Abstract
Sclerosing odontogenic carcinoma is a rare locally destructive neoplasm with many histologic mimics. Here the diagnostic challenges are presented of a case of sclerosing odontogenic carcinoma with variable histologic features, including unusual and unexpected negative immunostaining for CK19.
Keywords: Sclerosing odontogenic carcinoma, Case report, Review of literature
Introduction
Sclerosing odontogenic carcinoma (SOC) is a rare primary intra-osseous carcinoma of the jaw which has been added to the most recent 4th edition World Health Organization (WHO) classification of Head and Neck tumors [1, 2]. Despite its inclusion in the WHO as a distinct entity, it is a tumor which remains poorly defined in literature with only 10 reported cases to date [3–9, 11, 12]. The histomorphology consists of mixed epithelial and mesenchymal components, resembling other odontogenic neoplasms, although it is unclear whether this represents a bona-fide biphasic tumor or simply an epithelial neoplasm with a reactive mesenchymal proliferation [11]. The histologic overlap/resemblance of this tumor with other head and neck entities also renders it a diagnostic challenge and it should therefore currently be regarded as a diagnosis of exclusion [2, 6, 7, 9, 10]. Additionally, the rarity of this entity to date and locally aggressive behavior makes it difficult to standardize treatment. Here, we present a case of SOC and a review of previously reported cases in the literature, with a focus on the differential diagnosis, especially when faced with a limited biopsy.
Case Report
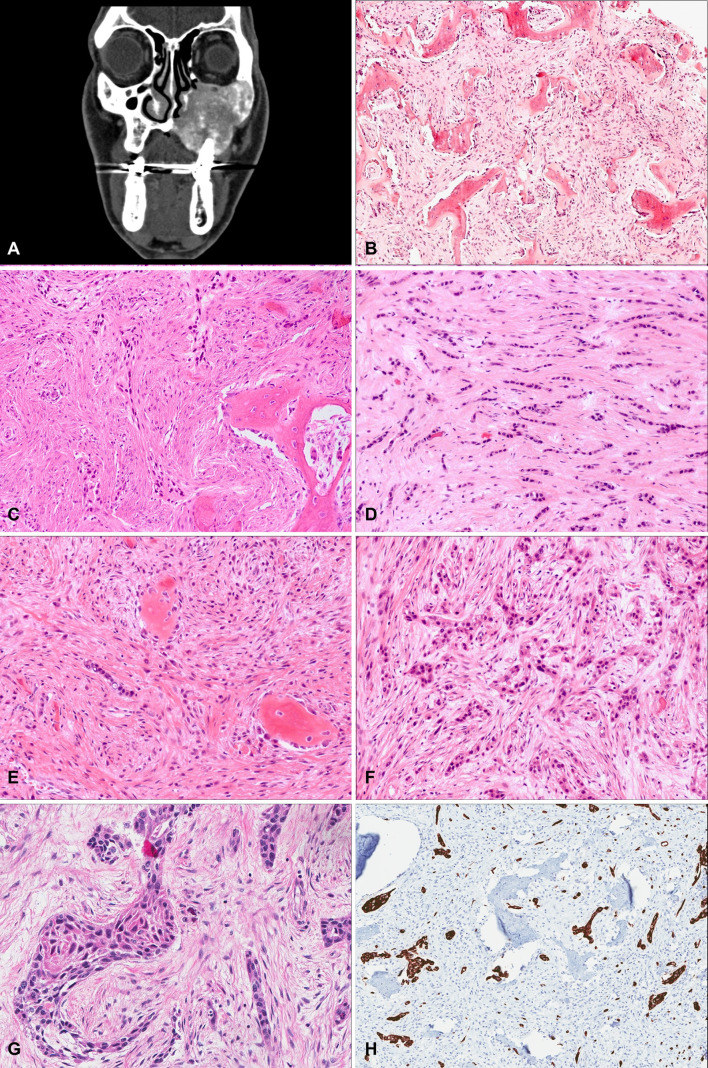
A 62-year-old male ex-smoker presented to his dentist with a 6-month history of progressive left upper jaw swelling, recurrent sinus infections, and loosening of the teeth (#21–26). His past medical history was otherwise notable for hepatocellular carcinoma. Initial physical examination revealed a hard expansion of the left maxilla on the buccal and palatal side intraorally. Radiography showed loss of trabeculations in the left maxillary bone with a ground glass appearance and a diagnosis of fibrous dysplasia was suspected. Computed tomography (CT) scan of the maxillary region revealed an infiltrating lesion concerning for malignancy (Fig. 1A). An incisional biopsy was performed and routine hematoxylin & eosin (H&E) sections showed areas of irregular trabeculae of woven bone rimmed by osteoblasts. The intervening stroma showed a moderately cellular fibroblastic proliferation with no evidence of malignancy. It was diagnosed as a benign fibro-osseous lesion (Fig. 1B). However, as the radiologic features on the CT scan were not deemed typical of fibrous dysplasia, a subsequent PET-CT was performed which showed features concerning for a malignant process. A debulking biopsy was performed; this was histologically diagnosed as a sclerosing odontogenic carcinoma (SOC) (Fig. 1C). The patient underwent a left maxillectomy and removal of the skull base involving the infratemporal fossa in order to achieve complete resection of the tumor.
Fig. 1.
A CT scan (axial view) showing an infiltrating lesion expanding the left maxillary bone with extension to the left zygomatic arch, medial and lateral pterygoid plate, left alveolar ridge and hard palate. B H&E stained section of the incisional biopsy of the maxillary lesion showing fragments of irregular trabeculae of woven bone surrounded by a moderately cellular fibroblastic stroma without evidence of malignancy. C Islands of tumor cells embedded in a sclerotic stroma adjacent to trabeculae of woven bone. D Islands and cords of epithelial cells with low-grade cytologic features surrounded by a dense sclerotic stroma. E Sparse epithelial cells with a reactive fibro-osseus proliferation with cellular stroma showing large fibroblasts. F Areas of higher density of epithelial cells with squamoid features. G Epithelial islands showing overt squamous pearl-like changes with a glassy abundant cytoplasm. H Immunohistochemistry demonstrating strong and diffuse CK5/6 staining of the squamous component
Gross pathological examination showed a firm expansile mass completely filling the maxillary sinus with a gritty flesh-like surface and ill-defined pushing borders eroding the surrounding bone, closely approaching all resection margins. Histologic examination revealed a non-encapsulated tumor with mixed epithelial and mesenchymal components. The epithelial component consisted of highly infiltrative nests and cords of small polygonal and cuboidal cells with eosinophilic cytoplasm and mild-moderate nuclear atypia, usually associated with a dense background stroma (Fig. 1D). There was, however, significant intratumoral variability, with areas predominantly showing a reactive fibro-osseous proliferation with a cellular stroma containing large fibroblasts, but only sparse epithelial cells (resembling the features seen in the prior biopsies) (Fig. 1E), while other areas showed a much higher density of epithelial cells with squamoid features (Fig. 1F) including some epithelial islands showing overt squamous pearl-like changes with a glassy abundant cytoplasm (Fig. 1G). The tumor expanded widely to involve surrounding bone with many irregular trabeculae of woven bone rimmed by osteoblasts. There were numerous reactive bone fragments in these areas of involved bone with accompanying large fibroblasts and loose connective tissue. There was no evidence of lymphovascular or perineural invasion. Mitotic activity was low with about 1 mitosis/10 HPFs, and no necrosis was present.
By immunohistochemistry, the epithelial component was strongly and diffusely positive for CK5/6 (Fig. 1H), CK14 and p63, and negative for CK7 and CK20 (Table 1). CK19 was also negative in both the epithelial and stromal components. The tumor cells were negative for EBER ISH, ER, PAX8 and CDX2. The Ki67 index was approximately 10%. Hepatocyte antigen was negative. Fluorescence in-situ hybridization was also performed to rule out an EWSR1 rearrangement using a 22q12 break-apart probe, which was negative. Despite the relatively bland cytologic features, the epithelial component showed an infiltrative growth pattern extending close to surgical margins. This was interpreted as macroscopically clear but microscopically positive margins (R1 margin) with the tumor 0.1 mm away from the closest specimen surface.
Table 1.
Immunohistochemical staining of reported cases of sclerosing odontogenic carcinoma
| Reported case | CK7 | CK5/6 | CK8/18 | CK14 | CK19 | CK20 | p63 | p40 |
|---|---|---|---|---|---|---|---|---|
| Koutlas et al. [5] | Positive (focal) | Positive | Negative | – | Positive | Negative | Positive | – |
| Negative | Positive | Negative | – | Positive | Negative | Positive | – | |
| Negative | Positive | Negative | Positive | Negative | Positive | – | ||
| Irie et al. [11] | Positive (focal) | Positive | Negative | – | Positive | Negative | Positive | – |
| Hussain et al. [4] | – | Positive | – | – | Positive | – | – | – |
| Saxena et al. [12] | – | Positive | – | – | – | – | Positive | – |
| Wood et al. [8] | Negative | Positive | – | Positive | Positive | Negative | Positive | – |
| Tan et al. [3] | Positive | Positive | – | – | Positive | Negative | Positive | – |
| Hanisch et al. [7] | – | Positive | – | – | – | – | Positive | Positive |
| Current case report | Negative | Positive | – | Positive | Negative | Negative | Positive | Positive |
| Positive cases/total cases | 3/7 | 10/10 | 0/4 | 2/2 | 7/8 | 0/7 | 9/9 | 2/2 |
| Reported case | CAM5.2 | AE1/AE3 | CEA | SMA | Desmin | S100 protein | E-cadherin | Estrogen receptor |
|---|---|---|---|---|---|---|---|---|
| Koutlas et al. [5] | Negative | – | Negative | Negative | Negative | Negative | Positive | – |
| Negative | – | Negative | Negative | Negative | Negative | Positive | – | |
| Negative | – | Negative | Negative | Negative | Negative | Positive | – | |
| Irie et al. [11] | – | – | Negative | Negative | – | Negative | – | – |
| Hussain et al. [4] | – | Positive | – | – | – | – | – | – |
| Saxena et al. [12] | – | – | – | – | – | – | – | – |
| Wood et al. [8] | – | – | Negative | – | – | Negative | Positive | Negative |
| Tan et al. [3] | Positive | – | Negative | Negative | Negative | Negative | Positive | Negative |
| Hanisch et al. [7] | – | – | – | – | – | – | – | – |
| Current case report | – | – | – | – | – | – | – | Negative |
| Positive cases/total cases | 1/4 | 1/1 | 0/6 | 0/5 | 0/4 | 0/6 | 5/5 | 0/3 |
A follow-up CT and MRI scan 5 months post-surgery showed evidence of disease recurrence. High-dose radiation (66 Gy in 33 fractions) to areas of gross disease and the postoperative bed was attempted for local control of the disease. Radio-sensitizing chemotherapy was not recommended given the low mitotic rate of the tumor. Six months post-radiotherapy the patient had an MRI/CT which showed no evidence of FDG avid disease; this was confirmed by follow-up CT done 8 months after the radiotherapy. The patient was therefore considered to be in clinical remission 9 months following the radiotherapy for the recurrence. Unfortunately, he succumbed to end-stage hepatocellular carcinoma 19 months following this course of radiotherapy; no further interval imaging studies were performed to evaluate for progression of his SOC.
Discussion
Sclerosing odontogenic carcinoma is a rare and relatively new entity, recently added to the latest WHO classification of Head and Neck tumors [1, 2]. Thus far, there have been only 10 other documented cases of sclerosing odontogenic carcinoma in the literature (Table 2) [3–9, 11, 12]. With locally aggressive behavior and bland histology with discrete areas of tissue invasion which extend beyond what is expected clinically and intraoperatively, this entity deserves a review of the potential causes for the discrepancy and an approach to the differential diagnosis on biopsy. Moreover, we will also emphasize the difficulties in accurate margin status assessment on histopathology.
Table 2.
Demographics and clinical outcomes of literature reported cases
| Reported case | Age/gender | Tumor site | Outcome | Treatment |
|---|---|---|---|---|
| Koutlas et al. [5] | 46/F | Right mandible | No recurrence, 5 years | Resection |
| 73/M | Right maxilla | No recurrence, 3.5 years | Resection, radiotherapy | |
| 72/M | Left mandible | No recurrence, 5 years | Resection, radical neck dissection | |
| Ide et al. [9] | 47/M | Left lingual gingiva | No recurrence, 6 years | Resection, Radical neck dissection |
| Irie et al. [11] | 67/M | Left mandible |
Recurrence at 8 months following enucleation No recurrence 15 months after second surgery |
Enucleation Complete resection, adjuvant chemotherapy |
| Hussain et al. [4] | 54/M | Right maxilla | No recurrence, 19 months | Resection |
| Saxena et al. [12] | 42/M | Right mandible | No recurrence, 10 months | Resection, Radical neck dissection, Radiotherapy |
| Wood et al. [8] | 43/F | Right hard palate | No recurrence, 17 months | Resection |
| Tan et al. [3] | 31/F | Right mandible | No recurrence, 12 months | Enucleation |
| Hanisch et al. [7] | 60/M | Left mandible | No recurrence, 9 months | Resection |
| Current case report (2018) | 62/M | Left maxilla |
Recurrence at 5 months following resection No clinical recurrence 19 months after radiotherapy for recurrence |
Resection (maxillectomy with removal of skull base) Radiotherapy for recurrence |
Of the cases reported thus far, patient age is widely variable in literature, ranging between 31 and 73 years (median age 54 years) [3–5, 7–9, 11, 12] (Table 2). The male to female ratio is 8:3. Six of the cases reported in literature occurred in the mandible, three in the maxilla and one each in the hard palate and lingual gingiva. Most cases presented with swelling and pain or paresthesia [3–5, 7–9, 11, 12].
All case reports in literature to date [3–5, 7–9, 11, 12] have described a primarily lytic lesion on radiographic imaging. Two case reports described sclerotic changes in association with the lesions on imaging [3, 9]. Our case showed loss of trabeculations in the maxillary bone with a ground-glass appearance on the initial X-ray. CT images demonstrated an aggressive growth pattern with cortical bone thinning, seen in other cases of SOCs as well [3–5, 7–9, 11, 12].
Histologically, this entity was first described in 2008 by Koutlas et al. [5] with a biphasic histomorphology characterized by a dense sclerotic stroma and multiple infiltrating cords, islands and trabeculae of epithelioid cells with low-grade nuclei and rare mitotic figures [5, 7, 8]. On low-power examination all of the tumors lack a capsule and show an infiltrative tumor front. The epithelial cells are polygonal with low-moderately atypical nuclei and an eosinophilic cytoplasm [7]. The appearance of these epithelial cells was thought to be reminiscent of cells of odontogenic epithelial rests when first described [5]. Some islands of the epithelial cells show central keratinization-like changes with obvious desmosomes on high-power while other cells demonstrate a more polygonal appearance. There may be focal cytoplasmic clearing. As per previous case reports, extensive perineural invasion is a common feature in SOC [4, 5, 8, 11, 12]. However, perineural invasion is not a mandatory feature of SOC; no evidence of perineural invasion was seen in our case, similar to that reported in other studies [7, 9]. Immunohistochemically, these tumors are typically CK5/6, CK14, p63 and CK19 positive, which can be suggestive of squamous-type differentiation in the epithelial component [3–5, 7, 8, 11, 12] (Table 1), although the CK5/6 and CK19 positive phenotype is also seen in odontogenic epithelium [5]. Our case demonstrated negative staining for CK19, which was an unexpected finding as this has not been reported in previous cases (Table 1). Although this calls the diagnosis into question, we believe SOC remains the best diagnosis for this case, based on the consistent histomorphologic features, the expected low proliferative index, the positivity of other expected immunohistochemical markers, and the absence of another more suitable differential diagnosis.
Based on histomorphology, the differential diagnosis for this tumor is broad, and as per recommendation in the latest WHO, care must be taken to exclude other diagnostic entities before arriving at a diagnosis of an SOC, including metastases [2, 4, 5, 9, 10, 12]. By morphology, SOC has shown to be a great mimicker of other neoplasms in the head and neck. In fact, all case reports of SOCs in the literature to date, save for one [5], have been initially diagnosed as other entities on biopsy material [3–5, 7–9, 11, 12]. The epithelial component of SOC, showing apparent keratinization and intercellular desmosomes, can closely mimic an invasive squamous cell carcinoma. It also resembles primary intraosseous carcinoma of the jaw (PIOC), a rare tumor with poor prognosis [3]; however, it does not have the significant cellular atypia seen in PIOCs. Hussain et al. reported the initial diagnosis of their SOC by core biopsy as a poorly differentiated squamous cell carcinoma while Tan et al. reported an initial diagnosis of a PIOC. Similarly, Wood et al. reported an initial diagnosis of an adenocarcinoma. Consideration must also be given to metastatic malignancies and in particular neoplasms of epithelial origin [13].
The stromal component of SOCs can similarly raise a broad differential diagnosis in the biopsy setting. In fact, the mesenchymal component is commonly the predominant feature in a lesion [3–5, 7–9, 11, 12]. This also increases the chance that the epithelial component may not be sampled, obscuring the diagnosis at the time of biopsy. In our case, a conspicuous fibro-osseous reaction was seen in the stromal component, and a diagnosis of a benign fibro-osseous lesion was made at biopsy. In addition, a fibrous-dysplasia-like low-grade osteosarcoma remained in the differential diagnosis, although the absence of atypia and a negative MDM2 stain helped to exclude this possibility [14]. The correct diagnosis relied upon the radiologic findings, which showed an infiltrative and destructive lesion that prompted the repeat biopsy required to achieve sufficient sampling of the epithelial component. Of the reported cases in the literature, only Irie et al. [11] has described a case where a fibro-osseous lesion predominated in the resection specimen; the initial biopsy was also diagnosed as a benign fibro-osseous lesion with focal metaplastic change. The prominent fibroblasts described in our case were typically associated with adjacent bone which was destroyed by the tumour and likely do not represent a bona-fide stromal component of this neoplasm. However, as previously emphasized, this can make limited biopsies which do not sample the epithelial component particularly challenging. Nevertheless, this highlights the importance of actively considering SOC as a differential diagnosis when a bland mesenchymal or fibro-osseous proliferation is seen in a limited biopsy of an intraosseous jaw lesion, despite the rarity of this tumor type.
Sclerosing odontogenic carcinomas also mimic other odontogenic neoplasms, most notably the epithelium rich variant of central odontogenic fibroma (ERCOF) [3], clear cell odontogenic carcinomas (CCOC) [10], calcifying epithelial odontogenic tumors (CEOT) and desmoplastic ameloblastomas [9]. ERCOF is an unusual neoplasm which shares many overlapping histologic features with SOCs [3, 5]. The presence of infiltrating cords and nests of epithelium with cytoplasmic clearing as demonstrated in our case and others [5] may easily be confused with ERCOF [3]. A possible distinguishing feature between the two entities is the relative lack of sclerosis in the fibroblastic stroma between the epithelial cells in ERCOFs as compared to SOCs [5]. The distinction between these two entities is challenging and it has been suggested that there are no firm criteria (immunohistochemical or molecular) to help separate the two entities [3]. Although our tumor was intimately associated with fragments of bone and a prominent fibroblastic stroma which was poorly delineated from the SOC sclerotic stroma these changes likely represent a reactive bony proliferation to the neoplasm, rather than a component of the neoplasm itself. CK19 staining is negative in the stromal component, arguing against the possibility that this represents the stromal component of an epithelial-rich central odontogenic fibroma. However, the features seen in this lesion do support the notion that there is histomorphologic resemblance and possible clinical overlap with ERCOF. CCOC may harbor groups and sheets of epithelioid cells with focal eosinophilic cytoplasm [10] which may resemble SOC. However, the frequent presence of vacuolated clear cytoplasm with eccentric dark nuclei helps serve as a distinguishing feature for this rare, locally aggressive neoplasm. In addition, detection of the EWSR1-ATF1 fusion characteristic of CCOC [10, 15, 16] can be relied upon to resolve this differential diagnosis; EWSR1 break-apart FISH was indeed helpful in our case to rule out CCOC, as in other reported cases of SOC [8, 10]. CEOTs also show infiltrative trabeculae and sheets of polyhedral squamous tumor cells with clear cytoplasmic change [17]. However, unlike SOCs, these tumors show deposits of amyloid with concentric calcifications which can serve as useful distinguishing features [17]. Additionally, they tend to have a scantier stroma compared to the dense sclerosing stroma in SOCs. Desmoplastic ameloblastoma is another entity with histologic overlap. The compressed angulated islands and strands of odontogenic tumor cells in a densely fibrotic stroma are reminiscent of SOC. The distinction between SOCs and desmoplastic ameloblastomas is particularly problematic in small biopsies [8, 9]. However, desmoplastic ameloblastomas should show some degree of peripheral palisading with reverse polarization and central stellate reticulum [18]. The presence of perineural invasion also highly favors SOC, although this is not a universal finding [7, 9].
Aside from the morphologic overlap of SOCs with numerous other entities, a particular challenge has been the proper pathologic assessment of the tumor margin status in these cases. While radiologic findings of SOCs have varied in literature from the well-circumscribed sclerotic lesions to those with ill-defined areas [7], authors have agreed on the difficulty in determining the overall extent of the tumor epithelial islands because of the tendency of these tumors to microscopically spread beyond the clinically and radiologically-apparent margins [4–7]. An important cause for this, as in our case and previous case reports [7] is the very dense sclerotic stroma often engulfing thin epithelial cords of cells making them difficult to detect on H&E and without the help of cytokeratin staining [9]. In fact, areas of the tumor show a gradual transition from abundant epithelial nests to a very bland sclerotic stroma with practically no epithelial nests (that are apparent at least). Moreover, there is no firm delineation between the sclerotic stromal component of the tumor and the surrounding benign stroma of tissues as the transition between these areas is gradual. With the background reactive bone changes and abundant fibroblasts it can be a challenge to determine where the actual interface between the SOC and the surrounding uninvolved tissue lies. Although the tumor in our case grossly appeared to be a few millimeters away from the margin as an expansile fibrotic and gritty mass, these same areas actually extended much closer to the surgical margins on microscopy. As described already, it is particularly the sparsely cellular stromal component with few barely discernable epithelial cells which closely approached the margin. However, even the presence of close margins does not necessarily predict local recurrence; for example, Wood et al. reported a case of SOC with 1.9 mm margins that underwent subsequent revision surgery which was negative for tumor [8]. Nevertheless, the local recurrence in our case report following extensive tumor resection procedure may be attributable to the microscopic positive margin status and the locally infiltrative nature of the tumor in a limited anatomic space. Only one other reported case had local recurrence following curettage [11] where clear resection margins were not achieved [11] as it was the thought that the tumor was a fibro-osseous lesion on biopsy.
Another consistent finding based on the literature reported cases is that none of the SOCs demonstrated metastatic spread. It has been postulated that the dense sclerotic stroma surrounding the epithelial component may play a role in the prevention of metastases [12] however this is an area which requires further investigation. The overall low-grade nuclear features of both the epithelial and stromal component, including the lack of significant mitotic activity may also explain the lack of tendency of SOCs to metastasize to date.
Given the rarity of SOCs in the literature to date, there is no standardized treatment approach, with considerable variability in treatment approaches in the reported cases (Table 2). Four of the 11 cases including ours underwent adjuvant therapy in addition to surgery; one patient underwent chemotherapy following a local recurrence after enucleation [11]; two patients underwent radiotherapy for close margins [5, 12].
Conclusion
While SOC remains a recently described and rare entity with few case reports to date, it is an important differential diagnosis to consider for head and neck tumors and a mimicker of other tumors in this region, including metastatic tumors. Although immunohistochemistry can be helpful in arriving at the diagnosis, with similar staining patterns across the studies reported thus far, our case demonstrated unexpected absence of staining for CK19, suggesting that SOC may show variable immunohistochemical features. As our case demonstrates, with recurrence despite extensive surgery, prudent margin assessment is of clinical importance. Finally recognized as a distinct tumor type in the WHO, SOCs will require more reported cases in order to standardize a diagnostic approach and treatment of these locally aggressive neoplasms.
Funding
No associated funding.
Compliance with Ethical Standards
Conflict of interest
No conflicts of interest to disclose.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization Classification of Head and Neck tumours. 4. Lyon: IARC Press; 2017. [Google Scholar]
- 2.Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch. 2018;472:331–339. doi: 10.1007/s00428-017-2182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan SH, Yeo JF, Kheem Pang BN, Petersson F. An intraosseous sclerosing odontogenic tumor predominantly composed of epithelial cells: relation to (so-called) sclerosing odontogenic carcinoma and epithelial-rich central odontogenic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:119–125. doi: 10.1016/j.oooo.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Hussain O, Rendon AT, Orr RL, Speight PM. Sclerosing odontogenic carcinoma in the maxilla: a rare primary intraosseous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:283–286. doi: 10.1016/j.oooo.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Koutlas IG, Allen CM, Warnock GR, Manivel JC. Sclerosing odontogenic carcinoma: a previously unreported variant of a locally aggressive odontogenic neoplasm without apparent metastatic potential. Am J Surg Pathol. 2008;32:1613–1619. doi: 10.1097/PAS.0b013e31817a8a58. [DOI] [PubMed] [Google Scholar]
- 6.Richardson MS, Muller S. Malignant odontogenic tumors: an update on selected tumors. Head Neck Pathol. 2014;8(4):411–420. doi: 10.1007/s12105-014-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanisch M, Baumhoer D, Elges S, Frohlich LF, Kleinheinz J, Jung S. Sclerosing odontogenic carcinoma: current diagnostic and management considerations concerning a most unusual neoplasm. Int J Oral Maxillofac Surg. 2017;46:1641–1649. doi: 10.1016/j.ijom.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Wood A, Young F, Morrison J, Conn BI. Sclerosing odontogenic carcinoma presenting on the hard palate of a 43 year old female: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:204–208. doi: 10.1016/j.oooo.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Ide F, Mishima K, Saito I, Kusama K. Diagnostically challenging epithelial odontogenic tumors: a selective review of 7 jawbone lesions. Head Neck Pathol. 2009;3:18–26. doi: 10.1007/s12105-009-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yancoskie AE, Sreekantaiah C, Jacob J, Rosenberg A, Edelman M, Antonescu CR. EWSR1 and ATF1 rearrangements in clear cell odontogenic carcinoma: presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(4):e115-8. doi: 10.1016/j.oooo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Irie T, Ogawa I, Takata T, Toyosawa S, Isobe T, Hokazono C. Sclerosing odontogenic carcinoma. Pathol Int. 2012;62:75–76. doi: 10.1111/j.1440-1827.2011.02749.x. [DOI] [PubMed] [Google Scholar]
- 12.Saxena S, Kumar S. Sclerosing odontogenic carcinoma: an enigma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:840. doi: 10.1016/j.oooo.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Misra SR, Shankar YU, Rastogi V, Maragathavalli G. Metastatic hepatocellular carcinoma in the maxilla and mandible, an extremely rare presentation. Contemp Clin Dent. 2015;6(Suppl 1):26–31. doi: 10.4103/0976-237X.152966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dujardin F, Binh MB, Bouvier C, Gomez-Brouchet A, Larousserie F, Muret AD, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2011;24:624–637. doi: 10.1038/modpathol.2010.229. [DOI] [PubMed] [Google Scholar]
- 15.Kwon IJ, Kim SM, Amponsah EK, Myoung H, Lee JH, Lee SK. Mandibular clear cell odontogenic carcinoma. World J Surg Oncol. 2015;13:284. doi: 10.1186/s12957-015-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37:1001–1005. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 17.Philipsen HP, et al. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol. 2000;36:17–26. doi: 10.1016/S1368-8375(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 18.Eversole LR, Leider AS, Strub D. Ameloblastoma with pronounced desmoplasia. J Oral Maxillofac Surg. 1984;42:735–740. doi: 10.1016/0278-2391(84)90423-3. [DOI] [PubMed] [Google Scholar]