Abstract
Purpose of Review
Minimally invasive approaches to adult spinal deformity (ASD) surgery have seen a large increase in popularity over the last decade, largely because these techniques are viewed as a potential improvement to the lengthy recovery and high complication rates observed after traditional open surgery for this pathology. The purpose of this review is to present a summary of the latest minimally invasive techniques used in adult spinal deformity surgery, examine whether MIS surgery can accomplish the goals of ASD surgery, and investigate whether MIS surgery is safer than traditional approaches.
Recent Findings
While minimally invasive approaches have been able to achieve similar patient-reported outcomes as open approaches, they are associated with their own unique complications. Furthermore, they are limited in their ability to correct severe sagittal imbalance. Emerging techniques, such as anterior column realignment and mini-open posterior column osteotomy, have been developed to address these limitations. The minimally invasive spinal deformity surgery algorithm (MISDEF) can help guide surgeons on which approaches may be appropriate for a particular case.
Summary
To maximize the benefits of a minimally invasive approach without compromising the goals of ASD surgery, surgeons must be selective in choosing which cases are amenable to an MIS approach. Leading experts continue to develop algorithms to guide surgical decision-making. As we learn to better define our indications, understand treatment goals, and refine our techniques, MIS approaches will likely play an even larger role in a comprehensive ASD treatment strategy.
Keywords: Adult spinal deformity, Adult scoliosis, Minimally invasive spine surgery, Deformity algorithm
Introduction
Minimally invasive spine surgery has seen an enormous expansion in popularity over the last decade. Originally utilized for microdiscectomy and decompression, applications have expanded to include multilevel degenerative disc disease, cervical pathology, trauma, and correction of spinal deformity [1]. Minimally invasive surgery (MIS) is based on the concept of accomplishing the same surgical goals while minimizing dissection or utilizing smaller operative corridors. Theoretically, such an approach limits the composite trauma of surgery, leading to a reduction in complications and allowing for a quicker patient recovery. With regard to surgical management of adult spinal deformity (ASD), surgical goals include restoration of sagittal and coronal balance, construction of a solid fusion foundation, and decompression of the neural elements. When considering the morbidity of long-segment open fusions traditionally utilized in this patient population [2–6], the potential of a minimally invasive approach can appeal to both patients and surgeons alike. However, given that extensive tissue dissection and release has been traditionally indispensable to achieve ASD goals especially with regard to restoration of alignment, a minimally invasive approach inherently seems problematic. Thus, initial research has focused on proving the feasibility of new minimally invasive techniques—such as lateral lumbar interbody fusion (LLIF), percutaneous pedicle screws, and minimally invasive transforaminal interbody fusion (MIS-TLIF)—and subsequent applications in adult spinal deformity [7].
Initial feasibility studies showed that minimally invasive techniques could achieve acceptable patient-reported outcomes in the setting of ASD [7], However, as follow-up times have increased and more surgeons adopt minimally invasive techniques, questions remain whether the goals of ASD surgery are being met. While the majority of investigations have consistently reported similar clinical improvements in disability and pain relief up to 2 years, many studies have cast doubt on whether minimally invasive surgery can achieve the same alignment goals as open techniques [7–10]. Furthermore, for ASD correction, are the goals of minimally invasive surgery (decreased complications, quicker recovery) being realized? Finally, multiple investigations have even questioned whether MIS approaches have proven any less morbid [7, 9, 11•, 12]. Thus, determining which types of ASD are amenable to an MIS approach is the key question that researchers currently face [13].
In the following review, we will present a summary of the latest minimally invasive techniques used in adult spinal deformity surgery, examine the key controversies, and conclude by summarizing the minimally invasive spinal deformity surgery algorithm (MISDEF), a recently developed surgical decision-making tool [13].
Overview of Currently Utilized MIS Techniques
Radiographically, adult spinal deformity encompasses patients > 18 years old with a coronal Cobb angle greater than 20 degrees, a sagittal vertebral axis (SVA) greater than 5 cm, or a pelvic tilt (PT) greater than 20 degrees [14]. The vast majority of deformity in these patients is present in the lumbar spine. Clinically, patients may present with back pain, radiculopathy, neurogenic claudication, or progressive deformity. Surgical management is thus focused on alleviating the patient’s primary complaint while avoiding iatrogenic destabilization. MIS approaches can be classified into three broad categories: MIS decompression (direct or indirect), multilevel circumferential MIS surgery (cMIS), and hybrid surgery (combination of MIS and traditional posterior open approach) [13, 14, 15••]. For the patient with mild deformity in whom direct decompression is necessary, MIS approaches may mitigate the risk of iatrogenic destabilization by avoiding destruction of the posterior ligamentous structures. On the other hand, indirect foraminal decompression can be achieved through standalone interbody cage placement. For those patients who require fusion at multiple levels to correct alignment parameters, circumferential MIS (cMIS) surgery generally is comprised of multilevel interbody cages, with posterior instrumentation introduced through percutaneous methods, either as a single- or two-stage procedure. Lastly, hybrid surgeries incorporate both minimally invasive and traditional surgical approaches. This approach is often utilized when an open posterior approach is necessary to allow for decompression or osteotomies. Hybrid surgeries are often performed in a two-stage fashion, with the first stage consisting of interbody cage placement and the second stage consisting of open posterior instrumentation with or without osteotomy and/or decompression.
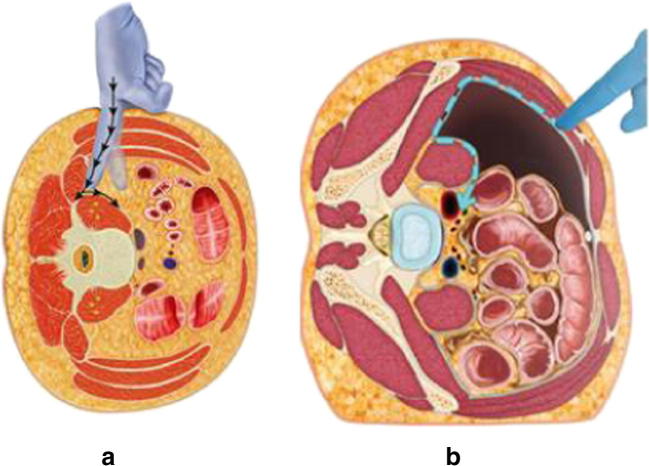
The type of approach utilized for the introduction of interbody cages is widely dependent on the surgeon and institution, as almost all approaches may be performed in a minimally invasive fashion. For example, anterior lumbar interbody fusion (ALIF) can be performed while obeying the principles of minimally invasive surgery. The lateral transpsoas approach (lateral lumbar interbody fusion [LLIF], extreme lateral interbody fusion [XLIF], direct lateral interbody fusion [DLIF]) has gained much popularity over the last decade as it avoids the morbidity associated with presacral dissection [16]. Many current minimally invasive spine surgeons rely on LLIF as their primary interbody approach, largely because it can be utilized at multiple levels in the lumbar and thoracic spine and allows for the insertion of a cage with a large footprint. However, the anatomy of the pelvis typically prohibits its use at the L5-S1 level. To overcome this limitation, the oblique lumbar interbody fusion technique (OLIF) was developed to allow access to the L5-S1 level while maintaining the advantages of a retroperitoneal dissection (Fig. 1) [17]. Transforaminal lumbar interbody fusions (TLIF) may be performed in a minimally invasive manner at all levels in the lumbar spine, also serving as an option to address the L5-S1 level [18]. Lastly, axial lumbar interbody fusion (AxiaLIF) has been utilized in adult deformity for L5-S1 or L4-S1 interbody fusions, primarily as a method to supplement S1 pedicle screws [19].
Fig. 1.
Illustrations comparing the LLIF (a) and OLIF (b) approaches. Note how the OLIF maintains a retroperitoneal blunt dissection at the L5-S1 level
Sagittal Balance Goals and Emerging MIS Techniques
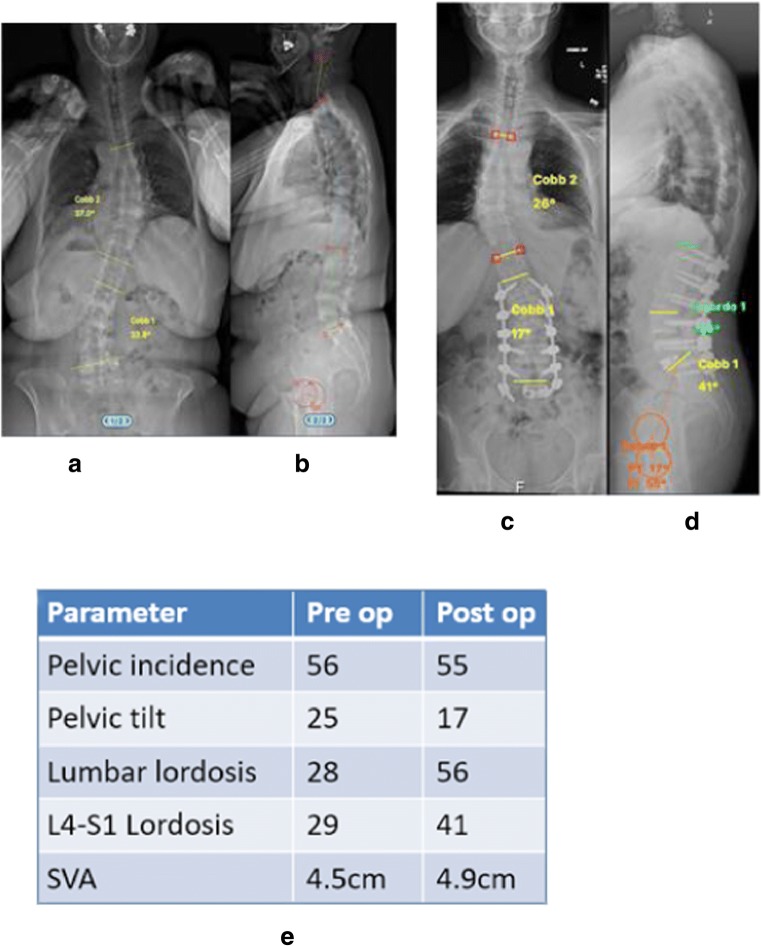
One of the most important aspects in the surgical management of adult spinal deformity is reestablishing global sagittal and coronal balance, as these parameters have been found to closely correlate with overall disability and patient-reported outcomes [20, 21]. Malalignment is generally defined as SVA > 5 cm or pelvic incidence-lumbar lordosis (PI-LL) mismatch of > 10 degrees [10, 22]. The most recently published studies on this topic demonstrate that patients with ASD who underwent MIS approaches are often left malaligned [8, 10, 23, 24••]. The ability of MIS-only approaches to achieve meaningful corrections was demonstrated in a retrospective review of 184 patients who underwent cMIS, hybrid, or open surgery for ASD [25]. While all groups demonstrated clinical improvements at 1 year, the SVA correction in the MIS group was − 0.3 mm, compared with − 33 mm in the open group (p < 0.001). The MIS group was left with an average PI-LL of 16 degrees, compared to 2.1 and 2.0 degrees in the hybrid and open groups, respectively. Similarly, Park et al. retrospectively reviewed 2-year radiographic and clinical outcomes for 185 ASD patients treated through cMIS or hybrid approaches, finding that 66.4% of patients were malaligned at 2 years. The correlation between malalignment and worse clinical outcomes [20] has also been demonstrated after MIS ASD surgery [24••]. In a retrospective review of 104 patients who underwent cMIS or hybrid surgery, postoperative sagittal alignment parameters of patients who experienced the greatest improvements in the Oswestry Disability Index (ODI) were compared to those who had the least improvement [24••]. The authors found that postoperative PI-LL mismatch and failure to correct SVA were associated with the least improvement after surgery. Lastly, in an analysis of 90 patients who underwent cMIS surgery for AD, Anand et al. demonstrated a “ceiling effect” of cMIS surgery [8]. The authors suggested that cMIS techniques were able to correct SVA only up to 89 mm, and that PI-LL mismatch could only be corrected in cases in which the preoperative PI-LL was 38 degrees or less (Fig. 2).
Fig. 2.
Case example of a patient with degenerative lumbar scoliosis who underwent correction with a cMIS approach. a, b Preoperative radiographs. c, d Postoperative radiographs. e Changes in radiographic parameters. Note that a cMIS approach was appropriate for this patient given the small preoperative SVA
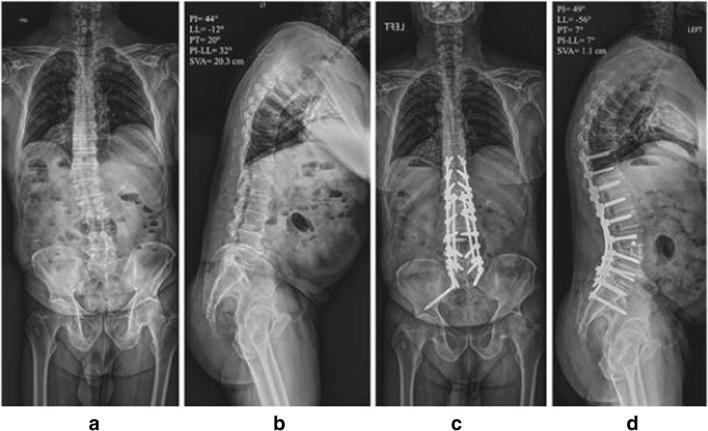
This limitation of MIS in correcting severe sagittal deformity has prompted the development of new MIS techniques over the last few years [26•, 27•]. Anterior column realignment (ACR) is identical to an LLIF approach but adds an anterior longitudinal ligament release to allow for the insertion of a hyperlordotic cage (usually 20 or 30 degrees) (Fig. 3). The appropriately sized cage is selected in order to allow for indirect foraminal decompression while maximizing lordosis, and a screw is often placed in order to avoid anterior displacement of the cage [28]. An initial series of eight patients reported segmental changes in lumbar lordosis of approximately 13–15 degrees [28]. Proponents of the technique argue that radiographic results are similar to that of a pedicle subtraction osteotomy. Utilizing an adult spinal deformity database, 17 patients who underwent ACR were propensity matched by pelvic incidence, lumbar lordosis, and thoracic kyphosis to a cohort of patients who underwent pedicle subtraction osteotomy (PSO). Though widespread conclusions were limited by the small patient sample, changes in radiographic outcomes were largely similar at 1 year [26•].
Fig. 3.
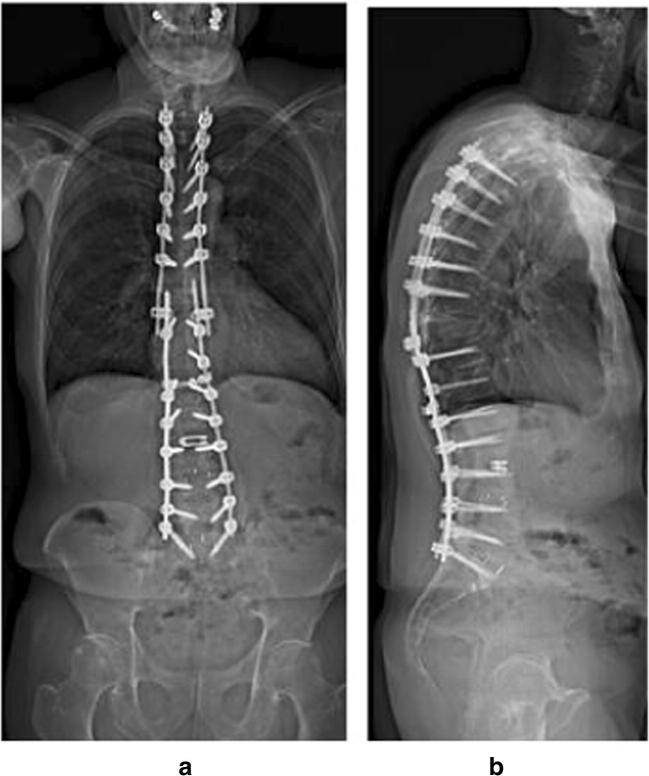
Case example of the use of anterior column realignment (ACR) to address adult spinal deformity. a, b preoperative radiographs. c, d Postoperative anteroposterior x-ray with L3–4 ACR and T10-pelvic posterior fusion. Note the high degree of lumbar lordosis created by the ACR
Expandable interbody cages offer another solution to address the sagittal alignment limitations of LLIF or TLIF (Fig. 4). Though expandable interbody cages have yet to be studied en masse in the setting of adult spinal deformity, investigators have published several series on their use in degenerative disc disease [29–31]. A small series of single-level MIS-TLIF with expandable cages demonstrated higher segmental lordosis compared to static cages, but neither technique affected the overall spinopelvic parameters [31]. On the other hand, a retrospective cohort study of 89 one-level TLIFs showed near identical changes in segmental and lumbar lordosis at 1 year, independent of the type of cage used [30]. Cadaveric studies have demonstrated higher contact forces found with expandable technology, raising concerns about cage subsidence, which has also been reported clinically [29, 32].
Fig. 4.
cMIS approach using multilevel LLIF at L1-L5 and MIS-TLIF at L5-S1 with an expandable cage to induce lordosis at the L5-S1 level. a, b Preoperative radiographs. c, d Intraoperative fluoroscopy
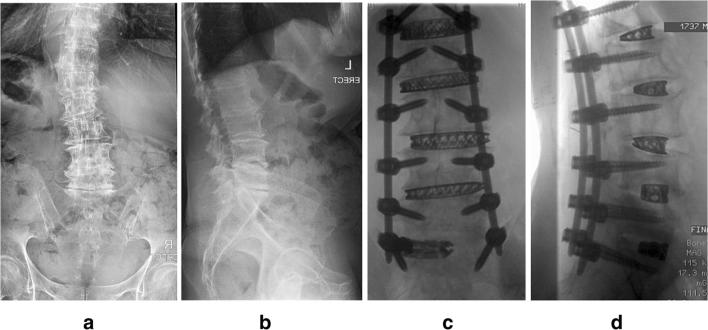
Lastly, investigators have developed ways to perform posterior osteotomies through smaller operative corridors. Originally performed in 2012, surgeons at the University of Miami developed a “mini-open” pedicle subtraction osteotomy (PSO) [33]. Pedicle screws are placed in a suprafascial or percutaneous technique at least three levels above and below the osteotomy site, and two rods are then placed proximal and distal to the osteotomy. Periosteal dissection is only performed at the level of the planned osteotomy. The osteotomy is then reduced through a cantilever technique and the two rods on either side are connected (Fig. 5). A recent case series in 16 patients demonstrated that the technique could achieve early radiographic results similar to open PSO [27•]. However, also similar to contemporary analyses of open PSO, high complication rates were observed (37.5%) [34, 35].
Fig. 5.
Radiographs of a four-rod cantilever technique to reduce kyphosis (a, b). The mini-open PSO technique utilizes a similar cantilever technique to reduce the osteotomy site
In conclusion, when compared to LLIF or MIS-TLIF, these new MIS techniques show promise in their ability to allow for larger corrections of sagittal deformity, but their inherently steep learning curves require longer-term follow up and wider adoption before conclusions can be made.
Comparing Complication Rates in MIS and Open Surgery
Traditional open techniques for surgical management of adult deformity have been associated with high rates of complications [2–6]. Major perioperative complications lead to increased patient disability as long as 1 year after surgery [4]. Even more concerning, an epidemiological analysis of adult deformity surgery reported an increase in inpatient complications from 2003 to 2010 [3]. The surgical trauma induced by extensive open posterior dissection also leads to a difficult recovery, with many patients staying in the hospital for a week or more [36, 37]. High rates of complications and prolonged hospital stays are linked, as lengthy recoveries put patients at risk for medical complications such as pneumonia, sepsis, and venous thromboembolism. Whether MIS surgery will serve as a panacea for these problems is still unclear.
The majority of studies have concluded that complication rates and length of stay for MIS approaches are similar to those of open approaches [7, 9, 11•, 12]. Bach et al. published a systematic review of 13 case series on outcomes after minimally invasive surgery for adult deformity [7]. The authors found that rates of complications after MIS surgery for ASD ranged from 14.3% to 87.5%. Out of the 258 patients included in the reviewed studies, the aggregate rate of complications was 46%, akin to previously reported rates in open surgery [2–6]. The most commonly reported complication was neurologic (14%), likely secondary to lumbar plexopathies unique to the transpsoas approach. The average length of stay was widely variable, ranging from 2.8 to 10 days, with two-stage surgeries falling on the higher end of the spectrum. A more recent meta-analysis specifically examined rates of complications after adult deformity surgery, comparing open, hybrid, and minimally invasive approaches [11•]. Perioperative major complication rates were comparable: 10.9% (95% confidence interval [CI 95%] 10.3–11.5) open surgery, 9.0% (CI 95% 6.2–12.8) MIS, and 14.7% (CI 95% 11.6–17.9) hybrid, p > 0.05. However, rates of overall complications (including minor perioperative and late complications) were found to be higher in hybrid surgeries compared to both open and MIS approaches 37.5% (CI 95% 36.7–38.4) open, 38.2% (CI 95% 32.9–43.8) MIS, and 51.8% (CI 95% 47.7–55.9) hybrid (p < 0.05 hybrid compared to MIS and open). The authors hypothesize that hybrid surgeries combine the morbidity of a lateral MIS and an open posterior approach, a theory that has been borne out in two retrospective cohort studies [9, 12] but contrasted by another [38]. Park et al. reported a complication rate of 55% in hybrid surgery, compared to 33% with a cMIS approach [9]. Similarly, in an analysis of patients who underwent open posterior fusion with L5-S1 TLIF, the addition of multilevel LIF increased major complication rates from 13% to 56% [12]. These two studies reported an average LOS of 8.6 and 9.2 days, similar to open procedures [36, 37].
One of the most problematic late complications after correction of sagittal spinal deformity is proximal junctional kyphosis (PJK). Proponents of MIS approaches argue that preservation of the posterior ligamentous structures may lead to a reduced incidence of (PJK). Unfortunately, the literature has not supported this hypothesis, instead reaffirming that PJK is likely related to the degree of correction more than surgical technique [39••, 40]. In a retrospective cohort analysis, 68 ASD patients who underwent LLIF with percutaneous posterior instrumentation were propensity score matched to patients who underwent LLIF with open posterior instrumentation [41]. In patients who had the same number of instrumented levels, rates of PJK were almost identical: 48.1% cMIS vs. 53.8%, p = 0.68. An analysis of rates of PJK after LLIF was also in line with previously reported rates after open surgery [42•]. Rates of PJK increased sequentially with the amount of correction, with patients who underwent ACR + PSO experiencing the highest rate of PJK (42.9% ACR + PSO vs. 30% ACR vs. 0% LLIF). Park et al. recently published 2-year radiographic results evaluating the effects of adding LLIF to open posterior spinal fusion for ASD [38]. Patients who underwent three-column osteotomies were excluded. The hybrid surgery cohort had a larger correction of lumbar lordosis and greater rate of PJK or PJA at 2 years (p < 0.05).
The MISDEF Algorithm
As we move forward in assessing the role of MIS surgery in ASD, patient selection will be the most important factor. Recent research has been dedicated to determine which ASD patients could derive the most benefit from an MIS approach and others from open surgery. Because multiple clinical studies have shown that a cMIS approach is unable to adequately correct a severe, rigid sagittal deformity, compromising sagittal alignment goals for reduced blood loss and a potentially quick recovery does not provide an overall benefit for this type of patient. On the other hand, in a patient with a mild deformity, the benefit of a cMIS approach may be realized, as alignment goals are more easily achieved. This may be particularly relevant for elderly patients, given that they do not require as much correction in order to obtain functionally similar results [10].
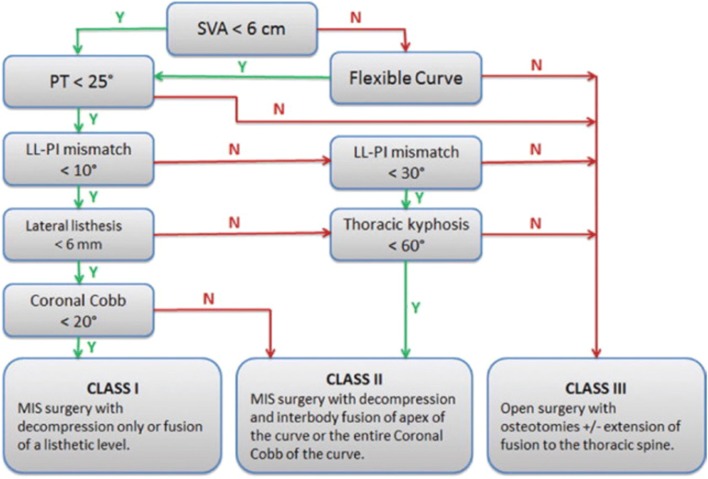
The MISDEF algorithm, first published by Mummaneni et al. in 2014, is a framework that surgeons can use to help guide their decision-making [13]. The branch points of the algorithm are based on preoperative spinopelvic parameters (SVA, PT, PI-LL mismatch, and thoracic kyphosis) and flexibility of the curve, dividing adult deformity into three treatment-based classes (Fig. 6). Of note, the algorithm does not take into account the patient’s health status or age-adjusted alignment goals. The class I approach is reserved for mild cases in which main pathology can be relieved with decompression, and thus includes direct or indirect MIS decompression. The class II approach addresses patients who suffer from stenosis/radiculopathy as well as deformity-induced back pain. This class includes multilevel surgeries, with fusion of multiple levels through exclusively MIS techniques (Figs. 2 and 4). Lastly, class III approaches are utilized for the most complex or severe deformities (especially those requiring long thoracolumbar fusions) and require open surgery. Eleven experienced spinal deformity surgeons were surveyed at two time points at least 2 months apart to validate the algorithm [13]. The surgeons reviewed 20 representative cases of adult patients with symptomatic deformities who had failed non-operative therapies. The algorithm was found to show moderate to substantial agreement, with an interobserver kappa of 0.58 and 0.69 in the first and second round surveys, respectively.
Fig. 6.
Original iteration of the MISDEF algorithm. LL, lumbar lordosis; N, no; PI, pelvic incidence; PT, pelvic tilt; SVA, sagittal vertical axis; Y, yes. (From Choy W, Miller CA, Chan AK, Fu KM, Park P, Mummaneni P V. Evolution of the Minimally Invasive Spinal Deformity Surgery Algorithm: An Evidence-Based Approach to Surgical Strategies for Deformity Correction. Neurosurg Clin N Am 2018;29:399–406; with permission)
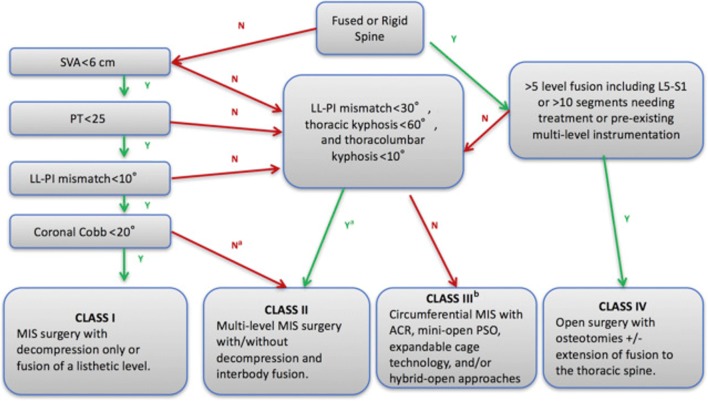
More effective techniques for the correction of sagittal alignment have been recently developed, which has prompted an update to the algorithm by some of the original authors [15]. The updated version takes into account new techniques such as ACR, mini-open PSO, and expandable cage technology. While the algorithm’s branch points still rely on the same original parameters, it incorporates if the spine is fused or rigid, a change from the first iteration, which asks about the SVA measurement. Most importantly, in the new version, a fourth treatment class is introduced for severe, non-rigid deformities which fall between the original class II and III (Fig. 7). In this treatment class, cMIS with ACR, mini-open PSO, expandable cages, or hybrid approaches are considered as an option to open surgery (Fig. 3). The authors make a point to note that such treatment strategies should be limited to those experienced with these techniques. The updated algorithm is currently being validated through similar methods as the original.
Fig. 7.
Revision of the MISDEF algorithm. ACR, anterior column realignment; N, no; PSO, pedicle subtraction osteotomy; Y, yes. a For curved greater than 60, double major curves, consider class III. b For experienced MIS surgeons. (From Choy W, Miller CA, Chan AK, Fu KM, Park P, Mummaneni P V. Evolution of the Minimally Invasive Spinal Deformity Surgery Algorithm: An Evidence-Based Approach to Surgical Strategies for Deformity Correction. Neurosurg Clin N Am 2018;29:399–406; with permission)
Conclusions
Akin to the introduction of many novel technologies, MIS approaches for adult deformity surgery have undergone much scrutiny for their applications, effect on clinical outcomes, and establishment of unique complications and limitations. New techniques are constantly being developed to overcome these barriers and push the limits of what an MIS approach can achieve, but wider adoption will be necessary before definitive conclusions can be made on their efficacy. A consistent theme supported by the most recent literature, and one that will continue to be true, is that not all ASD patients are candidates for an MIS approach. A new generation of surgeons is currently being trained to learn minimally invasive techniques as a standard component of their armamentarium. Thus, as we learn to better define our indications, understand treatment goals, and refine our techniques, MIS approaches will only play a larger role in a comprehensive ASD treatment strategy.
Funding Statement
No funding was received for this study.
Compliance with Ethical Standards
Human and Animal Rights Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Minimally Invasive Spine Surgery
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francis Lovecchio, Email: lovecchiof@HSS.EDU.
Sheeraz A. Qureshi, Phone: 212-606-1585, Email: qureshis@HSS.EDU
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9. doi: 10.3171/2009.7.FOCUS09121. [DOI] [PubMed] [Google Scholar]
- 2.Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976) 2007;32:2238–2244. doi: 10.1097/BRS.0b013e31814cf24a. [DOI] [PubMed] [Google Scholar]
- 3.Passias PG, Jalai CM, Worley N, Vira S, Marascalchi B, McClelland S, et al. Adult spinal deformity: national trends in the presentation, treatment, and perioperative outcomes from 2003 to 2010. Spine Deform. 2017;5:342–350. doi: 10.1016/j.jspd.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Glassman SD, Hamill CL, Bridwell KH, Schwab FJ, Dimar JR, Lowe TG. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976) 2007;32:2764–2770. doi: 10.1097/BRS.0b013e31815a7644. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Iyer S, Zebala LP, Kelly MP, Sciubba D, Protopsaltis TS, Gupta M, Neuman BJ, Mundis GM, Ames CP, Smith JS, Hart R, Burton D, Klineberg EO, International Spine Study Group (ISSG) Perioperative neurologic complications in adult spinal deformity surgery. Spine (Phila Pa 1976) 2017;42:420–427. doi: 10.1097/BRS.0000000000001774. [DOI] [PubMed] [Google Scholar]
- 6.Soroceanu A, Burton DC, Oren JH, Smith JS, Hostin R, Shaffrey CI, Akbarnia BA, Ames CP, Errico TJ, Bess S, Gupta MC, Deviren V, Schwab FJ, Lafage V, International Spine Study Group Medical complications after adult spinal deformity surgery. Spine (Phila Pa 1976) [Internet] 2016;41:1718–1723. doi: 10.1097/BRS.0000000000001636. [DOI] [PubMed] [Google Scholar]
- 7.Bach K, Ahmadian A, Deukmedjian A, Uribe JS. Minimally invasive surgical techniques in adult degenerative spinal deformity: a systematic review. Clin Orthop Relat Res. 2014;472:1749–1761. doi: 10.1007/s11999-013-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand N, Baron EM, Khandehroo B. Limitations and ceiling effects with circumferential minimally invasive correction techniques for adult scoliosis: analysis of radiological outcomes over a 7-year experience. Neurosurg Focus. 2014;36:E14. doi: 10.3171/2014.3.FOCUS13585. [DOI] [PubMed] [Google Scholar]
- 9.Park P, Wang MY, Lafage V, Nguyen S, Ziewacz J, Okonkwo DO, Uribe JS, Eastlack RK, Anand N, Haque R, Fessler RG, Kanter AS, Deviren V, la Marca F, Smith JS, Shaffrey CI, Mundis GM Jr, Mummaneni PV, International Spine Study Group Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J Neurosurg Spine. 2015;22:374–380. doi: 10.3171/2014.9.SPINE131004. [DOI] [PubMed] [Google Scholar]
- 10.Park P, Fu K, Mummaneni PV, Uribe JS, Wang MY, Tran S, et al. The impact of age on surgical goals for spinopelvic alignment in minimally invasive surgery for adult spinal deformity. J Neurosurg Spine. 2018;29:560–564. doi: 10.3171/2018.4.SPINE171153. [DOI] [PubMed] [Google Scholar]
- 11.Zanirato A, Damilano M, Formica M, Piazzolla A, Lovi A, Villafañe JH, Berjano P. Complications in adult spine deformity surgery: a systematic review of the recent literature with reporting of aggregated incidences. Eur Spine J. 2018;27:2272–2284. doi: 10.1007/s00586-018-5535-y. [DOI] [PubMed] [Google Scholar]
- 12.Theologis AA, Jr GMM, Nguyen S, Okonkwo DO, Mummaneni PV, Smith JS, et al. Utility of multilevel lateral interbody fusion of the thoracolumbar coronal curve apex in adult deformity surgery in combination with open posterior instrumentation and L5–S1 interbody fusion: a case-matched evaluation of 32 patients. J Neurosurg Spine. 2017;26:208–219. doi: 10.3171/2016.8.SPINE151543. [DOI] [PubMed] [Google Scholar]
- 13.Mummaneni PV, Shaffrey CI, Lenke LG, Park P, Wang MY, La Marca F, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus. 2014;36:E6. doi: 10.3171/2014.3.FOCUS1413. [DOI] [PubMed] [Google Scholar]
- 14.Kanter AS, Tempel ZJ, Ozpinar A, Okonkwo DO. A review of minimally invasive procedures for the treatment of adult spinal deformity. Spine (Phila Pa 1976) 2016;41:s59–s65. doi: 10.1097/BRS.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 15.Choy W, Miller CA, Chan AK, Fu KM, Park P, Mummaneni PV. Evolution of the minimally invasive spinal deformity surgery algorithm: an evidence-based approach to surgical strategies for deformity correction. Neurosurg Clin N Am [Internet]. Elsevier Inc. 2018;29:399–406. doi: 10.1016/j.nec.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6(2):89–97. doi: 10.4184/asj.2012.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karikari IO, Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion: a review of techniques and outcomes. Spine (Phila Pa 1976) 2010;35(26 Suppl):S294–S301. doi: 10.1097/BRS.0b013e3182022ddc. [DOI] [PubMed] [Google Scholar]
- 19.Boachie-Adjei O, Cho W, King AB. Axial lumbar interbody fusion (AxiaLIF) approach for adult scoliosis. Eur Spine J. 2013;22(Suppl 2):S225–S231. doi: 10.1007/s00586-012-2351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab FJ, Blondel B, Bess S, Hostin R, Shaffrey CI, Smith JS, Boachie-Adjei O, Burton DC, Akbarnia BA, Mundis GM, Ames CP, Kebaish K, Hart RA, Farcy JP, Lafage V, International Spine Study Group (ISSG) Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine (Phila Pa 1976) 2013;38:E803–E812. doi: 10.1097/BRS.0b013e318292b7b9. [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, Klineberg E, Schwab F, Shaffrey CI, Moal B, Ames CP, Hostin R, Fu KMG, Burton D, Akbarnia B, Gupta M, Hart R, Bess S, Lafage V, International Spine Study Group Change in classification grade by the SRS-Schwab adult spinal deformity classification predicts impact on health-related quality of life measures. Spine (Phila Pa 1976) 2013;38:1663–1671. doi: 10.1097/BRS.0b013e31829ec563. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Shaffrey CI, Bess S, Shamji MF, Brodke D, Lenke LG, et al. Recent and emerging advances in spinal deformity. Clin Neurosurg. 2017;80:S77–S85. doi: 10.1093/neuros/nyx028. [DOI] [PubMed] [Google Scholar]
- 23.Mundis GM, Turner JD, Deverin V, Uribe JS, Nunley P, Mummaneni P, et al. A critical analysis of sagittal plane deformity correction with minimally invasive adult spinal deformity surgery: a 2-year follow-up study. Spine Deform. 2017;5:265–271. doi: 10.1016/j.jspd.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Than KD, Park P, Fu K, Nguyen S, Wang MY, Chou D, et al. Clinical and radiographic parameters associated with best versus worst worst clinical outcomes in minimally invasive spinal deformity surgery. J Neurosurg Spine. 2016;25:1–5. doi: 10.3171/2015.11.SPINE151036. [DOI] [PubMed] [Google Scholar]
- 25.Haque RM, Mundis GM, Ahmed Y, El Ahmadieh TY, Wang MY, Mummaneni PV, et al. Comparison of radiographic results after minimally invasive, hybrid, and open surgery for adult spinal deformity: a multicenter study of 184 patients. Neurosurg Focus. 2014;36:E13. doi: 10.3171/2014.3.FOCUS1424. [DOI] [PubMed] [Google Scholar]
- 26.Mundis GM, Turner JD, Kabirian N, Pawelek J, Eastlack RK, Uribe J, et al. Anterior column realignment has similar results to pedicle subtraction osteotomy in treating adults with sagittal plane deformity. World Neurosurg. 2017;105:249–256. doi: 10.1016/j.wneu.2017.05.122. [DOI] [PubMed] [Google Scholar]
- 27.Wang MY, Bordon G. Mini-open pedicle subtraction osteotomy as a treatment for severe adult spinal deformities: case series with initial clinical and radiographic outcomes. J Neurosurg Spine. 2016;24:769–776. doi: 10.3171/2015.7.SPINE15188. [DOI] [PubMed] [Google Scholar]
- 28.Demikiran G, Theologis AA, Pekmezci M, Ames C, Deviren V. Adult spinal deformity correction with multi-level anterior column releases. Clin Spine Surg. 2016;29:141–149. doi: 10.1097/BSD.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 29.Massie LW, Zakaria HM, Schultz LR, Basheer A, Buraimoh MA, Chang V. Assessment of radiographic and clinical outcomes of an articulating expandable interbody cage in minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis. Neurosurg Focus. 2018;44(1):E8. doi: 10.3171/2017.10.FOCUS17562. [DOI] [PubMed] [Google Scholar]
- 30.Yee TJ, Joseph JR, Terman SW, Park P. Expandable vs static cages in transforaminal lumbar interbody fusion: radiographic comparison of segmental and lumbar sagittal angles. Neurosurgery. 2017;81(1):69–74. doi: 10.1093/neuros/nyw177. [DOI] [PubMed] [Google Scholar]
- 31.Hawasli AH, Khalifeh JM, Chatrath A, Yarbrough CK, Ray WZ. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus. 2017;43(2):E10. doi: 10.3171/2017.5.FOCUS17197. [DOI] [PubMed] [Google Scholar]
- 32.Pekmezci M, Tang JA, Cheng L, Modak A, McClellan RT, Buckley JM, et al. Comparison of expandable and fixed interbody cages in a human cadaver corpectomy model: fatigue characteristics. Clin Spine Surg. 2016;29(9):387–393. doi: 10.1097/BSD.0b013e31826eb0f7. [DOI] [PubMed] [Google Scholar]
- 33.Wang MY, Madhavan K. Mini-open pedicle subtraction osteotomy: surgical technique. World Neurosurg. 2014;81(5–6):843.e11–843.e14. doi: 10.1016/j.wneu.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Daubs MD, Brodke DS, Annis P, Lawrence BD. Perioperative complications of pedicle subtraction osteotomy. Glob Spine J. 2016;6:630–635. doi: 10.1055/s-0035-1570088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho SK, Bridwell KH, Lenke LG, Yi J-S, Pahys JM, Zebala LP, Kang MM, Cho W, Baldus CR. Major complications in revision adult deformity surgery. Spine (Phila Pa 1976) 2012;37:489–500. doi: 10.1097/BRS.0b013e3182217ab5. [DOI] [PubMed] [Google Scholar]
- 36.Klineberg EO, Passias PG, Jalai CM, Worley N, Sciubba DM, Burton DC, Gupta MC, Soroceanu A, Zebala LP, Mundis GM, Jr, Kim HJ, Hamilton DK, Hart RA, Ames CP, Lafage V. Predicting extended length of hospital stay in an adult spinal deformity surgical population. Spine (Phila Pa 1976) 2016;41:E798–E805. doi: 10.1097/BRS.0000000000001391. [DOI] [PubMed] [Google Scholar]
- 37.Pitter FT, Lindberg-Larsen M, Pedersen AB, Dahl B, Gehrchen M. Readmissions, length of stay and mortality after primary surgery for adult spinal deformity. Spine (Phila Pa 1976) 2019;44(2):E107–E116. doi: 10.1097/BRS.0000000000002782. [DOI] [PubMed] [Google Scholar]
- 38.Park HY, Ha KY, Kim YH, Chang DG, Kim SI, Lee JW, et al. Minimally invasive lateral lumbar interbody fusion for adult spinal deformity. Spine (Phila Pa 1976) 2018;43:E813–E821. doi: 10.1097/BRS.0000000000002507. [DOI] [PubMed] [Google Scholar]
- 39.Lafage R, Schwab F, Glassman S, Bess S, Harris B, Sheer J, Hart R, Line B, Henry J, Burton D, Kim H, Klineberg E, Ames C, Lafage V, International Spine Study Group Age-adjusted alignment goals have the potential to reduce PJK. Spine (Phila Pa 1976) 2017;42:1275–1282. doi: 10.1097/BRS.0000000000002146. [DOI] [PubMed] [Google Scholar]
- 40.Liu FY, Wang T, Yang SD, Wang H, Yang DL, Ding WY. Incidence and risk factors for proximal junctional kyphosis: a meta-analysis. Eur Spine J. 2016;25:2376–2383. doi: 10.1007/s00586-016-4534-0. [DOI] [PubMed] [Google Scholar]
- 41.Mummaneni PV, Park P, Fu KM, Wang MY, Nguyen S, Lafage V, et al. Does minimally invasive percutaneous posterior instrumentation reduce risk of proximal junctional kyphosis in adult spinal deformity surgery? A propensity-matched cohort analysis. Neurosurgery. 2015;78:101–108. doi: 10.1227/NEU.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 42.• Gandhi S V, Januszewski J, Bach K, Graham R, Vivas AC, Paluzzi J, et al. Development of proximal junctional kyphosis after minimally invasive lateral anterior column realignment for adult spinal deformity. Neurosurgery 2018. Epub ahead of print. 10.1093/neuros/nyy061. Analysis suggesting that the degree of correction is the most important known factor in the genesis of PJK, and that preservation of the posterior elements alone does not prevent PJK. [DOI] [PubMed]