prematurity and low educated mothers were highly significant predictors of below average cognitive development. Mode of feeding in the 1st six months was an important predictor for infant cognitive development. Father’s occupation, as the main source of household income, appeared as an influential variable for cognitive development.
Table 6.
Logistic Regression of Factors Affecting cognitive composite score
| B | S.E. | Wald | df | Sig. | AOR | 95%CI for AOR | |
|---|---|---|---|---|---|---|---|
| Feeding in the first six months of life | |||||||
| Breastfeeding | ® | ||||||
| Mixed | 1.293 | 0.553 | 5.464 | 2 | 0.019 | 0.275 | 0.098-0.873 |
| Bottle | 0.628 | 0.237 | 6.991 | 0.008 | 1.88 | 1.27-2.91 | |
| Gestational age | |||||||
| Full term | ® | ||||||
| Preterm | 0.636 | 0.237 | 7.230 | 1 | 0.007 | 0.529 | 0.33-0.84 |
| Mother education | |||||||
| High | ® | ||||||
| Illiterate, R&W | 0.782 | 0.270 | 8.415 | 1 | 0.004 | 0.458 | 0.27-0.78 |
| Complicated labour | |||||||
| No | ® | ||||||
| Yes | 0.721 | 0.256 | 7.623 | 1 | 0.003 | 0.489 | 0.75-2.31 |
| Father income | |||||||
| Upper middle | ® | ||||||
| Lower middle | 0.943 | 0.643 | 3.531 | 1 | 0.03 | 0.834 | 1.23-3.71 |
| Constant | 5.268 | 1.168 | 20.332 | 1 | 0.000 | 94.065 | |
Discussion
Breastfeeding initiation and duration are the points of concern for many researchers due to its relation and influence on early growth and development, especially cognitive and brain development. Breastfeeding could benefit development through nutrients in breast milk, especially essential fatty acids, reduced infant morbidity, or closer mother-child relations as it was found that there are improvements in motor development with longer duration of exclusive breastfeeding and that early introduction of supplementary bottle feeding was associated with poorer motor and cognitive function [25].
The first years of life constitute a critical period of rapid personal change, and the events of this phase prepare the child for subsequent developmental competency [26] In the current study, infants were classified according to the cognitive composite score cutoff point [85] into 2 groups (Below average group and average and above average group). The risk of having below average cognitive composite score was significantly associated with low father’s income and low maternal education. Other social variables as maternal age, maternal occupation and child order of birth had no association with a cognitive composite score of these infants; this is in agreement with Andrade & Shaffer et al., who found that family income, maternal education and socioeconomic factors indirectly affect children’s early cognitive development. The lower the maternal schooling and family income, the poorer the psychosocial stimulation, as children are deprived of play materials and school stimulation, negatively affecting their cognitive development. The study findings corroborate those described in the literature, indicating that maternal schooling affects children cognitive development using environmental organisation, parental expectations and practices, provision of materials for child’s cognitive stimulation, and variety in daily stimulation [27], [28]. As regards perinatal medical circumstances, preterm infants were more at risk of having below average cognitive composite score, this finding may be contributed to a lack of breastfeeding in preterm infants due to admission in incubator and some preterm infants are not physically or developmentally able to suckle, swallow and breathe in a coordinated manner this is in agreement with Kandeel et al., who found that mothers with a preterm newborn had a higher tendency toward artificial feeding than exclusive breastfeeding [29]. Infants born by cesarean section were at risk of having below average cognitive composite score than vaginally born infant although, this result is in agreement with Cain Polidano et al., who found through a longitudinal study that Cesarean birth may be directly and indirectly associated with negative child cognitive outcomes [30], cesarean procedures also poses postnatal maternal health risks [31] also there is potential knock-on effects for the child’s development through altered mother-child interactions [32] and lower rates of breastfeeding and its beneficial effects on early stage of brain development [33].
Complicated labour had a highly significant effect on cognitive composite score outcome as infants who experienced delivery problems were twice at risk of having below average cognitive composite score this result is in agreement with Pappas et al., who found that moderate to severe neonatal encephalopathy resulting from complicated labour contributes to a wide range of neurodevelopmental and cognitive impairments among survivors [34]. As regards chronic maternal illness before and during pregnancy didn’t appear to have a significant effect on the infant cognitive composite score outcome in the current study which disagrees with Thach et al., who found that child cognitive development is affected by antenatal iron deficiency anaemia and common mental problems [35]. The risk of having below average cognitive composite score in bottle-fed infants was near twice times higher than breastfed infants. At the same occasion, bottle-fed infants had the probability to get below average cognitive composite score 1.70 time greater than mixed fed infants , this result support and explain the importance of breastfeeding and also exclusive breastfeeding in the first 6 months of life the period of early brain development and agreed with James W Anderson et al., who found through a meta-analysis study that breastfeeding was associated with significantly higher scores for cognitive development than was formula feeding [36].
Time of introduction of complementary food could not be considered as a predictor of the cognitive composite score in this study. Starting CF before six months seemed to carry a non-significant risk of getting below the average cognitive composite score. This result is with an agreement with Sargoor et al., who found that there is no relation between age of introduction of complementary foods, and cognitive function [37]. We found that there were significantly lower levels of serum zinc and vitamin B12 in infants with below average cognitive composite score; it is believed that zinc is a vital nutrient for the brain. It has an important role in neurogenesis, maturation, migration of neurons and synapse formation [38]. Deficits in vitamin B12 (cobalamin) have negative consequences on the developing brain during infancy. Maureen et al. examined two mechanisms linking folate and vitamin B12 deficiency to abnormal behaviour and development in infants: disruptions to myelination and inflammatory processes [39]. In the current study, logistic regression analysis revealed the multifactorial interaction between infant feeding variables, socio-economic variables, maternal and perinatal health variables in predicting the cognitive developmental outcome. Complicated labour, prematurity and low educated mothers were highly significant predictors of below average cognitive development. Mode of feeding in the 1st six months was an important predictor for infant cognitive development as a nutritive value of breastfeeding in this period is essential for brain development. These results are in agreement with that of Metwally et al., who found that incorporative variables affected the infant social-emotional development. Breastfeeding, the level of maternal education and micronutrient sufficiency were the most important predictors of the infant mental health [40].
There may be some possible limitations in this study: Cross-sectional design is commonly preferred owing to its reasonable cost and feasibility. However, it cannot support a causal relationship. All participants in the current study were confined to the middle social class, as the majority of attendants of our clinic. Infants of low and high social classes were not included, which may constrain the generalisation of results. Time was a constraint, preventing the detailed recording of complementary feeding quality and parenting behaviour, which may reveal important influences on cognitive development.
In conclusion, multiple factors appear to interact, affecting the early cognitive development of Egyptian infants. Prematurity, complicated labour, poor maternal education, low family income and micronutrient deficiency are the main risk factors. Studying these factors is of great value in directing governmental intervention efforts. Providing good antenatal and natal care for all pregnant mothers especially poor ones, attention towards woman education, raising awareness about the importance of breastfeeding and providing adequate health care services for children will promote cognitive development in Egyptian children.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Nurliyana AR, Shariff ZM, Taib MN, Gan WY, Tan KA. Early nutrition, growth and cognitive development of infants from birth to 2 years in Malaysia:a study protocol. BMC pediatrics. 2016;16(1):160. doi: 10.1186/s12887-016-0700-0. https://doi.org/10.1186/s12887-016-0700-0 PMid:27687906 PMCid:PMC5043613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos LM, Santos DN, Bastos AC, Assis AM, Prado MS, Barreto ML. Determinants of early cognitive development:hierarchical analysis of a longitudinal study. Cadernos de Saúde Pública. 2008;24:427–37. doi: 10.1590/s0102-311x2008000200022. https://doi.org/10.1590/S0102-311X2008000200022. [DOI] [PubMed] [Google Scholar]
- 3.Tucker-Drob EM, Briley DA. Continuity of genetic and environmental influences on cognition across the life span:a meta-analysis of longitudinal twin and adoption studies. Psychol Bull. 2014;140(4):949–79. doi: 10.1037/a0035893. https://doi.org/10.1037/a0035893 PMid:24611582 PMCid:PMC4069230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng Zhu, Meng-Sha Sun, Jia-Hu Hao, Yu-Jiang Chen, Xiao-Min Jiang, Rui-Xue Tao, Kun Huang, Fang-Biao Tao. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Developmental Medicine & Child Neurology. 2014;56(3):283–289. doi: 10.1111/dmcn.12378. https://doi.org/10.1111/dmcn.12378 PMid:24512346. [DOI] [PubMed] [Google Scholar]
- 5.Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman SL, Baker-Henningham H, Chang SM, Hamadani JD, Lozoff B, Gardner JM. Inequality in early childhood:risk and protective factors for early child development. The lancet. 2011;378(9799):1325–38. doi: 10.1016/S0140-6736(11)60555-2. https://doi.org/10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 6.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72(4):267–84. doi: 10.1111/nure.12102. https://doi.org/10.1111/nure.12102 PMid:24684384. [DOI] [PubMed] [Google Scholar]
- 7.de La Rochebrochard E, Joshi H. Children born after unplanned pregnancies and cognitive development at 3 years:social differentials in the United Kingdom Millennium Cohort. American journal of epidemiology. 2013;178(6):910–920. doi: 10.1093/aje/kwt063. https://doi.org/10.1093/aje/kwt063 PMid:23887043 PMCid:PMC3775543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali SS. A brief review of risk-factors for growth and developmental delay among preschool children in developing countries. Advanced Biomedical Research. 2013;2:91. doi: 10.4103/2277-9175.122523. https://doi.org/10.4103/2277-9175.122523 PMid:24520553 PMCid:PMC3908499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu LH, Dapretto M, O'hare ED, Kan E, McCourt ST, Thompson PM, Toga AW, Bookheimer SY, Sowell ER. Relationships between brain activation and brain structure in normally developing children. Cerebral cortex. 2009;19(11):2595–604. doi: 10.1093/cercor/bhp011. https://doi.org/10.1093/cercor/bhp011 PMid:19240138 PMCid:PMC2758677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spratt EG, Friedenberg SL, Swenson CC, Larosa A, De Bellis MD, Macias MM, Summer AP, Hulsey TC, Runyan DK, Brady KT. The Effects of Early Neglect on Cognitive, Language, and Behavioral Functioning in Childhood. Psychology (Irvine) 2012;3(2):175–182. doi: 10.4236/psych.2012.32026. https://doi.org/10.4236/psych.2012.32026 PMid:23678396 PMCid:PMC3652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein SM, Berten S, Olson P, Paul R, Williams LM, Cooper N, Gordon E. Development and validation of a World-Wide-Web-based neurocognitive assessment battery:WebNeuro. Behavior Research Methods. 2007;39(4):940–9. doi: 10.3758/bf03192989. https://doi.org/10.3758/BF03192989 PMid:18183911. [DOI] [PubMed] [Google Scholar]
- 12.William D. Schlesselman: Case-control Studies: Design, Conduct, Analysis. New York: Oxford U. Press; 1982. pp. 144–152. On line calculation http://biostat.mc.vanderbilt.edu/PowerSampleSize. Version 3.0, January 2009. [Google Scholar]
- 13.El-Shakhs A. Social level and the economic scale of the family:the scale manual [Google Scholar]
- 14.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 15.WHO. Anthro Plus for personal computers. Manual Software for assessing growth of the world's children and adolescents. Geneva. 2009. http://www.who.int/growthref/tools/en/
- 16.Bayley N. Bayley scales of infant and toddler development-third edition. 2. Vol. 25. San Antonio, TX: Harcourt Assessment Journal of psychoeducational assessment; 2006. pp. 180–90. https://doi.org/10.1177/0734282906297199. [Google Scholar]
- 17.Chou PP. Zinc. In: Pesce AJ, Kaplan LA, editors. Methods in clinical chemistry. St. Louis, Missouri: The C.V. Mosby Company; 1987. pp. 596–602. [Google Scholar]
- 18.Taylor A, Bryant TN. Comparison of procedures for determination of copper and zinc serum by atomic absorption spectroscopy. Clin Chim Acta. 1981;110:83–90. doi: 10.1016/0009-8981(81)90304-1. https://doi.org/10.1016/0009-8981(81)90304-1. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization; 2011. [Google Scholar]
- 20.WHO C. Assessing the iron status of populations:including literature reviews:report of a Joint World Health Organization. Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland. 2004:6–8. [Google Scholar]
- 21.de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food and nutrition bulletin. 2008;29(2_suppl 1):S238–44. doi: 10.1177/15648265080292S129. https://doi.org/10.1177/15648265080292S129 PMid:18709899. [DOI] [PubMed] [Google Scholar]
- 22.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food and nutrition bulletin. 2007;28(Suppl 3):S403–29. doi: 10.1177/15648265070283S303. https://doi.org/10.1177/15648265070283S303 PMid:17988005. [DOI] [PubMed] [Google Scholar]
- 23.Murray RK, Jacob M, Varghese J. Plasma Proteins & Immunoglobulins. In: Bender DA, Botham KM, Weil PA, Kennelly PJ, Murray RK, Rodwell VW, editors. Harper's Illustrated Biochemistry. 29th. New York: McGraw-Hill; 2011. [Google Scholar]
- 24.CIOMS/WHO. International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva: CIOMS; 1993. [PubMed] [Google Scholar]
- 25.Shereen Jegtvig. Longer breastfeeding tied to better development. Health News. December 25. 2013 [Google Scholar]
- 26.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood:a follow-up study. Lancet. 2002;359:564–71. doi: 10.1016/S0140-6736(02)07744-9. https://doi.org/10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 27.Andrade AS, Santos DN, Bastos AC, Pedromônico MR, Almeida-Filho N, Barreto ML. Ambiente familiar e desenvolvimento cognitivo infantil:uma abordagem epidemiológica. Rev Saúde Pública. 2005;39:606–11. doi: 10.1590/s0034-89102005000400014. https://doi.org/10.1590/S0034-89102005000400014 PMid:16113911. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer D. Psicologia do desenvolvimento: infância e adolescência. São Paulo: Pioneira Thomson Learning; 2005. [Google Scholar]
- 29.Kandeel WA, Rabah TM, Zeid DA, El-Din EM, Metwally AM, Shaalan A, El Etreby LA, Shaaban SY. Determinants of Exclusive Breastfeeding in a Sample of Egyptian Infants. Open access Macedonian journal of medical sciences. 2018 Oct 25;6(10):1818–1823. doi: 10.3889/oamjms.2018.359. https://doi.org/10.3889/oamjms.2018.359 PMid:30455755 PMCid:PMC6236050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polidano C, Zhu A, Bornstein JC. The relation between cesarean birth and child cognitive development. Scientific reports. 2017;7(1):11483. doi: 10.1038/s41598-017-10831-y. https://doi.org/10.1038/s41598-017-10831-y PMid:28904336 PMCid:PMC5597642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Heaman M, Joseph KS, Liston RM, Huang L, Sauve R, Kramer MS. Risk of maternal postpartum readmission associated with mode of delivery. Obstetrics & Gynecology. 2005;105(4):836–42. doi: 10.1097/01.AOG.0000154153.31193.2c. https://doi.org/10.1097/01.AOG.0000154153.31193.2c PMid:15802414. [DOI] [PubMed] [Google Scholar]
- 32.Kelly Y, Sacker A, Del Bono E, Francesconi M, Marmot M. What role for the home learning environment and parenting in reducing the socioeconomic gradient in child development?Findings from the Millennium Cohort Study. Archives of Disease in Childhood. 2011;96(9):832–7. doi: 10.1136/adc.2010.195917. https://doi.org/10.1136/adc.2010.195917 PMid:21666278. [DOI] [PubMed] [Google Scholar]
- 33.Zanardo V, Svegliado G, Cavallin F, Giustardi A, Cosmi E, Litta P, Trevisanuto D. Elective cesarean delivery: does it have a negative effect on breastfeeding? Birth. 2010;37(4):275–9. doi: 10.1111/j.1523-536X.2010.00421.x. https://doi.org/10.1111/j.1523-536X.2010.00421.x PMid:21083718. [DOI] [PubMed] [Google Scholar]
- 34.Pappas A, Korzeniewski SJ. Long-Term Cognitive Outcomes of Birth Asphyxia and the Contribution of Identified Perinatal Asphyxia to Cerebral Palsy. Clin Perinatol. 2016;43(3):559–72. doi: 10.1016/j.clp.2016.04.012. https://doi.org/10.1016/j.clp.2016.04.012 PMid:27524454. [DOI] [PubMed] [Google Scholar]
- 35.Tran TD, Biggs BA, Tran T, Simpson JA, Hanieh S, Dwyer T, Fisher J. Impact on infants'cognitive development of antenatal exposure to iron deficiency disorder and common mental disorders. PLoS One. 2013;8(9):e74876. doi: 10.1371/journal.pone.0074876. https://doi.org/10.1371/journal.pone.0074876 PMid:24086390 PMCid:PMC3781140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. The American journal of clinical nutrition. 1999;70(4):525–35. doi: 10.1093/ajcn/70.4.525. https://doi.org/10.1093/ajcn/70.4.525 PMid:10500022. [DOI] [PubMed] [Google Scholar]
- 37.Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Hill JC, Kurpad AV, Muthayya S, Karat SC, Nalinakshi M, Fall CH. Infant feeding practice and childhood cognitive performance in South India. Archives of disease in childhood. 2010;95(5):347–54. doi: 10.1136/adc.2009.165159. https://doi.org/10.1136/adc.2009.165159 PMid:19946010 PMCid:PMC3428883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prado EL, Dewey KG Nutrition and brain development in early life. Nutrition Reviews. 2014;72(4):267–284. doi: 10.1111/nure.12102. https://doi.org/10.1111/nure.12102 PMid:24684384. [DOI] [PubMed] [Google Scholar]
- 39.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food and nutrition bulletin. 2008;29(2_suppl1):S126–31. doi: 10.1177/15648265080292S117. https://doi.org/10.1177/15648265080292S117 PMid:18709887 PMCid:PMC3137939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metwally AM, El-Din EM, Shehata MA, Shaalan A, El Etreby LA, Kandeel WA, Shaaban SY, Rabah TM. Early Life Predictors of Socio-Emotional Development in a Sample of Egyptian Infants. PloS one. 2016;11(7):e0158086. doi: 10.1371/journal.pone.0158086. https://doi.org/10.1371/journal.pone.0158086 PMid:27379907 PMCid:PMC4933375. [DOI] [PMC free article] [PubMed] [Google Scholar]


