Abstract
Purpose
To review the relevant recent literature regarding minimally invasive, lateral, and oblique approaches to the anterior lumbar spine, with a particular focus on the operative and postoperative complications.
Methods
A literature search was performed on Pubmed and Web of Science using combinations of the following keywords and their acronyms: lateral lumbar interbody fusion (LLIF), oblique lateral interbody fusion (OLIF), anterior-to-psoas approach (ATP), direct lateral interbody fusion (DLIF), extreme lateral interbody fusion (XLIF), and minimally invasive surgery (MIS). All results from January 2016 through January 2019 were evaluated and all studies evaluating complications and/or outcomes were included in the review.
Recent Findings
Transient neurological deficit, particularly sensorimotor symptoms of the ipsilateral thigh, remains the most common complication seen in LLIF. Best available current literature demonstrates that approximately 30–40% of patients have postoperative deficits, primarily of the proximal leg. Permanent symptoms are less common, affecting 4–5% of cases. Newer techniques to reduce this rate include different retractors, direct visualization of the nerves, and intraoperative neuromonitoring. OLIF may have lower deficit rates, but the available literature is limited. Subsidence rates in both LLIF and OLIF are comparable to ALIF (anterior lumbar interbody fusion), but further study is required. Supplemental posterior fixation is an active area of investigation that shows favorable biomechanical results, but additional clinical studies are needed.
Summary
Minimally invasive lumbar interbody fusion techniques continue to advance rapidly. As these techniques continue to mature, evidence-based risk-stratification systems are required to better guide both the patient and clinician in the joint decision-making process for the optimal surgical approach.
Keywords: LLIF, XLIF, DLIF, OLIF, Anterior to psoas, MIS
Introduction
One of the most significant developments in lumbar spine surgery over the past two decades has been the advent of minimally invasive approaches to the anterior lumbar spine. The technique typically relies on a lateral window to the anterior lumbar spine through the psoas and is now commonly known as the lateral lumbar interbody fusion (LLIF, Fig. 1) [1]. It is also sometimes referred to by the industry-associated names direct lateral interbody fusion (DLIF, Medtronic) or extreme lateral interbody fusion (XLIF, NuVasive). Recently, a variant has been developed which takes advantage of the window between the peritoneum and the psoas muscle in lieu of splitting the muscle, which is called the oblique lumbar interbody fusion (OLIF) or the anterior to psoas (ATP) approach [2]. As with other minimally invasive techniques, the theoretical advantages include reduced blood loss, improved postoperative pain due to less retraction and smaller incisions, faster recovery, and the obviation for the need of an approach surgeon [3].
Fig. 1.
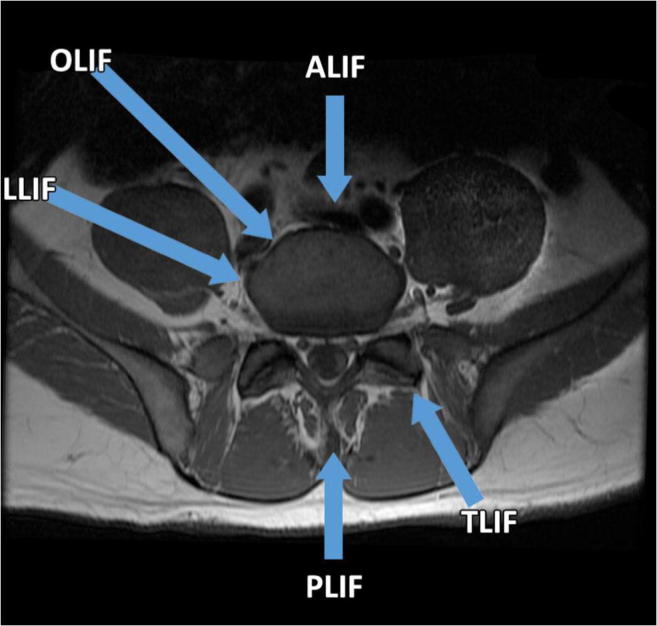
Approaches to the lumbar spine. Diagrammatic representation of the various open and minimally invasive approaches to lumbar interbody fusion (LIF). Options include the anterior, transforaminal, posterior, latera, and oblique approaches
Despite these compelling advantages, LLIFs have their own unique complications, primarily related to the approach itself [3]. The most commonly described complication has been transient, and less commonly permanent, sensorimotor deficits of the groin and/or thigh. However, critically interpreting earlier studies is difficult due to a general lack of standardization of reporting neurological deficits in the literature [4]. For example, one of the first published studies of LLIF patients showed 14% incidence of postop psoas weakness and 3.5% incidence of slight thigh atrophy in a series of 85 patients [5], while a subsequent retrospective study of 59 patients found 62.7% of patients had postoperative sensory or motor thigh symptoms, with over 90% resolving within a year of surgery [6]. Conversely, one of the largest reported LLIF series reported no intraoperative complications, with only 4 in 600 patients reporting transient postoperative nerve deficits [7]. Further difficulties in parsing the literature include the use of different terminology for the techniques, including some papers which refer to OLIF as LLIF. Here we present a concise review of the relevant recent literature regarding minimally invasive approaches to the anterior lumbar spine, with a particular focus on the operative and postoperative complications.
Methods
A literature search was performed on PubMed and Web of Science using combinations of the following keywords and their acronyms: lateral lumbar interbody fusion (LLIF), oblique lateral interbody fusion (OLIF), anterior-to-psoas approach (ATP), direct lateral interbody fusion (DLIF), extreme lateral interbody fusion (XLIF), and minimally invasive surgery (MIS). All results from January 2016 through January 2019 were first evaluated by reading the titles and abstracts. If the article met the criterion of studying of the above procedures for complications and/or outcomes, the full text was accessed, read in its entirety, and included in the review if relevant to the specific subheadings of the discussion.
Thigh symptoms
The sensorimotor deficits observed in patients undergoing lateral or oblique access surgery have been thought to be related to traction of the nerve(s) during surgery. An MRI evaluation of nearly 60 patients found that the lumbar plexus was approximately 5 to 13 mm from the center of the lumbar disc space, while the distance from the genitofemoral nerve was on average less than 1 mm at the L2/L3 level [8]. Historically, LLIF has been associated with 20–40% rate of thigh numbness or pain associated with prolonged muscle retraction of over 20–40 min per level [9]. In light of larger studies having shown a correlation between retraction and nerve symptoms [10], surgeons are still advised to minimize retractor time during this procedure.
A recent PRISMA-compliant systematic review and meta-analysis of older data through June 2016 identified 63 articles describing 6714 patients undergoing 11,325 levels of lumbar fusion (average 1.69 levels per patient) [11]. Due to study heterogeneity, most complications were analyzed for far fewer patients. Neurological deficit was the most common complication, with a pooled transient postoperative deficit rate of 36% in an aggregate sample of 5046 patients. Persistent symptoms, again primarily thigh sensorimotor loss, were seen in 4–5% of patients with the difference in definition between transient and persistent somewhat arbitrary at a cut-off of 6 months [12]. A small series of 18 patients in which LLIF was used to treat spondylolisthesis patients found a third (33%) of the patients developed sensory deficit of the thigh but also that all had resolved within 6 months postoperatively [13]. Similarly, another small series from a different institution evaluating this same procedure and pathology found 3 in 16 (19%) patients developed transient sensory loss that resolved within 1 year. [14]
In contrast to these high rates, a more recent, single-institution study of single-level LLIFs found that 2.6% (6 in 230 patients) suffered severe thigh weakness (as defined by strength of 3/5 or lower in either hip flexion or knee extension) over 6 weeks in duration. Twenty-two patients (9.6%) sustained sensory loss consistent with the surgery [12]. One possible explanation for the lower complication rate may be an improvement of the techniques with time, with newer retractors possibly associated with a reduced the rate of LLIF-related thigh symptoms [15, 16].
Other proposed advancements also show promise in decreasing the incidence of these complications with the direct visualization of the nerves. Two recent studies proposing this modification to the LLIF technique have demonstrated positive data. One found that by utilizing direct endoscopic visualization, the genitofemoral nerve was found within the surgical corridor in 33% (7/21) of patients. The nerve could be freely mobilized, with only one patient requiring intramuscular dissection for mobilization of the nerve [17]. Similarly, Levi and colleagues found that after implementing routine visualization of the lumbosacral plexus during their LLIFs, sensory deficits decreased from 60 to 19% and psoas weakness reduced from 23.7 to 3.1% [18]. Both of these studies are limited by the use of historical comparisons, but this topic clearly merits further study. Lastly, although intraoperative neuromonitoring of motor-evoked potentials has been found to be effective in preventing postoperative lumbar plexus deficits during LLIF, [19] its routine use is controversial [20].
An impetus for the development of the oblique approach was to reduce this complication [2] avoiding the psoas and subsequent traction injury to the nerves. Because of its novelty, there is a paucity of data, and a recent analysis of OLIF-related complications found only 16 papers of sufficient quality and relevance [21]. Notably, the overall combined rate of postoperative thigh symptoms was 1–3%, which is markedly lower than what has been observed in the literature for trans-psoas approaches. However some caution is warranted, as the review generally identified very low incidence rates for all complications. More recent patient series are reporting thigh weakness or numbness in approximately 5–14% of patients, [22–24], but one study has found no deficits in any patients with a minimum 6 months of follow-up [25]. Critically, direct comparisons of LLIF and OLIF have not yet been performed with scientific rigor. A small retrospective series [26] comparing LLIF (n = 31) to OLIF (n = 14) found that OLIF was associated with lower risk of thigh numbness. Likewise, a similar comparison of OLIF to LLIF in 43 total patients found OLIF to be significantly superior in preventing nerve deficits of the thigh [23]. However, the heterogeneity of the patient populations between the two groups in these papers makes it difficult to draw strong clinical conclusions.
Incomplete Decompression
An additional drawback of LLIF/OLIF is that the decompression of the neurological elements is generally achieved indirectly, via ligamentotaxis, risking incomplete relief. This concern has been confirmed in a retrospective series of 28 patients (53 levels) undergoing OLIF. The patients were evaluated with intraoperative CT myelograms to assess neural decompression after cage placement, and in 9 patients, (11 levels) the CT revealed inadequate radiographic decompression of the nerve root. All of these 9 patients then underwent additional direct posterior decompression as per the authors’ institutional protocol, with satisfactory outcomes [27]. Conversely, it is unclear if the improvement in direct decompression is clinically significant and affects outcomes. For example, a small prospective series (n = 55) comparing TLIF and LLIF with 2-year follow-up found that the TLIF group had improved decompression radiographically but did not detect a meaningful postoperative clinical difference, with a notable exception of hip flexion weakness only being seen in the LLIF group [28, 29].
Consequently, there is interest in defining risk factors for successful or failed indirect decompression through the lateral or oblique windows. A prospective multicenter trial evaluated several radiographic variables and their ability to predict a failure of indirect decompression during LLIF, as defined by need for revision surgery or inadequate improvement of symptoms at 6 months [30]. Disc height, foramen height, foramen area, central canal diameter, subarticular diameters, severity of facet arthropathy, and the presence of a bony lateral recess stenosis were evaluated. Of these, only bony lateral recess stenosis was found to be a significant predictor of indirect decompression. This is a significant finding that we believe is more clinically relevant to surgeons, as typically the adequacy of decompression is reported by radiographic parameters [31]. A separate study evaluating the radiographic appearance of the facet joints, specifically joint degeneration or facet angulation, did not find a relationship between the preoperative measurements and postoperative radiographic and clinical outcomes [32].
Sagittal Balance, Subsidence, and Fusion
There may also be technique-related issues with achieving proper decompression as well as sagittal balance. A retrospective patient series demonstrated that anterior positioning of the cage improved sagittal balance in LLIF as expected, with equivalent decompression when compared with placing the implant in the middle of the disk space [33]. Specially designed cages for improving lordosis may additionally be beneficial in restoring proper sagittal balance in LLIF [34], but open approaches may be superior in achieving the desired alignment [35]. When utilizing the minimally invasive lateral window in adult spine deformities, release of the ALL for anterior column reconstruction will improve lordosis versus LLIF alone [36]. Surgeons should also keep in mind that intraoperative fluoroscopy may not provide adequate accuracy in judging cage placement in OLIF when compared against the postoperative CT [37].
Because the approach allows for wider exposure of the disc space, cages used in OLIF and LLIF have a much wider footprint than implants typically utilized in posterior approach surgeries. Therefore, they are thought to better resist subsidence. An analysis of aggregated FDA-submitted biomechanical data indeed shows that LLIF implants are more resistant to subsidence compared with PLIF or TLIF, and equivalent to ALIF implants [38]. However, a different biomechanical study suggested that LLIF with cage alone, without posterior supplemental fixation, may not be sufficient in providing adequate stability for 3 or more levels of fusion, perhaps contributing to subsidence [39]. Clinically, the subsidence rate is poorly defined and poorly reported, making comparisons difficult. A study of nearly 300 patients who underwent LLIF without posterior instrumentation found a relatively low rate (11%) but determined that this was significantly correlated with risk for revision surgery [40]. In contrast, another series of 63 patients who underwent OLIF demonstrated that there was a very high subsidence rate of 33% at 1-year postop detected by CT, and these authors did not find a correlation between these radiographic findings with clinical outcomes [41]. While a meta-analysis found the pooled subsidence rate to be 6.6% [11], due to the large differences in how subsidence is defined, its clinical significance is unclear. Regardless, this is a clear concern that requires further observation and study, and the more recent data do appear to correlate with previously reported subsidence rates of 11–30% [42].
The overall successful fusion rates of LLIF/OLIF are comparable with that of established anterior and posterior fusion techniques. [43, 44] One active area of investigation currently is determining the role of supplemental fixation to increase rigidity and decrease the risk of pseudarthrosis, which is thought to be around 6% in the short- to medium-term [11]. As noted above, it appears that for 3 or more levels of fusion, an LLIF cage without supplementation is insufficient to maintain the necessary rigidity for solid fusion [39]. However, in a separate cadaveric study of 3 lumbar levels, supplemental lateral or posterior fixation was found to be stiffer as expected, but the LLIF cage alone demonstrated adequate stability [45]. There is ultimately a paucity of data to answer this question and further studies are required.
Uncommon Complications
Previously described arterial injury rates in open anterior lumbar spine surgery are 0.3–2.4% [46] and both LLIF and OLIF compare relatively favorably to this rate [11, 21, 47]. An important difference, however, is that the injuries in the minimally invasive approaches appear to primarily involve the segmental arteries [22, 25]. This has led to a newly described and relatively rare complication, symptomatic contralateral psoas hematomas, likely due to injury to the segmental artery intraoperatively during release of the contralateral annulus [48]. While the approach is theoretically designed to avoid the great vessels entirely, in contrast with the ALIF [49], injury to these vascular structures has been reported, including an injury to the iliac veins leading to perioperative mortality [50]. A recent anatomic study utilizing MRI data from 180 patients demonstrated that there is less anatomic variance on the left side, as well as the concave side of the deformity in the case of scoliosis [51]. Surgeons are also reminded that more caudal levels narrow the safe corridor significantly with respect to the great vessels, and care should be taken to not violate the anterior longitudinal ligament (ALL) inadvertently [50, 51].
Other complications, such as visceral injuries or intraoperative durotomies, are very uncommon and generally reported in the 1% or below range [21, 23, 52]. One rare complication more recently being reported is delayed incisional hernias and pseudohernia through the incision site [53, 54]. Because these are almost always operative, and sometimes urgently so, spine surgeons are encouraged to maintain a high index of suspicion. [54]
Conclusion and Commentary
The outcomes from lateral lumbar interbody fusion techniques have led to advocates for the procedure to be performed on an outpatient basis in ambulatory surgery centers [55]. Although our institutional experience has mirrored excellent patient tolerance with reasonable complication risk, we do not perform this in the outpatient setting for the potential emergent complication of vascular or visceral injury. We routinely use intraoperative neuromonitoring, direct visualization during trans-psoas dissection, and aim for retractor times below 20 min. We also now routinely utilize an anterior to psoas technique, particularly in the multilevel setting. For the deformity indication, we have found that the use of multilevel lateral interbody fusion is more effective for the coronal plane rather than the sagittal plane deformity, although sagittal correction can be obtained with attention to anterior cage placement accompanied by posterior column osteotomy. Anecdotally, indirect decompression may be more successful in patients who have relief of symptoms with sitting or lying down. Transient thigh numbness and weakness is observed, and further work needs to be done to compare the relative risks of neurologic symptoms in the different lumbar interbody strategies. As this technique continues to mature, evidence-based risk-stratification systems are required to better guide both the patient and clinician in the joint decision-making process for the optimal surgical approach.
Funding
Dr. Hah has not received funding for this work. Dr. Kang has received research support from The Eli and Edythe Broad Foundation during the completion of this work.
Compliance with Ethical Standards
Conflict of Interest
Dr. Hah has received personal fees as a consultant for NuVasive. Dr. Kang declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Minimally Invasive Spine Surgery
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raymond Hah, Phone: 323-442-5565, Email: Ray.Hah@med.usc.edu.
H. Paco Kang, Email: Hyunwoo.Kang@med.usc.edu.
References
- 1.Mobbs, R., Phan, K., Malham, G., Seex, K., Rao,P. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. Journal of Spine Surgery 2015; 1:2–18. [DOI] [PMC free article] [PubMed]
- 2.Silvestre C, Mac-Thiong J, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6:89–97. doi: 10.4184/asj.2012.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal A, Kerezoudis P, Alvi MA, Goncalves S, Bydon M. Outcomes following minimally invasive lateral transpsoas interbody fusion for degenerative low grade lumbar spondylolisthesis: a systematic review. Clin Neurol Neurosurg. 2018;167:122–128. doi: 10.1016/j.clineuro.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadian A, Deukmedjian AR, Abel N, Dakwar E, Uribe JS. Analysis of lumbar plexopathies and nerve injury after lateral retroperitoneal transpsoas approach: diagnostic standardization a review. J Neurosurg -Spine. 2013;18:289–297. doi: 10.3171/2012.11.SPINE12755. [DOI] [PubMed] [Google Scholar]
- 5.Pimenta L, Figueredo F, DaSilva M. The lateral endoscopic transpsoas retroperitoneal approach (LETRA): a new technique for accessing the lumbar spine. AANS/CNS joint section on disorders of the spine and peripheral nerves. San Diego, CA; 2004.
- 6.Cummock MD, Vanni S, Levi AD, Yu Y, Wang MY. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg -Spine. 2011;15:11–18. doi: 10.3171/2011.2.SPINE10374. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion an analysis of 600 cases. Spine. 2011;36:26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 8.He L, Kang Z, Tang WJ, Rong LM. A MRI study of lumbar plexus with respect to the lateral transpsoas approach to the lumbar spine. Eur Spine J. 2015;24:2538–2545. doi: 10.1007/s00586-015-3847-8. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien JR. Nerve injury in lateral lumbar interbody fusion. Spine. 2017;42:S24. doi: 10.1097/BRS.0000000000002034. [DOI] [PubMed] [Google Scholar]
- 10.Uribe JS, Isaacs RE, Youssef JA, Khajavi K, Balzer JR, Kanter AS, et al. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur Spine J. 2015;24(Suppl 3):378–385. doi: 10.1007/s00586-015-3871-8. [DOI] [PubMed] [Google Scholar]
- 11.Hijji FY, Narain AS, Bohl DD, Ahn J, Long WW, DiBattista JV, et al. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine Journal. 2017;17:1412–1419. doi: 10.1016/j.spinee.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Abel NA, Januszewski J, Vivas AC, Uribe JS. Femoral nerve and lumbar plexus injury after minimally invasive lateral retroperitoneal transpsoas approach: electrodiagnostic prognostic indicators and a roadmap to recovery. Neurosurg Rev. 2018;41:457–464. doi: 10.1007/s10143-017-0863-7. [DOI] [PubMed] [Google Scholar]
- 13.Campbell PG, Nunley PD, Cavanaugh D, Kerr E, Utter PA, Frank K, Stone M. Short-term outcomes of lateral lumbar interbody fusion without decompression for the treatment of symptomatic degenerative spondylolisthesis at L4-5. Neurosurg Focus. 2018;44:E6. doi: 10.3171/2017.10.FOCUS17566. [DOI] [PubMed] [Google Scholar]
- 14.Xu DS, Bach K, Uribe JS. Minimally invasive anterior and lateral transpsoas approaches for closed reduction of grade II spondylolisthesis: initial clinical and radiographic experience. Neurosurg Focus. 2018;44:E4. doi: 10.3171/2017.10.FOCUS17574. [DOI] [PubMed] [Google Scholar]
- 15.Sedra F, Lee R, Dominguez I, Wilson L. Neurological complications using a novel retractor system for direct lateral minimally invasive lumbar interbody fusion. J Clin Neurosci. 2016;31:81–87. doi: 10.1016/j.jocn.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Nunley P, Sandhu F, Frank K, Stone M. Neurological complications after lateral Transpsoas approach to anterior interbody fusion with a novel flat-blade spine-fixed retractor. Biomed Res Int. 2016:8450712. 10.1155/2016/8450712. [DOI] [PMC free article] [PubMed]
- 17.Lee C, Yoon K, Ha S. Which approach is advantageous to preventing development of adjacent segment disease? Comparative analysis of 3 different lumbar interbody fusion techniques (ALIF, LLIF, and PLIF) in L4-5 spondylolisthesis. World Neurosurg. 2017;105:612–622. doi: 10.1016/j.wneu.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Sellin JN, Brusko GD, Levi AD. Lateral lumbar interbody fusion revisited: complication avoidance and outcomes with the mini-open approach. World Neurosurg. 2019;121:E653–e653. doi: 10.1016/j.wneu.2018.09.180. [DOI] [PubMed] [Google Scholar]
- 19.Riley MR, Doan AT, Vogel RW, Aguirre AO, Pieri KS, Scheid EH. Use of motor evoked potentials during lateral lumbar interbody fusion reduces postoperative deficits. Spine Journal. 2018;18:1763–1778. doi: 10.1016/j.spinee.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Cheng I, Acosta F, Chang K, Pham M. Point-counterpoint: the use of neuromonitoring in lateral transpsoas surgery. Spine. 2016;41:S151. doi: 10.1097/BRS.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 21.Li JXJ, Phan K, Mobbs R. Oblique lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg. 2017;98:113–123. doi: 10.1016/j.wneu.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 22.Abe K, Orita S, Mannoji C, Motegi H, Aramomi M, Ishikawa T, Kotani T, Akazawa T, Morinaga T, Fujiyoshi T, Hasue F, Yamagata M, Hashimoto M, Yamauchi T, Eguchi Y, Suzuki M, Hanaoka E, Inage K, Sato J, Fujimoto K, Shiga Y, Kanamoto H, Yamauchi K, Nakamura J, Suzuki T, Hynes RA, Aoki Y, Takahashi K, Ohtori S. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery perspectives and indications from a retrospective, multicenter survey. Spine. 2017;42:55–62. doi: 10.1097/BRS.0000000000001650. [DOI] [PubMed] [Google Scholar]
- 23.Jin J, Ryu K, Hur J, Seong J, Kim J, Cho H. Comparative study of the difference of perioperative complication and radiologic results MIS-DLIF (minimally nvasive direct lateral lumbar interbody fusion) versus MIS-OLIF (minimally invasive oblique lateral lumbar interbody fusion) Clin Spine Surg. 2018;31:31–36. doi: 10.1097/BSD.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Z, Xu Z, He D, Zhao X, Ma W, Ni W, Song YX, Zhang JQ, Yu W, Fang XQ, Zhou ZJ, Xu NJ, Huang WJ, Hu ZC, Wu AL, Ji JF, Han JF, Fan SW, Zhao FD, Jin H, Pei F, Fan SY, Sui DX. Complications and prevention strategies of oblique lateral interbody fusion technique. Orthop Surg. 2018;10:98–106. doi: 10.1111/os.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods KRM, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine Journal. 2017;17:545–553. doi: 10.1016/j.spinee.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Miscusi M, Ramieri A, Forcato S, Giuffre M, Trungu S, Cimatti M, et al. Comparison of pure lateral and oblique lateral inter-body fusion for treatment of lumbar degenerative disk disease: a multicentric cohort study. Eur Spine J. 2018;27:222–228. doi: 10.1007/s00586-018-5596-y. [DOI] [PubMed] [Google Scholar]
- 27.Hayama S, Nakano A, Nakaya Y, Baba I, Fujiwara K, Fujishiro T, Yano T, Usami Y, Kino K, Obo T, Neo M. The evaluation of indirect neural decompression after lateral lumbar interbody fusion using intraoperative computed tomography myelogram. World Neurosurg. 2018;120:E718–e718. doi: 10.1016/j.wneu.2018.08.146. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs RE, Sembrano JN, Tohmeh AG, Degenerative Study Grp SOLAS. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part II: radiographic findings. Spine. 2016;41:S144. doi: 10.1097/BRS.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 29.Sembrano JN, Tohmeh A, Isaacs R, SOLAS Degenerative Study Group. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis part I: clinical findings spine 2016; 41:S132. doi: 10.1097/BRS.0000000000001471. [DOI] [PubMed]
- 30.Wang TY, Nayar G, Brown CR, Pimenta L, Karikari IO, Isaacs RE. Bony lateral recess stenosis and other radiographic predictors of failed indirect decompression via extreme lateral interbody fusion: multi-institutional analysis of 101 consecutive spinal levels. World Neurosurg. 2017;106:819–826. doi: 10.1016/j.wneu.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 31.Lang G, Perrech M, Navarro-Ramirez R, Hussain I, Pennicooke B, Maryam F, Avila MJ, Härtl R. Potential and limitations of neural decompression in extreme lateral interbody fusion-a systematic review. World Neurosurg. 2017;101:99–113. doi: 10.1016/j.wneu.2017.01.080. [DOI] [PubMed] [Google Scholar]
- 32.Navarro-Ramirez R, Lang G, Moriguchi Y, Elowitz E, Corredor JA, Avila MJ, Gotfryd A, Alimi M, Gandevia L, Härtl R. Are locked facets a contraindication for extreme lateral interbody fusion? World Neurosurg. 2017;100:607–618. doi: 10.1016/j.wneu.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Lee C, Chung S, Kang S, Park H, Kim S. The ideal cage position for achieving both indirect neural decompression and segmental angle restoration in lateral lumbar interbody fusion (LLIF) Clin Spine Surg. 2017;30:E790. doi: 10.1097/BSD.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 34.Sembrano JN, Horazdovsky RD, Sharma AK, Yson SC, Santos ERG, Polly DW, Jr. Do lordotic cages provide better segmental lordosis versus nonlordotic cages in lateral lumbar interbody fusion (LLIF)?. Clin Spine Surg. 2017; 30:E343. [DOI] [PubMed]
- 35.Lee TK, Yazdi JS, Floro KE, Arenos PT, Lee JR. Protection of the genitofemoral nerve using endoscopic assistance in minimally invasive lateral lumbar fusion. Interdiscip Neurosurg. 2017;8:4–7. doi: 10.1016/j.inat.2016.12.006. [DOI] [Google Scholar]
- 36.Turner JD, Akbarnia BA, Eastlack RK, Bagheri R, Nguyen S, Pimenta L, Marco R, Deviren V, Uribe J, Mundis GM. Radiographic outcomes of anterior column realignment for adult sagittal plane deformity: a multicenter analysis. Eur Spine J. 2015;24:427–432. doi: 10.1007/s00586-015-3842-0. [DOI] [PubMed] [Google Scholar]
- 37.Chung N, Lee H, Jeon C. Accuracy of the lateral cage placement under intraoperative C-arm fluoroscopy in oblique lateral interbody fusion. J Orthop Sci. 2018;23:918–922. doi: 10.1016/j.jos.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Peck JH, Kavlock KD, Showalter BL, Ferrell BM, Peck DG, Dmitriev AE. Mechanical performance of lumbar intervertebral body fusion devices: an analysis of data submitted to the Food and Drug Administration. J Biomech. 2018;78:87–93. doi: 10.1016/j.jbiomech.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Ma J, Park P, Huang X, Xie N, Ye X. Biomechanical comparison of multilevel lateral interbody fusion with and without supplementary instrumentation: a three-dimensional finite element study. BMC Musculoskelet Disord. 2017;18:63. doi: 10.1186/s12891-017-1387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tempel ZJ, McDowell MM, Panczykowski DM, Gandhoke GS, Hamilton DK, Okonkwo DO, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg -Spine. 2018;28:50–56. doi: 10.3171/2017.5.SPINE16427. [DOI] [PubMed] [Google Scholar]
- 41.Bocahut N, Audureau E, Poignard A, Delambre J, Queinnecc S, Lachaniette C-F, et al. Incidence and impact of implant subsidence after stand-alone lateral lumbar interbody fusion. Orthop Traumatol -Surg Res. 2018;104:405–410. doi: 10.1016/j.otsr.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. Clinical article J Neurosurg -Spine. 2013;19:110–118. doi: 10.3171/2013.4.SPINE12319. [DOI] [PubMed] [Google Scholar]
- 43.Teng I, Han J, Phan K, Mobbs R. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci. 2017;44:11–17. doi: 10.1016/j.jocn.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Malham GM, Parker RM, Blecher CM, Chow FY, Seex KA. Choice of approach does not affect clinical and radiologic outcomes: a comparative cohort of patients having anterior lumbar interbody fusion and patients having lateral lumbar interbody fusion at 24 months. Glob Spine J. 2016;6:472–481. doi: 10.1055/s-0035-1569055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reis MT, Reyes PM, Altun I, Newcomb AGUS, Singh V, Chang SW, et al. Biomechanical evaluation of lateral lumbar interbody fusion with secondary augmentation. J Neurosurg -Spine. 2016;25:720–726. doi: 10.3171/2016.4.SPINE151386. [DOI] [PubMed] [Google Scholar]
- 46.Fantini GA, Pawar AY. Access related complications during anterior exposure of the lumbar spine. World J Orthop. 2013;4:19–23. doi: 10.5312/wjo.v4.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan K, Maharaj M, Assem Y, Mobbs RJ. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF) J Clin Neurosci. 2016;31:23–29. doi: 10.1016/j.jocn.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 48.Beckman JM, Vincent B, Park MS, Billys JB, Isaacs RE, Pimenta L, Uribe JS. Contralateral psoas hematoma after minimally invasive, lateral retroperitoneal transpsoas lumbar interbody fusion: a multicenter review of 3950 lumbar levels. J Neurosurg -Spine. 2017;26:50–54. doi: 10.3171/2016.4.SPINE151040. [DOI] [PubMed] [Google Scholar]
- 49.Mobbs RJ, Phan K, Daly D, Rao PJ, Lennox A. Approach-related complications of anterior lumbar interbody fusion: results of a combined spine and vascular surgical team. Global spine journal. 2016;29:147–154. doi: 10.1055/s-0035-1557141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assina R, Majmundar NJ, Herschman Y, Heary RF. First report of major vascular injury due to lateral transpsoas approach leading to fatality: case report. J Neurosurg Spine. 2014;21:794–798. doi: 10.3171/2014.7.SPINE131146. [DOI] [PubMed] [Google Scholar]
- 51.Mai HT, Schneider AD, Alvarez AP, Hashmi SZ, Smith JT, Freshman RD, et al. Anatomic considerations in the lateral transpsoas interbody fusion: the impact of age, sex, BMI, and scoliosis. Clin Spine Surg. 2018. doi: 10.1097/BSD.0000000000000760 [doi]. [DOI] [PubMed]
- 52.Chang J, Kim J, Jo H. Ventral dural injury after oblique lumbar interbody fusion. World Neurosurg. 2017; 98:UNSP 881.e1s doi: 10.1016/j.wneu.2016.11.028 [DOI] [PubMed]
- 53.Gundanna M, Shah K. Delayed incisional hernia following minimally invasive trans-psoas lumbar spine surgery: report of a rare complication and management. Int J Spine Surg. 2018;12:126–130. doi: 10.14444/5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plata-Bello J, Roldan H, Brage L, Rahy A, Garcia-Marin V. Delayed abdominal pseudohernia in young patient after lateral lumbar interbody fusion procedure: case report. World Neurosurg. 2016; 91:UNSP 671.e13. doi: 10.1016/j.wneu.2016.04.010. [DOI] [PubMed]
- 55.Chin KR, Pencle FJR, Coombs AV, Brown MD, Conklin KJ, O'Neill AM, et al. Lateral lumbar interbody fusion in ambulatory surgery centers: patient selection and outcome measures compared with an Inhospital cohort. Spine. 2016;41:686–692. doi: 10.1097/BRS.0000000000001285. [DOI] [PubMed] [Google Scholar]


