Abstract
Major depressive disorder is a chronic debilitating mental illness. Its pathophysiology at cellular and molecular levels is incompletely understood. Increasing evidence supports a pivotal role of the mitogen-activated protein kinase (MAPK), in particular the extracellular signal-regulated kinase (ERK) subclass of MAPKs, in the pathogenesis, symptomatology, and treatment of depression. In humans and various chronic animal models of depression, the ERK signaling was significantly downregulated in the prefrontal cortex and hippocampus, two core areas implicated in depression. Inhibiting the ERK pathway in these areas caused depression-like behavior. A variety of antidepressants produced their behavioral effects in part via normalizing the downregulated ERK activity. In addition to ERK, the brain-derived neurotrophic factor (BDNF), an immediate upstream regulator of ERK, the cAMP response element-binding protein (CREB), a transcription factor downstream to ERK, and the MAPK phosphatase (MKP) are equally vulnerable to depression. While BDNF and CREB were reduced in their activity in the prefrontal cortex and hippocampus of depressed animals, MKP activity was enhanced in parallel. Chronic antidepressant treatment readily reversed these neurochemical changes. Thus, ERK signaling in the depression-implicated brain regions was disrupted during the development of depression, which contributes to the long-lasting and transcription-dependent neuroadaptations critical for enduring depression-like behavior and the therapeutic effect of antidepressants.
Keywords: Depression, antidepressant, ERK, MAPK phosphatase, BDNF, CREB, frontal cortex, hippocampus
Introduction
Major depressive disorder is one of the most common neuropsychiatric illnesses. As a chronic debilitating disorder, depression affects millions of people worldwide yearly and represents a major economic and medical burden. Despite its high prevalence, brain mechanisms underlying the development of depression-like behavior are far from clear. Accumulating evidence from extensive studies on humans and experimental animals indicates that adaptations of distinctive signaling pathways in neurons of brain regions implicated in depression occur during the progression of depression. These long-lasting adaptive changes in the signaling pathways participate in the remodeling of different forms of neuronal and synaptic plasticity critical for enduring depression-like behavior.
An essential family of serine/threonine protein kinases is the mitogen-activated protein kinase (MAPK). These kinases function to regulate cellular growth, differentiation, and survival in proliferative cells [1]. In addition, MAPKs are expressed in postmitotic neurons of the adult mammalian brain, where MAPKs respond to changing synaptic input and regulate neuronal activity and synaptic plasticity via a transcription-dependent or –independent manner [2]. Activation of the MAPK cascade requires four sequential events involving small GTPases (Ras and Rac proto-oncogenes), MAPK kinase kinases (Raf or MEKK), MAPK kinases (MEK), and MAPKs. After activation, MAPKs become a highly efficient signaling pathway linking a variety of extracellular signals to cytoplasmic, intranuclear, or synaptic responses [2-4].
A prototypic subfamily of MAPKs is the extracellular signal-regulated kinase (ERK) [1]. The Ras-Raf-MEK1/2 pathway is responsible for activating ERK via the dual function MEK-mediated threonine and tyrosine phosphorylation of ERK, whereas the dual-specificity MAPK phosphatase (MKP) and the serine/threonine protein phosphatase (PP) such as PP1 and PP2A dephosphorylate and thereby deactivate ERK [5-7]. Among several ERK isoforms (ERK1/2/3/4/5/7), ERK1/2 have been most thoroughly investigated and characterized in the central nervous system [8]. Available data show that ERK1/2 play a pivotal role in various neuropsychiatric disorders, including depression.
Increasing evidence shows that the ERK pathway in the brain regions implicated in major depression is vulnerable to chronic stressors. ERK activity in the prefrontal cortex and hippocampus was reduced in suicide subjects. Various chronic stressors caused a reduction of the cortical and hippocampal ERK signaling in experimental rodents. The MEK inhibitors induced depression-like behavior and blocked the effect of antidepressants. Various antidepressants reversed the hypoactivity of ERK and alleviated depression-like behavior. Altogether, these data support a model that the ERK pathway is downregulated in the cortex and hippocampus of depressed humans and animals, which contributes to the development of depression and serves as a substrate of antidepressants. In addition, brain-derived neurotrophic factor (BDNF), an immediate upstream regulator of ERK, and cAMP response element-binding protein (CREB), a transcription factor downstream to ERK, were downregulated in parallel and play a similar role as ERK in depression. This review, by focusing on recent data, discusses the sensitivity and responsiveness of forebrain ERK to depression and roles of ERK in the pathogenesis of depression and antidepressant action.
Changes in the ERK pathway in response to depression
Among initial attempts toward understanding the possible roles of ERK in depression, Dwivedi et al. assayed changes in expression and catalytic activity of ERK1/2 and expression of MKP-2 in various postmortem brain regions of suicide subjects with major depression as compared to non-psychiatric control subjects [9]. They found that ERK1/2 expression at both mRNA and protein levels and kinase activity of ERK1/2 were reduced in the prefrontal cortex and hippocampus, two major brain regions implicated in depression [10], but not in the cerebellum. In parallel, MKP-2 that dephosphorylates and deactivates ERK1/2 was enhanced in its expression in the prefrontal cortex and hippocampus. Similarly, the upstream activator of the ERK pathway, i.e., a Raf kinase, was altered by depression. Among three Raf kinases, i.e., A-Raf, B-Raf and C-Raf (Raf-1), B-Raf but not Raf-1 was selectively reduced in its catalytic activity and protein expression in the prefrontal cortex and hippocampus of suicide subjects [11]. In addition to Raf kinases, MEK1, an immediate upstream activator of ERK1/2, was downregulated in its catalytic activity and phosphorylation and in its interactions with B-Raf [12]. These results from a series of human studies demonstrate the abnormality of the ERK pathway in defined brain regions of subjects with major depression.
In preclinical animal studies, the responsiveness of the ERK pathway to depression was also investigated mainly in the frontal cortex and hippocampus. ERK phosphorylation and expression in the hippocampus were not significantly altered in an acute animal model of depression (acute restraint stress) [13]. In different studies, a single acute session of restraint stress or other stressors increased ERK phosphorylation in the frontal cortex and hippocampus [14,15]. In contrast to the acute model, ERK activity in the hippocampus was inhibited in a chronic animal model of depression [16]. A large number of subsequent studies reported the similar downregulation of ERK in various chronic models of depression. For instance, ERK2 phosphorylation was reduced in the prefrontal cortex and hippocampus of rats showing depression-like behavior following chronic forced swim stress [17,18]. A downregulated level of ERK phosphorylation and expression occurred in the frontal cortex and hippocampus of adult rats that have received neonatal treatment with clomipramine, a chronic model of depression persisting throughout adulthood [19]. These neurochemical changes in the ERK system correlated well with depression-like sexual behavior[19]. Many other stressors that consistently induced depression-like behavior reduced ERK1/2 phosphorylation in the frontal cortex and/or hippocampus [14, 20-34]. These data support a notion that the ERK pathway was downregulated in the prefrontal cortex and hippocampus following chronic stress. In further support of this notion, MKP-1 expression was elevated in the hippocampus of depressed mice [23]. Intra-hippocampal infusion of the MKP-1 inhibitor prevented depression-like behavior and normalized local MKP-1 expression and ERK phosphorylation. However, while prenatal restraint stress induced depression-like behavior and reduced ERK2 expression in the prefrontal cortex and hippocampus of one-month offspring rats [35], ERK phosphorylation and expression in the frontal cortex and hippocampus remained unchanged at 3 months of age following the prenatal stress [36]. There were the two other subclasses of MAPKs, the Jun N-terminal kinase (JNK) and p38 kinase, which exhibited a decrease in their phosphorylation in the frontal cortex or hippocampus. Thus, MAPK subclasses may differentially respond to depression, depending upon the specific model of depression, the developmental stage of depression, and other experimental conditions.
In addition to the prefrontal cortex and hippocampus, the lateral septum is a central site implicated in depression. As a major target of monoaminergic projections, the lateral septum receives the strongest noradrenergic and serotonergic input [37,38]. Early evidence indicates the association of deficiencies in monoaminergic transmission with depression. Antidepressant treatment markedly altered serotonin release and serotonin receptor signaling in the lateral septum in different animal paradigms of depression [39,40]. As a common signaling pathway downstream to serotonin receptors, ERK in the serotonin receptor-enriched lateral septum is reasoned to be vulnerable to depression. In fact, in a rat model of depression, ERK phosphorylation was reduced in the lateral septum [13]. This indicates that inhibition of ERK in the lateral septum is also implicated in the development of depression.
Mechanisms underlying plastic changes in the ERK pathway
How adaptive changes in the ERK pathway in response to depression occur is incompletely studied. The less activation of ERK seen in depressed humans and animals could occur as a result of the concurrent downregulation of upstream elements that are responsible for activating ERK. At the receptor level, ERK is coupled to various neurotransmitter receptors, including serotonin, adrenergic, dopamine and glutamate receptors [8,41]. Activation of distinct subtypes of these receptors leads to phosphorylation of ERK. Intracellularly, ERK is subjected to the positive regulation by several common protein kinases, such as protein kinase A (PKA) and protein kinase C (PKC), and generally PKA and PKC activators activated ERK1/2 in the hippocampus [42]. Thus, the hypoactive state of any receptors or effectors (PKA or PKC) in response to depression may subsequently result in the less activation of ERK. Consistent with this, the activity level of PKA, PKC, and adenylyl cyclase was reduced in the postmortem brain of suicide subjects or patients with major depression [43-46] or in the brain of depressed animals [47,48]. The PKA activator exhibited the antidepressant activity [49], whereas the PKA and PKC inhibitors blocked the effect of antidepressants [50,51]. In addition, MEK1 was downregulated in its phosphorylation and catalytic activity in the prefrontal cortex and hippocampus of suicide subjects [12]. MEK1/2 phosphorylation was decreased in the medial orbital cortex and dorsal endopiriform nuclei of the prefrontal cortex of stressed mice [24]. These data indicate that abnormal MEK1/2 activity may be linked to aberrant responses of ERK1/2 to depression.
In addition to the elements catalyzing phosphorylation of ERK, dephosphorylation of ERK could be another layer of mechanisms underlying the downregulation of the ERK pathway. The phosphatases that dephosphorylate and thereby deactivate ERK could undergo adaptive changes in response to depression, which thereby results in corresponding changes in ERK. It has been reported that expression of phosphatases (MKP-1, MKP-2, PP1) was elevated in the prefrontal cortex and hippocampus of depressed humans or animals [9,19,23,52,]. Intracranial injection of the MKP-1 inhibitor reversed the reduced hippocampal ERK phosphorylation and reduced behavioral responses to stress [23,53]. Chronic antidepressant treatment normalized the responses of MKP-1 and behavior to stress, and mice lacking MKP-1 were resilient to stress [52]. Thus, accelerated dephosphorylation of ERK due to hyperactive phosphatases contributes to the inactivation of the ERK pathway during the development of depression. It is likely that both activation and deactivation mechanisms work in concert to accurately control the responsiveness of ERK and depression-like behavior.
Roles of ERK in depression-like behavior and antidepressant effects
Plastic changes in the ERK pathway in depressed humans and animals imply a possible role of ERK in the development of depression. It is reasoned that direct inhibition of ERK may induce depression-like behavior if the loss of ERK activity is causally linked to the pathogenesis and symptomatology of depression. Indeed, while the acute behavioral effect of the ERK inhibition was inconsistent [54-58], chronic pharmacological inhibition of the ERK pathway by repeated infusions of U0126, a specific MEK inhibitor, into the dorsal hippocampus induced anhedonia and anxiety-like behavior [59]. U0126 after infusions into the medial prefrontal cortex also produced anhedonia. These results support that the lowered ERK activity in the frontal cortex and hippocampus contributes to mediating depression-like behavior. Consistent with this, while conventional ERK2 knockouts were not viable [60], conditional and region-specific ERK2 knockout in the central nervous system caused deficits in social behavior [61]. Overactivation of ERK2 in ERK1-deficient mice reduced depression-like behavior, which was fully reversed by the MEK inhibitor SL327 [56].
If inducible inhibition of ERK plays a role in mediating depression, restoration of the reduced ERK activity may be of an antidepressant property. In fact, a number of studies reveal that various antidepressants possess the common ability to reverse the loss of ERK activity in the frontal cortex and hippocampus of depressed animals. Antidepressants (amitriptyline and fluoxetine) reversed the inhibition of ERK1/2 phosphorylation in the frontal cortex and hippocampus when they suppressed depression-like behavior [18,20,21, although 62]. The antidepressant quetiapine in combination with transcranial magnetic stimulation also reversed the diminished hippocampal ERK1/2 phosphorylation and produced antidepressant effects [63]. Other antidepressants showed similar effects [24,27,28,31,33,34, 64-67]. In addition to antidepressants, a selective MKP-1 inhibitor sanguinarine injected into the hippocampus or ventrolateral orbital cortex increased ERK activation and reduced depressive immobility [23,53]. The effect of several antidepressants was blocked by a systemic, intracerebroventricular, or intrahippocampal injection of an MEK inhibitor U0126, SL327, or PD98059 [58,68-74]. The MEK inhibitor PD184161 also blocked the antidepressant effect of desipramine and sertraline [55]. These data altogether support the role of ERK in mediating the effect of antidepressants.
The ERK pathway serves as an information superhighway between the surface membrane and the nucleus and effectively links environmental signals to genomic responses. After activation, cytoplasmic ERK translocates to the nucleus where ERK activates specific transcription factors to regulate gene transcription [75]. The transcription factor Elk-1 is a nuclear substrate of ERK [75]. Another transcription factor CREB is also a downstream target of ERK [76]. Several studies reveal a role of the ERK-CREB coupling in depression-like behavior and antidepressant action. Chronic stress induced depression behavior and reduced ERK and CREB phosphorylation (activation) in the rat prefrontal cortex and hippocampus, which was reversed by fluoxetine [18]. Other studies also found that CREB phosphorylation was decreased in the frontal cortex and/or hippocampus of stressed humans and animals, which was usually accompanied by a decrease in ERK activity [30,34,65,77-80]. Antidepressants reversed the reduction of CREB phosphorylation in stressed animals [25,34,66,69,78,] or increased CREB phosphorylation in naive rats [81-84]. Infusion of U0126 into the medial prefrontal cortex or hippocampus induced depression-like behavior and reduced local CREB phosphorylation [59]. There results support a model that the ERK-CREB pathway is downregulated in the prefrontal cortex and hippocampus during the development of depression, which might participate in mediating depression-like behavior. As such, restoration of downregulated CREB could yield an antidepressant effect. Of note, the role of CREB in the nucleus accumbens seems to be different. Prolonged social isolation induced anxiety- and anhedonia-like symptoms in adult rodents [85]. Only the anxiety phenotype and its reversal by an antidepressant were mediated by CREB in the nucleus accumbens shell, while the anhedonia-like symptoms were not.
The BDNF-ERK pathway in depression
Neurotrophic factors are critical for the etiology and treatment of depression [86]. The MAPK cascade, including the ERK pathway, is one of the best-characterized signaling transduction pathways downstream to the BDNF-activated TrkB receptor [3]. Since ERK was downregulated in the prefrontal cortex and hippocampus of depressed humans and animals (see above), the BDNF signaling is likely reduced in depression. In fact, BDNF expression or TrkB phosphorylation was reduced in the prefrontal cortex and/or hippocampus of depressed humans and animals [25,29,34,74,87-91]. The reduction of TrkB phosphorylation occurred along with a decrease in ERK phosphorylation [20,66]. These results imply that BDNF could serve as a biomarker of depression as reduced BDNF indicates a higher state of vulnerability to depression. Indeed, a higher vulnerability to stress and depression was seen in humans with a decreased release of BDNF due to carrying a BDNF polymorphism (Val66/Met) [92]. A decreased volume of the hippocampus in depressed patients is consistent with the likelihood of a reduced neurotrophic factor support in the brain [93,94].
Antidepressants normalized the reduction of BDNF expression in the hippocampus of depressed patients and animals [25,29,34,70,74,91,95-97] or upregulated hippocampal BDNF expression [98-102], indicating a role of BDNF in the behavioral response to antidepressants. The role of BDNF is further supported by the following findings. Chronic peripheral administration of BDNF enhanced ERK and CREB phosphorylation in the mouse hippocampus and produced antidepressant effects in cellular and behavioral models of depression [103]. Direct injection of BDNF into the midbrain or hippocampus mimicked the antidepressant effect of BDNF administered systemically [104-106] and increased local ERK phosphorylation [106]. Inhibition of ERK with U0126 blocked the antidepressant effect of BDNF directly infused into the hippocampus [105]. The TrkB inhibitor K252a also caused a loss of effects of antidepressants [28,66,70]. In addition, heterozygous BDNF null mice were resistant to antidepressants [107] and displayed a depressive phenotype when combined with a low-dose of the MEK inhibitor or mild stress exposure [55]. Loss of function of BDNF in transgenic mice [108,109] or depletion of hippocampal BDNF by transfecting lentivirus-derived shBDNF [110] suppressed the behavioral response to antidepressants, indicating that normal BDNF signaling is required for the effect of antidepressants. Collectively, BDNF has a potential to serve as an etiological and therapeutic biomarker for depression. While the precise mechanisms(s) underlying BDNF involvements are unclear, the ERK pathway seems play a role in linking BDNF to depression as well as to antidepressant properties.
Conclusions
ERK is enriched in postmitotic neurons in brain regions implicated in major depression. Long-lasting adaptive changes in ERK phosphorylation, expression, and function occur in the prefrontal cortex and hippocampus during the course of the development of depression. In addition to ERK, the ERK-linked BDNF and CREB are sensitive to depression and display comparable changes in their expression and function (Fig. 1). In fact, as sequential and coherent events, BDNF may be initially reduced in its expression and function in the prefrontal cortex and hippocampal, which subsequently leads to a decrease in downstream elements, i.e., ERK and CREB. Through the BDNF-ERK-CREB pathway as well as other pathways, extracellular signals are transmitted to the nucleus to regulate a network of the depression-associated genes, which transcriptionally determines the pathogenesis and severity of depression-like behavior [111]. In addition, the BDNF-ERK-CREB cascade is a substrate of antidepressants. Various antidepressants act to reverse the downregulated BDNF-ERK-CREB pathway to alleviate depression-like behavior. Thus, the BDNF-ERK-CREB system represents a current target for developing new pharmacotherapies for depression.
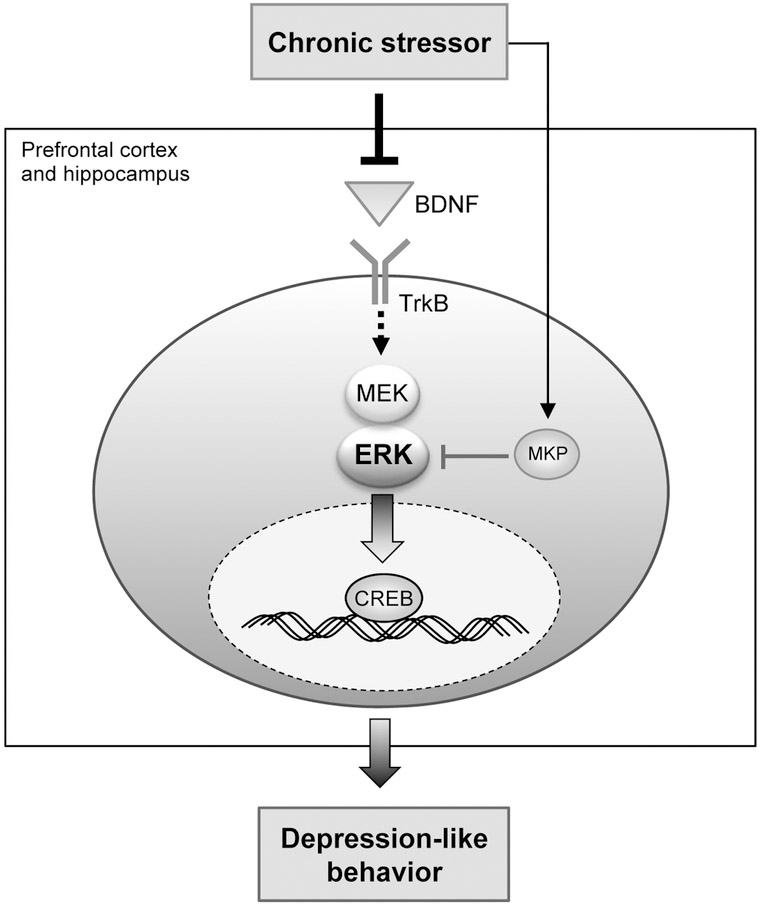
Figure 1. A schematic diagram illustrating the role of the BDNF-ERK-CREB pathway in the development of depression-like behavior.
Chronic stressors that cause depression-like behavior induce a reduction of BDNF expression in the brain regions implicated in the pathogenesis of depression, including the prefrontal cortex and hippocampus. This leads to downregulation of the ERK pathway downstream to TrkB, a receptor which BDNF interacts with. Chronic stressors also induce an increase in MKP activity in the same brain regions, which contributes to the downregulation of the ERK pathway. The downregulated ERK pathway results in a less amount of active ERK translocating from the cytoplasm to the nucleus, leading to hypoactivation of transcription factors such as CREB. Lowered CREB activity causes long-lasting adaptive changes in expression of a discrete set of genes associated with depression and transcriptionally contributes to enduring depression-like behavior.
While a traditional view is that ERK once activated translocates into the nucleus to regulate gene expression and thereby transcriptionally regulate cellular and synaptic activities, a sub-pool of ERK also notably resides in peripheral structures of neurons, such as postsynaptic dendritic spines, in various brain regions surveyed [112-115]. A complete set of all MAPK cascade components are present in the postsynaptic density microdomain [116,117]. Moreover, synaptic ERK is readily activated in response to changing synaptic input [2]. Functionally, ERK interacts with and regulate a number of synaptic proteins, including scaffold proteins, ion channels and G protein-coupled receptors, to determine the strength and efficacy of synaptic plasticity [2]. Apparently, ERK resides and functions at synaptic sites in addition to the nuclear location. To date, whether and how synaptic ERK responds to depression and plays a role in the pathophysiology of depression and antidepressant action is unclear. Future studies need to elucidate accurate roles of synaptic ERK in the reshape of excitatory transmission and plasticity critical for the progression of depression.
Acknowledgements
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Volmat V, Pouyssegur J (2001) Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell 93:71–79. [DOI] [PubMed] [Google Scholar]
- 2.Mao LM, Wang JQ (2016) Synaptically localized mitogen-activated protein kinases: local substrates and regulation. Mol Neurobiol 53:6309–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14:311–317. [DOI] [PubMed] [Google Scholar]
- 4.Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS (2000) cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci 20:4030–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverstein AM, Barrow CA, Davis AJ, Mumby MC (2002) Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci USA 99:4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY (2002) The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem 277:31818–31825. [DOI] [PubMed] [Google Scholar]
- 7.Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE, Wang JQ (2005) Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem 280:12602–12610. [DOI] [PubMed] [Google Scholar]
- 8.Wang JQ, Fibuch EE, Mao L (2007) Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem 100:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN (2001) Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem 77:916–928. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, Cui R (2017) The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast 2017:6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwivedi Y, Rizavi HS, Conley RR, Pandey GN (2006) ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry 11:86–98. [DOI] [PubMed] [Google Scholar]
- 12.Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN (2009) Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1). Int J Neuropsychopharmacol 12:1337–1354. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan TP, Neve RL, Duman RS, Russell DS (2003) Antidepressant effect of the calcium-activated tyrosine kinase Pyk2 in the lateral septum. Biol Psychiatry 54:540–551. [DOI] [PubMed] [Google Scholar]
- 14.Meller E, Shen C, Nikolao TA, Jensen C, Tsimberg Y, Chen J, Gruen RJ (2003) Region-specific effects of acute and repeated restraint streee on the phosphorylation of mitogen-activated protein kinases. 979:57–64. [DOI] [PubMed] [Google Scholar]
- 15.Galeotti N, Ghelardini C (2012) Regionally selective activation and differential regulation of ERK, JNK and p38 MAP kinase signalling pathway by protein kinase C in mood modulation. Int J Neuropsychopharmacol 15:781–793. [DOI] [PubMed] [Google Scholar]
- 16.Schultz H, Sheehan TP, Duman RS, Russell DS (2001) Regulation of ERK activity in brain by chronic stress and antidepressants. Soc Neurosci Abs 906:p6. [Google Scholar]
- 17.Qi X, Lin W, Li J, Pan Y, Wang W (2006) The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav Brain Res 175:233–240. [DOI] [PubMed] [Google Scholar]
- 18.Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M (2008) Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis 31:278–285. [DOI] [PubMed] [Google Scholar]
- 19.Feng P, Guan Z, Yang X, Fang J (2003) Impariments of ERK signal transduction in the brain in a rat model of depression induced neonatal exposure of clomipramine. Brain Res 991:195–205. [DOI] [PubMed] [Google Scholar]
- 20.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR (2008) Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 63:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A (2011) The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci 45:246–255. [DOI] [PubMed] [Google Scholar]
- 22.Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, Hu ZL, Fu H, Wang F, Chen JG (2011) Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull 34:253–259. [DOI] [PubMed] [Google Scholar]
- 23.Jia W, Liu R, Shi J, Wu B, Dang W, Du Y, Zhou Q, Wang J, Zhang R (2013) Differential regulation of MAPK phosphorylation in the dorsal hippocampus in response to prolonged morphine withdrawal-induced depressive-like symptoms in mice. PLoS One 8:e66111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leem YH, Yoon SS, Kim YH, Jo SA (2014) Disrupted MEK/ERK signaling in the medial orbital cortex and dorsal endopiriform nuclei of the prefrontal cortex in a chronic restraint stress mouse model of depression. Neurosci Lett 580:163–168. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H, Pang Q (2014) Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res 271:116–121. [DOI] [PubMed] [Google Scholar]
- 26.Luo YW, Xu Y, Cao WY, Zhong XL, Duan J, Wang XQ, Hu ZL Li F, Zhang JY, Zhou M, Dai RP, Li CQ (2015) Insulin-like growth factor 2 mitigates depressive behavior in a rat model of chronic stress. Neuropharmacology 89:318–324. [DOI] [PubMed] [Google Scholar]
- 27.Shibata S, Linuma M, Soumiya H, Fukumitsu H, Furukawa Y, Furukawa S (2015) A novel 2-decenoic acid thioester ameliorates corticosterone-induced depression- and anxiety-like behaviors and normalizes reduced hippocampal signal transduction in treated mice. Pharmacol Res Perspect 3:e00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Zhou W, Zhou X, Zhuang F, Chen Q, Li M, Ma T, Gu S (2015) Antidepressant-like effects of alarin produced by activation of TrkB receptor signaling pathways in chronic stress mice. Behav Brain Res 280:128–140. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Xie Y, Zhang T, Bo S, Bai X, Liu H, Li T, Liu S, Zhou Y, Cong X, Wang Z, Liu D (2016) Resveratrol reverses chronic restraint stress-induced depression-like behavior: involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res Bull 125:134–143. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Shao F, Wang W (2018) Region-dependent alterations in cognitive function and ERK1/2 signaling in the PFC in rats after social defeat stress. Neural Plast 2018:9870985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li E, Deng H, Wang B, Fu W, You Y, Tian S (2016) Apelin-13 exerts antidepressant-like and recognition memory improving activities in stressed rats. Eur Neuropsychopharmacol 26:420–430. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Chen Y, Gao X, Zhang Z (2017) The behavioral deficits and cognitive impairment are correlated with decreased IGF-II and ERK in depressed mice induced by chronic unpredictable stress. Int J Neurosci 127:1096–1103. [DOI] [PubMed] [Google Scholar]
- 33.Oh DR, Yoo JS, Kim Y, Kang H, Lee H, Lm SJ, Choi EJ, Jung MA, Bae D, Oh KN, Hong JA, Jo A, Shin J, Kim J, Kim YR, Cho SS, Lee BJ, Choi CY (2018) Vaccinium bracteatum leaf extract reverses chronic restraint stress-induced depression-like behavior in mice: regulation of hypothalamic-pituitary-adrenal axis, serotonin turnover systems, and ERK/Akt phosphorylation. Front Pharmacol 9:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Nakagawasai O, Nemoto W, Kadota S, Odaira T, Sakuma W, Arai Y, Tadano T, Tan-No K (2018) Memantine ameliorates depressive-like behaviors by regulating hippocampal cell proliferation and neuroprotection in olfactory bulbectomized mice. Neuropharmacology 137:141–155. [DOI] [PubMed] [Google Scholar]
- 35.Guan L, Jia N, Zhao X, Zhang X, Tang G, Yang L, Sun H, Wang D, Su Q, Song Q, Cai D, Cai Q, Li H, Zhu Z (2013) The involvement of ERK/CREB/Bcl-2 in depression-like behavior in prenatally stressed offspring rats. Brain Res Bull 99:1–8. [DOI] [PubMed] [Google Scholar]
- 36.Budziszewska B, Szymanska M, Leskiewicz M, Basta-Kaim A, Jaworska-Feil L, Kubera M, Jantas D, Lason W (2010) The decrease in JNK- and p38-MAP kinase activity is accompanied by the enhancement of PP2A phosphate level in the brain of prenatally stressed rats. J Physiol Pharmacol 61:207–215. [PubMed] [Google Scholar]
- 37.Kohler C, Chan-Palay V, Steinbusch H (1982) The distribution and origin of serotonin-containing fibers in the septal area: A combined immunohistochemical and fluorescent retrograde tracing study in the rat. J Comp Neurol 209:91–111. [DOI] [PubMed] [Google Scholar]
- 38.Gall C, Moore RY (1984) Distribution of enkephalin, substance P, tyrosine hydroxylase, and 5-hydroxytryptamine immunoreactivity in the septal region of the rat. J Comp Neurol 225:212–227. [DOI] [PubMed] [Google Scholar]
- 39.Kirby LG, Lucki I (1997) Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther 282:967–976. [PubMed] [Google Scholar]
- 40.Shen C, Li H, Meller E (2002) Repeated treatment with antidepressants differentially alters 5-HT1A agonist-stimulated [35S] GTPγS binding in rat brain regions. Neuropharmacology 42:1031–1038. [DOI] [PubMed] [Google Scholar]
- 41.Watts SW (1998) Activation of the mitogen-activated protein kinase pathway via the 5-HT2A receptor. Ann NY Acad Sci 861:162–168. [DOI] [PubMed] [Google Scholar]
- 42.Robertson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD (1999) The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 19:4337–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA (1997) Protein kinase C in the postmortem brain of teenage suicide victims. Neurosci Lett 228:111–114. [DOI] [PubMed] [Google Scholar]
- 44.Reiach JS, Li PP, Warsh JJ, Kish SJ, Young LT (1999) Reduced adenylyl cyclase immunolabeling and activity in postmortem temporal cortex of depressed suicide victims. J Affect Discord 56:141–151. [DOI] [PubMed] [Google Scholar]
- 45.Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, Palkovits M, Sarosi A, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2004) Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry 55:234–243. [DOI] [PubMed] [Google Scholar]
- 46.Shelton RC, Manier DH, Lewis DA (2009) Protein kinase A and C in postmortem prefrontal cortex from persons with major depression and normal controls. Int J neuropsychopharmacol 12:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Qi Y, Cheng Z, Zhu X, Fan C, Yu SY (2016) The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience 322:358–369. [DOI] [PubMed] [Google Scholar]
- 48.Fan J, Wei W, Liao X, Wang S (2017) Chronic social defeat stress leads to changes of behaviour and memory-associated proteins of young mice. Behav Brain Res 316:136–144. [DOI] [PubMed] [Google Scholar]
- 49.Branski P, Palucha A, Szewczyk B, Wieronska JM, Pilc A, Nowak G (2008) Antidepressant-like activity of 8-Br-cAMP, a PKA activator, in the forced swim test. J Neural Transm (Vienna) 115:829–830. [DOI] [PubMed] [Google Scholar]
- 50.Zeni AL, Zomkowski AD, Maraschin M, Rodrigues AL, Tasca CI (2012) Involvement of PKA, CaMKII, PKC, MAPK/ERK and PI3K in the acute antidepressant-like effect of ferulic acid in the tail suspension test. Pharmacol Biochem Behav 103:181–186. [DOI] [PubMed] [Google Scholar]
- 51.Manosso LM, Moretti M, Ribeiro CM, Goncalves FM, Leal RB, Rodrigues ALS (2015) Antideppressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation. Prog Neuropsychopharmacol Biol Psychiatry 59:59–67. [DOI] [PubMed] [Google Scholar]
- 52.Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS (2010) A negative regulator o MAP kinase causes depressive behavior. Nat Med 16:1328–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Wang H, Zhang R, Wang H, Peng Z, Sun R, Tan Q (2012) Microinjection of sanguinarine into the ventrolateral orbital cortex inhibits Mkp-1 and exerts an antidepressant-like effect in rats. Neurosci Lett 506:327–331. [DOI] [PubMed] [Google Scholar]
- 54.Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G (2003) The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 23:7311–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS (2007) A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry 61:661–670. [DOI] [PubMed] [Google Scholar]
- 56.Tronson NC, Schrick C, Fischer A, Sananbenesi F, Pages G, Pouyssegur J, Radulovic J (2008) Regulatory mechanisms of fear extinction and depression-like behavior. Neuropsychopharmacology 33:1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todorovic C, Sherrin T, Pitts M, Hippel C, Rayner M, Spiess J (2009) Suppression of the MEK/ERK signaling pathway reverses depression-like behaviors of CRF2-deficient mice. Neuropsychopharmacology 34:1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz JR, Dal-Pizzol F, Quevedo J (2014) MAPK signaling correlates with the antidepressant effects of ketamine. J Psychiatr Res 55:15–21. [DOI] [PubMed] [Google Scholar]
- 59.Qi X, Lin W, Wang D, Pan Y, Wang W, Sun M (2009) A role for the extracellular signal-regulated kinase signal pathway in depressive-like behavior. Behav Brain Res 199:203–209. [DOI] [PubMed] [Google Scholar]
- 60.Saba-El-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S (2003) An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep 4:964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satoh Y, Endo S, Nakata T, Kobayashi Y, Yamada K, Ikeda T, Takeuchi A, Hiramoto T, Watanabe Y, Kazama T (2011) ERK2 contributes to the control of social behaviors in mice. J Neurosci 31:11953–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA (2005) Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem 93:1551–1560. [DOI] [PubMed] [Google Scholar]
- 63.Chen YH, Zhang RG, Xue F, Wang HN, Chen YC, Hu GT, Peng Y, Peng ZW, Tan QR (2015) Quetiapine and repetitive transcranial magnetic stimulation ameliorate depression-like behaviors and up-regulate the proliferation of hippocampal-derived neural stem cells in a rat model of depression: the involvement of the BDNF/ERK signal pathway. Pharmacol Biochem Behav 136:39–46. [DOI] [PubMed] [Google Scholar]
- 64.Islam MR, Moriquchi S, Tagashira H, Fukunaga K (2014) Revastigmine improves hippocampal neurogenesis and depression-like behaviors via 5-HT1A receptor stimulation in olfactory bulbectomized mice. Neuroscience 272:116–230. [DOI] [PubMed] [Google Scholar]
- 65.Liu D, Zhang Q, Gu J, Wang X, Xie K, Xian X, Wang J, Jiang H, Wang Z (2014) Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 49:21–29. [DOI] [PubMed] [Google Scholar]
- 66.Yan T, Wu B, Liao ZZ, Liu B, Zhao X, Bi KS, Jia Y (2016) Brain-derived neurotrophic factor signaling mediates the antidepressant-like effect of the total flavonoids of alpiniae oxyphyllae fructus in chronic unpredictable mild stress mice. Phytother Res 30:1493–1502. [DOI] [PubMed] [Google Scholar]
- 67.Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L, Xu T, Wang S, Yu L, Liu D, Zhan R, Yu SY (2012) Curcumin produces antidepressant effects via activating MAPK/ERK-dependent brain-derived neurotrophic factor expression in the amygdala of mice. Behav Brain Res 235:67–72. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Luo Y, Zhang R, Shi H, Zhu W, Shi J (2015) Neuropeptide trefoil factor 3 reverses depressive-like behaviors by activation of BDNF-ERK-CREB signaling in olfactory bulbectomized rats. Int J Mol Sci 16:28386–28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li CF, Chen XM, Chen SM, Mu RH, Liu BB, Luo L, Liu XL, Geng D, Liu D, Yi LT (2016) Activation of hippocampal BDNF signaling is involved in the antidepressant-like effect of the NMDA receptor antagonist 7-chlorokynurenic acid. Brain Res 1630:73–82. [DOI] [PubMed] [Google Scholar]
- 71.Domin H, Szewczyk B, Pochwat B, Wozniak M, Smialowska M (2017) Antidepressant-like activity of the neuropeptide Y Y5 receptor antagonist Lu AA33810: behavioral, molecular, and immunohistochemical evidence. Psychopharmacology (Berl) 234:631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goncalves FM, Neis VB, Rieger DK, Lopes MW, Heinrich IA, Costa AP, Rodrigues ALS, Kaster MP, Leal RB (2017) Signaling pathways underlying the antidepressant-like effect of inosine in mice. Purinergic Signal 13:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pochwat B, Rafalo-Ulinska A, Domin H, Misztak P, Nowak G, Szewczyk B (2017) Involvement of extracellular signal-regulated kinase (ERK) in the short and long-lasting antidepressant-like activity of NMDA receptor antagonist (zinc and Ro 25–6981) in the forced swim test in rats. Neuropharmacology 125:333–342. [DOI] [PubMed] [Google Scholar]
- 74.Sawamoto A, Okuyama S, Amakura Y, Yoshimura M, Yamada T, Yokogoshi H, Nakajima M, Furukawa Y (2017) 3,5,6,7,8,3',4',-Heptamethoxyflavone ameliorates depressive-like behavior and hippocampal neurochemical changes in chronic unpredictable mild stressed mice by regulating the brain-derived neurotrophic factor: requirement for ERK activation. Int J Mol Sci 18:E2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L (2004) Glutamate signaling to Ras-MAPK in striatal neurons. Mol Neurobiol 29:1–14. [DOI] [PubMed] [Google Scholar]
- 76.Xing J (1996) Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959–963. [DOI] [PubMed] [Google Scholar]
- 77.Yamada S, Yamamoto H, Qzawa H, Riederer P, Saito T (2003) Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J Neural Transm 110:671–680. [DOI] [PubMed] [Google Scholar]
- 78.Laifenfeld D, Karry R, Grauer E, Klein E, Ben-Shachar D (2005) Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis 20:432–441. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y, Ku BS, Tie L, Yao HY, Jiang WG, Ma X, Li XJ (2006) Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res 1122:56–64. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi K, Nakagawasai O, Nemoto W, Odaira T, Arai Y, Hisamitsu T, Tan-No K (2017) Time-dependent role of prefrontal cortex and hippocampus on cognitive improvement by aripiprazole in olfactory bulbectomized mice. Eur Neuropsychopharmacol 27:1000–1010. [DOI] [PubMed] [Google Scholar]
- 81.Dowlatshahi D, MacQueen GM, Wang JF, Young LT (1998) Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet 352:1754–1755. [DOI] [PubMed] [Google Scholar]
- 82.Thomas GM, Huganir RL (2004) MAPK cascade signaling and synaptic plasticity. Nat Rev Neurosci 5:173–183. [DOI] [PubMed] [Google Scholar]
- 83.Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M (2004) Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology 29:1831–1840. [DOI] [PubMed] [Google Scholar]
- 84.Yuan S, Jiang X, Zhou X, Zhang Y, Teng T, Xie P (2018) Inosine alleviates depression-like behavior and increases the activity of the ERK-CREB signaling in adolescent male rats. Neuroreport 29:1223–1229. [DOI] [PubMed] [Google Scholar]
- 85.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniquez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ (2009) CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci 12:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt HD, Banasr M, Duman RS (2008) Future antidepressant targets: neurotrophic factors and related signaling cascades. Drug Discov Today Ther Strateg 5:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith MA, Makino S, Kvetnansky R, Post RM (1995) Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15:1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nibuya M, Takahashi M, Russell DS, Duman RS (1999) Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett 267:81–84. [DOI] [PubMed] [Google Scholar]
- 89.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2003) Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60:804–815. [DOI] [PubMed] [Google Scholar]
- 90.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R (2005) Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Mol Brain Res 136:29–37. [DOI] [PubMed] [Google Scholar]
- 91.First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A (2013) The effects of reboxetine treatment on depression-like behavior, brain neurotrophins, and ERK expression in rats exposed to chronic mild stress. J Mol Neurosci 50:88–97. [DOI] [PubMed] [Google Scholar]
- 92.Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM (2009) Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry 14:681–695. [DOI] [PubMed] [Google Scholar]
- 93.Sheline Y, Wany P, Gado MH, Csemansky JG, Vannier MW (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bremner J, Narayan M, Anderson ER, Staib LH, Miller H, Charney DS (2000) Smaller hippocampal volume in major depression. Am J Psychiatry 157:115–117. [DOI] [PubMed] [Google Scholar]
- 95.Ge JF, Gao WC, Cheng WM, Lu WL, Tang J, Peng L, Li N, Chen FH (2014) Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur Neuropsychopharmacol 24:172–180. [DOI] [PubMed] [Google Scholar]
- 96.Sawamoto A, Okuyama S, Yamamoto K, Amakura Y, Yoshimura M, Nakajima M, Furukawa Y (2016) 3,5,6,7,8,3',4',-Heptamethoxyflavonel, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules 21:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ye M, Ke Y, Liu B, Yuan Y, Wang F, Bu S, Zhang Y (2017) Root bark of morus alba ameliorates the depression-like behavior in diabetic rats. Neurosci Lett 637:136–141. [DOI] [PubMed] [Google Scholar]
- 98.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT (2001) Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50:260–265. [DOI] [PubMed] [Google Scholar]
- 99.Wu R, Tao W, Zhang H, Xue W, Zou Z, Wu H, Cai B, Doron R, Chen G (2016) Instant and persistent antidepressant response of gardenia yellow pigment is associated with acute protein synthesis and delayed upregulation of BDNF expression in the hippocampus. ACS Chem Neurosci 7:1068–1076. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Ge JF, Wang FF, Liu F, Shi C, Li N (2017) Crassifoside H improve the depressive-like behavior of rats under chronic unpredictable mild stress: possible involved mechanisms. Brain Res Bull 135:77–84. [DOI] [PubMed] [Google Scholar]
- 101.Zhao J, Luo D, Liang Z, Lao L, Rong J (2017) Plant natural product puerarin ameliorates depressive behaviors and chronic pain in mice with spared nerve injury (SNI). Mol Neurobiol 54:2801–2812. [DOI] [PubMed] [Google Scholar]
- 102.Sawamoto A, Okuyama S, Amakura Y, Yamada R, Yoshimura M, Nakajima M, Furukawa Y (2018) Sansoninto as evidence-based remedial medicine for depression-like behavior. J Nat Med 72:118–126. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt HD, Duman RS (2010) Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35:2378–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siuciak JA, Lewis DR, Wiegand SJ, Lundsay R (1997) Antidepressant-like effect of brain derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 56:131–137. [DOI] [PubMed] [Google Scholar]
- 105.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sirianni RW, Olausson P, Chiu AS, Taylor JR, Saltzman WM (2010) The behavioral and biochemical effects of BDNF containing polymers implanted in the hippocampus of rats. Brain Res 1321:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E (2003) Activation of the TrkB neurotrophic receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ (2004) Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA 101:10827–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS (2006) Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin HJ, Pei L, Li YN, Zheng H, Yang S, Wan Y, Mao L, Xia YP, He QW, Li M, Yue ZY, Hu B (2017) Alleviative effects of fluoxetine on depressive-like behaviors by epigenetic regulation of BDNF gene transcription in mouse model of post-stroke depression. Sci Rep 7:14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marsden WN (2013) Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropscyhopharmacol Biol Psychiatry 43:168–184. [DOI] [PubMed] [Google Scholar]
- 112.Boggio EM, Putignano E, Sassoe-Pognetto M, Pizzorusso T, Glustetto M (2007) Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS One 2:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Casar B, Arozarena I, Sanz-Moreno V, Pinto A, Agudo-Ibanez L, Marais R, Lewis RE, Berciano MT, Crespo P (2009) MTRas subcellular localization defines extracellular signal-regulated kinase 1 and 2 substrate specificity through distinct utilization of scaffold proteins. Mol Cell Biol 29:1338–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ (1995) Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci 15:1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ (2013) Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res 1494:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki T, Mitake S, Murata S (1999) Presence of up-stream and downstream components of a mitogen-activated protein kinase pathway in the PSD of the rat forebrain. Brain Res 840:36–44. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki T, Okumura-Noji K, Nishida E (1995) ERK2-type mitogen-activated protein kinase (MAPK) and its substrates in postsynaptic density fractions from the rat brain. Neurosci Res 22:277–285. [DOI] [PubMed] [Google Scholar]