Abstract
Purpose of Review
Overview the outcomes of the latest use of platelet-rich plasma (PRP) for the treatment of knee lesions in the clinics and discuss the challenges and limitations.
Recent Findings
Recent clinical studies mainly indicate there may be benefit of PRP usage for the treatment of knee lesions. As an autologous source of bioactive components, PRP has been shown to be typically safe, free of major adverse outcomes. The use of PRP has been continuously increasing, and some well-designed, double-blinded, placebo-controlled clinical trials have been published.
Summary
Clinical outcomes relating to PRP usage are multifactorial and depend on the severity of the lesion and patient characteristics. Although PRP is safe to use and it can be easily applied in the clinics, case-specific considerations are needed to determine whether PRP could be beneficial or not. If the use of PRP is favored, then, the configuration/optimization of the preparation and administration/delivery strategy with or without a concomitant treatment may further enhance the clinical outcomes and patients’ experience.
Electronic supplementary material
The online version of this article (10.1007/s12178-019-09573-3) contains supplementary material, which is available to authorized users.
Keywords: Platelet-rich plasma, Knee, Osteoarthritis, Meniscus, Ligament, Biologics
Introduction
Knee joint disorders and injuries are common in orthopedics and can affect millions of people. The knee comprises several different entities with different biology and biomechanics [1–5]. Knee lesions can be asymptomatic or symptomatic with different etiologies that might lead to joint degeneration. Meniscal, chondral, osteochondral, and ligamentous lesions are among the frequent lesions. Orthopedics is a dynamic field that evolves with the basic and applied/clinical evidence while it has the ability to incorporate technological and scientific novelties. Despite the critical advances in science and surgical approaches, the treatments of orthopedic disorders/diseases and managing the associated pain of the patient remain as an outstanding challenge [1, 4, 6–8]. Various repair/regenerative approaches have been applied in orthopedics, including but not limited to cellular therapies with or without supporting biomaterials [1, 6, 9–11], biologics [12, 13], and gene therapy [14, 15].
The current literature indicates that these current strategies have not yet achieved the required efficiency to fully satisfy all clinical needs, and there is a vast room for further research and development. Biologics are biologically active natural components in an isolated or concentrated form such as growth factors, cytokines, stem cells, bone marrow aspirate concentrates, and platelet-rich plasma (PRP). Herein, we evaluate some recent works dealing with PRP and summarized the latest clinical outcomes that have been reported when treating knee lesions.
Platelet-Rich Plasma
PRP is a blood-derived concentrate that is known to enhance the healing of an injured tissue via modulation of cellular signaling pathways [12, 16–18]. Growth factors bind to the specific receptors of the cells and influence the cell activities such as gene expression, growth, and differentiation [19, 20]. Platelets are small cytoplasmic fragments of megakaryocytes in the peripheral blood. Upon an injury, platelets rapidly arrive to the site and release growth factors and mediate the healing process with various proteins in their α-granules [21–23]. This is linked with the recruitment of inflammatory cells and stem cells to the injury site.
PRP is an autologous blood fraction that is prepared from anti-coagulated blood with a supraphysiologic platelet concentration of 1 million platelets/μL or more, while the baseline comprises around 0.15–0.35 million platelets/μL [24–27]. Typical PRP preparation involves the collection of blood from the patient with anti-coagulant followed by centrifugation to separate the red blood cells, platelet-poor plasma, and the buffy coat that contains the white blood cells and concentrated platelets. For leukocyte-poor PRP, the leukocytes needed to be further separated. A meta-analysis of 6 randomized controlled trials [28–33] and 3 prospective comparative studies [34–36] that involve 1055 patients in total indicated that leukocyte-poor PRP might provide better functional outcome scores for the treatment of knee osteoarthritis, than the leukocyte-rich PRP [37•]. However, the dependency of the local adverse reactions on leukocyte concentration was not detected. Platelets are activated by either in situ by contact with collagen, or prior to application for instance by using calcium chloride or bovine thrombin. Upon activation, and degranulation of the α-granules, the growth factors are released. On YouTube (www.youtube.com), there are many step-by-step PRP preparation videos available, as well as on the company (e.g., Harvest Technologies, MTF Biologics, DePuy Synthes Mitek Sports Medicine, Biomet Orthopedics, and Arthrex) websites of the commercially available PRP preparation systems (Appendix 1).
PRP provides various growth factors at physiological proportions for a topical application. The growth factors include platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), and insulin-like growth factor-1 (IGF-1). In addition to the growth factors, cytokines, chemokines, adhesive proteins, clotting factors, fibrinolytic factors, proteases, anti-proteases, and anti-microbial proteins are also present [22, 24]. Given the fact that PRP is obtained from the autologous blood, it is intrinsically safe without immune response and disease transmission risk, and it is not carcinogenic since the growth factors do not enter the cell but bind to the receptors on the surface of the cells. Employing PRP to support tissue healing is a rational approach that has been extensively studied in orthopedics (Fig. 1) [13, 38–40, 41••, 42–44].
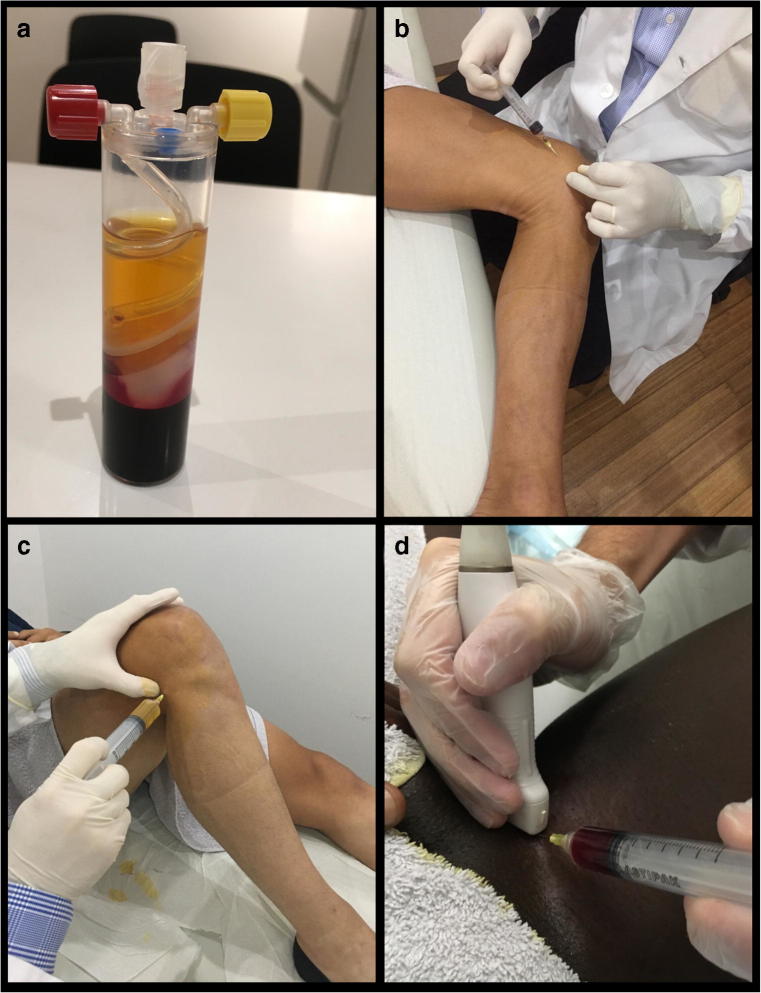
Fig. 1.
A commercial system (Biomet Orthopedics, USA) for preparation of PRP (A), PRP application on medial collateral ligament (B), PRP application on lateral collateral ligament (C), and ultrasound-guided PRP application (D)
Filardo et al. [41••] systematically reviewed the in vitro, animal studies, and clinical outcomes of PRP treatment for cartilage lesions and osteoarthritis. Based on the available evidence, potential benefits of PRP is supported by the preclinical studies, it is safe and without major adverse incidents while clinical improvements are good in the short term. Intra-articular application of PRP may contribute to the health of the entire joint, down-regulate the inflammation, relieve the pain, decelerate degenerative events. This systematic analysis also shows that there is an overall pre-clinical advantage of using PRP; however, here are few published high-quality clinical trials, and they indicate a benefit, particularly in young patients without advanced degeneration.
Latest Outcomes: PRP for Ligamentous Lesions, Patellar Tendinopathy, and Meniscal Lesions
Partial anterior cruciate ligament tears in 42 patients were treated by intra-ligament PRP injection (Fig. 2A). 71.1% of the included patients returned to pre-injury sports activity after 5.8 months in average. With a mean follow-up of 33 months, good to excellent outcomes were obtained with the failure rate of 9.5% [46]. PRP has also been used to augment the trephination of the ACL origin [47]. Twenty-four patients were treated, and good to excellent clinical outcomes were reported at a mean of 25.1 M follow-up. However, the lack of a control group (i.e., non-PRP group) hinders the contribution of intra-ligament PRP injection.
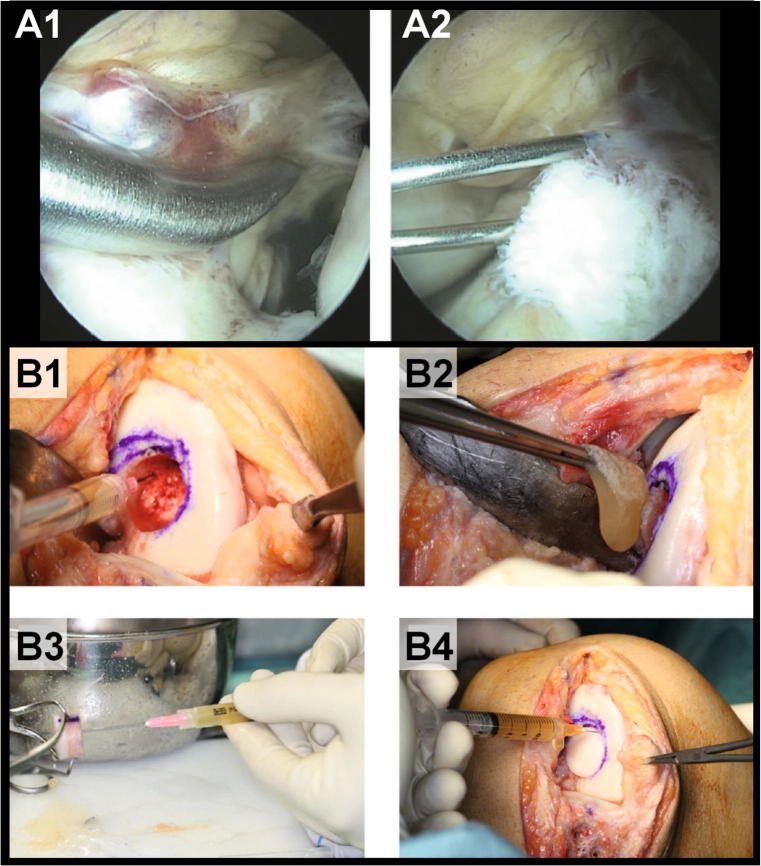
Fig. 2.
Healing response technique (A1), and intra-ligament injection of PRP in a partial ACL rupture (A2), (adapted from [39]); infiltration of PRP into subchondral bone (B1), PRP membrane was placed into the wound bed (B2), the femoral plug osteochondral allograft was infiltrated with PRP (B3), sealing the interface around the allograft with PRP (B4) (adapted from [45])
In a single-blind randomized controlled trial involving a total of 57 athlete patients, with patellar tendinopathy of Blazina stage IIIB, received a single injection of either leukocyte-rich or -poor PRP or saline injection with a subsequent supervised rehabilitation for 6 weeks [48••]. With a single injection, no significant differences in the outcomes were detected between the groups with a follow-up of 12 months. The primary outcome was Victorian Institute of Sport Assessment (patellar; Victorian Institute of Sport Assessment-P) at week 12, while pain during activity and patients’ global rating of change were the secondary outcomes [48••].
Lateral discoid meniscus tears of 29 patients were arthroscopically repaired by meniscal suturing with and without PRP injection directly on the repair site with thrombin to form a gel clot [49]. At mid-term follow-up, significant differences in pain relief, functional improvement, or failure rate between the groups were not detected. PRP injections were used to augment allograft transplantation [45, 50] (Fig. 2B). Recently, Zhang et al. [50] reported the outcomes of 31 patients that 90.7% of the patients had significant improvements in all functional and pain score patients have upon treatment. However, since there was no control group, all patients underwent an allograft transplantation combined with PRP injection, the efficacy of the PRP cannot be identified.
In a non-randomized study, platelet-rich fibrin and PRP were incorporated into the arthroscopic meniscal repair of 17 patients, while not in 5 patients in the control group [51]. The groups have similar Tegner Activity Level Scale, Lysholm Knee Scoring Scale, and International Knee Documentation Committee (IKDC); and the improvements with the biologics were not detected. It should be noted that the meniscal tears and their locations were different [51], and this may be one of the factors in evaluating the outcomes [1, 52–54].
In a very recent randomized, placebo-controlled study with 72 patients [55•], improved rate of meniscus healing, better functional outcomes, and higher visual analog scale (VAS) scores were obtained in patients with degenerative meniscus lesions that were treated with percutaneous trephination and PRP injection as compared to patients treated without PRP. The concomitant PRP injection also lowered the need for a future arthroscopy [55•].
In another randomized, placebo-controlled study, PRP-augmented repair of bucket handle meniscal tears (n = 18) provided better outcomes than the control group (n = 17) that received saline injection [56•]. At intra-repair site, injection of PRP lead to higher meniscus healing than the controls by being 85% and 47% respectively. The scores of IKDC, Knee Injury and Osteoarthritis Outcome Score (KOOS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were better in the PRP-treated group. Assessed with second-look arthroscopy and MRI, the cumulative outcomes indicated that in the PRP-treated group, 14 menisci healed, 3 menisci partially healed, and 3 cases failed; while in the control group, only 7 menisci healed, with 1 meniscus partially healed, and 9 cases failed [56•].
Latest Outcomes: PRP for Knee Osteoarthritis
In a double-blind randomized trial, 18 patients with knee osteoarthritis with Dejour grades II–IV were treated with injections of bone marrow–derived mesenchymal stem cells with (n = 9) and without PRP (n = 9) [57]. The KOOS scores were better in both groups; the improvement was higher in the PRP group (22.6 vs. 26.4). The pain, function, and daily activities were improved throughout 12 months follow-up for both patient groups. Inclusion of PRP lead to an improvement in KOOS pain sub-score from 26.5 to 57.1%, in KOOS-quality of life from 22.4 to 30.7%, and 28.0 to 32.2%. However, from the statistical point of view, a significant additional benefit of inclusion of PRP could not be detected within that trial with a small number of patients.
In a trial with 366 younger patients with knee osteoarthritis (18–30 years old) [58], significant alleviation of inflammation was observed after intra-lesional injections of PRP as compared with the placebo [58]. Intra-articular injection of PRP (leukocyte-poor) was compared with oral non-steroidal anti-inflammatory drugs (NSAID) or intra-articular hyaluronic acid for the treatment of early knee osteoarthritis in a randomized controlled trial with a total of 98 patients [59]. PRP treatment provided higher improvement in WOMAC pain and VAS than NSAID and hyaluronic acid treatments as evaluated at 52-week follow-up, while none of the three treatments provided thickening of the cartilage tissue or reduction of Kellgren–Lawrence scores. Upon 3 weekly intra-articular injections of leukocyte-rich PRP, no better overall clinical outcomes were achieved. In addition, no superior symptomatic functional scores or longer effect duration were obtained with PRP as compared with hyaluronic acid injections at any follow-up points based on the long-term clinical results [60••]. The study was a double-blind, randomized controlled trial with patients having chronic symptomatic knee osteoarthritis having Kellgren–Lawrence grade of 0–3 [60••]. It was shown that the reintervention rate at 24 months follow-up was significantly lower in the PRP group (22.6%) as compared with the hyaluronic acid group (37.1%). It should also be noted that at the final evaluation, the PRP group’s IKDC subjective score was significantly better than the baseline while the hyaluronic acid group’s not [60••]. The superiority of PRP over hyaluronic acid regarding clinical outcomes, pain relief and functional status of the patients, was also reported in another randomized clinical trial with 89 patients that received in total 3 intra-articular injections with a 2-week gap between injections [61]. While in another study with a total of 54 patients, a single injection of PRP or hyaluronic acid treatments performed without a significant difference between each other and both provided significantly better outcomes compared with baseline [62]. To further investigate the synergy of PRP and hyaluronic acid, these were combined and compared with each of them alone and with placebo for the treatment of knee osteoarthritis of a total of 360 patients [63]. Combination of PRP and hyaluronic acid provided improved results (WOMAC, pain, and physical function) as compared with the two components alone.
A non-randomized study with 115 patients with mild to moderate osteoarthritis indicated that intra-articular injection of methylprednisolone prior PRP injection provided better outcomes regarding VAS and WOMAC at 3 months post-treatment when compared with PRP or methylprednisolone alone. However, the differences between groups were not maintained at 12 months follow-up [64]. In another study, 57 patients with knee osteoarthritis were treated with a single large volume (8.8 ± 1.1 mL) leukocyte-poor PRP injection with a short-term follow-up [65]. The results indicated that PRP was beneficial for 84.2% of the patients at 3 months follow-up regarding functional improvement and pain relief; and at 6 months follow-up, the KOOS total score was significantly increased although the MRI analysis did not provide significant differences compared with the baseline. However, there was no inclusion of a control group in the study; thus, the placebo effect should be considered.
Outcomes of intra-articular injections of platelet-rich plasma, hyaluronic acid, and corticosteroids for the treatment of symptomatic early-stage knee osteoarthritis were compared in a randomized controlled study with a total of 120 patients [66]. Compared with the baseline, significant improvements in WOMAC and VAS were observed in all groups. For pain relief, the PRP group provided superior outcomes. At 3 months follow-up, the WOMAC scores were not significantly different between the groups, while at 6, 9, and 12 months of follow-ups, the PRP group had significantly lower (favorable) WOMAC scores compared with the other two groups.
For the treatment of hemophilic arthroplasty of the knee of 22 patients, single intra-articular PRP injection was compared with five weekly intra-articular injections of hyaluronic acid [67]. PRP treatment provided better outcomes regarding pain relief and knee function improvement as compared with hyaluronic acid. PRP also help reduce synovial hyperanemia. The 3 weekly intra-articular injections of leukocyte-poor PRP provided better outcomes in comparison with hyaluronic acid for the treatment of mild to moderate osteoarthritis of the knees (53 patients, 87 knees) as studied in a randomized, double-blind, placebo-controlled clinical trial [68]. At 1-month treatment, all groups including the sham group, showed significant improvements in WOMAC and IKDC as compared with the baseline; while at 12 months, only the PRP group showed functional improvements.
For the treatment of mild to moderate osteoarthritis of the knee with intra-articular injection of PRP, the additional beneficial contribution of concomitant intra-osseous PRP injections into the subchondral bone was shown in a study with a total of 86 patients [69]. The study also employed hyaluronic acid in one of three groups that are (i) combined intra-articular and intra-osseous injections of PRP, (ii) only intra-articular injections of PRP, and (iii) injections of hyaluronic acid. Inclusion of intra-osseous injections leads to improvements in the subscales of WOMAC and self-reported pain [69]. A study with 30 geriatric patients with moderate to severe osteoarthritis [70] indicated that simultaneous intra-articular and perimeniscal pes anserinus PRP injections can provide favorable proteomic changes and better functional and pain scores. With this method, PRP can be brought in contact with pes anserine tendons, bursa, medial collateral ligament, and medial meniscus [70]. The study recommends the multiple monthly, at least two monthly injections.
Challenges and Limitations
Several challenges and limitations are affecting the clinical outcomes when comparing the results of trials of the PRP and the experience of the patient. These include the following:
PRP-related issues: Given the fact that PRP is derived from autologous blood, the therapeutic features depend on the donor, and there are significant variations between PRP obtained from different donors [71]. PRP can be prepared either by manual centrifugation or by using one of the commercially available systems which provide different PRPs with different in the number of platelets and leukocyte number [72, 73].
Patient- and lesion-related issues: The outcomes depend on the type and the severity of the lesions, as well as the age and condition of the patient.
Placebo effect: Placebo effect can have a clinical meaning [74•], and thus, the inclusion of a placebo group is highly valuable to understand the effects of PRP.
Contraindications: The contraindications to use PRP include platelet/blood disorders, systemic infections, acute viral infection, hepatorenal syndrome, immunosuppression, and injection site infection [75].
Study/treatment design-related issues: Regarding the PRP application, there are several considerations to be made, including but not limited to: the preparation method, location of injection, the volume of applied PRP, frequency (single injection or a series of injections with a frequency), use/effects of anesthetics, being applied to augment a surgical procedure, and the presence of any concomitant treatment. Interpretation of the outcomes/treatment effect should be made correctly to avoid misconceptions about the better results compared with baseline [48, 76]. Although the delivery of PRP can be beneficial, its effects are limited, and thus, the lifespan of the platelets and the growth factor release kinetics should be considered to define that last time-point that an effect is expected. It is also highly beneficial to characterize the applied PRP and report it along with the treatment outcomes.
Conclusions and Take-Home Messages
PRP treatments are safe for the patients, and the studies mainly acknowledge its theoretical and practical benefits. PRP has a place for treatment of knee lesions alone, as an augmentation, as a supplementary component of the conventional treatment, or as a part of tissue engineering construct. Several, but not all clinical studies showed a clinical benefit of PRP, particularly for patients with mild-moderate degenerative cartilage lesions of the knee. PRP preparation and application is typically time-efficient and uncomplicated. In addition to the fact that different PRPs can be prepared using different commercial systems and patient response can be dependent on a multitude of factors. Patients respond differently to the bioactive substances, while the lesion types, severity, locations, and etiologies are variable. Nonetheless, the trend in the literature is expected to continue, and more PRP clinical studies will be published. It should be clearly noted that we need well-controlled statistically powered studies to determine the efficacy of PRP in the long term.
Electronic supplementary material
(DOCX 15 kb)
Acknowledgments
The authors would like to thank the Portuguese Foundation for Science and Technology (FCT) project M-ERA-NET/0001/2014 project. This work is also a result of the project FROnTHERA
(NORTE-01-0145-FEDER-000023), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). IFC thanks the FCT for the grant SFRH/BD/99555/2014. JMO also thanks the FCT for the funds provided under the program Investigador FCT 2015 (IF/01285/2015).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This paper does not contain any studies with human or animal subjects performed the authors.
Footnotes
This article is part of the Topical Collection on Outcomes Research in Orthopedics
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Cengiz IF, Pereira H, Espregueira-Mendes J, Oliveira JM, Reis RL. Treatments of meniscus lesions of the knee: current concepts and future perspectives. Regen Eng Transl Med. 2017;3(1):32–50. [Google Scholar]
- 2.Gomoll A, Filardo G, De Girolamo L, Esprequeira-Mendes J, Marcacci M, Rodkey W, et al. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):450–466. doi: 10.1007/s00167-011-1780-x. [DOI] [PubMed] [Google Scholar]
- 3.Heijink A, Gomoll AH, Madry H, Drobnič M, Filardo G, Espregueira-Mendes J, van Dijk CN. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):423–435. doi: 10.1007/s00167-011-1818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira H, Cengiz IF, Vilela C, Ripoll PL, Espregueira-Mendes J, Oliveira JM et al. Emerging concepts in treating cartilage, osteochondral defects, and osteoarthritis of the knee and ankle. Osteochondral Tissue Engineering. Springer; 2018. p. 25–62. [DOI] [PubMed]
- 5.Vannini F, Spalding T, Andriolo L, Berruto M, Denti M, Espregueira-Mendes J, Menetrey J, Peretti GM, Seil R, Filardo G. Sport and early osteoarthritis: the role of sport in aetiology, progression and treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1786–1796. doi: 10.1007/s00167-016-4090-5. [DOI] [PubMed] [Google Scholar]
- 6.Cengiz IF, Pereira H, de Girolamo L, Cucchiarini M, Espregueira-Mendes J, Reis RL, Oliveira JM. Orthopaedic regenerative tissue engineering en route to the holy grail: disequilibrium between the demand and the supply in the operating room. J Exp Orthop. 2018;5(1):14. doi: 10.1186/s40634-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delanois RE, Etcheson JI, Sodhi N, Henn RF III, Gwam CU, George NE, et al. Biologic therapies for the treatment of knee osteoarthritis. J Arthroplast. 2018. [DOI] [PubMed]
- 8.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira H, Frias AM, Oliveira JM, Espregueira-Mendes J, Reis RL. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy. 2011;27(12):1706–1719. doi: 10.1016/j.arthro.2011.08.283. [DOI] [PubMed] [Google Scholar]
- 11.Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol. 2015;11(4):213–222. doi: 10.1038/nrrheum.2015.27. [DOI] [PubMed] [Google Scholar]
- 12.Cengiz IF, Oliveira JM, Ochi M, Nakamae A, Adachi N, Reis RL. “Biologic” treatment for meniscal repair. Injuries and health problems in football. Springer; 2017. p. 679–686
- 13.Wasserman A, Matthewson G, MacDonald P. Platelet-rich plasma and the knee—applications in orthopedic surgery. Curr Rev Musculoskelet Med. 2018;11(4):607–615. doi: 10.1007/s12178-018-9521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cucchiarini M, Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat Rev Rheumatol. 2018;(1). [DOI] [PubMed]
- 15.Venkatesan JK, Rey-Rico A, Cucchiarini M. Current trends in viral gene therapy for human orthopaedic regenerative medicine. Tissue Eng Regen Med. 2019:1–11. [DOI] [PMC free article] [PubMed]
- 16.Cengiz IF, Oliveira JM, Reis RL. PRP therapy. Osteochondral Tissue Engineering. Springer; 2018. p. 241–53.
- 17.Le AD, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasterlain AS, Braun HJ, Dragoo JL. The systemic effects of platelet-rich plasma. Platelet rich plasma in musculoskeletal practice: Springer; 2016. p. 199–222.
- 19.Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1–12. doi: 10.1016/j.actbio.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbiah R, Guldberg RE. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv Healthc Mater. 2019;8(1):1801000. doi: 10.1002/adhm.201801000. [DOI] [PubMed] [Google Scholar]
- 21.Everts PA, Knape JT, Weibrich G, Schonberger J, Hoffmann J, Overdevest EP, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174–187. [PMC free article] [PubMed] [Google Scholar]
- 22.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;(13):3532–48. [DOI] [PubMed]
- 23.Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 24.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 25.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Metcalf KB, Mandelbaum BR, McIlwraith CW. Application of platelet-rich plasma to disorders of the knee joint. Cartilage. 2013;4(4):295–312. doi: 10.1177/1947603513487553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 29.Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13(1):229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart R, Safi A, Komzák M, Jajtner P, Puskeiler M, Hartová P. Platelet-rich plasma in patients with tibiofemoral cartilage degeneration. Arch Orthop Trauma Surg. 2013;133(9):1295–1301. doi: 10.1007/s00402-013-1782-x. [DOI] [PubMed] [Google Scholar]
- 31.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez M, Fiz N, Azofra J, Usabiaga J, Recalde EA, Gutierrez AG, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Spakova T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 34.Filardo G, Kon E, Ruiz MTP, Vaccaro F, Guitaldi R, Di Martino A, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single-versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082–2091. doi: 10.1007/s00167-011-1837-x. [DOI] [PubMed] [Google Scholar]
- 35.Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Say F, Gürler D, Yener K, Bülbül M, Malkoc M. Platelet-rich plasma injection is more effective than hyaluronic acid in the treatment of knee osteoarthritis. Acta Chir Orthop Traumatol Cechoslov. 2013;80(4):278–283. [PubMed] [Google Scholar]
- 37.Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800. doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- 38.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 39.Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11(4):583–592. doi: 10.1007/s12178-018-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fice MP, Miller JC, Christian R, Hannon CP, Smyth N, Murawski CD, Cole BJ, Kennedy JG. The role of platelet-rich plasma in cartilage pathology: an updated systematic review of the basic science evidence. Arthroscopy. 2019;35:961–976.e3. doi: 10.1016/j.arthro.2018.10.125. [DOI] [PubMed] [Google Scholar]
- 41.•• Filardo G, Kon E, Roffi A, Di Matteo B, Merli M, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2013:1–16. This is a valuable systematic review on thein vitro,in vivo, and the clinical studies about PRP for joint degeneration. [DOI] [PMC free article] [PubMed]
- 42.Kennedy MI, Whitney K, Evans T, LaPrade RF. Platelet-rich plasma and cartilage repair. Curr Rev Musculoskelet Med. 2018;11(4):573–582. doi: 10.1007/s12178-018-9516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kia C, Baldino J, Bell R, Ramji A, Uyeki C, Mazzocca A. Platelet-rich plasma: review of current literature on its use for tendon and ligament pathology. Curr Rev Musculoskelet Med. 2018;11(4):566–572. doi: 10.1007/s12178-018-9515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage. 2017;8(4):341–364. doi: 10.1177/1947603516670709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez M, Delgado D, Garate A, Sánchez P, Padilla S, Azofra J. Platelet-rich plasma combined with allograft to treat osteochondritis dissecans of the knee: a case report. J Med Case Rep. 2019;13(1):105. doi: 10.1186/s13256-019-2027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch M, Mayr F, Achenbach L, Krutsch W, Lang S, Hilber F, Weber J, Pfeifer CG, Woehl R, Eichhorn J, Zellner J, Nerlich M, Angele P. Partial anterior cruciate ligament ruptures: advantages by intraligament autologous conditioned plasma injection and healing response technique—midterm outcome evaluation. Biomed Res Int. 2018;2018:1–9. doi: 10.1155/2018/3204869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch M, Matteo BD, Eichhorn J, Zellner J, Mayr F, Krutsch W, Achenbach L, Woehl R, Nerlich M, Angele P. Intra-ligamentary autologous conditioned plasma and healing response to treat partial ACL ruptures. Arch Orthop Trauma Surg. 2018;138(5):675–683. doi: 10.1007/s00402-018-2885-1. [DOI] [PubMed] [Google Scholar]
- 48.•• Scott A, LaPrade RF, Harmon KG, Filardo G, Kon E, Della Villa S et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med 2019:0363546519837954. This work compares leukocyte-rich and -poor PRP for patellar tendinopathy treatment. [DOI] [PubMed]
- 49.Dai W-L, Zhang H, Lin Z-M, Shi Z-J, Wang J. Efficacy of platelet-rich plasma in arthroscopic repair for discoid lateral meniscus tears. BMC Musculoskelet Disord. 2019;20(1):113. doi: 10.1186/s12891-019-2500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Chen S, Qiu M, Zhou A, Yan W, Zhang J. Lateral meniscus allograft transplantation with platelet-rich plasma injections: a minimum two-year follow-up study. Knee. 2018;25(4):568–576. doi: 10.1016/j.knee.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Kemmochi M, Sasaki S, Takahashi M, Nishimura T, Aizawa C, Kikuchi J. The use of platelet-rich fibrin with platelet-rich plasma support meniscal repair surgery. J Orthop. 2018;15(2):711–720. doi: 10.1016/j.jor.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cengiz IF, Pereira H, Silva-Correia J, Ripoll PL, Espregueira-Mendes J, Kaz R et al. Meniscal lesions: from basic science to clinical management in footballers. Injuries and health problems in football. Springer; 2017. p. 145–163.
- 53.Pereira H, Cengiz IF, Silva-Correia J, Cucciarini M, Gelber PE, Espregueira-Mendes J et al. Histology-ultrastructure-biology. Surgery of the Meniscus. Springer; 2016. p. 23–33.
- 54.Pereira H, Cengiz IF, Silva-Correia J, Ripoll PL, Varatojo R, Oliveira JM et al. Meniscal repair: indications, techniques, and outcome. Arthroscopy. Springer; 2016. p. 125–42.
- 55.Kaminski R, Maksymowicz-Wleklik M, Kulinski K, Kozar-Kaminska K, Dabrowska-Thing A, Pomianowski S. Short-term outcomes of percutaneous trephination with a platelet rich plasma intrameniscal injection for the repair of degenerative meniscal lesions. A prospective, randomized, double-blind, parallel-group, placebo-controlled study. Int J Mol Sci. 2019;20(4):856. doi: 10.3390/ijms20040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.• Kaminski R, Kulinski K, Kozar-Kaminska K, Wielgus M, Langner M, Wasko MK, et al. A prospective, randomized, double-blind, parallel-group, placebo-controlled study evaluating meniscal healing, clinical outcomes, and safety in patients undergoing meniscal repair of unstable, complete vertical meniscal tears (bucket handle) augmented with platelet-rich plasma. Biomed Res Int. 2018;2018. This study analyzing the effects of PRP on the repair of bucket handle meniscal lesions in a limited number of patients. [DOI] [PMC free article] [PubMed]
- 57.Bastos R, Mathias M, Andrade R, Bastos R, Balduino A, Schott V, Rodeo S, Espregueira-Mendes J. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3342–3350. doi: 10.1007/s00167-018-4883-9. [DOI] [PubMed] [Google Scholar]
- 58.Huang G, Hua S, Yang T, Ma J, Yu W, Chen X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp Ther Med. 2018;15(3):3096–3102. doi: 10.3892/etm.2018.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buendia-Lopez D, Medina-Quirós M, Marín MÁF-V. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol. 2018;19(1):3. doi: 10.1186/s10195-018-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347–354. doi: 10.1177/0363546518814532. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad HS, Farrag SE, Okasha AE, Kadry AO, Ata TB, Monir AA, Shady I. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis. 2018;21(5):960–966. doi: 10.1111/1756-185X.13315. [DOI] [PubMed] [Google Scholar]
- 62.Louis ML, Magalon J, Jouve E, Bornet CE, Mattei JC, Chagnaud C, Rochwerger A, Veran J, Sabatier F. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: a randomized double blind noninferiority trial compared with viscosupplementation. Arthroscopy. 2018;34(5):1530–1540. doi: 10.1016/j.arthro.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med. 2018;16(3):2119–2125. doi: 10.3892/etm.2018.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camurcu Y, Sofu H, Ucpunar H, Kockara N, Cobden A, Duman S. Single-dose intra-articular corticosteroid injection prior to platelet-rich plasma injection resulted in better clinical outcomes in patients with knee osteoarthritis: a pilot study. J Back Musculoskelet Rehabil. 2018(Preprint):1–8. [DOI] [PubMed]
- 65.Guillibert C, Charpin C, Raffray M, Benmenni A, Dehaut F-X, El Ghobeira G, et al. Single injection of high volume of autologous pure PRP provides a significant improvement in knee osteoarthritis: a prospective routine care study. Int J Mol Sci. 2019;20(6):1327. doi: 10.3390/ijms20061327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis. Orthopade. 2019;48(3):239–247. doi: 10.1007/s00132-018-03659-5. [DOI] [PubMed] [Google Scholar]
- 67.Li TY, Wu YT, Chen LC, Cheng SN, Pan RY, Chen YC. An exploratory comparison of single intra-articular injection of platelet-rich plasma vs hyaluronic acid in treatment of haemophilic arthropathy of the knee. Haemophilia. 2019;25:484–492. doi: 10.1111/hae.13711. [DOI] [PubMed] [Google Scholar]
- 68.Lin K-Y, Yang C-C, Hsu C-J, Yeh M-L, Renn J-H. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy. 2019;35(1):106–117. doi: 10.1016/j.arthro.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 69.Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018;37(5):1341–1350. doi: 10.1007/s10067-018-3985-6. [DOI] [PubMed] [Google Scholar]
- 70.Chen CP, Chen J-L, Hsu C-C, Pei Y-C, Chang W-H, Lu H-C. Injecting autologous platelet rich plasma solely into the knee joint is not adequate in treating geriatric patients with moderate to severe knee osteoarthritis. Exp Gerontol. 2019;119:1–6. doi: 10.1016/j.exger.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 71.Fréchette J-P, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 72.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266–271. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 73.Magalon J, Bausset O, Serratrice N, Giraudo L, Aboudou H, Veran J, Magalon G, Dignat-Georges F, Sabatier F. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30(5):629–638. doi: 10.1016/j.arthro.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 74.Bishop FL, Coghlan B, Geraghty AW, Everitt H, Little P, Holmes MM, et al. What techniques might be used to harness placebo effects in non-malignant pain? A literature review and survey to develop a taxonomy. BMJ Open. 2017;7(6):e015516. doi: 10.1136/bmjopen-2016-015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Domzalski ME, Szkutnik P. Emerging orthobiologic approach to fractures. Bio-orthopaedics. Springer; 2017. p. 473–478.
- 76.Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45(1):226–233. doi: 10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)