Abstract
Azo dyes are the largest class of synthetic dyes and are utilized in several industries. Effluents containing dyes are released to the environment and pose harm to humans who might be exposed to these contaminants. This study aims to investigate the removal of methylene blue (MB) dye using duckweed (Lemna minor). L. minor (2 g) was exposed into 50 mg/L of MB dyes for 24 h. The absorbance values were measured at 0, 0.5, 1, 2, 3, 4, 5, 6, and 24 h with a maximum wavelength of 665 nm. The dye removal percentage and relative growth rate of L. minor during exposure to MB were observed. The removal percentage was 80.56 ± 0.44% for 24 h with a relative growth rate of 0.006/h. L. minor has potential as a phytoremediation agent to remove dyes from wastewater.
Keywords: Environmental engineering, Environmental science, Green engineering, Waste treatment, Water pollution, Aquatic plants, Azo dyes, Biosorption, Decolorization, Relative growth rate, Wastewater
1. Introduction
The utilization of azo dyes in industries can potentially lead to dye waste (Chung, 2016; Sarkar et al., 2017; Tahir et al., 2016). Azo dyes are the largest class of synthetic dyes (Brüschweiler and Merlot, 2017; Chung, 2016). Effluent containing dyes is released to the environment, thereby seriously affecting the environment by damaging ecosystems, causing water pollution, and reducing light penetration for aquatic ecosystems (Al Farraj et al., 2019). Azo dyes are also toxic and carcinogenic, thereby possibly affecting human health and living organisms (Khataee et al., 2012).
Three categories of technology are commonly used to degrade dyes, namely, chemical, physical, and biological (Al Farraj et al., 2019; El Hassani et al., 2019; Hadibarata et al., 2018; Imron and Titah, 2018). Chemical processes generally involve the use of chemicals via the oxidation process to degrade dyes (Hassaan et al., 2017). However, the by-products of the chemical process, such as sludge, require further processing, because they also contain hazardous chemicals. In addition, the chemical process requires high costs, even if it has low efficiency to degrade dyes. Physical processes, such as coagulation-flocculation, adsorption, ion exchange, and membrane filter, release a large amount of sludge as a by-product, consume a high amount of energy, and require high costs (Azimi et al., 2017; Verma et al., 2012). The biological process is more suitable for degrading dyes than chemical and physical processes; biological process produces nontoxic by-products, such as CO2 and H2O (Al Farraj et al., 2019; Imron et al., 2019a; Titah et al., 2019). Moreover, the biological process is eco-friendly, effective, easy to operate, and releases nontoxic by-products to the environment. The biological process utilizes living organisms, such as microorganisms and plants, to transform pollutants via the metabolic pathway (Imron et al., 2019b).
The use of plants as bioremediation agents is very promising for degrading various dyes and organic and inorganic pollutants (Ali, 2010; Singh and Singh, 2017). Plants are very sensitive to polluted environments, including organic and inorganic pollutants (Purwanti et al., 2018; Tangahu et al., 2019; Titah et al., 2018). Plants have the potential to degrade textile dyes (Khataee et al., 2012). Some aquatic plants, such as Azolla pinnata and Lemna minor, have the potential to degrade azo dyes (Al-Baldawi et al., 2018; Khataee et al., 2012; Reema et al., 2011). Based on Al-Baldawi et al. (2018), the maximum degradation efficiency of methylene blue (MB) is 90%, which can be achieved by A. pinnata for 5 days at 25 mg/L initial concentration. According to Khataee et al. (2012), L. minor can degrade Acid Blue 92 dyes up to 80% for 6 days. Zhou and Xiang (2013) reported that Medicago sativa L. and Sesbania cannabina Pers are salt-plants that have the potential to degrade azo dyes.
MB is a heterocyclic aromatic used in biological and chemical industries, textile industries, and medicine (Al-Baldawi et al., 2018; Contreras et al., 2019). MB dye produces serious effects, such as headache, vomiting, and high blood pressure when consumed by humans. However, studies on the phytoremediation of MB dye using duckweed (L. minor) are lacking in the scientific literature. To fill this research gap, this study aims to investigate the removal of MB dye using duckweed (L. minor). The results of this study will be useful for industries. Alternative biotechnology using plants can be used to treat dye-contaminated water.
2. Materials and methods
2.1. Plant cultivation
L. minor was collected from Surabaya River, Indonesia. The plant was acclimatized for 7 days before the main experiment. All used plants were healthy, as indicated by no-withered leaves on plants.
2.2. Dye analysis
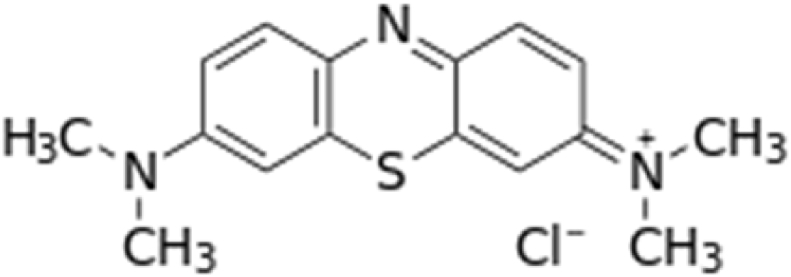
Methylene Blue (C16H18N3SCl.3H2O) (Merck, Germany) was used as the pollutant to be removed in this experiment. Its characteristics and chemical structure are presented in Fig. 1 and Table 1. The initial pH of the dyes solution ranged from 6.1 to 7.9, and the initial temperature was ranged from 28.6 °C to 30.3 °C in all reactors. At several time intervals of the biological treatment, the remaining MB was measured with UV/Vis Microplate Spectrophotometer (Thermo Fisher Scientific, USA) at maximum absorption wavelength, λmax = 665 nm (Al-Baldawi et al., 2018; Low et al., 2012).
Fig. 1.
Chemical structure of methylene blue.
Table 1.
Characteristics of methylene blue.
| Parameters | Properties |
|---|---|
| Molecular formula | C16H18N3SCl |
| Molecular weight (g/mol) | 319.851 |
| Colour index number | 52015 |
| Density (g/mL) | 1.0 |
| Melting point (°C) | 190 |
| λmax (nm) | 665 |
2.3. Decolorization experiments
Decolorization experiments were conducted in a glass container containing 50 mL of MB at 50 mg/L (Pego et al., 2017). Two grams of L. minor (Liu et al., 2018) were exposed to MB-contaminated water at room temperature (20 °C–25 °C) under white lamp light at the approximation of 10,000–25,000 lux (Al-Baldawi et al., 2018). The reactor was designed to replicate the floating treatment wetland under batch system reactor (Lucke et al., 2019). The researchers ensured that all plant roots reached the bottom of the reactor during the test to accommodate the root sorption capability and the rhizosphere removal mechanisms (Almuktar et al., 2018).
Samples (1 mL) were collected every 0, 0.5, 1, 2, 3, 4, 5, 6, and 24 h to measure the absorbance value. The absorbance value was analyzed using UV/Vis Microplate Spectrophotometer (Thermo Fisher Scientific, USA) at maximum absorption wavelength, λmax = 665 nm with distilled water used as blank (Al-Baldawi et al., 2018; Low et al., 2012). Before the absorbance was measured, the sample was filtered using 40 μm Wattman filter paper to remove unwanted particles. All measurements were conducted in three replicates. For further clarification, the hourly absorbance of MB (50 mg/L) in contaminated water in various wavelengths (400–750 nm) after treatment with L. minor was also determined using UV/Vis spectra (Al Farraj et al., 2019). The removal of MB was approached by using absorbance analysis in terms of decolorization. The decolorization percentage was calculated based on Eq. (1) (Al Farraj et al., 2019; Khataee et al., 2012), as follows:
| (1) |
where,
A0 = the initial absorbance at 0 hour
At = the final absorbance at t-hour
2.4. Growth rate determination
The plant growth rate was calculated using the relative growth rate (RGR) equation given in Eq. (2) based on Radić et al. (2011). RGR equation was determined based on increasing fresh weight (FW) after exposure by MB. All measurements were carried out in three replicates.
| (2) |
where,
FW0 = the initial weight at 0 hour (g)
FWt = the final weight at t-hour (g)
t = time exposed (h)
2.5. Statistical analysis
The results of this experiment were analyzed by one-way analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons test using Minitab 16 Statistical software to determine the significance of the result with α = 0.05. All data were presented as the average ±standard deviation (SD) of three replicates. The different letters above the graph indicate a significant difference among the results.
3. Results and discussion
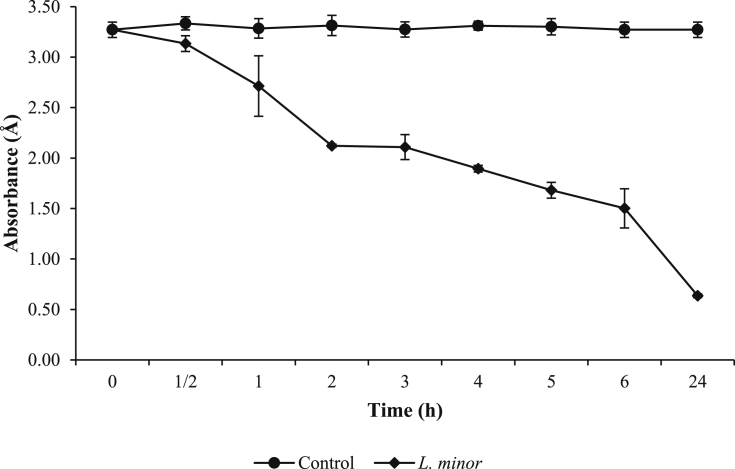
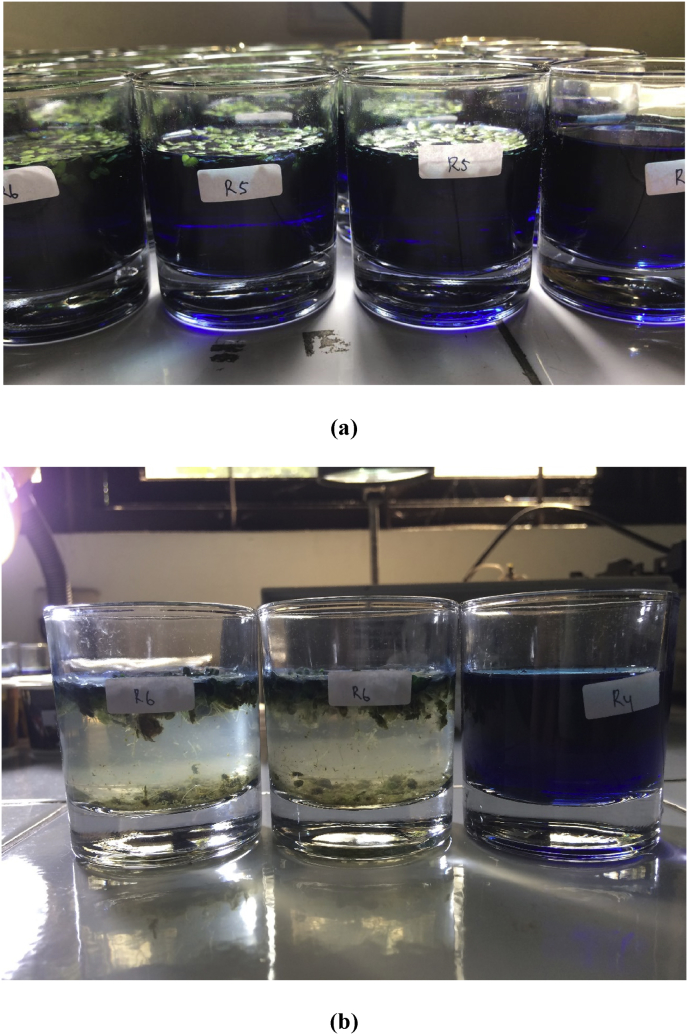
L. minor‘s removal of MB (50 mg/L) was investigated for 24 h. The results of the absorbance values after treatment by L. minor are shown in Fig. 2. Based on Fig. 2, the absorbance values were 3.27 ± 0.08 Å at 0 h and decreased to 3.13 ± 0.08, 2.71 ± 0.3, 2.12 ± 0.02, 2.11 ± 0.12, 1.90 ± 0.03, 1.68 ± 0.08, 1.50 ± 0.19, and 0.64 ± 0.02 Å at 0.5, 1, 2, 3, 4, 5, 6, and 24 h, respectively. The absorbance value decreased with increasing duration of exposure. The physical observed reactor can be seen in Fig. 3. The absorbance value of control did not decrease. The decrease of absorbance on the main reactors indicated that the MB dyes were decolorized by L. minor. Reema et al. (2011) reported that the dye uptake capacity increased with increasing contact time. Based on the decreasing absorbance value, it was indicated that L. minor can adsorb and accumulate MB. Similar to results obtained by Reema et al. (2011) and Khataee et al. (2012), L. minor removed azo dye-contaminated water by up to 80% for 3 days.
Fig. 2.
The absorbance of methylene blue (MB) after water contaminated with 50 mg/L of MB dye was treated with L. minor for 24 hours. Values are presented in the average of triplicates ±SD.
Fig. 3.
Physical observation of methylene blue before (a) and after (b) L. minor treatment for 24 hours.
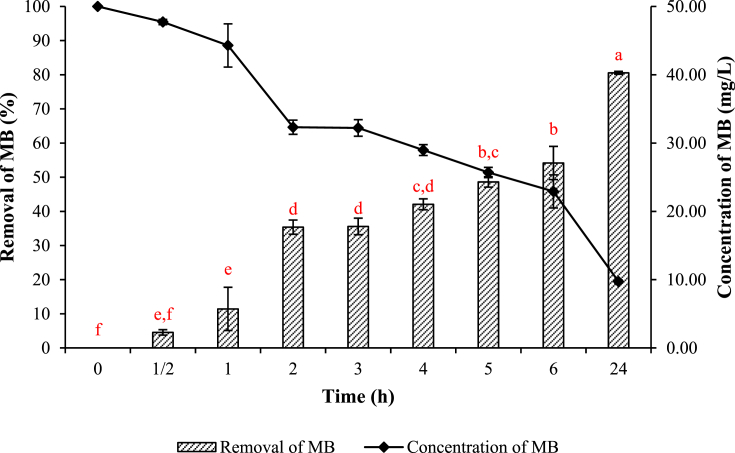
The removal of MB by L. minor and the remaining concentration are calculated and presented in Fig. 4. It shows that the removal percentage of MB dye was 0 ± 0.00% at 0 h and increased to 4.56 ± 0.79, 11.43 ± 6.33, 35.36 ± 2.08, 35.58 ± 2.43, 42.05 ± 1.59, 48.62 ± 1.51, 54.16 ± 4.86, and 80.56 ± 0.44% at 0.5, 1, 2, 3, 4, 5, 6, and 24 h, respectively. The concentration of MB at 0 h was 50 mg/L and decreased to 47.72 ± 0.39, 44.29 ± 3.16, 32,32 ± 1.04, 32.21 ± 1.22, 28.97 ± 0.79, 25.69 ± 0.76, 22.92 ± 2.43, and 9.72 ± 0.22% at 0.5, 1, 2, 3, 4, 5, 6, and 24 h, respectively. Based on Fig. 4, the contact times at 0.5 h compared with 1 h, at 2 h compared with 4 h, and at 5 h compared with 6 h were not significantly different in terms of MB removal (p < 0.05). However, at 6 h compared with 24 h, the removal of MB showed a significant difference (p < 0.05). The removal of MB was increased with increasing exposure time (Pathania et al., 2017; Vieira et al., 2009). It fact, increasing the exposure time of the dye on plants increases the probability of contact between dye molecules and plant surface (Khataee et al., 2012, 2010; Tangahu et al., 2019). The surface area for sorption of the dye molecule is greater when the exposure time is increased. This result was in agreement with that obtained by Nasrullah et al. (2015) and Pathania et al. (2017), in which the increasing incubation time resulted in high decolorization efficiency.
Fig. 4.
Removal and concentration of methylene blue (MB) after water contaminated with 50 mg/L of MB was treated with L. minor for 24 hours. Values are presented in the average of triplicates ±SD. Different letters (a, b, c, d, e, and f) indicate significant differences among the results (p < 0.05).
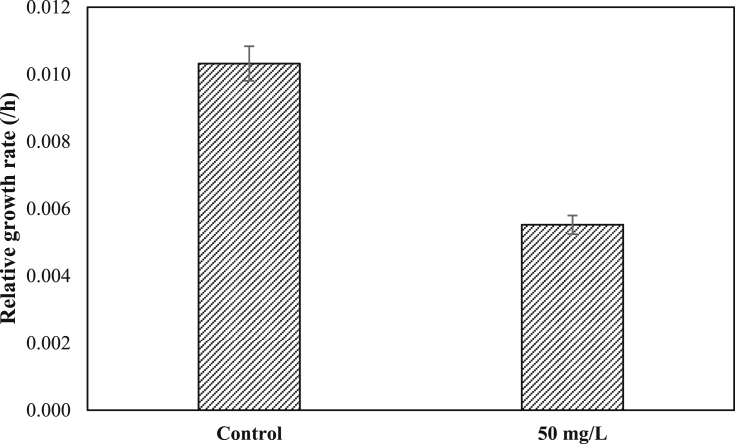
The relative growth rate (RGR) was observed for 24 h of exposure time and can be seen in Fig. 5. Based on Fig. 5, the RGR was affected by 50 mg/L of MB compared with the control. The RGR value of L. minor in 50 mg/L MB dye-contaminated water is 0.006/h, whereas this was 0.01/h in the control. Based on the results, MB had a negative effect on RGR, i.e., decreased by 46.52% compared with the control, after 24 h of exposure. The MB affected the growth rate of the plant. The RGR value decreased with increasing dye concentration (Wu, 2016). Khataee et al. (2012) showed that the RGR value of L. minor decreased by 68.8% after exposure to AB92 dyes. The same effect was also reported by Cleuvers and Ratte (2002) and Reema et al. (2011) who showed that the growth of L. minor was strongly inhibited after exposure to MB and Brilliant Blue spezial.
Fig. 5.
Relative growth rate of L. minor on 50 mg/L of MB dye.
The final pH in all reactor ranged from 6 to 6.8, and the final temperature ranged from 28.6 °C to 29 °C. The observed pH decreased throughout the 24 h research period, whereas the observed temperature was not significantly altered. The decreasing in pH occurred due to the secretion of some plant metabolites. The initial step of the degradation mechanism of MB is the cleavage of the bonds of C–S=C into two benzene rings. The hydroxylation process of an aromatic ring produced a phenolic compound instead of a toxic aromatic compound (Pandey et al., 2007; Sarkar et al., 2017). The phenolic compound is important in biodegradation related to antioxidant and enzyme properties (Khataee et al., 2012). Afterward, the enzyme produced by plants oxidized the phenolic compounds to generate a phenoxy radical and carbonium ion (Singh et al., 2015; Sudha et al., 2014). The production and secretion of these ions increased the acidity of the living environment, thereby resulting in lower observed pH at the end of the test period.
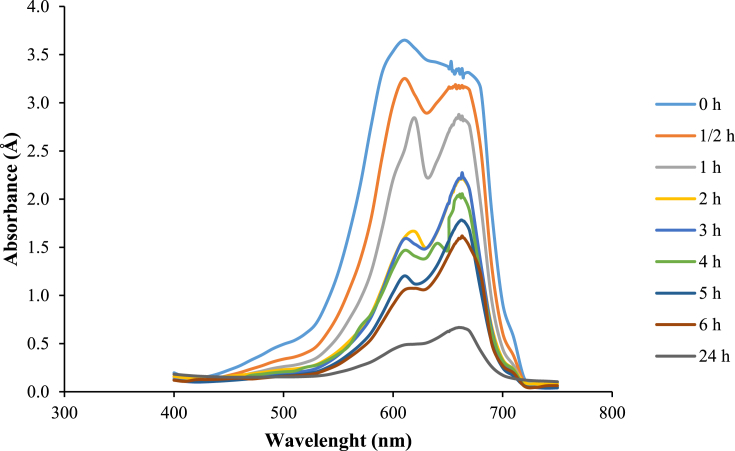
The approximate approach to the removal of MB by bio-decolorization process can be examined using UV/Vis spectra based on Al Farraj et al. (2019) and as presented in Fig. 6. It is clear that the peak at λ = 665 nm shows a significant decrease. Furthermore, the major absorbance peak can be decreased up to 80.56 ± 0.44% for 24 h after L. minor treatment. Based on Fig. 6, the cleavage of the aromatic ring on MB was examined at peaks λ = 630 nm and λ = 700 nm (Al Farraj et al., 2019) and decreased after L. minor treatment throughout the test period. The decrease of peaks throughout the test period can indicate the decrease of the aromatic ring in the solution. The spectra within 600–630 nm can indicate the formation of a new metabolite. Similar to Khataee et al. (2012), L. minor degraded azo dye into intermediates compounds, such as phenol and benzoic acid, which were analyzed using GC-MS. Reema et al. (2011) showed FTIR results indicating that C = O carbonyl in carboxylic groups, amine group N–H, and phosphonate group P–O contained in MB are bound to the surface of L. minor.
Fig. 6.
UV spectra data of methylene blue (MB) in various wavelengths after L. minor treatment in water contaminated by 50 mg/L of MB dye.
In a floating wetland system, the rhizosphere of plants that have a direct contact with wastewater plays an important role in some removal mechanisms. Important mechanisms underlying the removal of dyes with phytoremediation include phyto-uptake, phyto-stimulation, rhizodegradation, phyto-extraction, phyto-degradation, and phyto-volatilization (Chandra et al., 2017; Tangahu et al., 2011). Three of the mentioned mechanisms (phyto-uptake, phyto-stimulation, and rhizodegradation) occurred in the rhizosphere. During treatment, the pollutant is adsorbed by plants via phyto-uptake mechanism through the roots; this is the main reason why the length of the root should reach the bottom of the reactor (Tangahu et al., 2011). Plants also secrete some chemical compounds known as exudates to initiate and support the root bacterial community to perform degradation (phyto-stimulation). The exudate can also perform direct degradation if the plants have the capability to secrete degradation enzyme/metabolites (rhizodegradation) (Chandra et al., 2017). The adsorbed compound then accumulated inside plants cell in any location regardless of the type of pollutant and the capability of plants to perform phyto-extraction (Garbisu and Alkorta, 2001). Despite only being accumulated, several compounds enter internal enzymatic reactions and are converted into useful compound for plants metabolism or into less toxic compounds (phyto-degradation) (Al-Baldawi et al., 2015). The product of complete enzymatic degradation will be volatilized by plants as a volatile form into the atmosphere (phyto-volatilization) (Limmer and Burken, 2016).
Although studies on enzymatic removal mechanism of MB by plants are lacking, several studies have elucidated the degradation of MB through phytoremediation. Aquatic plants use MB as their sole carbon or nitrogen source (Sarkar et al., 2017; Sudha et al., 2014), because it contains carbon bonds. Geoffroy et al. (2004) reported that during the degradation of dye by plants and microorganism, different enzymes convert the pollutant into a less toxic compound. According to Paczkowska et al. (2007), L. minor can produce superoxide dismutase (SOD), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX). The presence of these enzymes in the medium is seen in L. minor‘s response to contaminants; these enzymes can be used as bioindicators of stress caused by pollutants (Tovar-Sánchez et al., 2019). Superoxide containing MB dye will be converted to H2O2 by SOD, and reactive oxygen species (ROS) are released (Dikalov and Harrison, 2014). This by-product will stimulate another enzyme, such as catalase (CAT) in L. minor that detoxifies ROS (Sofo et al., 2015). The activities of enzymes produced by plants in the medium were used to increase the resistance of plant to pollutants (Nigam, 2013; Ojuederie and Babalola, 2017). The activities of these enzymes in the biodegradation process depend on the types of plants and dyes (Chen, 2006; Karigar and Rao, 2011).
The utilization of aquatic plants as phytoremediation agent of dyes is considered an environmentally friendly technology. Phytoremediation of dyes using aquatic plants has gained attention as an alternative technology due to its low operational cost, eco-friendliness, nontoxic by-products, and low amount of sludge production. These findings were limited to one type of dye and one type of aquatic plant. Thus, further study on different wastewater, combination of different types of aquatic plants, and the extended period of treatment is needed. These findings will be very helpful for industries and an introduce an alternative biotechnology to treat dye-contaminated wastewater using plants (phytoremediation).
4. Conclusion
The removal of Methylene Blue (MB) by Lemna minor is significantly affected by the contact time. L. minor showed a significant removal increase from 3.27 ± 0.08 Å to 0.64 ± 0.02 Å after 24 h. Physical observation showed a clear indication of bio-decolorization by L. minor after 24 h of exposure to the dye. UV/Vis spectra analysis also illustrated the decrease of MB absorbance throughout the 24 h of the test period and showed up to 80.56 ± 0.44% of decolorization. The decolorization of MB by L. minor increased with increasing of exposure time. Our findings suggest that L. minor has the potential to be used as phytoremediation agent in treating dye-contaminated wastewater. Further study related to different dyes types, combination of different aquatic plants, and extended time exposure will greatly contribute to the knowledge on phytoremediation of dye-contaminated wastewater.
Declarations
Author contribution statement
Muhammad Fauzul Imron: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Setyo Budi Kurniawan: Analyzed and interpreted the data; Wrote the paper.
Agoes Soegianto: Contributed reagents, materials, analysis tools or data.
Febri Eko Wahyudianto: Conceived and designed the experiments; Performed the experiments.
Funding statement
This work was supported by the Faculty of Science and Technology, Universitas Airlangga through the scheme of RKAT 2019 No. 2420/UN3.1.8/LT/2019.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Al-Baldawi I.A., Abdullah S.R.S., Anuar N., Hasan H.A. Phytotransformation of methylene blue from water using aquatic plant ( Azolla pinnata ) Environ. Technol. Innov. 2018;11:15–22. [Google Scholar]
- Al-Baldawi I.A., Abdullah S.R.S., Anuar N., Suja F., Mushrifah I. Phytodegradation of total petroleum hydrocarbon (TPH) in diesel-contaminated water using Scirpus grossus. Ecol. Eng. 2015;74:463–473. [Google Scholar]
- Al Farraj D.A., Elshikh M.S., Al Khulaifi M.M., Hadibarata T., Yuniarto A., Syafiuddin A. Biotransformation and detoxification of antraquione dye green 3 using halophilic Hortaea sp. Int. Biodeterior. Biodegrad. 2019;140:72–77. [Google Scholar]
- Ali H. Biodegradation of synthetic dyes—a review. Water, Air. Soil Pollut. 2010;213:251–273. [Google Scholar]
- Almuktar S.A.A.A.N., Abed S.N., Scholz M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: a review. Environ. Sci. Pollut. Res. 2018;25:23595–23623. doi: 10.1007/s11356-018-2629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi A., Azari A., Rezakazemi M., Ansarpour M. Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev. 2017;4:37–59. [Google Scholar]
- Brüschweiler B.J., Merlot C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharmacol. 2017;88:214–226. doi: 10.1016/j.yrtph.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Chandra R., Dubey N.K., Kumar V. first ed. CRC Press; Boca Raton: 2017. Phytoremediation of Environmental Pollutants. [Google Scholar]
- Chen H. Recent advances in azo dye degrading enzyme research. Curr. Protein Pept. Sci. 2006;7:101–111. doi: 10.2174/138920306776359786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.-T. Azo dyes and human health: a review. J. Environ. Sci. Heal. C. 2016;34:233–261. doi: 10.1080/10590501.2016.1236602. [DOI] [PubMed] [Google Scholar]
- Cleuvers M., Ratte H.-T. Phytotoxicity of coloured substances: is Lemna Duckweed an alternative to the algal growth inhibition test? Chemosphere. 2002;49:9–15. doi: 10.1016/s0045-6535(02)00193-5. [DOI] [PubMed] [Google Scholar]
- Contreras M., Grande-Tovar C.D., Vallejo W., Chaves-López C. Bio-removal of methylene blue from aqueous solution by Galactomyces geotrichum KL20A. Water. 2019;11:282. [Google Scholar]
- Dikalov S.I., Harrison D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014;20:372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hassani K., Kalnina D., Turks M., Beakou B.H., Anouar A. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst. Separ. Purif. Technol. 2019;210:764–774. [Google Scholar]
- Garbisu C., Alkorta I. Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001;77:229–236. doi: 10.1016/s0960-8524(00)00108-5. [DOI] [PubMed] [Google Scholar]
- Geoffroy L., Frankart C., Eullaffroy P. Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Environ. Pollut. 2004 doi: 10.1016/j.envpol.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Hadibarata T., Syafiuddin A., Al-Dhabaan F.A., Elshikh M.S., Rubiyatno Biodegradation of Mordant orange-1 using newly isolated strain Trichoderma harzianum RY44 and its metabolite appraisal. Bioproc. Biosyst. Eng. 2018;41:621–632. doi: 10.1007/s00449-018-1897-0. [DOI] [PubMed] [Google Scholar]
- Hassaan M.A., El Nemr A., Madkour F.F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 2017;43:11–19. [Google Scholar]
- Imron M.F., Kurniawan S.B., Soegianto A. Characterization of mercury-reducing potential bacteria isolated from Keputih non-active sanitary landfill leachate, Surabaya, Indonesia under different saline conditions. J. Environ. Manag. 2019;241:113–122. doi: 10.1016/j.jenvman.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Imron M.F., Kurniawan S.B., Titah H.S. Potential of bacteria isolated from diesel-contaminated seawater in diesel biodegradation. Environ. Technol. Innov. 2019;14:100368. [Google Scholar]
- Imron M.F., Titah H.S. Optimization of diesel biodegradation by Vibrio alginolyticus using Box-Behnken design. Environ. Eng. Res. 2018;23:374–382. [Google Scholar]
- Karigar C.S., Rao S.S. Role of microbial enzymes in the bioremediation of pollutants: a review. Enzym. Res. 2011;2011:1–11. doi: 10.4061/2011/805187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khataee A.R., Dehghan G., Ebadi A., Zarei M., Pourhassan M. Biological treatment of a dye solution by Macroalgae Chara sp.: effect of operational parameters, intermediates identification and artificial neural network modeling. Bioresour. Technol. 2010;101:2252–2258. doi: 10.1016/j.biortech.2009.11.079. [DOI] [PubMed] [Google Scholar]
- Khataee A.R., Movafeghi A., Torbati S., Salehi Lisar S.Y., Zarei M. Phytoremediation potential of duckweed (Lemna minor L.) in degradation of C.I. Acid Blue 92: artificial neural network modeling. Ecotoxicol. Environ. Saf. 2012;80:291–298. doi: 10.1016/j.ecoenv.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Limmer M., Burken J. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016;50:6632–6643. doi: 10.1021/acs.est.5b04113. [DOI] [PubMed] [Google Scholar]
- Liu C., Gu W., Dai Z., Li J., Jiang H., Zhang Q. Boron accumulation by Lemna minor L. under salt stress. Sci. Rep. 2018;8:8954. doi: 10.1038/s41598-018-27343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low L.W., Teng T.T., Morad N., Azahari B. Studies on the adsorption of methylene blue dye from aqueous solution onto low-cost tartaric acid treated bagasse. APCBEE Procedia. 2012;1:103–109. [Google Scholar]
- Lucke T., Walker C., Beecham S. Experimental designs of field-based constructed floating wetland studies: a review. Sci. Total Environ. 2019;660:199–208. doi: 10.1016/j.scitotenv.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Nasrullah A., Khan H., Khan A.S., Man Z., Muhammad N., Khan M.I., Abd El-Salam N.M. Potential biosorbent derived from calligonum polygonoides for removal of methylene blue dye from aqueous solution. Sci. World J. 2015;2015:1–11. doi: 10.1155/2015/562693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam P. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules. 2013;3:597–611. doi: 10.3390/biom3030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojuederie O., Babalola O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int. J. Environ. Res. Public Health. 2017;14:1504. doi: 10.3390/ijerph14121504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowska M., Kozłowska M., Goliński P. Oxidative stress enzyme activity in Lemna minor L. exposed to cadmium and lead. Acta Biol. Cracov. Ser. Bot. 2007 [Google Scholar]
- Pandey A., Singh P., Iyengar L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007 [Google Scholar]
- Pathania D., Sharma S., Singh P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017;10:S1445–S1451. [Google Scholar]
- Pego M.F.F., Carvalho J., Borges W., Bianchi M.L. Impact of corona treated activated carbon in anionic and cationic dye adsorption. Cerne. 2017;23:219–228. [Google Scholar]
- Purwanti I.F., Simamora D., Kurniawan S.B. Toxicity test of tempe industrial wastewater on cyperus rotundus and scirpus grossus. Int. J. Civ. Eng. Technol. 2018 [Google Scholar]
- Radić S., Stipaničev D., Cvjetko P., Marijanović Rajčić M., Širac S., Pevalek-Kozlina B., Pavlica M. Duckweed Lemna minor as a tool for testing toxicity and genotoxicity of surface waters. Ecotoxicol. Environ. Saf. 2011;74:182–187. doi: 10.1016/j.ecoenv.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Reema R.M., Saravanan P., Kumar M.D., Renganathan S. Accumulation of methylene blue dye by growing Lemna minor. Separ. Sci. Technol. 2011;46:1052–1058. [Google Scholar]
- Sarkar S., Banerjee A., Halder U., Biswas R., Bandopadhyay R. Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv. Sci. Eng. 2017;2:121–131. [Google Scholar]
- Singh P.K., Singh R.L. Bio-removal of azo dyes: a review. Int. J. Appl. Sci. Biotechnol. 2017;5:108–126. [Google Scholar]
- Singh R.L., Singh P.K., Singh R.P. Enzymatic decolorization and degradation of azo dyes - a review. Int. Biodeterior. Biodegrad. 2015 [Google Scholar]
- Sofo A., Scopa A., Nuzzaci M., Vitti A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015;16:13561–13578. doi: 10.3390/ijms160613561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha M., Saranya A., Selvakumar G., Sivakumar N. Microbial degradation of azo dyes: a review. Int. J. Curr. Microbiol. Appl. Sci. 2014 [Google Scholar]
- Tahir U., Yasmin A., Khan U.H. Phytoremediation: potential flora for synthetic dyestuff metabolism. J. King Saud Univ. Sci. 2016;28:119–130. [Google Scholar]
- Tangahu B.V., Ningsih D.A., Kurniawan S.B., Imron M.F. Study of BOD and COD removal in batik wastewater using scirpus grossus and Iris pseudacorus with intermittent exposure system. J. Ecol. Eng. 2019;20:130–134. [Google Scholar]
- Tangahu B.V., Sheikh Abdullah S.R., Basri H., Idris M., Anuar N., Mukhlisin M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011;2011:1–31. [Google Scholar]
- Titah H.S., Abdullah S.R.S., Idris M., Anuar N., Basri H., Mukhlisin M., Tangahu B.V., Purwanti I.F., Kurniawan S.B. Arsenic resistance and biosorption by isolated rhizobacteria from the roots of Ludwigia octovalvis. Int. J. Microbiol. 2018;2018:1–10. doi: 10.1155/2018/3101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titah H.S., Purwanti I.F., Tangahu B.V., Kurniawan S.B., Imron M.F., Abdullah S.R.S., Ismail N. Izzati. Kinetics of aluminium removal by locally isolated Brochothrix thermosphacta and Vibrio alginolyticus. J. Environ. Manag. 2019;238:194–200. doi: 10.1016/j.jenvman.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Tovar-Sánchez E., Suarez-Rodríguez R., Ramírez-Trujillo A., Valencia-Cuevas L., Hernández-Plata I., Mussali-Galante P. Environmental Biosensors. IntechOpen. 2019. The use of biosensors for biomonitoring environmental metal pollution. [Google Scholar]
- Verma A.K., Dash R.R., Bhunia P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012;93:154–168. doi: 10.1016/j.jenvman.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Vieira A.P., Santana S.A.A., Bezerra C.W.B., Silva H.A.S., Chaves J.A.P., de Melo J.C.P., da Silva Filho E.C., Airoldi C. Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp. J. Hazard Mater. 2009;166:1272–1278. doi: 10.1016/j.jhazmat.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Wu H. Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta) BioMed Res. Int. 2016;2016:1–8. doi: 10.1155/2016/7383918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Xiang X. Effect of different plants on azo-dye wastewater bio-decolorization. Procedia Environ. Sci. 2013;18:540–546. [Google Scholar]