Abstract
Human papillomavirus (HPV)-related multiphenotypic sinonasal carcinoma (HMSC) is a recently described distinctive clinicopathologic entity defined by association to high risk HPV, localization to sinonasal tract and close histologic resemblance to salivary gland tumors. Lack of awareness of its pathologic features and biology among pathologists and oncologists make this entity susceptible to misdiagnosis and erroneous management. Herein, we illustrate a case of HMSC of the nasal cavity associated with heretofore unreported subtype HPV-52 and discuss the challenges associated with diagnosis and management of this rare tumor. A 48-year-old woman with intermittent epistaxis for 6 months presented with a nasal mass and underwent middle turbinectomy. Histology showed a tumor with features typical of adenoid cystic carcinoma (ACC) in the form of basaloid cells and cribriform architecture. However, careful inspection revealed findings uncommon in ACC; such as surface pagetoid tumor spread, areas of solid sheets of myoepithelial cells accompanied by increased mitotic figures which prompted immunohistochemistry. Multidirectional differentiation into ductal (CK7, AE1/AE3) and myoepithelial (p63, p40, S100, calponin) lineage together with strong and diffuse immunopositivity for p16 distinguished this tumor from ACC. HPV genotyping was positive for high risk HPV subtype HPV52, which confirmed the diagnosis of HMSC. HPV-related multiphenotypic sinonasal carcinoma is an under-recognized unique clinicopathologic entity that needs awareness to avoid mistaking it for commoner salivary gland tumors. Making accurate diagnosis of this newly-described tumor is imperative in order to understand its biology and to develop optimal therapeutic strategies.
Keywords: Human papillomavirus-related multiphenotypic sinonasal carcinoma, HPV type 52, Immunohistochemistry, Adenoid cystic-like carcinoma, Differential diagnosis
Introduction
Human papillomavirus (HPV) is well known for its association with carcinomas at various sites. In the head and neck region, the oropharynx is the most common location for HPV-related cancers accounting for 80% of cancers in western population [1]. While non-keratininzing squamous cell carcinoma is the commonest histology described for HPV-related cancers in the head and neck region, various other types are also reported such as papillary squamous, lymphoepithelial, small cell neuroendocrine, and ciliated carcinomas to name a few [2]. Recently, the sinonasal tract has emerged as the second ‘hot-spot’ for HPV with upto 21% of sinonasal cancers harboring high risk HPV [2].
HPV-related multiphenotypic carcinoma (HMSC) is the latest addition to the family of sinonasal tract carcinomas with an established etiological role of HPV infection, especially with type 33. HMSC bears a distinctive clinicopathologic profile characterized by high-risk HPV association, sinonasal tract localization, salivary gland tumor-like appearance, high-grade histologic appearance, and a paradoxical indolent clinical course [3–5]. However, close morphological resemblance to common salivary gland tumors, exacerbated by a lack of awareness of this entity by pathologists and oncologists make this entity susceptible to misdiagnosis, erroneous management and eventual skewing of clinical and epidemiologic data. Not much is known about the biology and optimal treatment of this newly recognized tumor. Herein, we illustrate a case of nasal HMSC associated with HPV 52, a, to the best of our knowledge heretofore unreported subtype of HPV and discuss the diagnostic pitfalls supplemented with an updated review of literature.
Case Report
A 48-year-old woman presented with complaints of intermittent episodes of epistaxis and pain in the medial canthus of eye for 6 months. Local examination revealed a mass completely obstructing the nasal cavity. Contrast-enhanced computed tomography (CECT) and magnetic resonance imaging (MRI) scans showed a well-defined, heterogeneously isointense to hyperintense lobulated, soft tissue density lesion on T2W involving left nasal cavity, measuring 9.5 × 3.4 × 2.7 cm (Fig. 1). The mass arose from the middle turbinate, extending into the nasopharynx without invading any sinuses. Endoscopic tumor debulking with middle turbinectomy was performed and the tissue was sent to our hospital.
Fig. 1.
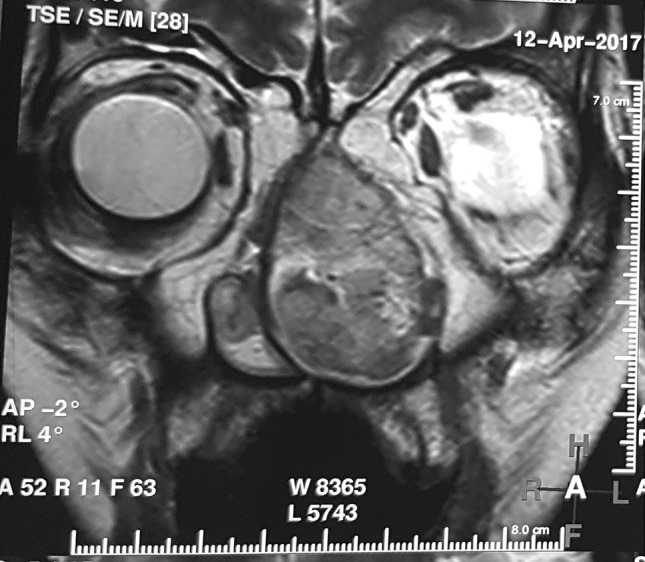
Magnetic resonance imaging (MRI) showing expansile, well defined, lobulated mass lesion in left nasal cavity which was isointense to hyperintense of T2W, bowing the nasal septum medially and deviating the medial wall of maxillary antrum laterally without invasion
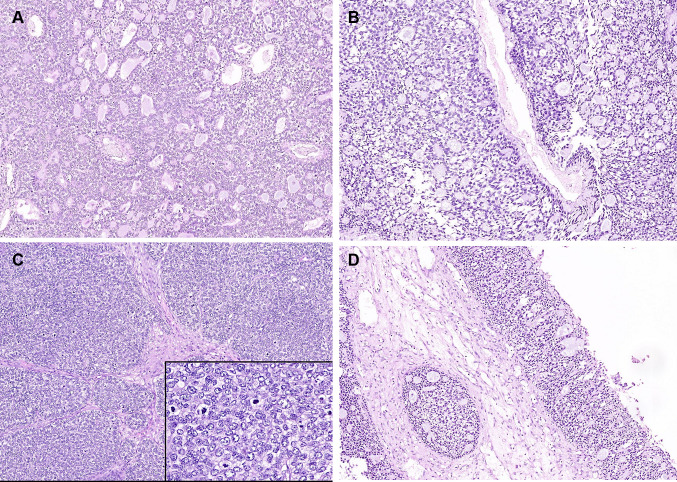
Histology revealed multiple tissue fragments focally lined by ulcerated respiratory epithelium, with squamous metaplasia at places. A submucosal tumor was seen, separated by bands of fibrosis and hyalinization. Two distinct architectural patterns were noted within the tumor at low power; cribriform pattern with striking resemblance to adenoid cystic carcinoma (ACC), and a solid pattern with high grade histological features (Fig. 2a). The former pattern accounted for 60% of the tumour. In the cribriform areas, basaloid tumor cells lined cylindromatous microcystic spaces that were filled with basophilic mucoid material (Fig. 2b). Focal clear cell change was noted. Tumor cells in the solid areas showed moderate nuclear pleomorphism with vesicular nuclei and scant cytoplasm (Fig. 2c). Occasional bizarre cells with multinucleation and prominent nucleoli were also noted. In addition, solid areas showed individual cell and confluent areas of necrosis. Mitotic activity was brisk in these areas with 50–55 mitoses per 10 high power field (Fig. 2c inset). Lymphovascular tumor emboli and perineural infiltration were absent. The overlying epithelium revealed occasional foci of surface epithelial colonization by the tumor (Fig. 2d); overt surface squamous dysplasia was not seen. The tumor lacked overt bi-layering by ductal and myoepithelial cells, ductular or squamous differentiation and mesenchymal chondro-osseous or sarcomatoid dedifferentiation.
Fig. 2.
a Tumor showing intimately admixed solid and cribriform areas (hematoxylin and eosin, × 50). b Cribriform spaces reminiscent of those seen in adenoid cystic carcinoma (hematoxylin and eosin, × 200). c Solid sheets of tumor cells with vesicular nuclei (hematoxylin and eosin, × 200). Inset: brisk mitoses in the solid areas (hematoxylin and eosin, × 400). d Pagetoid tumor spread in the surface epithelium (hematoxylin and eosin, × 100)
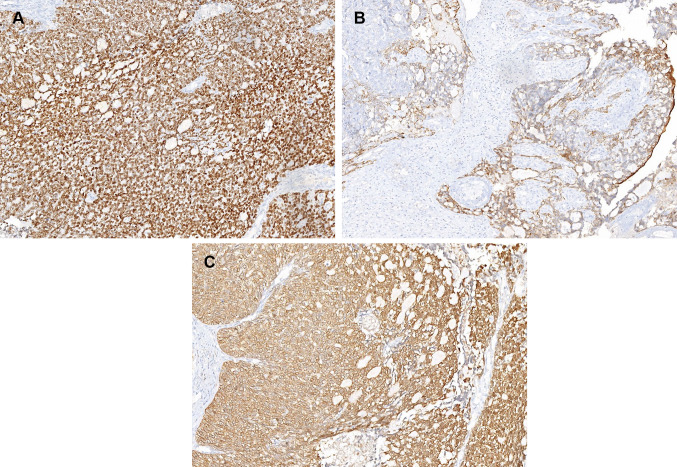
Immunohistochemistry (IHC) was performed on 4 µm thick sections on Ventana Benchmark autostainer. The following antibodies were utilized: AE1/AE3 (Clone: AE1/AE3, 1:300, Biocare, Concord, CA), CK7 (OV-TL, 1:30, Dako, Carpinteria, CA), p40 (MRQ 40, 1:75, Biocare), p63 (BC4A4, 1:250, Biocare), p16 (E6H4, 1:25, Ventana), S100 (Polyclonal, 1:1000, Dako), Calponin (CALP, 1:150. Dako), CD117 (Polycloncal, 1:1000, Dako), NUT (Polyclonal 1:50, Cell Signalling, Danvers, MA), Androgen receptor (SP107, 1:50, Cell Marque, CA), Synaptophysin (Polyclonal, 1:300, Cell Marque, CA), Chromogranin (DAL-A3, 1:250, Dako). Immunohistochemistry for ductal and myoepithelial markers revealed a dual differentiation; strong CK7 immunoreactivity highlighted the ductal cells while the predominant cellular component and abluminal cells stained diffusely for myoepithelial markers namely, p40, S100p, AE1/AE3, calponin and weakly for CK7 (Fig. 3a, b). CD117 (c-kit) was negative in our case. Tumor cells were immunonegative for c-erbB2, androgen receptor, desmin, Fli-1, NUT protein, synaptophysin, chromogranin and CD56. Immunohistochemistry for p16 (Fig. 3c) exhibited a strong and diffuse nuclear as well as cytoplasmic staining in almost all tumor cells.
Fig. 3.
Immunohistochemistry showing diffuse staining for myoepithelial marker, p40 (a); focal epithelial marker expression, CK7 (b); diffuse and strong immunostaining for p16 was noted in tumour (c)
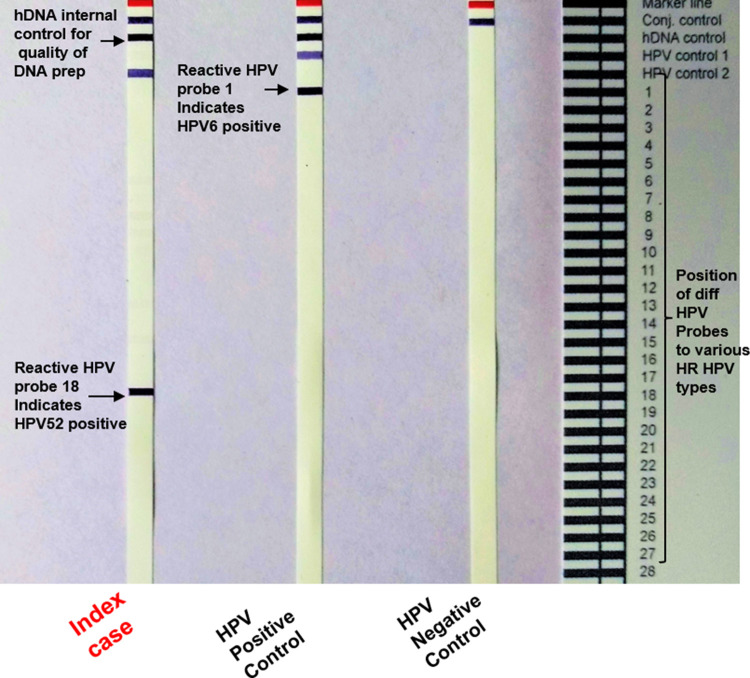
HPV genotyping was performed using real time polymerase chain reaction (RT PCR) using HPV PCR assay (Fujirebio-Europe, Gent, Belgium). The genotyping revealed presence of HPV 52 in the tumor (Fig. 4). Thus, a final diagnosis of HPV type 52-related multiphenotypic sinonasal carcinoma was rendered. The patient refused any adjuvant treatment and is currently without evidence of disease at 12 months following surgery.
Fig. 4.
INNO-LIPA HPV PCR genotyping assay illustrating HPV positivity on LiPA strips, showing respective subtypes which appear as specific purple bands. Reactive Probe 18, HPV 52 positive test sample (on left strip); reactive probe 1, HPV 6 positive sample used as control (middle strip), HPV negative control affirming conjugate- substrate reaction (right strip). Reading card indicating the location of specific probes on the INNO-LIPA HPV genotyping assay
Discussion
HPV-related multiphenotypic sinonasal carcinoma is a recently described entity associated with HPV genotypes and a peculiar proclivity for the sinonasal tract. Limited data in the form of case reports [4, 6] and a few small case series [7–9] builds together a clinicopathologic sketch of a unique tumor that needs urgent attention of pathologists and treating oncologists. The largest series of HMSC is by Bishop et al. [3] which is a multi-institutional collection of 49 cases aimed at gaining a better understanding of the biology and clinical features of this rare tumor. The present case is not only an addition to the existing data base of this tumor but also has a unique finding of association with high risk HPV 52, which has, to the best of our knowledge, not been previously reported.
The patient had a nasal mass at presentation with symptoms of mass effect in the form of nasal obstruction, pain, and epistaxis, which are by far the most common site for HMSC (57%) and symptoms of this tumor. A wide age range of 28–90 years (mean age 54 years) has been noted with a slight female preponderance (F:M = 1.56:1). On radiology, the current case is 9.5 cm in maximum dimension, which is the largest of all reported case (range 0.7–8.5 cm) in the literature to date [3, 4]. This maybe attributable to delayed presentation of the patient to the hospital.
On histology, the current case showed a combination of cribriform (ACC-like) architecture and solid areas, which on immunohistochemistry showed dual ductal (focal) and myoepithelial (predominant) differentiation. The literature describes multiple lines of differentiation towards epithelial (ductal, myoepithelial, squamous), myoepithelial or mixed epithelial-myoepithelial and mesenchymal differentiation. The mesenchymal differentiation in the form of chondro-osseous (6.1%, 3/49) or sarcomatoid differentiation (10.2%, 5/49) has been reported [3]. Cytoplasmic clearing, being one of the histological features of myoepithelial differentiation, was noted in 89.8% cases (44/49) [3]. HMSC is commonly reported to display surface squamous dysplasia [3] fueling speculations of origin from surface epithelium. Although this feature was not seen in our case, a pagetoid tumor colonization of surface epithelium was observed.
The vast morphological variation with basaloid, cribriform, and solid sheeted pattern and mesenchymal differentiation leads to a wide range of differential diagnoses which are discussed in Table 1. Thus, careful assessment of morphological features and a wide range of immunohistochemical panel is essential to arrive at the correct diagnosis. Positivity for ductal (CK7), myoepithelial (p40 and/or p63, S100p, calponin) markers and diffuse cytoplasmic and nuclear p16 positivity (> 95% of tumour cells), potentiated by molecular HPV subtyping, segregates this entity from all the other relevant differential diagnoses. Pattern and extent of p16 immunostaining is of utmost importance, as diagnosis of HMSC necessitates almost every tumour cell to have nuclear and cytoplasmic positivity for p16. This is different from the 70% tumour cell p16 positivity (nuclear and cytoplasmic) used for HPV-related oropharyngeal carcinoma, as per recent College of American Pathologist (CAP) guidelines [10]. Focal p16 expression has been well documented in many salivary gland tumors, especially ACC with at least some cases reaching the CAP threshold of p16 positivity [11, 12]. While HPV DNA testing supports the diagnosis, the virus could still be a passenger without transcriptional activity. RNA insitu hybridization for detecting HPV E6/E7 transcripts is confirmatory as it permits identification of integrated and transcriptionally active virus, allows visualization of viral copies in tissues, and is amenable to light microscopic evaluation in a routine clinical laboratory [13]. Various high risk HPV has been associated with HMSC, type 33 being the commonest (34/50) with rare cases of HPV 35 (3/50) and type HPV 56 (1/50) and HPV 16 (1/50) [3, 4, 9]. Interestingly, HPV 26, classified as an intermediate risk HPV was recently reported to be associated with HMSC [5]. We describe the type 52 in the present case. Though HPV 52 infection has been linked with cancers in the sinonasal tract and larynx, [14] none of the reported cases of HMSC, till date, have shown HPV 52 infection. The extent to which HPV subtype influences the biology and outcome of this cancer is currently unknown. Studies are needed to elucidate the exact route of infection and etio-pathogenesis of HPV in HMSC. A brief review of literature has been summarized in Table 2.
Table 1.
Features of distinction in HMSC mimickers
| HMSC | Basaloid squamous carcinoma | Adenoid cystic carcinoma | High grade myoepithelial carcinoma | SMARCB-1 (INI-1) deficient sinonasal carcinoma | NUT midline carcinoma | |
|---|---|---|---|---|---|---|
| Histological features | ||||||
| Basaloid cells | + | + | + | ± | + | + |
| Ductal cells | + | − | + | ± | − | − |
| Myoepithelial cells | + | − | + | + | − | − |
| Rhabdoid cells | − | − | − | − | + | − |
| Round to undifferentiated cells | − | − | − | − | + | + |
| Squamous differentiation | May be present | Usually, present, focal | Uncommon | Uncommon | Not seen | May be present, abrupt and focal |
| Heterologous differentiation | May be present, rare | Absent | Absent | Absent | Absent | Absent |
| Immunoprofile of the tumours | ||||||
| p16 | Positive in almost every cell | Mostly negative | Patchy ductal, never diffuse | Patchy | Mostly negative | Mostly negative, Never diffuse |
| Ductal | May be positive | May be patchy | Diffuse | May be patchy | May be patchy | May be patchy |
| Myoepithelial | Positive | Negative | Positive | Positive | Negative | Negative |
| NUT protein | Negative | Negative | Negative | Negative | Negative | Positive |
| INI-1 protein | Retained | Retained | Retained | Retained | Lost | Retained |
| Diagnostic molecular evaluation | HPV genotyping-RT PCR, ISH | − | MYB, NFIB, MYBL-1 rearrangement | − | Homo-/heterozygous deletion of SMARCB1 | NUT translocation |
| Therapeutic options | Surgery, role of adjuvant unknown | Surgery with adjuvant radiotherapy | Radical excision with adjuvant radiotherapy | Surgery, non- sensitive to radiotherapy | Surgery with neoadjuvant therapy. Targeted therapy being tried (EZH2 and histone deacetylase inhibitors) | Surgery ± chemo-radiation |
| Clinical course | Indolent, locally recurrent (38.5%), very rare metastasis | Aggressive with common nodal and distant metastasis | Locally recurrent, late metastasis | Aggressive with local and nodal recurrence | Highly aggressive with high mortality rate | Highly aggressive and commonly fatal |
HMSC HPV-related multiphenotypic sinonasal carcinoma
Table 2.
Review of literature
| Author name | Bishop et al. [3] | Hwang et al. [6] | Hang et al. [9] | Shah et al. [4] | Ruangritchankul et al. [5] | Current case |
| No. of cases | 49 | 1 | 5 | 1 | 1 | 1 |
| Age range (mean age (years)/gender | 28–90 (54), 28F:21M | 75/F | 43.8 (30–58), 5M | 69/F | 50/F | 48/F |
| Tumour site | Nasal cavity with/without sinuses | Nasal cavity | Nasal cavity and middle turbinate | Hard palate | Right nasal cavity | Nasal cavity mass |
| Clinical presentation | Nasal obstruction/stenosis | Recurrent epistaxis | Nasal obstruction, epistaxis | Palatal soreness | Recurrent epistaxis and nasal congestion | Epistaxis 6 with pain |
|
Tumour size (mean) cm |
0.7–8.5 (3.8) | NA | 3.7 (0.9–3.4) | 3.5 | NA | 9.5 |
| Stage | T1 (16/39), T2 (7/39), T3 (8/39), T4 (9/39) | T1N0M0 | T1 (2/4), T2 (1/4), T3 (1/4) | Recurrent mass | T2aN0M0 | T1N0M0 |
| Treatment | Surgery (18/38), surgery with post-operative radiotherapy (15/38), Surgery f/b chemoradiation 3/38 | Endoscopic inferior turbinectomy | Surgery (n = 4), Surgery with radiotherapy (n = 1) | Maxillectomy f/b partial palatectomy | Endoscopic excision with adjuvant radiotherapy | Endoscopic debulking with middle turbinectomy |
| Surface involvement | 69% | Present | Present in 80% (4/5) | Present | Present | Present |
| Histological features | Solid and cribriform growth pattern with myoepithelial differentiation, squamous differentiation within the invasive tumor, sarcomatoid and chondo-osseous differentiation | Nests and cribriform architecture of basaloid cells | Solid and cribriform growth pattern with their fusion, squamous differentiation within tumour, tumour giant cells and necrosis | High-grade neoplasm with a salivary tumor-like appearance | Malignant basaloid tumour in cribriform pattern with focal duct formation | Solid sheets and cribriform pattern lined by basaloid cells with inconspicuous ductal formations present |
| Immunohistochemistry | AE1/AE3 (39/39), CK (31/39), p40/p63 (48/49), Calponin (29/30), SMA (30/37), CKIT (41/43 with ductal staining pattern in 29), S100p (44/46) | ND | CK7 and C-KIT positive in Ductal and p63, p40, Calponin, SMA in abluminal component | AE1/AE3, CK 7, SOX-10, p40 (Diffuse); CK 5/6, SMA, SMMS-1, S-100 (Focal); CD117 (Rare, weak) | (AE1/AE3), EMA, vimentin, SMA, calponin,, calponin, S-100 protein, CD117, p63 | AE1/AE3, p63, p40, S100p (Diffuse), CK7 & calponin (Focal), |
| p16 IHC | p16 (49/49) | Positive | Positive (5/5) | Positive | Positive | Positive |
| PCR and In-situ Hybridization (ISH) | HPV 33 (33/49) by ISH, HPV 35 (3/14), HPV 56 (1/14), HPV 16 (1/1) | Positive for high risk cocktail | HPV 33 (4/5), HPV 16 (1/5) | HPV 33 | HPV 26 | HPV 52 |
| Any other molecular evaluation | FISH rearrangements: MYB (0/15), MYBL1 (0/6), and NFIB ((0/6). | ND | MYB FISH negative (4/4) | MYB negative | ND | ND |
| Follow up (months) | 1–256 (42): local recurrence (14/39) Distant metastasis (2/39). 30Alive with NED, 9 Alive with disease | 12, NED | 3–184 (mean = 52, median 12.5), NED (4/5) | Two recurrences, after 2 years and 30 years | 85, NED | 12, NED |
M male, F female, NA available, ND not done, f/b followed by, NED no evidence of disease
Being a recently recognized entity with only few reported cases, clinical behavior, and optimal treatment
protocols are not established for HMSC. Surgery with or without adjuvant chemo-radiotherapy have been tried in these cases with varied outcomes [3]. Limited studies available hint at an indolent course however local recurrences, as late as 30 years after diagnosis, [4] have been seen in up to 40% (18 of 44) patients [3, 4, 9], while distant metastasis is infrequent albeit documented (to lung and hand) [3]. Hence, a complete excision and a long term follow up is warranted. A better awareness amongst oncologists and a well documented follow up is necessary in this tumor. Whether therapeutic benefit of radiation or prognostic influence of HPV extends to this entity as well remains to be seen.
In conclusion, HMSC is a newly recognized, rare tumor, with a vast histological spectrum and association with HPV infection. It is a close mimicker of more commonly occurring salivary gland neoplasms, especially ACC in the sinonasal tract, thus a potential diagnostic pitfall. Awareness of this newly recognized entity and judicious use of immunohistochemistry is imperative in avoiding an erroneous diagnosis, and hence guiding accurate patient management. Though indolent, this tumor appears to have a locally aggressive behavior in the form of local recurrences. Hence a complete surgical resection at the time of presentation is of utmost importance. Distant metastases, though described, are distinctly uncommon. The current case, the first HMSC case reported from our country, additionally displayed a hitherto unreported association with HPV type 52. Accurate diagnosis and pooling of cases will help gather information on the cell of origin, epidemiology, treatment response and long term biologic behavior of this unique tumor which is essential to discover potential therapeutic targets.
Compliance with Ethical Standards
Conflict of interest
All authors declare no conflicts of interest.
References
- 1.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 2.Stevens TM, Bishop JA. HPV-related carcinomas of the head and neck: morphologic features, variants, and practical considerations for the surgical pathologist. Virchows Arch. 2017;471(2):295–307. doi: 10.1007/s00428-017-2118-y. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JA, Andreasen S, Hang JF, Bullock MJ, Chen TY, Franchi A, et al. HPV-related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as HPV-related carcinoma with adenoid cystic carcinoma-like features. Am J Surg Pathol. 2017;41:1690–1701. doi: 10.1097/PAS.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AA, Lamarre ED, Bishop JA. Human papillomavirus-related multiphenotypic sinonasal carcinoma: a case report documenting the potential for very late tumor recurrence. Head Neck Pathol. 2018 doi: 10.1007/s12105-018-0895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruangritchankul K, Jitpasutham T, Kitkumthorn N, Thorner PS, Keelawat S. Human papillomavirus-related multiphenotypic sinonasal carcinoma: first case report associated with an intermediate-risk HPV type and literatures review. Hum Pathol. 2018;14:20–24. [Google Scholar]
- 6.Hwang SJ, Ok S, Lee HM, Lee E, Park IH. Human papillomavirus-related carcinoma with adenoid cystic-like features of the inferior turbinate: a case report. Auris Nasus Larynx. 2015;42:53–55. doi: 10.1016/j.anl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen S, Bishop JA, Hansen TV, Westra WH, Bilde A, von Buchwald C, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features of the sinonasal tract: clinical and morphological characterization of six new cases. Histopathol. 2017;70:880–888. doi: 10.1111/his.13162. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hang JF, Hsieh MS, Li WY, Chen JY, Lin SY, Liu SH, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a series of five cases expanding the pathological spectrum. Histopathology. 2017;71(6):887–896. doi: 10.1111/his.13301. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JS, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the college of american pathologists. Arch Pathol Lab Med. 2018;142(5):559–597. doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 11.Boland JM, McPhail ED, Garcia JJ, et al. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 12.Jour G, West K, Ghali V, Shank D, Ephrem G, Wenig BM. Differential expression of p16(INK4A) and cyclin D1 in benign and malignant salivary gland tumors: a study of 44 Cases. Head Neck Pathol. 2013;7(3):224–231. doi: 10.1007/s12105-012-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, Taube JM, Koch WM, Westra WH. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36(12):1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isayeva. T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6:10420. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]