Abstract
Background
Acute promyelocytic leukemia (APL) is commonly characterized by the fusion of retinoic acid receptor alpha (RARA) with promyelocytic leukemia (PML). Most APL patients acquire long-term survival after treatment with all-trans retinoic acid (ATRA) or arsenic agents-based chemotherapy.
Case presentation
A rare case of APL was reported after IRF2BP2-RARA was detected in the relapsed process using next-generation RNA-sequencing analysis. In addition, the mutation of NRAS was also detected. ATRA and arsenic trioxide combined with daunorubicin were used during induction treatment. The patient acquired complete remission but relapsed in 12 months. The patient was resistant to all other chemotherapies and refused any further therapy. The literature review indicated that allogeneic hematopoietic stem cell transplantation might be a therapeutic method to treat APL with IRF2BP2-RARA fusion.
Conclusion
Atypical APL should be considered even if the patients present with normal chromosomal karyotype and no classic PML-RARA fusions, but classical clinical features and bone marrow cell morphology. We reported a case of APL with IRF2BP2-RARA fusion was shown to harbor the NRAS mutation at relapse.
Keywords: acute promyelocytic leukemia, IRF2BP2-RARA, variant translocation, gene fusion, NRAS, mutation
Background
Acute promyelocytic leukemia (APL) is characterized by the fusion of retinoic acid receptor alpha (RARA) with promyelocytic leukemia (PML). In addition, about 2% APL patients present variant fusions.1 Hitherto, >10 variant translocations have been reported to share the same C-terminal domains of RARA but different N-terminal sequences.2 In 2015, Yin et al, for the first time, reported the occurrence of IRF2BP2-RARA.3 To date, four reports of IRF2BP2-RARA have been published worldwide.3–6 Herein, we reported a case of relapse APL with IRF2BP2-RARA fusion from China.
Case presentation
A 32-year old woman was first hospitalized for menorrhagia and severe tiredness. Anemia with a hemoglobin of 64 g/L and low platelet count of 33×109/L were noted. Blast cells were also observed in the peripheral blood smear. However, a normal activated partial prothrombin time (aPTT), fibrinogen level, and thrombin time were observed in addition to the prolonged prothrombin time (PT) of 14 s. Her bone marrow was hypercellular with 57% promyelocytes, frequent Auer rods, and strong myeloperoxidase (Figure 1). All-trans retinoic acid (ATRA) and arsenic trioxide (ATO) were administered immediately after APL was suspected. Flow cytometry showed the immature cells expressing CD13, CD33, CD117, and CD64, and was negative for HLA-DR and CD34, CD3, CD4, CD56, CD5, CD10, CD19, CD20, CD7, CD11b, CD16, CD15, CD14, CD36, CD41, and CD71. Chromosomal examination of leukemia cells revealed the karyotype 45, X, -X (Figure 2). Reverse transcription-polymerase chain reaction (RT-PCR) did not detect any translocations that were commonly detected in APL and other acute myelogenous leukemia including PML-RARA, PLZF-RARA, and NPM-RARA. Fluorescence in situ hybridization (FISH) failed to detect the fusion of RARA with PML or other gene partners. Also, no mutations were detected in FLT3, NPM1, CEBPA, WT1, or IDH1/2. Due to the inconsistency in morphology, immunological, cytogenetical, and molecular tests, the levels of PML-RARA, NPM-RARA, NuMA-RARA, FIPIL-RARA, PLZF-RARA, PPK-RARA, and STAT5b-RARA were examined for the second time by RT-PCR in the clinical laboratory of Huaxi Hospital. It was slightly positive for PML-RARA, thus leading to the diagnosis of APL.
Figure 1.
Bone marrow examination at diagnosis.
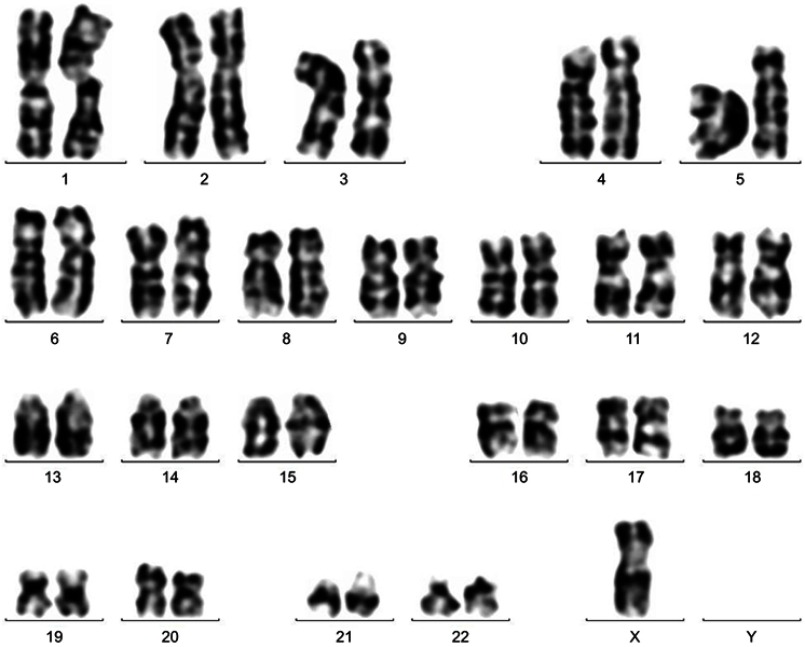
Figure 2.
Chromosome karyotype analysis at first diagnosis: 45, X, -X [16].
After receiving ATRA (10 mg po. tid) and ATO (10 mg ivgtt qd) for 35 days, along with daunorubicin (45 mg/m2 on days 1–3), the patient acquired complete remission (CR). The consolidation chemotherapy comprised of treatment with DA (daunorubicin 45 mg/m2/day on days 1–3; cytarabine 100 mg/m2/day on days 1–7), and HA (homoharringtonine 2 mg/m2/day on days 1–5, cytarabine 100 mg/m2/day on days 1–7). ATRA (45 mg/m2/d) was administered on days 1–15 in combination with three single-agent chemotherapy courses. Subsequently, the patient who tested negative for PML/RARA was started on oral ATRA (10 mg tid) for 15 days, intravenous ATO (10 mg) for 15 days, and oral methotrexate (15 mg/m2/week) every 3 months. Methotrexate (10 mg), cytarabine (50 mg), and dexamethasone (10 mg) were administered four times by spinal puncture to prevent the central nervous system leukemia. After 12 months, she presented with fever, blast cells were found in peripheral blood, and relapse was confirmed by bone marrow aspirate. RT-PCR and FISH still failed to detect the RARA arrangement. Cytogenetics revealed 45, X, -X [8]/45, idem, t(9;12)(q13;p11) [12], which differed from that during the initial diagnosis (Figure 3). The putative fusion gene was investigated by next-generation RNA-sequencing analysis. NRAS, BMP4 mutations, and the fusion between RARA and interferon regulatory factor 2 binding proteins 2 (IRF2BP2) were verified. This confirmed the variant case of APL. The patient received re-induction therapy, including ATRA, ATO, and daunorubicin; however, CR was not achieved. Then, CLAG protocol (cladribine 4 mg/m2 on days 1–5; cytarabine 1000 mg/m2 q12h on days 1–5; G-CSF 300 µg on days 1–5) was administered. However, the patient still did not respond to the treatment and refused further therapy, and died eventually.
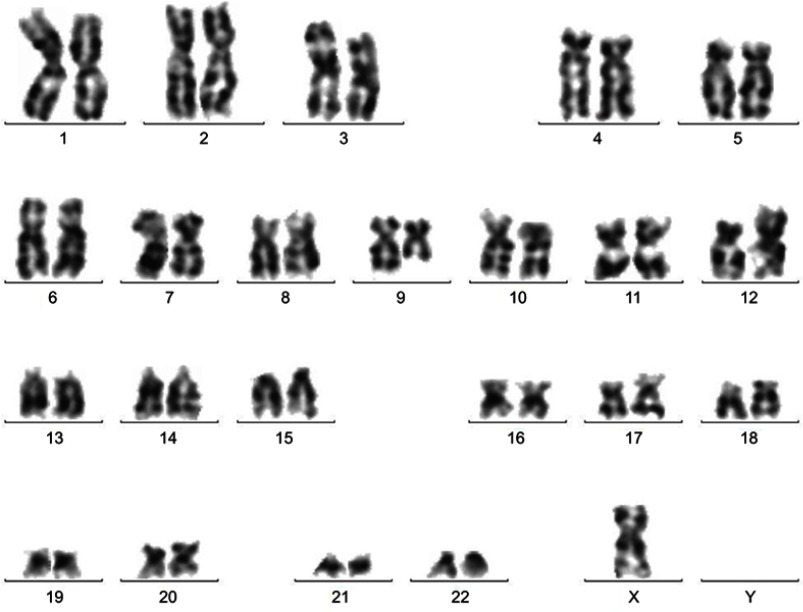
Figure 3.
Chromosome karyotype analysis at relapse: 45, X, -X [8]/45, idem, t(9; 12) (q13; p11) [12].
Discussion
APL is characterized by the fusion of RARA with PML or, rarely, other gene partners including PLZF, NPM, NuMA, STAT5b, PRKAR1A, FLP1L1, BCOR, OBFC2A, TBLR1, GTF2I, and STAT3. Herein, we reported a rare fusion IRF2P2-RARA detected in relapsed process. IRF2BP2 is localized at chromosome 1q42.3 and encodes a nuclear protein consisting of an N-terminal zinc finger and a C-terminal RING finger domain that interacts specifically with the C-terminal transcriptional repression domain of IRF2.9 IRF2BP2 has also been involved in breast cancer cell lines, monoclonal gammopathy of undetermined significance, and other solid tumors.7,10,11
To date, four reports have been published on IRF2BP2-RARA.3–6 Clinical features of the APL with IRF2BP2-RARA were compared (Table 1). These 5 cases comprised of males and females; 4 were <40-years-old. . All the patients presented different degrees of cytopenia or pancytopenia. Only two patients showed abnormal coagulant function with hypofibrinogenemia. Morphologically, four cases were identified as APL, except the case that was reported as atypical by Shimomura et al.3–6 Four cases exhibited classic immunophenotype of promyelocyte by flow cytometry (CD13, CD33, CD64, CD117-positive, and HLA-DR-negative), except one patient, who was HLA-DR-positive. The chromosome karyotype of (1;17)(q42; q21) was found in two cases, from which IRF2BP2-RARA was derived. In the present study, a case of a young female with cytopenia, mild abnormal coagulant function, classic morphology, and immunophenotype of APL at diagnosis was reported. Before IRF2BP2-RARA was verified, several examinations of the chromosome failed to find (1;17) (q42; q21), and normal karyotype was reported. All the cases including the current were stratified into low-intermediate risk. IRF2BP2-RARA was confirmed by next-generation RNA-sequencing analysis. The breakpoint in the current case was at the same intron 2 in the RARA as reported by Yin et al in APL patients with IRF2BP2-RARA and respond differently to ATRA or ATO, which is usually inferior to typical APL. All the five cases were administered with ATRA at diagnosis, two combined with ATO, and three combined with anthracycline agents during the induction treatment. All cases acquired CR. The elderly patient from Japan failed to respond to ATRA, idarubicin, and cytarabine treatment. As reported, three patients relapsed and the event-free survival (RFS) was between 10 and 18 months.
Table 1.
Comparison of clinical features of the five cases of acute promyelocytic leukemia with IRF2BP2-RARA fusion
| Originate country/Publish time | Age/ gender |
Regular blood test | Coagulant function |
Bone marrow morphology | Flow cytometry | Chromosome karyotype | IRF2BP2-RARA breakpoint | Induction treatment | Achieve CR | EFS after CR1 | Allogeneic HSCT | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA, 2015 | 19 y/ Female |
4.5×109/L Hb 91 g/L Plt 2×109/L |
PT 19.6 s APTT 46.3 s D-dimer >20 µg/mL FIB 60 mg/dL |
Classic APL morphology | Aberrant promyelocytic immunophenotype (no detail) | Diploid karyotype | Exon 2 in IRF2BP2 and intron 2 in the RARA | ARAT+AS2O3+igemtuzumab ozogamicin | Yes | 8 months | Yes | CR2 |
| Japan, 2016 | 68 y/ Female |
WBC 1.65×109/L Hb 82 g/L Plt 48×109/L |
Normal | Classic APL morphology | CD33+, CD34+, CD64+, CD117+, HLA-DR+, CD2-, CD7-, CD13-, CD19- | -X | Exon 1 in IRF2BP2 and exons 3–9 in RARA | ATRA+idarubucin+Arac+gemtuzumab ozogamicin | Yes | 10 months | No | Died |
| UK, 2016 | 37 y/ Male |
WBC 3.8×109/L Hb 125 g/L Plt 7×109/L |
FIB 138mg/dL D-dimer >20 µg/mL |
Classic APL morphology No Auer rods |
CD34, HLADR-, CD117+ weak, CD13+, CD33+, CD11b, CD15-, CD45+ weak, CD71-, CD56-. | Normal karyotype | Intron 1 in IRF2BP2 and intron 2 in RARA | ATRA only | Yes | Not known | No | CR1 |
| USA, 2018 | 34 y/ Male |
WBC 4.1×109/L Hb 93 g/L Plt 23×109/L |
D-dimer 37.21 mg/L | Classic APL morphology | CD13+, CD33+CD38 (dim)+, CD117+, MPO+, HLA-DR- | T(1;17)(q42;q21) | Extron 1 in IRF2BP2 and extron 3 in RARA | ATRA+idarubucin+Arac | Yes | 18 months | No | CR1 |
| China, 2019 | 32 y/ Female |
WBC 5.14109/L Hb 64 g/L Plt 33×109/L |
PT 14 s | Classic APL morphology | CD13+, CD33+, CD117+, CD64+, HLA-DR-, CD34- | -X | Intron 1 in IRF2BP2 and intron 2 in the RARA NRAS and BMP4 mutation were detected at relapse |
ARAT+ATO+DNR+Arac | Yes | 12 months | No | Died |
Arsenic binds to the PML moiety of the PML-RARA fusion. ATRA binds to the RARA portion. Each drug initiates biochemically independent degradation pathways,12 which might partially explain the lack of response of APL with the variant fusions between RARA and other rare partners as compared to that of classic APL to arsenic agents due to the loss of binding sites.2,8 In addition, variant fusions can inhibit the target of retinoic acid but are not sensitive to ATRA. This phenomenon might lead to ATRA to fail to inhibit the apoptosis pathway induced by RARA efficiently.13 In the four cases with IRF2BP2-RARA reported previously, all patients acquired CR after ATRA-based therapy, one received allotransplantation and acquired long term survival, three patients relapsed in 12 months, and two maintained CR before the article was published. Reportedly, the RARA fusions occur with other genes including PLZF, STAT5b, and GTF2I that are resistant to ATRA.13–16 Whether IRF2BP2 might be a regulatory factor to enhance the effect of RARA and cause resistance to ATRA is yet unknown.
NRAS mutation was found in the patient in this study and the 19-year-old female from the USA. EFS after the first CR was 10 and 12 months, respectively. NRAS mutation was common in myeloid tumors, lymphoma, lymphocyte leukemia, melanoma, intestinal cancer, lung cancer, and pancreatic cancer.17–19 Furthermore, NRAS mutation was found in 14.7% of acute myeloid leukemia patients.20 Welch et al compared the most mutations in acute myeloid leukemia and APL. The research concluded that in many cases, only one or two additional, cooperating mutations were essential to generate the malignant founding clone. The cells from the founding clone can acquire additional cooperating mutations, yielding subclones that contribute to disease progression and/or relapse.21 Madan et al explored the mutational landscape using whole-exome (n=12) and subsequent targeted sequencing of 398 genes in 153 primary and 69 relapse APLs. Also, the recurrent alterations in FLT3, WT1, NRAS, and KRAS mutation were observed in the newly diagnosed APL, whereas mutations in the other genes commonly mutated in myeloid leukemia were rarely detected. NRAS mutation occurred at a higher frequency in newly diagnosed samples as compared to with the relapse (9.7% in newly diagnosed and 5.2% in relapse).22 Thus, these studies might explain the poor prognosis of the APL patient with IRF2BP2-RARA and NRAS mutation. However, the combined effects of IRF2BP2-RARA and NRAS mutation in APL need to be investigated further.
It is worthy of note that only 2 cases of APL with IRF2BP2-RARA presented with hypofibrinogenemia (40%). The incidence of abnormal coagulant function seemed less than typical APL. Wang X et al reviewed characteristics of STAT5b/RARa APL patients. 9 in 12 cases were diagnosed as disseminated intravascular coagulation. Whether the lower incidence of abnormal coagulant function was the clinical feature of APL with IRF2BP2-RARA, further observation is needed.23
Conclusion
Patients were suspected to exhibited APL based on the clinical features and bone marrow cell morphology. The abnormal chromosomal karyotype and classic PML-RARA fusion test were not sufficient to exclude APL. Thus, variant APL should be considered. IRF2BP2-RARA is a rare variant fusion in APL, and only five cases were reported worldwide. ATRA and arsenic agent-based induction treatment are still effective for APL with IRF2BP2-RARA fusion. However, allogeneic hematopoietic stem cell transplantation might be a therapeutic method to acquire long-term survival.
Ethics approval and informed consent
The study protocol was approved by the Ethics Committees of the Mianyang Central Hospital (P2019003), and the participant provided written informed consent.
Consent for publication
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Abbreviations
APL, Acute promyelocytic leukemia; RARA, retinoic acid receptor alpha; ATRA, all-trans retinoic acid; PML, promyelocytic leukemia; aPTT, activated partial prothrombin time; PT, prothrombin time; ATO, arsenic trioxide.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hematology CSo, Association CMD. [Chinese guidelines for diagnosis and treatment of acute promyelocytic leukemia (2018)]. Zhonghua Xue Ye Xue Za Zhi. 2018;39(3):179–183. doi: 10.3760/cma.j.issn.0253-2727.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan W, Molecular Characteristics ZG. Clinical significance of 12 fusion genes in acute promyelocytic leukemia: a systematic review. Acta Haematol. 2016;136(1):1–15. doi: 10.1159/000444514 [DOI] [PubMed] [Google Scholar]
- 3.Yin CC, Jain N, Mehrotra M, et al. Identification of a novel fusion gene, IRF2BP2-RARA, in acute promyelocytic leukemia. J Natl Compr Canc Netw. 2015;13(1):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimomura Y, Mitsui H, Yamashita Y, et al. New variant of acute promyelocytic leukemia with IRF2BP2-RARA fusion. Cancer Sci. 2016;107(8):1165–1168. doi: 10.1111/cas.12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jovanovic JV, Chillon MC, Vincent-Fabert C, et al. The cryptic IRF2BP2-RARA fusion transforms hematopoietic stem/progenitor cells and induces retinoid-sensitive acute promyelocytic leukemia. Leukemia. 2017;31(3):747–751. doi: 10.1038/leu.2016.338 [DOI] [PubMed] [Google Scholar]
- 6.Mazharuddin S, Chattopadhyay A, Levy MY, Redner RL. IRF2BP2-RARA t(1;17)(q42.3;q21.2) APL blasts differentiate in response to all-trans retinoic acid. Leuk Lymphoma. 2018;59(9):2246–2249. doi: 10.1080/10428194.2017.1421761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9(2):e88557. doi: 10.1371/journal.pone.0088557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao L, Wen L, Wang N, et al. Identification of novel recurrent STAT3-RARA fusions in acute promyelocytic leukemia lacking t(15;17)(q22;q12)/PML-RARA. Blood. 2018;131(8):935–939. doi: 10.1182/blood-2017-09-807370 [DOI] [PubMed] [Google Scholar]
- 9.Carneiro FR, Ramalho-Oliveira R, Mognol GP, Viola JP. Interferon regulatory factor 2 binding protein 2 is a new NFAT1 partner and represses its transcriptional activity. Mol Cell Biol. 2011;31(14):2889–2901. doi: 10.1128/MCB.00974-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blotta S, Tassone P, Prabhala RH, et al. Identification of novel antigens with induced immune response in monoclonal gammopathy of undetermined significance. Blood. 2009;114(15):3276–3284. doi: 10.1182/blood-2009-04-219436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyquist KB, Panagopoulos I, Thorsen J, et al. Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One. 2012;7(11):e49705. doi: 10.1371/journal.pone.0049705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alice V, Jehannine A. Resistance to therapy in acute promyelocytic leukemia. N Engl J Med. 2014;371(12):1170–1172. doi: 10.1056/NEJMc1409040 [DOI] [PubMed] [Google Scholar]
- 13.He LZ, Guidez F, Tribioli C, et al. Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18(2):126–135. doi: 10.1038/ng0298-126 [DOI] [PubMed] [Google Scholar]
- 14.Licht JD, Chomienne C, Goy A, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17). Blood. 1995;85(4):1083–1094. [PubMed] [Google Scholar]
- 15.Li J, Zhong HY, Zhang Y, et al. GTF2I-RARA is a novel fusion transcript in a t(7;17) variant of acute promyelocytic leukaemia with clinical resistance to retinoic acid. Br J Haematol. 2015;168(6):904–908. doi: 10.1111/bjh.13157 [DOI] [PubMed] [Google Scholar]
- 16.Dong S, Tweardy DJ. Interactions of STAT5b-RARalpha, a novel acute promyelocytic leukemia fusion protein, with retinoic acid receptor and STAT3 signaling pathways. Blood. 2002;99(8):2637–2646. doi: 10.1182/blood.v99.8.2637 [DOI] [PubMed] [Google Scholar]
- 17.Burd CE, Liu W, Huynh MV, et al. Mutation-specific RAS oncogenicity explains NRAS codon 61 selection in melanoma. Cancer Discov. 2014;4(12):1418–1429. doi: 10.1158/2159-8290.CD-14-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molnar B, Galamb O, Peterfia B, et al. Gene promoter and exon DNA methylation changes in colon cancer development - mRNA expression and tumor mutation alterations. BMC Cancer. 2018;18(1):695. doi: 10.1186/s12885-018-4242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsen JN, Santoni-Rugiu E, Grauslund M, Melchior L, Sorensen JB. Concomitant driver mutations in advanced EGFR-mutated non-small-cell lung cancer and their impact on erlotinib treatment. Oncotarget. 2018;9(40):26195–26208. doi: 10.18632/oncotarget.25490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borthakur G, Popplewell L, Boyiadzis M, et al. Activity of the oral mitogen-activated protein kinase kinase inhibitor trametinib in RAS-mutant relapsed or refractory myeloid malignancies. Cancer. 2016;122(12):1871–1879. doi: 10.1002/cncr.29986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchberger MC, Ugurel S, Mangana J, et al. MEK inhibition may increase survival of NRAS-mutated melanoma patients treated with checkpoint blockade: results of a retrospective multicentre analysis of 364 patients. Eur J Cancer. 2018;98:10–16. doi: 10.1016/j.ejca.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang F, Chen X, et al. Mutation profiling of 16 candidate genes in de novo acute myeloid leukemia patients. Front Med. 201. 9;13(2):229–237. doi:10.1007/s 11684-018-0616-1 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Wang J, Zhang L. Characterization of atypical acute promyelocytic leukaemia: three cases report and literature review. Medicine (Baltimore). 2019;98(19):e15537. doi: 10.1097/MD.0000000000015537 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request