Abstract
The global contamination with persistent organic pollutants (POPs), or compounds with similar characteristics, is well known. Still there are data gaps for POP concentrations from many areas in the world. The aim of the present study is to assess several legacies POPs and also hexabromocyclododecane (HBCDD) and methoxylated polybrominated diphenyl ethers (MeO-PBDEs) in shellfish from three locations in the Yellow Sea and East China Sea. The sources of the contaminants are discussed. Pooled samples were treated by liquid-liquid extraction and acid and column cleanup prior to analysis by gas chromatogram equipped with electron capture detector (GC-ECD) and gas chromatography-mass spectrometry (GC-MS). The by far most abundant environmental contaminant originates from dichlorodiphenyltrichloroethane (DDT), independent of species analyzed or sampling site. The results indicate ongoing or at least recent discharges of DDT. The second highest concentrations were reported for HBCDD (21–40 ng/g fat) in the shellfish, independent of sampling sites. The two natural products, 6-MeO-BDE-47 and 2′-MeO-BDE-68, were also present in the shellfish (1.3–22 and 1–14 ng/g fat, respectively). The polychlorinated biphenyl (PCB) congener CB-153 (0.8–6.5 ng/g fat), hexachlorobenzene (HCB) (1.1–3.6 ng/g fat), and β-hexachlorocyclohexane (β-HCH) (2.3–4.9 ng/g fat) were all higher than the concentrations of other HCH isomers, β-endosulfan, PBDE congeners, and mirex. Apart from the DDTs and HBCDDs, it is evident that the pollution of shellfish was similar to, or lower than, the contamination of shellfish in other parts of the world.
Electronic supplementary material
The online version of this article (doi:10.1007/s11356-014-3198-8) contains supplementary material, which is available to authorized users.
Keywords: Shellfish, Pesticides, HBCDD, DDT, MeO-PBDE, China
Introduction
Persistent organic pollutants (POPs) are lipophilic organic chemicals with low chemical reactivity allowing them to undergo long-range transport, making them bioaccumulative and toxic to wildlife and humans (UNEP 2014). POPs can be classified in three categories according to their sources: pesticides, industrial chemicals, and by-products. Large amount of organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) have been manufactured and used in China for several decades.
Being the country with the world’s largest population, China has produced and consumed large amount of pesticides for crop protection and disease vector control (Wong et al. 2005). For instance, 400 thousand tonnes of dichlorodiphenyltrichloroethane (DDT) and 4.9 million tonnes of hexachlorocyclohexanes (HCHs) from the 1950s to 1983 were produced for agricultural use (Fu et al. 2003; Li et al. 1998). Even though China initiated bans of pesticides for certain uses from 1980s and ratified the Stockholm Convention in 2004, some specific exemptions were requested. For example, DDT is still used for malaria control while mirex and chlordane have been used as termiticides (Wong et al. 2005). Pesticides residue level detected in China environment and human are still relatively high even if some data indicated descending temporal trends (Wong et al. 2002). There is, however, a shortage of data from China to make any firm conclusions on general levels spatial and temporal trends of POPs in China.
PCBs have been used for their good electrical insulating properties since the 1930s. However, it was not until 1966 that PCBs were detected in wildlife from the marine environment (Jensen et al. 1969). In 1968, the Yusho incident occurred in Japan (Yoshimura 2003). One thousand and eight hundred people were poisoned by PCB-contaminated rice oil. It was reported that 8,000 tons of PCBs had been manufactured from the 1960s to 1970s (Jiang et al. 1997; Qiu et al. 2012) in China, and technical mixtures named #1 PCB (chlorine content 42 % similar to Aroclor 1242) and #2 PCB (chlorine content 56 % similar to Aroclor 1254) were produced. PCBs were restricted from the 1970s in China. PCB concentrations in environmental media in China are relatively low on a national scale, and it still could be a risk for humans and wildlife due to exposure to PCB-containing equipment and by-product from combustion (Xing et al. 2005).
PBDEs and hexabromocyclododecanes (HBCDDs) are brominated flame retardants used in primarily polymeric materials for improving their fire safety. It was reported that the domestic production demand of brominated flame retardants was 10,000 tonnes in 2000 and has increased by an annual rate of 8 % (Mai et al. 2005). Materials and goods are sources of general PBDE and HBCDD contamination, while e-waste recycling has created severe hot spots.
Methoxylated PBDEs (MeO-PBDEs) are neutral chemicals that seem to primarily originate from natural sources, particularly if the methoxy group is located to the ortho-position to the diphenyl ether bridge. They can be also formed from the metabolism of PBDEs (Feng et al. 2010). MeO-PBDEs have been identified in algae, blue mussel, and seals (Haglund et al. 1997; Lofstrand et al. 2011; Malmvarn et al. 2005). It has been suggested as a potential source of hydroxylated PBDEs (Wan et al. 2009).
Shellfish (e.g., mussels and clams) are popular seafood for people living around the coastline of China. Mussels have been widely used as a biomarker for detecting pollutants and monitoring the contamination all over the world, due to their wide distribution, low mobility, and high filtration capacity (Goldberg et al. 1978; O’Connor 2002; Ramu et al. 2007a). They live attached to rocks by its byssus and feed on plankton and other primary producers. Similar to mussels, clams feed on plankton by filter feeding. However, clams are kinds of cave sediment bivalves rather than attaching to rocks.
The Yellow Sea (YS) and East China Sea (ECS) are two of the continental seas of China. The two longest rivers, Yangtze River and Yellow River, are flowing into the ECS and YS, respectively. The coasts of the YS and ECS are densely populated and the most developed in China. The seawater has been used for fishing for a long time. For example, Zhoushan fishing ground, located in ECS, is the biggest fish ground in China. It is famous for croceine croaker, octopus, and cuttlefish. However, the seawater quality in YS and ECS according to national seawater quality standard has become increasingly worse. Moreover, there is limited information on levels of POPs in seafood and accordingly health risk assessments are hampered.
The aim of the present study was integrating a high consumption rate of shellfish and their contamination degree of a selected number of POPs, to determine residual concentrations of OCPs, PCBs, PBDEs, HBCDDs, and MeO-PBDEs in shellfish from a few locations along the eastern coastline of China. In addition, the potential sources and the extent of pollution are discussed.
Materials and methods
Samples
The shellfish were collected from three locations from eastern China. The sampling information is shown in Fig. 1 and Table S1. Blue mussels (Mytilus edulis) (ME) were taken from the North Yellow Sea, Weihai city (WH) and the East China Sea, Zhoushan city (ZS) in July 2010 and 2011, respectively. The blue mussels from ZS were collected from natural (ZS1) and cultivated source (ZS2). Three kinds of clams which are Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) were taken from the south Yellow Sea, Nantong city (NT) in July 2011. The clams, CS and RP, are the predominant clam species in the Yangtze River Estuary. All samples were packed in aluminum foil and kept frozen at −20 °C until the start of analysis.
Fig. 1.
Sampling locations of shellfish for the present study
Chemicals
All solvents and chemicals used were of highest commercially available quality. Authentic reference standards of dichlorodiphenyltrichloroethanes (DDTs), including 4,4′-DDT, 4,4′-DDE, 4,4′-DDD, 2,4′-DDT, 2,4′-DDE, and 2,4′-DDD; HCHs, including α-HCH, β-HCH, γ-HCH, δ-HCH, and ε-HCH; hexachlorobenzene (HCB); mirex; α-endosulfan; and β-endosulfan were purchased as a mixture from Larodan Fine Chemicals (Malmö, Sweden). The PCB congeners: CB-28, CB-52, CB-101, CB-118, CB-138, CB-153, and CB-180, and PBDE congeners: BDE-47, BDE-85, BDE-90, BDE-99, BDE-100, BDE-153, BDE-154, and BDE-183, used as external standards were purchased from Larodan Fine Chemicals and LGC Promochem (Wesel, Germany), respectively. MeO-PBDEs (including 6-MeO-BDE-47, 2′-MeO-BDE-68, 6-MeO-BDE-85, 6-MeO-BDE-90, 6-MeO-BDE-99, 2-MeO-BDE-123, and 6-MeO-BDE-137) were synthesized in-house (Marsh et al. 2003; Marsh et al. 2005). Technical HBCDD was obtained from the Dead Sea Bromine Group (Israel). The full name of DDTs, PCBs, PBDEs, and MeO-PBDEs and their abbreviations are listed in Supplemental Material. Silica gel (0.063–0.2 mm) from Merck (Darmstadt, Germany) was activated at 300 °C in oven overnight before use.
Extraction and cleanup
The shellfish meat was homogenized as an individual pool (n = 30) for each species and sampling site. The samples (10 g) were extracted as described elsewhere (Jensen et al. 2009), except that n-hexane was exchanged for cyclohexane due to its lower toxicity (for detailed description, see Supplemental Material). The lipid weight was determined gravimetrically for all the extracted samples after solvent removal. CB-200 (30 ng), CB-207 (1 ng), BDE-138 (1 ng), and 4-MeO-BDE-121 (1 ng) were added to the samples and blanks as surrogate standards. Lipids were removed by concentrated sulfuric acid (1 mL/0.1 g lipid) treatment, a procedure that was repeated once. After sulfuric acid treatment, the samples were further cleaned up on two different types of chromatographic columns. The first column was packed with activated silica (0.9 g) impregnated with concentrated sulfuric acid (2:1 w/w). The column was conditioned with cyclohexane (10 mL), and the analytes were eluted with dichloromethane (15 mL). The second column was packed with silica gel (0.5 g). It was pre-eluted with dichloromethane (5 mL) and then the analytes were eluted with dichloromethane (10 mL). The solvent volume was reduced by a gentle flow of nitrogen gas and changed to n-hexane. Prior to instrumental analysis, CB-189 (2 ng) and BDE-139 (1 ng) were added as volumetric standard. The final volumes were 0.5 mL for DDT analysis and 0.1 mL for the other compounds.
Instrumental analysis
The analysis of PCBs and pesticide mixture was performed on a Varian 450 gas chromatogram equipped with electron capture detector (GC-ECD) maintained at 360 °C and a Varian CP-8400 autosampler. The injector (1 μL) was operated in the splitless mode, and the temperature for the injector was 260 °C. The non-polar Varian CP-Sil 8 CB (25 m × 150 μm × 0.12 μm) column (Middleburg, the Netherlands) was used. Helium was used as a carrier gas and nitrogen as the makeup gas. The column oven temperature was programmed from 80 °C for 1 min, 20 °C/min to 300 °C and held constant for 5 min.
The PBDEs, MeO-PBDEs, and HBCDDs were analyzed by gas chromatography-mass spectrometry (GC-MS) in selected ion monitoring mode (bromide ions m/z 79 and 81) and were identified by retention time using authentic reference standards. Automated 1 μL injections with a CTC GC Pal autosampler were conducted on a Varian 450-GC connected to a Varian 320-MS. A programmable temperature vaporizing (PTV) injector was used with a DB-5 HT capillary column (15 m × 250 μm × 0.1 μm) from J&W Scientific (Folsom, USA). Helium was used as carrier gas at a set constant flow of 1.2 mL/min. The ion source temperature was 200 °C and the transfer line temperature to 290 °C. Methane (scientific 5.5, AGA Stockholm, Sweden) was used as reagent gas. The oven was programmed as follows: 55 °C for 2 min, 15 °C/min to 320 °C and held constant for 5 min. The PTV injector temperature was 280 °C with a splitless mode. The PTV injector was also pressure programmed with a pressure pulse of 10 psi for 0.5 min upon injection.
Quality control
One procedure blank was run for each batch of six samples to assess any potential contamination during laboratory work. Except for BDE congeners, no other analyte was present in blank samples. Three duplicates from each location and species were analyzed to determine the analytical precision. The range of recoveries (mean value ± standard deviation) for surrogate standards CB-207, BDE-138, and 4-MeO-BDE-121 were 79–115 % (103 ± 8 %), 106–118 % (112 ± 7 %), and 61–101 % (88 ± 19 %), respectively. All external calibration curves have a good correlation coefficient. Limit of detection was defined as three times the signal-to-noise (S/N) in the chromatogram and limit of quantification (LOQ) as ten times the S/N. If the blank samples were contaminated, the LOQ was defined as three times the average amount found in the procedure blanks. More information on LOQ is given in Supplementary Material and in Table S5.
Results
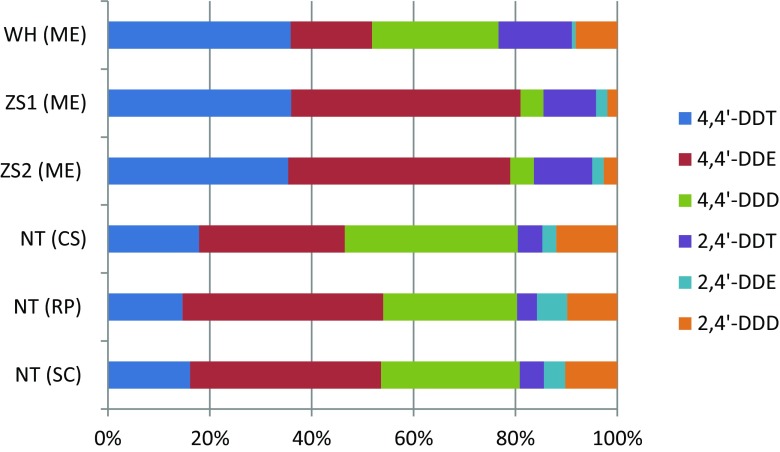
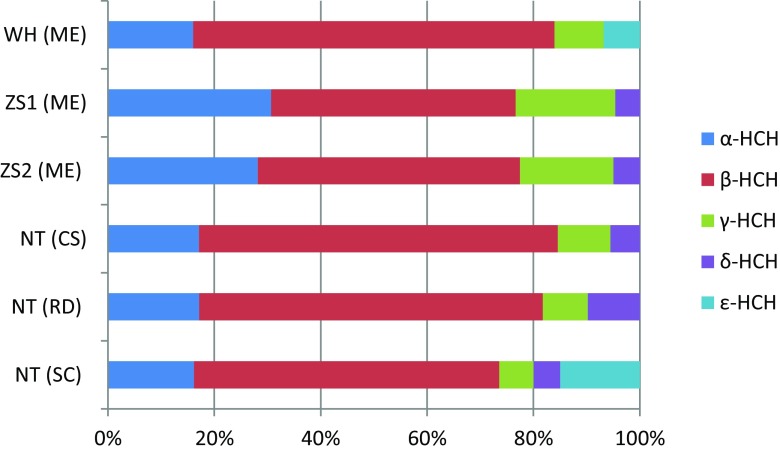
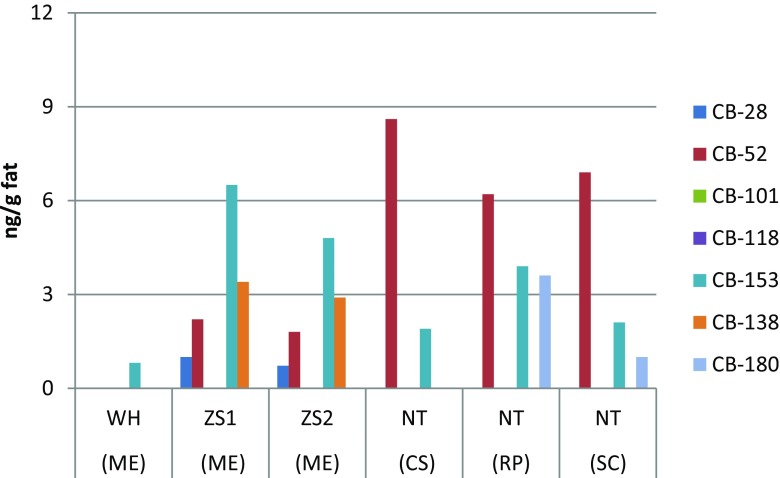
Mean concentrations of the individual main chlorinated and brominated organic analytes from the DDTs, HCHs, PCBs, PBDEs, HBCDD, and MeO-PBDEs in shellfish are presented in Table 1. Detailed concentration data of all the analytes are given in the Tables S2–4 and S6 of the Supplementary Material, including sum data of DDTs, HCHs, PCBs, PBDEs, and MeO-PBDEs. The relative contribution of the main three 4,4′-DDTs and the 2,4′-DDTs are given from each sampling site in Fig. 2. Similarly, the HCH isomer relative distribution in the sampled matrices is shown in Fig. 3, while the congener concentration patterns of PCBs, PBDEs, and MeO-PBDEs are presented in Figs. 4–6, respectively.
Table 1.
Concentrations (ng/g fat) of some prioritized polychlorinated and brominated organic pollutants in Mytilus edulis (ME), Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) from Weihai (WH), Zhoushan (ZS), and Nantong (NT); ZS1 and ZS2 are natural and cultivated mussel, respectively
| Compound | WH (ME) (n = 3) | ZS1 (ME) (n = 3) | ZS2 (ME) (n = 3) | NT (CS) (n = 3) | NT (RP) (n = 3) | NT (SC) (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| Lipid (%) | 2.6 | 0.11 | 1.6 | 0.19 | 2.0 | 0.27 | 1.6 | 0.050 | 0.97 | 0.030 | 2.2 | 0.090 |
| 4,4′-DDT | 1100 | 99 | 160 | 8.0 | 130 | 15 | 100 | 2.4 | 67 | 0.74 | 190 | 4.3 |
| 4,4′-DDE | 490 | 25 | 200 | 9.8 | 160 | 13 | 160 | 6.1 | 180 | 3.7 | 440 | 5.3 |
| 4,4′-DDD | 760 | 30 | 20 | 1.7 | 17 | 1.3 | 190 | 6.8 | 120 | 1.9 | 320 | 3.6 |
| α-HCH | 0.66 | 0.028 | 1.8 | 0.22 | 1.6 | 0.14 | 0.99 | 0.15 | 1.3 | 0.57 | 0.65 | 0.070 |
| β-HCH | 2.8 | 0.21 | 2.7 | 0.29 | 2.8 | 0.19 | 3.9 | 0.50 | 4.9 | 0.19 | 2.3 | 0.23 |
| γ-HCH | 0.38 | 0.017 | 1.1 | 0.053 | 1.0 | 0.022 | 0.57 | 0.073 | 0.64 | 0.18 | 0.26 | 0.062 |
| β-endosulfan | 1.8 | 0.22 | 0.78 | 0.14 | 0.70 | 0.056 | 1.0 | 0.15 | 1.9 | 0.16 | 0.35 | 0.058 |
| HCB | 1.1 | 0.45 | 2.4 | 0.085 | 1.7 | 0.12 | 1.5 | 0.48 | 3.4 | 0.045 | 3.6 | 0.31 |
| CB-153 | 0.81 | 0.096 | 6.5 | 0.77 | 4.8 | 0.74 | 1.9 | 0.24 | 3.9 | 0.24 | 2.1 | 0.43 |
| BDE-47 | 3.3 | 0.54 | 2.0 | 0.24 | 1.3 | 0.24 | 1.6 | 0.50 | 1.6 | 0.22 | 1.9 | 0.46 |
| HBCDD | 40 | 7.8 | 32 | 7.1 | 21 | 8.3 | 42 | 8.1 | 34 | 4.5 | 38 | 0.34 |
| 6-MeO-BDE-47 | 22 | 2.1 | 8.2 | 2.4 | 5.5 | 1.8 | 1.9 | 0.65 | 1.3 | 0.35 | 4.6 | 0.41 |
| 2′-MeO-BDE-68 | 14 | 1.6 | 4.2 | 1.3 | 2.4 | 1.0 | 0.96 | 0.32 | 1.3 | 0.34 | 2.1 | 0.17 |
Fig. 2.
Patterns of DDTs in Mytilus edulis (ME), Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) from Weihai (WH), Zhoushan (ZS), and Nantong (NT). ZS1 natural mussel and ZS2 cultivated mussel
Fig. 3.
Patterns of HCHs in Mytilus edulis (ME), Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) from Weihai (WH), Zhoushan (ZS), and Nantong (NT). ZS1 natural mussel and ZS2 cultivated mussel
Fig. 4.
Concentrations of PCBs in Mytilus edulis (ME), Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) from Weihai (WH), Zhoushan (ZS), and Nantong (NT). ZS1 natural mussel and ZS2 cultivated mussel
Fig. 6.
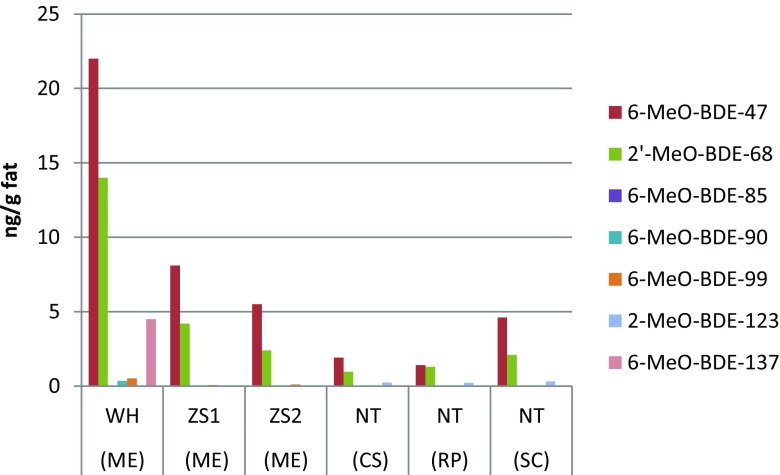
Concentrations of MeO-PBDEs in Mytilus edulis (ME), Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) from Weihai (WH), Zhoushan (ZS), and Nantong (NT). ZS1 natural mussel and ZS2 cultivated mussel
Discussion
It is notable that the three 4,4′-DDTs are present at high level in shellfish from all sites in the present study, making up approximately 80 % of the total concentrations of DDTs. The data confirm recent or even ongoing releases of 4,4′-DDT (cf. below). However, another interesting result is the relatively high levels of HBCDD independent of sampling site and species analyzed (Table 1). The concentrations of HBCDD are coming up as the second most important pollutant in any of the shellfish species, being higher than even the sum concentrations of any of the other pollutants analyzed, except the DDTs. This observation is supported by the levels reported of HBCDD in oysters and blue mussels sampled from coastal areas of Japan (Table 2) (Ueno et al. 2010). Shellfish contaminant levels, as determined in the present study are put in perspective to reports from other sites in Table 2. Unfortunately, it is not possible to compare all reported data due to different basis for presenting the levels in the international literature, but for those studies permitting recalculations to present either wet weight or lipid weight based concentrations, data are presented in Table 2. The results of the present study are put in perspective for each of the contaminant congener group of pollutants or of individual contaminants, which are discussed groupwise, below.
Table 2.
Concentration ranges of chlorinated and brominated pollutants in different shellfish species around the world
| Location | Sampling year | Species | Unit (ng/g) | Lipid content (%) | Compounds | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4,4′-DDT | 4,4′-DDE | 4,4′-DDD | 2,4′-DDT | DDTsa | HCHsb | HCB | CB-153 | 7CBsc | BDE-47 | HBCDD | ||||||
| Weihai, China | 2010 | Blue mussel | w.w. | 2.6 | 29 | 13 | 20 | 11 | 0.11 | 0.029 | 0.021 | 0.021 | 0.086 | 1.0 | Present study | |
| l.w. | 1,100 | 490 | 760 | 440 | 4.1 | 1.1 | 0.81 | 0.81 | 3.3 | 40 | ||||||
| Zhoushan, China | 2011 | Blue mussel | w.w. | 1.6–2.0 | 2.6 | 3.2 | 0.32–0.34 | 0.67–0.80 | 0.09–0.11 | 0.03–0.04 | 0.10 | 0.21 | 0.03 | 0.42–0.51 | Present study | |
| l.w. | 130–160 | 160–200 | 17–20 | 42–46 | 5.7–5.8 | 1.5–1.7 | 4.8–6.5 | 11–15 | 1.3–2.0 | 21–32 | ||||||
| Nantong, China | 2011 | Clam | w.w. | 0.97–2.2 | 0.65–4.2 | 1.8–9.7 | 1.2–7.0 | 0.17–1.2 | 0.07–0.09 | 0.03–0.08 | 0.03–0.05 | 0.13–0.22 | 0.02–0.04 | 0.33–0.84 | Present study | |
| l.w. | 67–190 | 160–440 | 120–320 | 18–56 | 4.0–7.6 | 2.4–3.4 | 1.9–3.9 | 10–14 | 1.6–1.9 | 34–42 | ||||||
| Shengsi, China | 2004 | Green mussel | l.w. | 0.88 | 1,500 | 24 | 5.0 | 3.1 | (Ramu et al. 2007b) | |||||||
| Chongming, China | 2004 | Blue mussel | l.w. | 0.79 | 13,000 | 96 | 4.2 | 6.1 | (Ramu et al. 2007b) | |||||||
| Jiaozhou Wan, China | 2004 | Blue mussel | l.w. | 0.78 | 10,000 | 79 | 5.5 | 5.9 | (Ramu et al. 2007b) | |||||||
| Qiantang River, China | 2006 | Mussel | w.w. | 3.0 | 6.8 | 15 | 3.8 | 1.20 | 12.2 | (Zhou et al. 2008) | ||||||
| Yangtze Estuary, China | 2002 | Clam | w.w. | 1.6–1.9 | 2.6–12 | 3–8.9 | n.d.–9.8d | 1–15 | 2–4.6 | (Yang et al. 2006) | ||||||
| Pearl River Delta, China | 2001–2002 | Green mussel | l.w. | n.d.–41d | n.d.–104d | n.d.–200d | n.d.–1.04d | (Fang 2004) | ||||||||
| Mariculture zones, Hong Kong | 2002 | Green mussel | l.w. | 0.90–210 | 5.8–1,400 | 0.7–230 | 12–780 | 26–430 | 0.90–118 | 44–460 | (So et al. 2005) | |||||
| Coastal area of Hong Kong | 2004 | Green mussel | l.w. | 0.64–1.7 | 720–58,000 | 3.8–18 | <1 | (Ramu et al. 2007b) | ||||||||
| East coast, India | 2004 | Green mussel | l.w. | 0.37–2.4 | 380–640 | 7.2–230 | <1 | (Ramu et al. 2007b) | ||||||||
| Coastal area of Korea | 2005 | Blue mussel | l.w. | 0.72–2.3 | 24–400 | 1.1–82 | 0.31–50 | 0.020–3.3 | (Ramu et al. 2007a) | |||||||
| Coastal areas of Japan | Oysters and blue mussel | l.w. | 6.9–760 | 18–570 | 5.3–350 | n.d.–44d | 3.8–420 | 16–1,900 | 1.6–23 | 12–5,200 | (Ueno et al. 2010) | |||||
| Ionian Sea, Italy | 2008 | Mussel | w.w. | n.d.d | n.d.–5.4d | n.d.–0.34d | n.d.d | n.d.–31d | n.d.–78d | (Giandomenico et al. 2013) | ||||||
| Scheldt estuary, Netherlands and Belgium | 2010 | Blue mussel | w.w. | 2.0–3.7 | 0.09–1.2 | 10–39 | 3.5–16 | 0.83–1.8 | 0.09–0.76 | 51–360 | 120–760 | 0.28–5.2 | (Van Ael et al. 2012) | |||
| Adriatic coast, Croatia | 2003–2008 | Blue mussel | w.w. | 0.56–2.0 | 0.15–0.62 | 0.02–0.17 | 0.28–2.1 | (Kozul et al. 2011) | ||||||||
| Baltic Sea, Finland | 2007 | Clam | w.w. | 0.13–0.47 | 1.0–3.0 | 0.23–1.0 | 0.24–0.49 | 0.07–0.15 | 0.56–1.6 | 1.7–4.3 | (Pikkarainen 2007) | |||||
| Usuk, Greenland | 2000 | Blue mussel | l.w. | 2.0 | 6.1 | 11 | 5.1 | 13 | 4.1 | 7.1 | 15 | 5.1 | (Christensen et al. 2002; Glasius et al. 2005) | |||
aSum of 4,4′-DDT, 4,4′-DDE, and 4,4′-DDD
bSum of α-HCH, β-HCH, and γ-HCH
cSum of CB-28, CB-52, CB-101, CB-118, CB-138, CB-153, and CB-180
dn. d. not detected
Organohalogen pesticides in shellfish
DDTs
The patterns of the DDTs in the shellfish, in the present study, are shown in Fig. 2 and Table S2. The mean concentrations of 4,4′-DDT in shellfish from the three sites were quite similar to other studies in the waters close to China, Japan, India, and Republic of Korea (Ramu et al. 2007b; Ramu et al. 2007a; Yang et al. 2006; Zhou et al. 2008), except for levels reported in green mussels from Hong Kong (Ramu et al. 2007b), showing 10 to a 100 times higher ∑DDT concentrations (Table 2). Contrast to the high 4,4′-DDE concentration in mussel from Zhoushan, 4,4′-DDD levels were comparable to 4,4′-DDE’s level in clam from Nantong. It might be due to the species-specific lifestyle. As mentioned, mussels are stick in the rock but clams live in the sediment where 4,4′-DDT is prior to degrade to form 4,4′-DDD under anaerobic condition. The levels of the three 4,4′-DDTs, as reported in shellfish from other sites, primarily Europe, were similar or lower than those from eastern Asia (Giandomenico et al. 2013; Pikkarainen 2007; Van Ael et al. 2012), c.f. Table 2.
The occurrence of 2,4′-DDT was in the expected range (0.21–0.24) for technical DDT products but with one sampling site, Weihai, showing a higher ratio (0.29) that is difficult to explain unless there were current discharges of another, less well known, DDT product. The ratio of 4,4′-DDT/(4,4′-DDT + 4,4′-DDE + 4,4′-DDD) indicated that there were fresh or at least recent input of DDT in Weihai and the Zhoushan areas (ratios are 0.42–0.47), while less so in the Nantong area (ratios here are 0.21–0.23).
The results on the DDTs from the Weihai and Zhoushan areas indicate that DDT is still used in China. DDT was banned as a pesticide in 1983 in China but it has been continuously manufactured for some non-agricultural purposes, i.e., malaria control, export, painting of boat hulls (ban implemented 2009), and synthesis of dicofol (UNEP 2007; Wong et al. 2005). It is reported that the majority of DDT produced has been used for dicofol synthesis since 1988 (Qiu et al. 2005). The ratio of 2,4′-DDT/4,4′-DDT was applied to explore the source of DDT (Qiu et al. 2005). The low 2,4′-DDT/4,4′-DDT ratio in this study implies the newly input DDTs could not only be attribute to the dicofol usage.
HCHs
The relative concentrations of five HCH isomers, determined for all shellfish analyzed, showed a rather even distribution between sampling sites, as visualized in Fig. 3 and with concentration data shown in Table S2. The high abundance of β-HCH indicates previous use of technical HCH, i.e., a mixture of the isomers α-HCH (60–70 %), β-HCH (5–12 %), γ-HCH (10–12 %), δ-HCH (6–10 %), and ɛ-HCH (3–4 %). However, β-HCH was the most persistent, due to its chemical stability, of the HCH isomers and accordingly the isomer to be expected in the highest concentrations if technical HCH was the source. The ratio of α-HCH/γ-HCH detected in the present study ranged from 1.0 to 2.5, which was lower than technical HCH which varies between 4 and 7 (Walker et al. 1999). This result indicated other sources, i.e., lindane (99 % γ-HCH) has been continuously used as an insecticide after technical HCH was banned. Comparing ∑(α-, β-, γ-HCH) levels in shellfish from around the world, it is notable that the present concentrations were in the lower end in relation to data from east Asian waters (Ramu et al. 2007b) and also compared to blue mussels from Greenland (Glasius et al. 2005) (Table 2). However, comparing ∑(α-, β-, γ-HCH) levels presented herein with the European sampling sites (Kozul et al. 2011; Van Ael et al. 2012) indicated similar or only slightly higher levels (Table 2). It is reasonable to conclude that the high proportion of β-HCH together with low ratio of α-HCH/γ-HCH indicates that HCHs profile in shellfish was due to contamination of technical HCH together with lindane.
Endosulfan
The α- and β-endosulfan were determined in all samples except for α-endosulfan in Weihai mussels (Table S2). The concentration of endosulfan present in this study varied from 0.57 to 2.3 ng/g fat, which is lower than another study on mollusks in China (Liu et al. 2010). β-Endosulfan dominated in the shellfish sampled independent of sampling site, accounting for 100, 71, and 50 % for Weihai, Zhoushan, and Nantong area, respectively. Technical endosulfan consists of 70 % α-endosulfan and 30 % β-endosulfan. It is worth to note that the β-endosulfan concentrations were similar or higher than those of the HCH isomers and HCB, assessed and reported herein (Table 1 and S2).
Mirex
Mirex was only detected in shellfish from Nantong in a concentration range of 0.6–2.5 ng/g fat (Table S2). This is similar as the one determined for β-endosulfan (cf. above). The occurrence of mirex in the shellfish from the Nantong area is not surprising since most, if not all, of the mirex manufacturing is located to the Jiangsu Province (Wang et al. 2010a). Accordingly, mirex reported herein might be from its production or application as a pesticide. China is the country that has suffered from termite damage most severely in the world, especially in the south. However, on a regional scale, the level of mirex determined in the present study was low compared with another study (Jia et al. 2011). Jia et al. measured mirex in oyster in north of China with a mean concentration of 2 ng/g w.w. China has started to substitute mirex for dechlorane plus (Shen et al. 2010).
Industrial organohalogens in shellfish
HCB
HCB levels in shellfish as reported in the present study (1.1–3.6 ng/g fat) are similar to most shellfish levels previously reported as summarized in Table 2 except for a couple of studies (Ramu et al. 2007b; So et al. 2005). Particularly, the latter was reporting on high HCB concentrations in green mussels from Hong Kong (26–430 ng/g fat) (So et al. 2005).
The HCB may come from several sources, i.e., if used as a pesticide, general combustion (Wang et al. 2010b), industrial by-product, and its use as a starting material for production of pentachlorophenol (PCP) and pentachlorophenol-Na (PCP-Na) (Wong et al. 2005). Qiu and coworkers has reported positive relation between concentration of pentachloroanisole, a metabolite of PCP, and HCB in fish samples from Taihu lake (Qiu et al. 2012). China has stopped producing and using HCB since 2004 (UNEP 2007). Nevertheless, temporal trend of HCB and PCP is needed for monitoring the development of these OCPs in the Chinese environment.
PCBs
PCB concentrations are presented in the diagram, Fig. 4, indicating overall low concentrations in the shellfish. It seems that there was a somewhat higher concentration of CB-52 in the Nantong area shellfish, while CB-153 was more evenly distributed between the sites and at a slightly lower concentration (Fig. 4). Tabulated concentrations are presented in Table S4. The PCB concentrations were in the lower end of all global reports used for reference purposes herein (Table 2). The data confirm limited uses of PCBs in China.
HBCDDs and PBDEs
As shown in Table 1, relatively high and evenly distributed HBCDD concentrations (21 to 42 ng/g fat) were determined in the shellfish analyzed in the present study. Still, these levels were lower than those reported for oysters and blue mussels from coastal areas of Japan (Table 2) (Ueno et al. 2010). In contrast, the presently analyzed shellfish had 2–4 times higher HBCDD levels than blue mussel from Sweden (NRM 2012) and reported in fish from Taihu Lake (Qiu et al. 2012). Due to the analytical methodology (GC-MS), it is unfortunately not possible to report the HBCDD isomer pattern. The data points at regional contamination of HBCDD but no details are yet known to us.
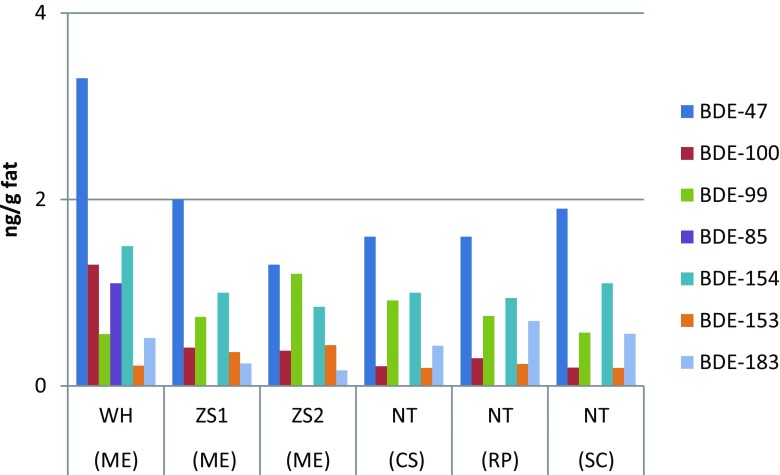
The PBDEs concentrations determined in shellfish in the present study were rather low (Fig. 5, Table S4), with BDE-47 showing the highest levels among the PBDEs. The levels reported in this study were similar to those reported in another study in China (Table 2) (Ramu et al. 2007b). Other congeners with concentrations in descending order were BDE-154, BDE-99, BDE-153, BDE-100, and BDE-183. The pattern observed was consistent with another study (Gao et al. 2009).
Fig. 5.
Concentrations of PBDEs in Mytilus edulis (ME), Cyclina sinensis (CS), Ruditapes philippinarum (RP), and Sinonovacula constricta (SC) from Weihai (WH), Zhoushan (ZS), and Nantong (NT). ZS1 natural mussel and ZS2 cultivated mussel
The reason for composition of PBDE congeners found in this study is not known. It could be explained by commercial PentaBDE product. However, it could also have been influenced by transformation and debromination. For instance, BDE-99 can be transformed to BDE-47 while BDE-154 is transformed from BDE-183 in common carp (Stapleton et al. 2004). Other studies show that BDE-47 could be formed through microbial reductive debromination (He et al. 2006) or photochemical degradation (Fang et al. 2008). So far, there is a lack of knowledge on PBDE metabolism and transformation in shellfish.
Natural compounds in shellfish
MeO-PBDEs
The two naturally produced compounds, 6-MeO-BDE-47 and 2′-MeO-BDE-68, were detected in 100 % of the shellfish samples, independent of sampling location (Fig. 6, Table S6). The highest concentration of MeO-PBDEs in shellfish was observed in blue mussels from Weihai and Zhoushan, while lower in the other shellfish species from Nantong. It is not possible to state if this is a species difference or a difference in the environmental concentrations at the different sites. The concentrations of MeO-PBDEs in this study were consistent with the other in bivalve from Liaohe Bay in China (16 ng/g fat) (Zhang et al. 2010). The level of MeO-PBDEs was comparable to those in mussel from Canada (14 ng/g d.w.) (Kelly et al. 2008) and was lower than those in the Baltic Sea (160–420 ng/g fat) (Lofstrand et al. 2011).
Besides 6-MeO-BDE-47 and 2′-MeO-BDE-68, also several other ortho-MeO-substituted MeO-PBDEs were detected and quantified (Table S6). MeO-PBDEs reported in marine biota species are mostly ortho-substituted and are considered as natural product originating from alga or its associated microflora (Malmvarn et al. 2005). Qiu et al. ((2012) reported that 6-MeO-BDE-47 in fish from Taihu Lake may come from cyanobacteria. The MeO-PBDE level measured in this study was similar to those reported in fish (Qiu et al. 2012).
Concluding remarks
The results from this study clearly show a severe pollution situation regarding the DDTs in the shellfish from the Yellow Sea and the East China Sea. The relatively high HBCDD concentrations in shellfish from all three sampling locations must be followed up by screening in other wildlife species and search for sources of contamination. HBCDD is indeed proposed as a new POP according to the Stockholm Convention, indicating the importance of controlling also this BFR. Further, it may be of interest to look closer into also the POPs not measured herein.
It is a serious obstacle that the scientific literature is not better coordinated in relation to reporting contaminant levels (Table 2). In this table, it is necessary to compare data both on wet and lipid weight basis due to the lack of proper reporting of lipid weight. Data from several studies had to be recalculated to enable comparison to wet weight concentrations since the original data are reported on dry weight. As authors of this article, we ask for actions in this context, possibly via scientist community development of weight of evidence for exposures to pollutants in wildlife.
The data presented herein include several shellfish species but it seems reasonable to propose more work on blue mussels since this is indeed a common species for environmental contamination levels around the world. The use of blue mussels will allow comparisons between truly wild mussels and cultivated blue mussels. This may be a possibility for locating point sources of POPs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 177 kb)
Acknowledgments
Financial supports from the SIDA Partner Driven Cooperation project (AKT 2010-015) and the VR Research Cooperation China project (639-2013-6913) are gratefully acknowledged. The financial support from Tongji University is also acknowledged. We would like to thank Ioannis Athanassiadis for performing the GC-MS analysis.
References
- Christensen JH, Glasius M, Pecseli M, Platz J, Pritzl G. Polybrominated diphenyl ethers (PBDEs) in marine fish and blue mussels from southern Greenland. Chemosphere. 2002;47:631–638. doi: 10.1016/S0045-6535(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Fang ZQ. Organochlorines in sediments and mussels collected from coastal sites along the Pearl River Delta, South China. J Environ Sci (China) 2004;16:321–327. [PubMed] [Google Scholar]
- Fang L, Huang J, Yu G, Wang L. Photochemical degradation of six polybrominated diphenyl ether congeners under ultraviolet irradiation in hexane. Chemosphere. 2008;71:258–267. doi: 10.1016/j.chemosphere.2007.09.041. [DOI] [PubMed] [Google Scholar]
- Feng CL, Xu YP, He Y, Luo QA, Zha JM, Wang ZJ. Debrominated and methoxylated polybrominated diphenyl ether metabolites in rainbow trout (Oncorhynchus mykiss) after exposure to decabromodiphenyl ether. J Environ Sci (China) 2010;22:1425–1434. doi: 10.1016/S1001-0742(09)60271-0. [DOI] [PubMed] [Google Scholar]
- Fu JM, Mai BX, Sheng GY, Zhang G, Wang XM, Peng PA, Xiao XM, Ran R, Cheng FZ, Peng XZ, Wang ZS, Tang UW. Persistent organic pollutants in environment of the Pearl River Delta, China: an overview. Chemosphere. 2003;52:1411–1422. doi: 10.1016/S0045-6535(03)00477-6. [DOI] [PubMed] [Google Scholar]
- Gao ZS, Xu J, Xian QM, Feng JF, Chen XH, Yu HX. Polybrominated diphenyl ethers (PBDEs) in aquatic biota from the lower reach of the Yangtze River, East China. Chemosphere. 2009;75:1273–1279. doi: 10.1016/j.chemosphere.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Giandomenico S, Spada L, Annicchiarico C, Assennato G, Cardellicchio N, Ungaro N, Di Leo A. Chlorinated compounds and polybrominated diphenyl ethers (PBDEs) in mussels (Mytilus galloprovincialis) collected from Apulia Region coasts. Mar Pollut Bull. 2013;73:243–251. doi: 10.1016/j.marpolbul.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Glasius M, Christensen JH, Platz J, Vorkamp K. Halogenated organic contaminants in marine fish and mussels from southern Greenland—pilot study on relations to trophic levels and local sources. J Environ Monit. 2005;7:127–131. doi: 10.1039/b411959b. [DOI] [PubMed] [Google Scholar]
- Goldberg ED, Bowen VT, Farrington JW, Harvey G, Martin JH, Parker PL, Risebrough RW, Robertson W, Schneider E, Gamble E. Mussel watch. Environ Conserv. 1978;5:101–125. doi: 10.1017/S0376892900005555. [DOI] [Google Scholar]
- Haglund PS, Zook DR, Buser HR, Hu JW. Identification and quantification of polybrominated diphenyl ethers and methoxy-polybrominated diphenyl ethers in Baltic biota. Environ Sci Technol. 1997;31:3281–3287. doi: 10.1021/es9702834. [DOI] [Google Scholar]
- He JZ, Robrock KR, varez-Cohen L. Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs) Environ Sci Technol. 2006;40:4429–4434. doi: 10.1021/es052508d. [DOI] [PubMed] [Google Scholar]
- Jensen S, Johnels AG, Olsson M, Otterlin G. DDT and PCB in marine animals from Swedish waters. Nature. 1969;224:247–250. doi: 10.1038/224247a0. [DOI] [PubMed] [Google Scholar]
- Jensen S, Lindqvist D, Asplund L. Lipid extraction and determination of halogenated phenols and alkylphenols as their pentafluorobenzoyl derivatives in marine organisms. J Agric Food Chem. 2009;57:5872–5877. doi: 10.1021/jf803971d. [DOI] [PubMed] [Google Scholar]
- Jia HL, Sun YQ, Liu XJ, Yang M, Wang DG, Qi H, Shen L, Sverko E, Reiner EJ, Li YF. Concentration and bioaccumulation of dechlorane compounds in coastal environment of Northern China. Environ Sci Technol. 2011;45:2613–2618. doi: 10.1021/es103723h. [DOI] [PubMed] [Google Scholar]
- Jiang K, Li LJ, Chen YD, Jin J. Determination of PCDD/Fs and dioxin-like PCBs in Chinese commercial PCBs and emissions from a testing PCB incinerator. Chemosphere. 1997;34:941–950. doi: 10.1016/S0045-6535(97)00397-4. [DOI] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Gobas FAPC. Hydroxylated and methoxylated polybrominated diphenyl ethers in a Canadian Arctic marine food web. Environ Sci Technol. 2008;42:7069–7077. doi: 10.1021/es801275d. [DOI] [PubMed] [Google Scholar]
- Kozul D, Romanic SH, Kljakovic-Gaspic Z, Veza J. Distribution of polychlorinated biphenyls and organochlorine pesticides in wild mussels from two different sites in central Croatian Adriatic coast. Environ Monit Assess. 2011;179:325–333. doi: 10.1007/s10661-010-1739-2. [DOI] [PubMed] [Google Scholar]
- Li YF, Cai DJ, Singh A. Technical hexachlorocyclohexane use trends in China and their impact on the environment. Arch Environ Contam Toxicol. 1998;35:688–697. doi: 10.1007/s002449900432. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang HM, Tao MH, Yang SB, Wang LW, Liu Y, Ma DD, He ZM. Organochlorine pesticides in consumer fish and mollusks of Liaoning Province, China: distribution and human exposure implications. Arch Environ Contam Toxicol. 2010;59:444–453. doi: 10.1007/s00244-010-9504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofstrand K, Liu XT, Lindqvist D, Jensen S, Asplund L. Seasonal variations of hydroxylated and methoxylated brominated diphenyl ethers in blue mussels from the Baltic Sea. Chemosphere. 2011;84:527–532. doi: 10.1016/j.chemosphere.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Mai BX, Chen SJ, Luo XJ, Chen LG, Yang QS, Sheng GY, Peng PG, Fu JM, Zeng EY. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ Sci Technol. 2005;39:3521–3527. doi: 10.1021/es048083x. [DOI] [PubMed] [Google Scholar]
- Malmvarn A, Marsh G, Kautsky L, Athanasiadou M, Bergman A, Asplund L. Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea. Environ Sci Technol. 2005;39:2990–2997. doi: 10.1021/es0482886. [DOI] [PubMed] [Google Scholar]
- Marsh G, Stenutz R, Bergman A (2003) Synthesis of hydroxylated and methoxylated polybrominated diphenyl ethers—natural products and potential polybrominated diphenyl ether metabolites. European Journal of Organic Chemistry 2566–2576
- Marsh G, Athanasiadou M, Athanassiadis I, Bergman A, Endo T, Haraguchi K. Identification, quantification, and synthesis of a novel dimethoxylated polybrominated biphenyl in marine mammals caught off the coast of Japan. Environ Sci Technol. 2005;39:8684–8690. doi: 10.1021/es051153v. [DOI] [PubMed] [Google Scholar]
- NRM (2012) Övervakning av metaller och organiska miljögifter i marin biota, 2012.
- O’Connor TP. National distribution of chemical concentrations in mussels and oysters in the USA. Mar Environ Res. 2002;53:117–143. doi: 10.1016/S0141-1136(01)00116-7. [DOI] [PubMed] [Google Scholar]
- Pikkarainen AL. Polychlorinated biphenyls and organochlorine pesticides in Baltic Sea sediments and bivalves. Chemosphere. 2007;68:17–24. doi: 10.1016/j.chemosphere.2006.12.079. [DOI] [PubMed] [Google Scholar]
- Qiu XH, Zhu T, Yao B, Hu JX, Hu SW. Contribution of dicofol to the current DDT pollution in China. Environ Sci Technol. 2005;39:4385–4390. doi: 10.1021/es050342a. [DOI] [PubMed] [Google Scholar]
- Qiu YL, Strid A, Bignert A, Zhu ZL, Zhao JF, Athanasiadou M, Athanassiadis L, Bergman A. Chlorinated and brominated organic contaminants in fish from Shanghai markets: a case study of human exposure. Chemosphere. 2012;89:458–466. doi: 10.1016/j.chemosphere.2012.05.099. [DOI] [PubMed] [Google Scholar]
- Ramu K, Kajiwara N, Isobe T, Takahashi S, Kim EY, Min BY, We SU, Tanabe S. Spatial distribution and accumulation of brominated flame retardants, polychlorinated biphenyls and organochlorine pesticides in blue mussels (Mytilus edulis) from coastal waters of Korea. Environ Pollut. 2007;148:562–569. doi: 10.1016/j.envpol.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Ramu K, Kajiwara N, Sudaryanto A, Isobe T, Takahashi S, Subramanian A, Ueno D, Zheng GJ, Lam PKS, Takada H, Zakaria MP, Viet PH, Prudente M, Tana TS, Tanabe S. Asian mussel watch program: contamination status of polyhrominated diphenyl ethers and organochlorines in coastal waters of Asian countries. Environ Sci Technol. 2007;41:4580–4586. doi: 10.1021/es070380p. [DOI] [PubMed] [Google Scholar]
- Shen L, Reiner EJ, MacPherson KA, Kolic TM, Sverko E, Helm PA, Bhavsar SP, Brindle ID, Marvin CH. Identification and screening analysis of halogenated norhornene flame retardants in the Laurentian great lakes: dechloranes 602, 603, and 604. Environ Sci Technol. 2010;44:760–766. doi: 10.1021/es902482b. [DOI] [PubMed] [Google Scholar]
- So MK, Zhang X, Giesy JP, Fung CN, Fong HW, Zheng J, Kramer MJ, Yoo H, Lam PK. Organochlorines and dioxin-like compounds in green-lipped mussels Perna viridis from Hong Kong mariculture zones. Mar Pollut Bull. 2005;51:677–687. doi: 10.1016/j.marpolbul.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Letcher RJ, Baker JE. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio) Environ Sci Technol. 2004;38:1054–1061. doi: 10.1021/es0348804. [DOI] [PubMed] [Google Scholar]
- Ueno D, Isobe T, Ramu K, Tanabe S, Alaee M, Marvin C, Inoue K, Someya T, Miyajima T, Kodama H, Nakata H. Spatial distribution of hexabromocyclododecanes (HBCDs), polybrominated diphenyl ethers (PBDEs) and organochlorines in bivalves from Japanese coastal waters. Chemosphere. 2010;78:1213–1219. doi: 10.1016/j.chemosphere.2009.12.058. [DOI] [PubMed] [Google Scholar]
- UNEP (2007) The People’s Republic of China National Implementation Plan for the Stockholm Convention on Persistent Organic Pollutants.
- UNEP (2014) Stockholm Convention Overview.
- Van Ael E, Covaci A, Blust R, Bervoets L. Persistent organic pollutants in the Scheldt estuary: environmental distribution and bioaccumulation. Environ Int. 2012;48:17–27. doi: 10.1016/j.envint.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Walker K, Vallero DA, Lewis RG. Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environ Sci Technol. 1999;33:4373–4378. doi: 10.1021/es990647n. [DOI] [Google Scholar]
- Wan Y, Wiseman S, Chang H, Zhang XW, Jones PD, Hecker M, Kannan K, Tanabe S, Hu JY, Lam MHW, Giesy JP. Origin of hydroxylated brominated diphenyl ethers: natural compounds or man-made flame retardants? Environ Sci Technol. 2009;43:7536–7542. doi: 10.1021/es901357u. [DOI] [PubMed] [Google Scholar]
- Wang B, Iino F, Yu G, Huang J, Wei YX, Yamazaki N, Chen JF, Chen XL, Jiang W, Morita M. HRGC/HRMS analysis of mirex in soil of Liyang and preliminary assessment of mirex pollution in China. Chemosphere. 2010;79:299–304. doi: 10.1016/j.chemosphere.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Wang G, Lu YL, Han JY, Luo W, Shi YJ, Wang TY, Sun YM. Hexachlorobenzene sources, levels and human exposure in the environment of China. Environ Int. 2010;36:122–130. doi: 10.1016/j.envint.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Wong CKC, Leung KM, Poon BHT, Lan CY, Wong MH. Organochlorine hydrocarbons in human breast milk collected in Hong Kong and Guangzhou. Arch Environ Contam Toxicol. 2002;43:364–372. doi: 10.1007/s00244-002-1210-7. [DOI] [PubMed] [Google Scholar]
- Wong MH, Leung AOW, Chan JKY, Choi MPK. A review on the usage of POP pesticides in China, with emphasis on DDT loadings in human milk. Chemosphere. 2005;60:740–752. doi: 10.1016/j.chemosphere.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Xing Y, Lu YL, Dawson RW, Shi YJ, Zhang H, Wang TY, Liu WB, Ren HC. A spatial temporal assessment of pollution from PCBs in China. Chemosphere. 2005;60:731–739. doi: 10.1016/j.chemosphere.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu M, Xu S, Hou L, Ou D, Liu H, Cheng S, Hofmann T. HCHs and DDTs in sediment-dwelling animals from the Yangtze Estuary, China. Chemosphere. 2006;62:381–389. doi: 10.1016/j.chemosphere.2005.04.102. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Yusho in Japan. Ind Health. 2003;41:139–148. doi: 10.2486/indhealth.41.139. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wan Y, An LH, Hu JY. Trophodynamics of polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers in a marine food web. Environ Toxicol Chem. 2010;29:2792–2799. doi: 10.1002/etc.334. [DOI] [PubMed] [Google Scholar]
- Zhou RB, Zhu LZ, Kong QX. Levels and distribution of organochlorine pesticides in shellfish from Qiantang River, China. J Hazard Mater. 2008;152:1192–1200. doi: 10.1016/j.jhazmat.2007.07.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 177 kb)