Abstract
Background
The malignant transformation of an ectopic pancreas in the duodenum is extremely rare. Herein, we report a case of an adenocarcinoma that arose from an ectopic pancreas. We also reviewed 14 cases of malignant transformations arising from an ectopic pancreas in the duodenum that were previously published.
Case presentation
An 81-year-old man with a 1-month history of vomiting was admitted to our institution. Esophagogastroduodenoscopy (EGD) and computed tomography (CT) scans revealed an obstruction at the first part of the duodenum. A distal gastrectomy was performed for diagnostic and therapeutic purposes. The histopathological examination of the resected specimen showed adenocarcinoma that arose from an ectopic pancreas (Heinrich type 1). The patient is alive without relapse at 18 months of follow-up.
Conclusions
Adenocarcinoma that arises from an ectopic pancreas should be considered when an obstruction is identified in the duodenum.
Keywords: Ectopic pancreas, Distal gastrectomy, Duodenal adenocarcinoma, Cancer-induced vomiting
Background
An ectopic pancreas, often found during surgery or biopsy, is defined as an uncommon pancreatic tissue outside the normal pancreas, which lacks any connection to the normal pancreas, and has its own vascular and ductal systems [1]. The frequency of the occurrence of ectopic pancreatic tissue is found in 0.25% of abdominal surgeries and 1.2% of gastrectomy operations, and its frequency at autopsy has been reported to be 0.55–13.7% [2]. Ectopic pancreatic tissue has been found in both abdominal and extra-abdominal locations, but is mainly encountered in the duodenum (25–35%) [3] and stomach (25–60%) [4], though mesocolon [5, 6] and Meckel’s diverticulum [7] are also other rare sites. Malignant transformations that arise from ectopic pancreatic tissue are extremely rare, and there are only 14 reported cases in the literature. Here, we report a case of adenocarcinoma that arose from an ectopic pancreas in the first part of the duodenum.
Case presentation
An 81-year-old Japanese man was admitted to our institution with a 1-month history of vomiting. Although the patient did not complain of any obvious weight loss, he experienced daily persistent vomiting and always felt full. Past medical history was positive for chronic atrial fibrillation, chronic heart failure, Graves’ disease, hyperlipidemia, and benign prostatic hyperplasia. The patient had no previous surgical history. Serum tumor markers, such as carbohydrate antigen (CA) 19–9, CA 125, α-fetoprotein (AFP), and carcinoembryonic antigen (CEA), were all within normal ranges. An esophagogastroduodenoscopy (EGD) revealed a submucosal tumor-like lesion with a smooth surface involving the entire circumference of the first part of the duodenum. The demarcation line of the lesion was unclear (Fig. 1). We could not pass the endoscope beyond the first part of the duodenum because of duodenal stenosis. An endoscopic ultrasound (EUS) was not performed; enhanced multi-detector row computed tomography (enhanced MDCT) revealed increased wall thickness in the first part of the duodenum (Fig. 2). No swollen lymph nodes were detected. The forceps biopsy specimen from the submucosal tumor-like lesion did not show evidence of malignancy. As the possibility of a malignant tumor could not be ruled out clinically, a surgical resection was planned for diagnostic and therapeutic purposes.
Fig. 1.
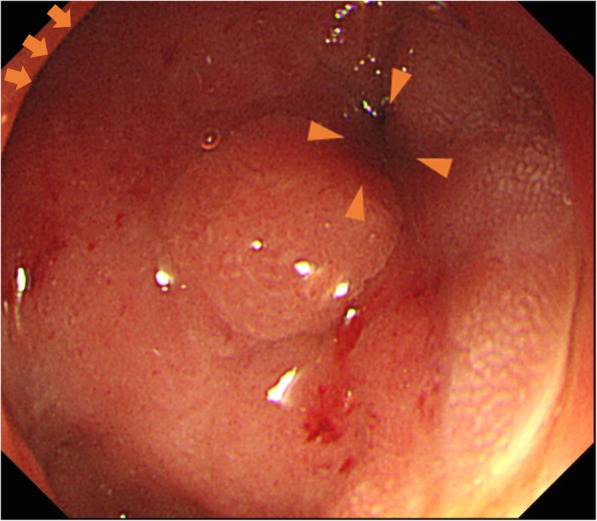
Esophagogastroduodenoscopy showing the obstruction at the first part of the duodenum. Arrow: pylorus ring. Arrow head: obstruction of the first part of the duodenum
Fig. 2.

Enhanced multi-detector row computed tomography (MDCT) image showing the wall thickness in the duodenum in the transverse (a) and the coronal (b) planes. Arrow heads: wall thickness of the first part of the duodenum
The patient subsequently underwent an open surgery. A hard mass was palpable in the duodenal bulb, which extended dorsally to the second part of the duodenum. After Kocherization of the duodenum, the area proximal to the pylorus ring to the end of the second part of the duodenum, where the tumor was not palpated, was resected (Fig. 3).
Fig. 3.
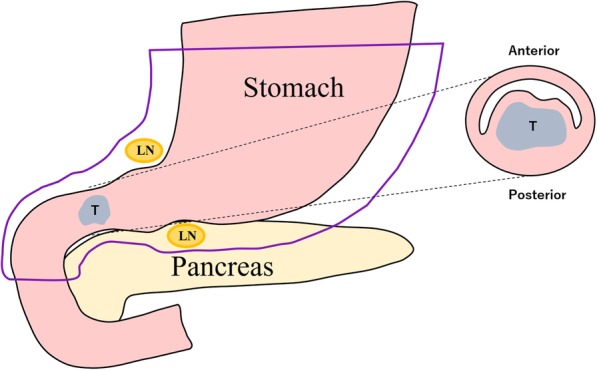
Schema of the operative findings. LN, Lymph node; T, Tumor
A distal gastrectomy was performed. The tumor was 30 × 10 mm and located in the first part of the duodenum (Fig. 4). It was not continuous with the normal pancreas as revealed first by imaging and later confirmed during surgery. Microscopically, the tumor was diagnosed as a moderately differentiated adenocarcinoma that extended from the submucosal layer to the muscularis propria of the duodenum. Normal pancreatic tissue was observed adjacent to the tumor, suggesting the presence of an ectopic pancreas (Fig. 5). Surgical margins were negative for the presence of tumor cells. Moderate lymphatic invasion, moderate venous invasion, marked neural invasion, and metastases to both superior and inferior pyloric lymph nodes were observed. The adjacent ectopic pancreatic tissue had a microscopic appearance consistent with Heinrich’s type 1 [8, 9] and was characterized by the presence of ducts, islets, and acini. On immunohistochemical staining, the islets of the ectopic pancreas and the normal pancreas showed positive staining for chromogranin A, synaptophysin, neural cell adhesion molecule (NCAM), insulin, glucagon, and somatostatin. Based on these findings, our final diagnosis was of a ductal adenocarcinoma arising from an ectopic pancreas in the first part of the duodenum.
Fig. 4.
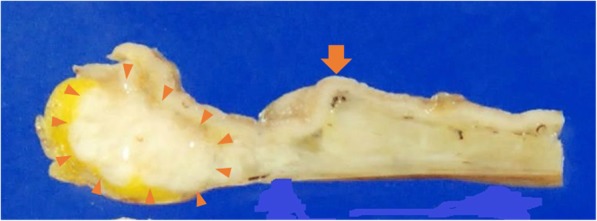
Resected specimen (gastric antrum and duodenum). Arrow heads: tumor. Arrow: pyloric ring
Fig. 5.
a Ectopic pancreas of the duodenum showing pancreatic ducts, acinar cells, and islets called Heinlich type1. b Adenocarcinoma in the duodenum mainly located in extra-mucosal layer, and combined with ectopic pancreas, which is consistent with carcinoma derived from ectopic pancreas. Magnified figures in the square are shown in c (yellow) and d (blue). c Papillary adenocarcinoma of the duodenum derived from ectopic pancreas. d Ectopic pancreatic tissue composed of pancreatic ducts and islets in duodenal muscle layer
The patient was discharged 18 days after surgery, with no complications. Postoperative adjuvant chemotherapy was not administered. We performed a follow-up blood exam including tumor markers (CEA, CA 19–9) every 3 months and CT images every 6 months. The patient is alive without relapse, at 18 months of follow-up.
Discussion
An ectopic pancreas is defined as an uncommon pancreatic tissue that exists outside the normal pancreas with no connection to it. The frequency of ectopic pancreatic tissue has been reported to be 0.25% in abdominal surgery [2], 25–35% of which was found in the duodenum [3].
Histopathologically, ectopic pancreatic tissue has been classified into four types by Heinrich [8, 9] depending on the presence or absence of pancreatic ducts, acini, and islet cells. The ectopic pancreas in our patient was located in the first part of the duodenum and contained ducts, acini, and islet cells, making it a Heinrich type 1 ectopic pancreas. The malignant transformation of ectopic pancreatic tissue is extremely rare, with a frequency that ranges from 0.7 to 1.8%, among all cases of ectopic pancreatic tissue [10, 11]. These tumors are usually located in the submucosal layer and only occasionally expand into the muscularis propria [1]. Jaervi and Lauren [12] have proposed three criteria for diagnosing carcinoma that arises from a heterotopic pancreas:
The tumor must be found within or close to the ectopic pancreatic tissue.
A direct transition must be observed between pancreatic structures and the carcinoma (malignant transformation of an ectopic pancreas must be differentiated from a metastatic deposit or a neoplastic invasion from a neighboring digestive cancer, especially from the stomach, the biliary tract, and the ectopic pancreas).
The non-neoplastic pancreatic tissue must comprise fully developed acini and ductal structures.
In this case, the adenocarcinoma was adjacent to the ectopic pancreas and located in the submucosal layer, away from the normal pancreas. No obvious cancer was seen in other organs. Therefore, we diagnosed the patient with adenocarcinoma of an ectopic pancreas in the duodenum.
To the best of our knowledge, 52 cases of malignant transformation arising from an ectopic pancreas, including the present case, have been reported in PubMed (keywords: ectopic OR heterotopic OR aberrant pancreas, carcinoma), 14 of which were malignant transformation arising from an ectopic pancreas in the duodenum (Table 1) [13–23]. The mean age of the patients in this group was 70.2 years (range 56–86 years), and eight patients were males and six were females. The mean tumor size was 27.9 mm (range 12–50 mm). Eleven of the 14 patients were pathologically diagnosed with adenocarcinoma (tubular adenocarcinoma, poorly differentiated adenocarcinoma, papillary adenocarcinoma, and mucinous adenocarcinoma), and about half of the carcinomas arose from Heinrich type 1 ectopic pancreatic tissue. In all except 2 of the 14 cases, the tumors were located in the first or second part of the duodenum.
Table 1.
Review of case reports of adenocarcinoma arising from a heterotopic pancreas in the duodenum
| Case | Year | Author | Age | Sex | Part of duodenum involved | Contrast effect on enhanced CT | Diagnostic approach | Operative method | Pathology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1993 | Tanaka | 72 | M | ND | ND | Operation | PD | Cancer | ND |
| 2 | 1996 | Inoue | 81 | F | ND | ND | Operation | DG | Adenocarcinoma + muc | ND |
| 3 | 2006 | Inoue | 75 | M | ND | ND | Operation | PPPD | Adenocarcinoma | ND |
| 4 | 2007 | Tison | 72 | M | Second portion, vater | ND | Operation | PD | Adenocarcinoma, CDHP | Death (16 months) |
| 5 | 2007 | Kawakami | 65 | F | Second portion, vater | Heterogeneously enhanced | Operation | SSPPD | Acinar cell carcinoma | Alive (19 months) |
| 6 | 2008 | Rosok | 59 | F | Proximal | Multi-cystic lesion | Operation | LR | IPMC | Alive (36 months) |
| 7 | 2010 | Inoue | 75 | M | Second portion | Homogeneously enhanced | Operation | PPPD | Adenocarcinoma | Alive (72 months) |
| 8 | 2010 | Bini | 56 | M | First portion | ND | Operation | PD | Adenocarcinoma | ND |
| 9 | 2011 | Stock | 79 | F | Fourth portion | ND | Operation | SD | Adenocarcinoma | ND |
| 10 | 2012 | Kinoshita | 62 | F | First portion | Heterogeneously enhanced | Operation | PD | Adenocarcinoma | Alive (12 months) |
| 11 | 2013 | Ginori | 86 | F | First portion | ND | Operation | STG + DR | Adenocarcinoma + muc | ND |
| 12 | 2014 | Endo | 75 | M | Second portion | ND | EUS-FNA | SSPPD | Adenocarcinoma | Alive (60 months) |
| 13 | 2015 | Fukino | 62 | M | Fourth portion | Poorly enhanced | Operation | SD | Adenocarcinoma | Death (33 months) |
| 14 | Present case | 81 | M | First portion | Same contrast effect as duodenum | Operation | DG | Adenocarcinoma | Alive (18 months) | |
Abbreviations: M male, F female, ND not described, muc mucinous carcinoma, CDHP cystic dystrophy in heterotopic pancreas, IPMC intraductal papillary-mucinous carcinoma, EUS-FNA endoscopic ultrasonography-guided fine-needle aspiration, PD pancreaticoduodenectomy, DG distal gastrectomy, PPPD pylorus-preserving pancreaticoduodenectomy, SSPPD subtotal stomach-preserving pancreaticoduodenectomy, LR laparoscopic resection of tumor and duodenal wall, SD segmental duodenectomy, STG + DR subtotal gastrectomy with duodenal bulb resection
Except for one patient, no other patient has reportedly been diagnosed with a malignant transformation arising from an ectopic pancreas prior to surgery. Endo et al. were able to diagnose an ectopic pancreas adenocarcinoma preoperatively using endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) [22]. They suggested that EUS-FNA is a useful procedure for preoperative diagnosis in such cases [22] probably because ectopic pancreatic tissue is usually situated in the submucosal layer [1]. In our case, due to the obstruction of the duodenum, which made curative or palliative surgery necessary, EUS-FNA was not performed. Ordinary pancreatic cancer is characterized by an ischemic mass on enhanced CT; however, only one in six cases presented with low contrast effects. In the present case, the tumor was not distinctive on enhanced MDCT and the wall of the first part of the duodenum looked similar to the uninvolved parts.
Because few reports of malignant transformations arising from ectopic pancreatic tissue are available, no reports have compared prognosis between these patients and those with ordinary pancreatic cancer. An analysis of the eight cases of ectopic pancreatic tissue that had reported on patient prognoses after surgery, including the present case, revealed a 5-year survival rate of 64.3%. The corresponding survival rate for ordinary pancreatic cancer is about 10% [24]. In malignant transformations that arise from ectopic pancreatic tissue, gastrointestinal symptoms due to stenosis are easier to identify than in ordinary pancreatic cancer, which may result in a better prognosis in the former.
Some patients underwent postoperative adjuvant chemotherapy similar to patients with ordinary pancreatic cancer, such as S-1 or gemcitabine monotherapy [23]. However, in the present case, no postoperative adjuvant chemotherapy was performed, because no evidence of the efficacy of postoperative adjuvant chemotherapy for adenocarcinoma arising from ectopic pancreatic tissue in an old patient with low ADL exists.
Conclusions
We reported an extremely rare case of an adenocarcinoma that arose from ectopic pancreatic tissue in the duodenum. Considering the rarity of this disease, gathering data from all cases of adenocarcinoma arising from ectopic pancreatic tissue in the duodenum will facilitate the development of diagnostic and treatment strategies.
Acknowledgements
Not applicable
Abbreviations
- ADL
Activities of daily living
- AFP
α-Fetoprotein
- CA
Carbohydrate antigen
- CEA
Carcinoembryonic antigen
- CT
Computed tomography
- EGD
Esophagogastroduodenoscopy
- Enhanced MDCT
Enhanced multi-detector row computed tomography
- EUS
Endoscopic ultrasound
- EUS-FNA
Endoscopic ultrasonography-guided fine-needle aspiration
- NCAM
Neural cell adhesion molecule
- PD
Pancreaticoduodenectomy
Authors’ contributions
TK wrote the manuscript and performed the literature search. MO, KO, AF, TY, RT, KK, MS, and NI treated and observed the patient. NK and HK performed the histological examination. DM, MK, TN, and SH supervised the preparation of this case report. All authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Ethics approval and consent to participate
The institutional ethics committee approved the publication of this case report.
Consent for publication
Informed consent was obtained from this patient to publish the details of the case, and his identity has been protected.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tsukasa Kaneko, Email: geckotk@gmail.com.
Masanori Ohara, Email: moohara@hnh.hosp.go.jp.
Kunishige Okamura, Email: kunishige-o@hotmail.co.jp.
Aki Fujiwara-Kuroda, Email: a-black@wj8.so-net.ne.jp.
Daisuke Miyasaka, Email: matue8686@yahoo.co.jp.
Takumi Yamabuki, Email: bukiyama@themis.ocn.ne.jp.
Ryo Takahashi, Email: rtakahashi@hnh.hosp.go.jp.
Kazuteru Komuro, Email: sakura-k@rainbow.plala.or.jp.
Masato Suzuoki, Email: msuzuoki@hnh2.host.jp.
Nozomu Iwashiro, Email: niwashir@hnh.hosp.go.jp.
Mototsugu Kato, Email: mkato@hnh.hosp.go.jp.
Noriko Kimura, Email: kimura-path@hnh.hosp.go.jp.
Hiroshi Kijima, Email: hkijima@cc.hirosaki-u.ac.jp.
Toru Nakamura, Email: torunakamura@med.hokudai.ac.jp.
Satoshi Hirano, Email: satto@med.hokudai.ac.jp.
References
- 1.Thoeni RF, Gedgaudas RK. Ectopic pancreas: usual and unusual features. Gastrointest Radiol. 1980;5:37–42. doi: 10.1007/BF01888597. [DOI] [PubMed] [Google Scholar]
- 2.Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974;109:762–765. doi: 10.1001/archsurg.1974.01360060032010. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno Y, Sumi Y, Nachi S, Ito Y, Marui T, Saji S, et al. Acinar cell carcinoma arising from an ectopic pancreas. Surg Today. 2007;37:704–707. doi: 10.1007/s00595-006-3384-5. [DOI] [PubMed] [Google Scholar]
- 4.Mulholland KC, Wallace WD, Epanomeritakis E, Hall SR. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004;5:498–501. [PubMed] [Google Scholar]
- 5.Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, Imaoka S, et al. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. 1990;85:597–601. [PubMed] [Google Scholar]
- 6.Tornóczky T, Kálmán E, Jáksó P, Méhes G, Pajor L, Kajtár GG, et al. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol. 2001;54:241–245. doi: 10.1136/jcp.54.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh HC, Page B, Black C, Brown I, Ballantyne S, Galloway DJ. Ectopic pancreatic-type malignancy presenting in a Meckel’s diverticulum: a case report and review of the literature. World J Surg Oncol. 2009;7:54. doi: 10.1186/1477-7819-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Heinrich H. Ein Beitrag zur Histologie des sogen, Akzessorischen Pankreas. Virchows Arch A. Pathol Anat Histopathol. 1909;198:392–401. doi: 10.1007/BF02085327. [DOI] [Google Scholar]
- 9.Gaspar Fuentes A, Campos Tarrech JM, Fernández Burgui JL, Castells Tejón E, Ruíz Rossello J, Gómez Pérez J, et al. Pancreatic ectopias. Rev Esp Enferm Apar Dig. 1973;39:255–268. [PubMed] [Google Scholar]
- 10.Makhlouf HR, Almeida JL, Sobin LH. Carcinoma in jejunal pancreatic heterotopia. Arch Pathol Lab Med. 1999;123:707–711. doi: 10.1043/0003-9985(1999)123<0707:CIJPH>2.0.CO:2. [DOI] [PubMed] [Google Scholar]
- 11.Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568–571. [PubMed] [Google Scholar]
- 12.Jaervi O, Lauren P. Gastric glandular tumors provided with excretory ducts, and criticism of the theory of the tumors arising in heterotopic pancreas; observations on the occurrence of atypical glands in the stomach. Acta Pathol Microbiol Scand. 1964;62:1–23. doi: 10.1111/apm.1964.62.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Stock C, Keutgen XM, Pisapia D, Crawford C, Zarnegar R. Heterotopic pancreatic neoplasm presenting as an obstructing mass at the fourth portion of the duodenum. JOP. 2011;12:241–243. [PubMed] [Google Scholar]
- 14.Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, et al. Diagnosis and management of heterotopic pancreas. Int Surg. 1993;78:32–35. [PubMed] [Google Scholar]
- 15.Inoue Y, Hayashi M, Arisaka Y, Higuchi K, Egashira Y, Tanigawa N. Adenocarcinoma arising in a heterotopic pancreas (Heinrich type III): a case report. J Med Case Rep. 2010;4:39. doi: 10.1186/1752-1947-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tison C, Regenet N, Meurette G, Mirallié E, Cassagnau E, Frampas E, et al. Cystic dystrophy of the duodenal wall developing in heterotopic pancreas: report of 9 cases. Pancreas. 2007;34:152–156. doi: 10.1097/01.mpa.0000246669.61246.08. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami H, Kuwatani M, Onodera M, Hirano S, Kondo S, Nakanishi Y, et al. Primary acinar cell carcinoma of the ampulla of Vater. J Gastroenterol. 2007;42:694–697. doi: 10.1007/s00535-007-2070-8. [DOI] [PubMed] [Google Scholar]
- 18.Røsok BI, Rosseland AR, Grzyb K, Mathisen O, Edwin B. Laparoscopic resection of an intraductal papillary mucinous carcinoma in ectopic pancreatic tissue. J Laparoendosc Adv Surg Tech A. 2008;18:723–725. doi: 10.1089/lap.2007.0168. [DOI] [PubMed] [Google Scholar]
- 19.Bini R, Voghera P, Tapparo A, Nunziata R, Demarchi A, Capocefalo M, et al. Malignant transformation of ectopic pancreatic cells in the duodenal wall. World J Gastroenterol. 2010;16:1293–1295. doi: 10.3748/wjg.v16.i10.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita H, Yamaguchi S, Shimizu A, Sakata Y, Arii K, Mori K, et al. Adenocarcinoma arising from heterotopic pancreas in the duodenum. Int Surg. 2012;97:351–355. doi: 10.9738/CC148.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginori A, Vassallo L, Butorano MA, Bettarini F, Di Mare G, Marrelli D. Pancreatic adenocarcinoma in duodenal ectopic pancreas: a case report and review of the literature. Pathologica. 2013;105:56–58. [PubMed] [Google Scholar]
- 22.Endo S, Saito R, Ochi D, Yamada T, Hirose M, Hiroshima Y, et al. Effectiveness of an endoscopic biopsy procedure using EUS-FNA and EMR-C for diagnosing adenocarcinoma arising from ectopic pancreas: two case reports and a literature review. Intern Med. 2014;53:1055–1062. doi: 10.2169/internalmedicine.53.1420. [DOI] [PubMed] [Google Scholar]
- 23.Fukino N, Oida T, Mimatsu K, Kuboi Y, Kida K. Adenocarcinoma arising from heterotopic pancreas at the third part of the duodenum. World J Gastroenterol. 2015;2(13):4082–4088. doi: 10.3748/wjg.v21.i13.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doi R, Imamura M, Hosotani R, Imaizumi T, Hatori T, Takasaki K, et al. Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer. Surg Today. 2008;38:1021–1028. doi: 10.1007/s00595-007-3745-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.



