Abstract
The present study reports the optimized production and purification of an extremely active fibrinolytic enzyme from newly isolated marine bacterium Fictibacillus sp. strain SKA27, with a specific activity of 125,107.85 U/mg and an apparent molecular weight of 28 kDa on SDS-PAGE. Wheat bran extract used for submerged production proved to be highly beneficial and enhanced fibrinolytic enzyme production when combined with yeast extract and CaCl2. Optimization of culture media by response surface methodology (RSM) resulted in high root mean square error (RMSE), which led to the training of a back propagation multilayer artificial neural network (ANN) with 3–5–1 topology for better prediction quality. The prediction and optimization capabilities of regression and ANN were critically examined and ANN displayed higher proficiency with R2 of 0.99 and RMSE of 2.0 compared to 0.98 R2 and 48.9 RMSE of the regression model. An adept ANN linked genetic algorithm (GA) optimized the medium components to achieve 1.8-fold higher enzyme production (4175.41 U/mL). Further, a new and improved in vitro qualitative analysis displayed high specificity of purified enzyme to fibrin.
Keywords: Fibrinolytic enzyme, Marine Fictibacillus sp., Wheat bran extract, Neural network, Genetic algorithm
Introduction
Venous thromboembolism comprising of deep vein thrombosis and pulmonary embolism is one of the primary cause of cardiovascular deaths around the world. It may also lead to acute myocardial infarction and stroke and is often the most common complication in cancer patients as they are at high risk of developing an intravascular thrombosis. The incidence of asymptomatic deep vein thromboembolism is also a key concern among patients undergoing major surgery (Suzuki et al. 2010). The fundamental cause of this deadly disability is the restriction of blood flow to vital organs due to thrombus formation. Rapid, complete, and sustained restoration of blood flow in the thrombotic arteries and veins can be achieved by thrombolytic therapy. However, even the most effective thrombolytic agents available today have significant limitations, which include re-obstruction, bleeding complications, low specificity to fibrin, the requirement of multiple massive dosages, and high cost. Hence, the present scenario demands a highly effective, safe, and economical thrombolytic agent.
The marine and coastal biodiversity is ecologically distinct and is composed of diverse bacterial communities, which are phylogenetically different from their terrestrial counterparts. From a biotechnological perspective, marine microbial biocatalysts are highly desirable due to their peculiar and inexplicable performance during bioprocesses which can be attributed to habitat-related characteristics such as salt tolerance, hyperthermostability, barophilicity, and cold adaptivity (Trincone 2011). Such incredible potential of marine bacteria which produce unique therapeutic compounds for their survival adaptations is mostly unexplored. Interestingly, even within the known compounds, where the chemical space has been extensively examined, marine bacterial natural products can provide a different and fresh insight (Williams 2008). Though initially it was believed that the high salinity of marine environment does not support significant bacterial growth, there is a renewed interest since the seawater salinity is chemically close to blood plasma and hence enable bioprocesses using biocatalysts like marine enzymes resulting in fewer side effects and byproducts (Lima and Porto 2016; Bi et al. 2013).
Majority of marine proteases are from Bacillus sp. (Cheng et al. 2015; Baweja et al. 2017) indicating it to be the predominant fibrinolytic enzyme-producing bacteria. However, this study reports a highly potent fibrinolytic enzyme from marine Fictibacillus sp., a close, yet distinct, relative of Bacillus sp.
Optimization of medium components plays a vital role in enhancing enzyme production. Cost-effective production of desired enzymes can be done using cheap agro-industrial residues, which aid in improving enzyme production by providing essential nutrients (Moharam et al. 2019; Pan et al. 2019). Wheat bran has been successfully used in the past for solid-state fibrinolytic enzyme production (Nascimento et al. 2016; Wang et al. 2009). However, submerged fermentation has the advantage of simpler extraction techniques and easier scale-up where the fermentation operations like agitation, aeration, temperature, and pH can be accurately monitored and controlled.
A considerable amount of literature has reported the optimization of fibrinolytic enzyme production using response surface methodology (de Souza et al. 2016; Taneja et al. 2017). However, most studies have focused on the general fitness of the model and added discussions on error parameters like RMSE, standard error of prediction (SEP), and prediction ability are greatly required. Statistical methods like artificial neural network (ANN) coupled with genetic algorithm (GA) are gaining high research interest in the recent past, because of its higher prediction capability and its ability to optimize non-linear problems. Recently, the ANN model strategy has been applied effectively to maximize the production of various enzymes (Salim et al. 2019; Erva et al. 2018; Singh et al. 2013), but have not been attempted for fibrinolytic enzyme production. Contrary to RSM, neural network does not require a statistically designed model for optimization, which is an advantage if a large number of independent variables is to be examined. ANN reduces errors by using all the data provided and, thereby, increases the model accuracy, unlike RSM. GA in turn imitates the process of evolution and finds the best fit based on the alternatives given. This research highlights the optimization and prediction efficiency of RSM and ANN, and in the process, the medium components were optimized through ANN-linked GA for maximum fibrinolytic enzyme production. This is the first report on the optimization of culture media for the production of fibrinolytic enzyme from Fictibacillus sp. using statistical methods such as response surface methodology (RSM) and artificial neural network (ANN)-linked genetic algorithm (GA).
Materials and methods
Isolation and screening of fibrinolytic enzyme producing strain from marine niche
The sand, seashells, and marine water from the Kozhikode beach area situated on the Malabar Coast of India (latitude 11°15′36″N, longitude 75°46′03″E) were screened for fibrinolytic enzyme producing bacterial strains. The samples were collected in sterile bottles and were plated on to skim milk agar media in the laboratory containing artificial seawater (ASW) and 0.5% tryptone, 0.3% yeast extract, 1.2% bacteriological agar, and 25% (v/v) skim milk. The plates were incubated for 48 h at 37 °C. The proteolytic isolates that hydrolyzed skim milk to produce a clear zone were selected and repeatedly streaked onto nutrient agar medium to obtain a pure culture. Further screening was done on fibrin agar plates composed of 0.5% fibrin, 0.2% ammonium sulfate, 0.1% CaCl2, 0.01% K2HPO4, 0.01% KH2PO4, 0.02% MgSO4. 7H2O, and 1.8% agar, pH 8.0. The strains that showed clear fibrinolysis on plates were selected and retained for further studies (Table 1). ASW was used for all the initial studies and contained 2.46% NaCl, 0.07% KCl, 0.5% MgCl2. 6H2O, 0.14% CaCl2, 0.63% MgSO4. 7H2O, and 0.02% NaHCO3, with pH adjusted to 8.
Table 1.
Fibrinolytic activity of marine bacterial isolates
| Bacterial isolate | Enzyme activity (U/mL) | Phenotypic identification |
|---|---|---|
| M1 | 249.18 | Bacillus sp. |
| M10/3 | 198.36 | Bacillus cereus |
| M10/4 | 139.34 | Pseudomonas aeroginosa |
| SS1a | 167.21 | Bacillus megaterium |
| SS1b | 227.87 | Bacillus sp. |
| SS10/5 | 211.48 | Bacillus sp. |
| SKA27 | 303.28 | Fictibacillus sp. |
Phenotypic characterization and 16S rRNA sequence analysis of selected strain
The isolated strain SKA27, which showed high fibrinolytic enzyme production was selected and identified based on its morphological, biochemical, and 16S rRNA sequencing.
Genomic DNA was isolated from an 18 h culture using Bacterial Genomic DNA Extraction Kit (Himedia, India) as per the manufacturer’s instructions. Universal primers 8F and 1492R were used to amplify approximately 1.5 kb of the 16S rRNA gene and then purified using Exonuclease I-Shrimp Alkaline Phosphatase (Exo-SAP). Amplification was carried out using Bio-Rad S1000 thermal cycler and Pfu polymerase (Thermo Scientific) under the following conditions: 5 min denaturation (94 °C), 30 s at 94 °C (35 cycles), 30 s at 55 °C, 1 min 30 s at 72 °C, and 1 min final extension (72 °C). After analyzing the purified amplicons on agarose gel, sequencing was performed bidirectionally using BDT v3.1 Cycle sequencing kit with primers 704F and 907R on ABI 3500XL Genetic Analyzer (Applied Biosystems, USA) at National Collection of Industrial Microorganisms, Pune, India. The sequences obtained were edited using CHROMASLITE (version 1.5) and further analyzed by BLASTn tool at NCBI and Ezbiocloud-e server (Yoon et al. 2017). Sequence homology studies were carried out and the 16S rRNA sequence obtained was deposited in the GenBank database.
Cultivation and production of the fibrinolytic enzyme
Inoculum preparation and production were done in broth medium prepared with ASW containing 1% tryptone and 0.5% yeast extract in 250 mL Erlenmeyer flasks with a working volume of 50 mL. A loopful of pure culture suspended in the culture medium served as inoculum and after 24 h, 10% of the inoculum was added to the production medium and cultivated for 48 h. Throughout the study, the production was carried out by submerged fermentation at 37 °C and 180 rpm on an orbital shaker. The cells were removed after 48 h by centrifugation (10,000×g, 10 min) and the supernatant was analyzed for fibrinolytic activity.
Fibrinolytic activity assay
Fibrinolytic activity was estimated by Anson (1939) method with suitable modifications. The reaction mixture consisted of 500 µl (1% w/v) of fibrin in 0.1 M Tris–HCl (pH 8.0) as a substrate, to which cell-free supernatant (suitably diluted) was added and incubated at 37 °C for 15 min. The reaction was stopped by adding one volume of trichloroacetic acid (20% w/v), and after keeping at room temperature for 15 min, the precipitate was removed by centrifuging at 10,000×g for 15 min. The absorbance was measured at 275 nm against a blank to which the enzyme solution was added after the addition of trichloroacetic acid. One unit of fibrinolytic activity was the amount of enzyme required to liberate 1 μg of tyrosine per minute under the experimental conditions.
Preparation of substrates and screening for fibrinolytic enzyme production
Agro residues like rice bran, wheat bran extract (WBE), coconut meal, soybean meal, sesame seed meal, and groundnut meal were investigated one at a time for high production of fibrinolytic enzyme by slurry fermentation. The slurry of substrates was prepared by adding 20 g/L substrate in a minimal medium composed of 0.1% K2HPO4.3H2O and 0.05% CaCl2. The autoclaved (121 °C, 30 min) medium was inoculated with SKA27 strain.
Apart from slurry fermentation, extracts prepared from wheat bran and rice bran were also examined for enzyme production. Extracts of rice bran and wheat bran were prepared by autohydrolysis at high temperatures. 2 g each of respective bran was added to 100 mL distilled water and heated to boil with continuous stirring for 1 h. After cooling, the solution was filtered and centrifuged at 10,000×g for 30 min. The clear supernatant was carefully removed, transferred to a conical flask with the minimal medium, autoclaved (121 °C, 15 min), and inoculated. After incubation for 48 h, the culture was centrifuged and the supernatant was analyzed for fibrinolytic activity.
The substrate that showed highest activity was selected as the single carbon source and kept constant, which was then supplemented with 1% nitrogen source (yeast extract, casitone, casein, and soy peptone) one at a time and analyzed for significant enzyme production. Similarly, to the selected carbon and nitrogen source, 0.1% each of mineral ions NaCl, K2HPO4, MgCl2, CaCl2, MgSO4, and NaHCO3 were investigated individually to identify its role in fibrinolytic enzyme production. All observations were carried out in triplicates and mean values were taken.
Optimization of significant factors by central composite design (CCD)
A five-level-three-factor CCD of RSM was used to identify the optimal concentration of critical components obtained from the preliminary analysis. The matrix design generation and statistical analysis were carried out using MINITAB 17. A full CCD design with three factors; wheat bran extract, yeast extract, and calcium chloride was studied at five levels (− α, − 1, 0, + 1, + α; where α = 2n/4 and n is the number of variables). The center points were selected based on the steepest ascent method and the design consisted of 20 sets of experiments with 14 random points and 6 center points (Table 2). All analyses were done in triplicates and average values were reported. The experiments designed by MINITAB were performed in random order to rule out bias and fitted to a second-order polynomial to predict the fibrinolytic enzyme activity (Y).
| 1 |
where Xi and Xj are input variables, β0 the intercept coefficient, βi the ith linear coefficient, β ii its quadratic coefficient, and β ij is the ijth interaction coefficient. The model was further checked for satisfactory fit using analysis of variance (ANOVA). The interactive influence of each significant variable on enzyme activity was visualized as 3D surface plots (Stat-Ease Design Expert 7.0.3).
Table 2.
Experimental design and the response of central composite design (CCD) and artificial neural network (ANN)
| Run | Wheat bran extract* | Yeast extract* | CaCl2* | Enzyme Activity (U/mL) | ||
|---|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | Experimental | RSM predicted | ANN predicted | |
| 1 | 0 (35) | 0 (15) | 0 (3) | 4172.13 | 4130.11 | 4172.11 |
| 2 | − 1 (20) | 1 (20) | − 1 (2) | 3639.34 | 3649.92 | 3639.31 |
| 3 | 0 (35) | 0 (15) | 0 (3) | 4172.13 | 4130.11 | – |
| 4 | 0 (35) | − 1.68 (6.59) | 0 (3) | 2483.61 | 2533.29 | 2483.59 |
| 5 | 1 (50) | − 1 (10) | − 1 (2) | 2909.84 | 2896.20 | 2909.83 |
| 6 | 0 (35) | 0 (15) | 0 (3) | 4114.75 | 4130.11 | – |
| 7 | − 1 (20) | − 1 (10) | − 1 (2) | 2655.74 | 2673.42 | 2655.89 |
| 8 | − 1.68 (9.77) | 0 (15) | 0 (3) | 3368.85 | 3390.17 | 3368.83 |
| 9 | 0 (35) | 0 (15) | 0 (3) | 4122.95 | 4130.11 | – |
| 10 | 0 (35) | 1.68 (23.41) | 0 (3) | 3713.11 | 3727.62 | 3712.86 |
| 11 | 1.68 (60.23) | 0 (15) | 0 (3) | 3508.2 | 3551.17 | 3508.20 |
| 12 | 1 (50) | − 1 (10) | 1 (4) | 3327.87 | 3270.00 | 3327.85 |
| 13 | − 1 (20) | 1 (20) | 1 (4) | 3778.69 | 3745.12 | 3778.67 |
| 14 | 0 (35) | 0 (15) | − 1.68 (0.07) | 3606.56 | 3560.41 | 3606.54 |
| 15 | 0 (35) | 0 (15) | 1.68 | 3844.26 | 3954.79 | 3844.24 |
| 16 | 0 (35) | 0 (15) | 0 (3) | 4040.98 | 4130.11 | – |
| 17 | 1 (50) | 1 (20) | − 1 (2) | 3934.43 | 3958.80 | 3942.18 |
| 18 | 0 (35) | 0 (15) | 0 (3) | 4172.13 | 4130.11 | – |
| 19 | − 1 (20) | − 1 (10) | 1 (4) | 3459.02 | 3387.42 | 3459.01 |
| 20 | 1 (50) | 1 (20) | 1 (4) | 3778.69 | 3713.80 | 3778.67 |
*Uncoded values (g/L) are shown in parentheses
Predictive modeling and optimization using artificial neural network and linked genetic algorithm
ANN is a mathematical model, which is inspired by the structural and functional aspects of the human brain. The complex computational network made up of neurons identifies the non-linear relationship between the input variables and the response output. A feed forward multilayer perceptron neural network was employed in this study using MATLAB R2017a (The Mathwork Inc., USA) and consisted of input, hidden, and output layers. Wheat bran extract (X1), yeast extract (X2), and CaCl2 (X3) were chosen as input parameters while fibrinolytic activity (U/mL) was the only output parameter. The set of experimental data used for RSM was used to train the ANN network (Table 2). The replicates were, however, not used since it does not improve the predictability of ANN. The successive layers, interconnected through connection weights (Wi) and biases (bi), formed the network topology and these layers ensure that the output best fits the incoming signal. From the input layer (Xi), the data were transferred to the output layer through the hidden layer in a hierarchical pattern, where the activated output of each layer served as input for the next layer. The neuron input (Xi) and weights were summed and transmitted along with bias as per Eq. 2. Different combinations of transfer functions ‘log-sigmoid’ (logsig (In) = 1/(1 + exp (− In)), ‘tan sigmoid’ (tansig (In) = 2/(1 + exp (− 2*In))− 1), and ‘pure linear’ (purelin (In) = In) were tested for the hidden and output layer to calculate the layer’s output from its net input (In). The activation functions that generated the least RMSE was selected and used further.
| 2 |
The network was trained using the Levenberg–Marquardt back propagation algorithm. Here, the error between the predicted output response and actual output is calculated and then transmitted back through the network and the weights were adjusted accordingly to reduce error in each successive layer. This error propagation was repeated until the errors between the networks’ predicted output and actual output met a specific error criterion. The network that provided better accuracy in terms of coefficient of determination (R2), RMSE, standard error of prediction (SEP), and absolute average deviation (AAD) using Eqs. 3–6 was chosen and the proficiency of the developed model was compared to RSM.
| 3 |
| 4 |
| 5 |
| 6 |
where ym is the experimental mean, yie and yip are the experimental and predicted value at the point i and n is the number of experimental variables.
A fitness function was defined from the trained neural network and was implemented with the objective of using genetic algorithm to find the optimal medium concentration, which provides the maximum fibrinolytic enzyme production. GA’s perform based on the principle of natural selection. A better solution to a problem was developed by the ‘survival of the fittest’ strategy. First, an initial population of a set of individuals was randomly generated based on the input variables and it was evaluated based on the objective function provided. Best individuals were then selected and a new set of individuals was created out of them by repeated crossover and mutation. After the predefined number of generations and by testing the results against the objective function, GA was terminated. The GA optimization was based on constraints so that the optimal was searched in the potential region. The data simulation for GA was performed using the MATLAB R2017a optimization toolbox.
Purification and molecular weight determination of fibrinolytic enzyme
The cells were separated and the supernatant was collected after centrifugation at 10,000×g for 10 min. The proteins in the crude supernatant were salted out by adding a gradual, specific amount of ammonium sulfate. The precipitated proteins at different fractions were centrifuged at 10,000×g for 20 min, resolubilized in 25 mM Tris–HCl buffer (pH 8.0), and then dialyzed using the same buffer. The portion that showed fibrinolytic activity was then loaded onto a DEAE Sephadex column (2 × 20 cm2) previously equilibrated with 25 mM Tris–HCl buffer (pH 8.0). Bound proteins were eluted out using a linear grade of NaCl (0.1–1.0 M) at a flow rate of 75 mL/h. The active portions were pooled, concentrated by ultracentrifugation using Amicons (10 kDa MW), and then applied on to Sephacryl S-100 HR gel filtration column (2 × 100 cm2), which was equilibrated and eluted (30 mL/h flow rate) with 0.1 M Tris–HCl buffer (pH 8.0). The fractions which showed fibrinolytic activity were pooled, concentrated, and stored for further analyses at − 20 °C. Molecular weight determination of purified enzyme was done by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970). 5% (w/v) gel was used for stacking and 12% (w/v) polyacrylamide was used as resolving gel. Coomassie brilliant blue (CBB) R-250 was used for staining and destaining was done with methanol:glacial acetic acid:distilled water (5:1:4). PageRuler Prestained Protein Ladder (Thermo Fisher Scientific Inc, USA) was used for molecular weight determination. Further, the visualization of enzyme activity was done by degradation of substrate (fibrin) using in gel overlay zymography technique (Ramsby 2004) with modifications. After SDS-PAGE, the gel was immersed in renaturation buffer containing 2.5% Triton X-100 in 0.1 M Tris–HCl (pH 8) for 40 min. The gel was then repeatedly washed in deionized water and 0.1 M Tris–HCl (pH 8), respectively to remove Triton X-100. An indicator gel was prepared separately by adding 1% (w/v) fibrin to 12% (w/v) polyacrylamide. The washed SDS-PAGE gel was then gently placed over the fibrin indicator gel and incubated at 37 °C for 2 h in a moist chamber containing cotton balls dipped in 0.1 M Tris–HCl (pH 8) to provide moisture. After incubation, the gel was stained with CBB R-250 for 3 h and destained for 1 h.
Evaluation of fibrinolytic activity in vitro
The ability of the purified enzyme to degrade blood clot was investigated in vitro by modifying the method described by Omura et al. (2005). 1 ml blood was collected from a healthy male volunteer with proper consent and a spontaneous blood clot of equal length was made in four catheters with 2 mm diameter, which resembled a human artery. It was made to stand for 1 h and then the clot was rinsed gently with PBS (phosphate-buffered saline, pH 7.0) without dislodging the clot. Various doses (10 U, 50 U, and 100 U in PBS) of purified enzyme from Fictibacillus sp. strain SKA27 was passed through the tubes and incubated for 18 h at 37 °C. Normal saline was used as control.
Further, a qualitative analysis was developed to detect fibrinolysis on fibrin plates using Congo red dye. The fibrinolytic activity of 1 U purified enzyme was tested against 1 U of plasmin, by spotting on to 2 mm wells punched on 1% (w/v) fibrin agarose gel and incubating at 37 °C for 18 h with normal saline as control. After incubation, the plates were flooded with 0.1% (w/v) Congo red solution and allowed to stand for 30 min. Congo red binds to fibrin to impart a red color to the gel and the plates were checked for clear halo zone of fibrinolysis against the colored background after destaining with 1 M NaCl for 15 min.
Results and discussion
Isolation and screening of marine strains for fibrinolytic enzyme production
The unique compounds produced by marine microbes under extreme conditions have contributed significantly to the discovery of various novel bioactive drugs and compounds of pharmacological value (Waters et al. 2010). With this viewpoint, an attempt was made to identify possible fibrinolytic enzyme producing bacteria from the marine source.
In the present study, over 16 proteolytic bacterial species were isolated on skim milk agar, out of which 7 showed fibrinolytic enzyme production on fibrin agar plates. The isolated bacterial cultures were preserved as glycerol stocks and revival was done in suitable media when required. The extracellular fibrinolytic enzyme activity was determined (Table 1), and the highest fibrinolytic enzyme producing isolate, designated as SKA27, was selected for further studies.
16S rRNA sequencing and phenotypic characterization of SKA27
The isolate SKA27 was found to be Gram-stain positive, motile, aerobic, and endospore-forming rods. Morphological features showed that the colonies were round and slightly irregular, smooth, glossy, butyrous, creamy off-white, and almost opaque on nutrient media.
16S rRNA gene sequence analysis indicated the close relation of the isolate SKA27 to the members of genus Fictibacillus. Fictibacillus sp. is a novel genus of bacteria, which has unique lipid patterns and can be differentiated physiologically and biochemically from its closest relatives of Bacillus sp. (Glaeser et al. 2013). The highest similarity of 16S rRNA gene sequence of strain SKA27 was with F. phosphorivorans Ca7T (99.65%), F. nanhaiensis DSM 23009T (99.65%) and F. barbaricus V2-BIII-A2T (99.65%) followed by F. arsenicus Con a/3T (99.52%) and F. halophilus AS8T (99.44%). Though species share a high level of 16S rRNA gene sequence similarity (> 99%), at the species level, a clear distinction can still exist (Rossello-Mora and Amann 2001). On that basis and various phenotypic and chemotaxonomic characteristics, the marine isolate (SKA27) was designated as Fictibacillus sp. strain SKA27. The 1472 bp 16S rRNA sequence was deposited in GenBank database with accession number KY385631.
Screening of substrates
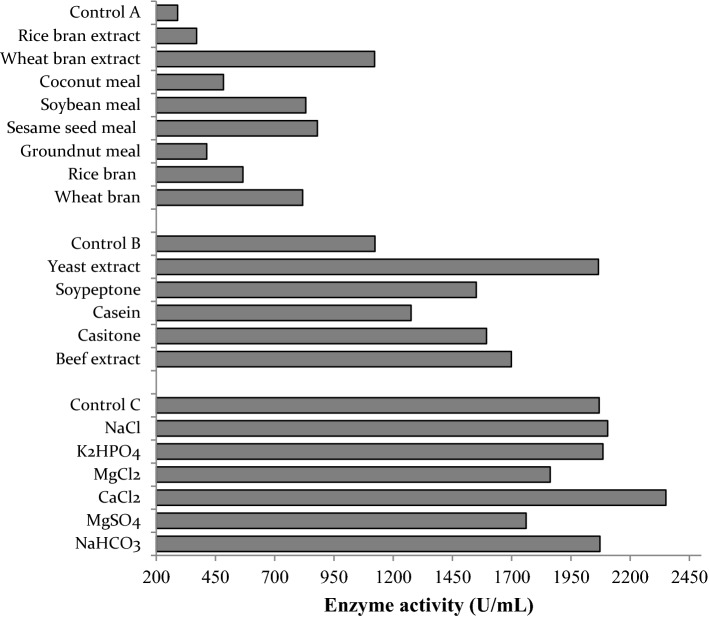
A step-by-step single factor optimization strategy was adopted to investigate the influence of different constituents on fibrinolytic enzyme production (Fig. 1). Various agro-residues were analyzed as sole carbon source and wheat bran extract showed the highest enzyme production (1121.31 U/mL) against other components. Determination of total carbohydrate by Nielsen (2010) and protein content by Bradford method in 5% wheat bran extract revealed the presence of 15.75 mg/mL water-soluble carbohydrates and 1.11 mg/mL proteins. Luria–Bertani medium with 0.1% K2HPO4.3H2O and 0.05% CaCl2 was used as control. Wheat bran has often been used as a substrate for solid-state fermentation, but the bacteria intake carbohydrates better in a soluble form and this could have enhanced the production during submerged fermentation. Moreover, wheat bran extract (WBE) is a rich source of water-soluble carbohydrates, mainly monosaccharides (glucose, xylose, and arabinose), small oligosaccharides (xylobiose and xylotriose), some larger oligosaccharides, apparently tetra-, penta-, and hexa-saccharides, and also soluble proteins (Sun et al. 2008). Higher production of fibrinolytic enzyme in WBE than the wheat bran slurry may be due to the presence of concentrated nutrients in the slurry that could not be taken up or the presence of inhibitory components in the slurry that repressed enzyme production. During fermentation, the delay in the release of elements that stimulated fibrinolytic enzyme production would also have resulted in lower yield in slurry than WBE. Residual oils from the meal were accumulated when the culture was grown on coconut meal and groundnut meal slurry, which blocked the bacterial growth and thereby decreased the fibrinolytic enzyme production. Organic nitrogen sources always induced high protease production than inorganic ones (Wang et al. 2008) and yeast extract was found to be the preferred nitrogen source with increased activity of 2065.57 U/mL when combined with WBE.
Fig. 1.
Effect of various components on fibrinolytic enzyme production. Control A: LB (Luria–Bertani) medium, Control B: 20 g/L WBE (wheat bran extract), Control C: 20 g/L WBE, and 10 g/L yeast extract
One of the crucial reasons the terrestrial isolates are preferred over marine organisms is because of the stigma that most marine organisms are obligate marine and it requires seawater or mainly sodium for growth. Conversely, it was noted here that marine bacterium Fictibacillus sp. strain SKA27 could grow and produce metabolites equally well with or without seawater or its major components like NaCl. Among the mineral ions investigated with WBE and yeast extract, CaCl2 (2350.82 U/mL) was preferred over NaCl (1977.05 U/mL) and other metal ions. CaCl2 plays a significant role in translocation of serine proteases across the plasma membrane by dislocating the membrane-bound serine protease, which inhibits the protein synthesis within the cells by inducing a feedback control response (Mahajan et al. 2012). A reduction in fibrinolytic enzyme production with Mg2+ ions (Fig. 1) may be due to lowered synthesis and release of the enzyme in the presence of Mg2+ ion or the result of autodegradation of protease.
Optimization of significant factors by central composite design (CCD)
The experimental and predicted results of RSM using CCD and the levels of variables are shown in Table 2. The dependent variable was fit to the second-order polynomial using Eq. 7.
| 7 |
where X1, X2, and X3 denote the coded values of wheat bran extract, yeast extract, and CaCl2.
F test (ANOVA) was used to check the statistical significance of the model equations. The ANOVA summary for response surface model and their significance level are shown in Table 3. All the three linear and quadratic terms had a significant P value (P < 0.05) indicating their influence on fibrinolytic enzyme production. The interactive effects of WBE and yeast extract with CaCl2 were significant, while the interaction of WBE with yeast extract did not show significant impact on enzyme production.
Table 3.
ANOVA and significance level of response surface quadratic model
| Source | Sum of squares | df | Mean square | F value | P value |
|---|---|---|---|---|---|
| Model# | 4,654,620 | 9 | 517,180 | 108.05 | < 0.0001 |
| X1 | 31,164 | 1 | 31,164 | 6.51 | 0.029 |
| X2 | 1,719,887 | 1 | 1,719,887 | 359.32 | < 0.0001 |
| X3 | 18,852 | 1 | 18,852 | 39.39 | < 0.0001 |
| X21 | 783,401 | 1 | 783,401 | 163.67 | < 0.0001 |
| X22 | 1,800,034 | 1 | 1,800,034 | 376.07 | < 0.0001 |
| X23 | 250,052 | 1 | 250,052 | 52.24 | < 0.0001 |
| X1 X2 | 3704 | 1 | 3704 | 0.77 | 0.4 |
| X1 X3 | 57,856 | 1 | 57,856 | 12.09 | 0.006 |
| X2 X3 | 191,489 | 1 | 191,489 | 40.01 | < 0.0001 |
| Residual | 47,865 | 10 | 4786 | ||
| Lack of fit | 34,371 | 5 | 6874 | 2.55 | 0.164 |
| Pure error | 13,493 | 5 | 2699 | ||
| Total | 4,702,485 | 19 |
#R2 = 0.9898, R2(adj) = 0.9807, R2(pred) = 0.9401
The high F value (108.05) of the model with a P value < 0.05 and an insignificant lack of fit assure the aptness of the model. In addition, the coefficient of determination (R2) value of 0.9898 indicated that this model could explain 98.98% variability in response data. Further, the R2 value was in good agreement with the adjusted R2 value of 0.9807 and that ruled out any bias in the regression output.
Three-dimensional plots of significant model terms (Fig. 2) depict the change in the response variable (fibrinolytic activity) with the interaction of two independent variables while the third variable is held at a fixed level. The elliptical distribution of the contour plot indicated a better response and the convex curvature of surface plot implied a peak in fibrinolytic activity with an increase in the concentration of variables and then a gradual decrease. Figure 2a shows the influence of wheat bran extract and CaCl2 on fibrinolytic enzyme production when yeast extract was fixed at a level of 15 g/L. It was noted that there was no significant activity at low levels of WBE. Fictibacillus sp. strain SKA27 grew better and had enhanced fibrinolytic enzyme production with WBE, and apparently, the soluble oligosaccharides present in the extract stimulated the synthesis when combined with yeast extract and CaCl2.
Fig. 2.
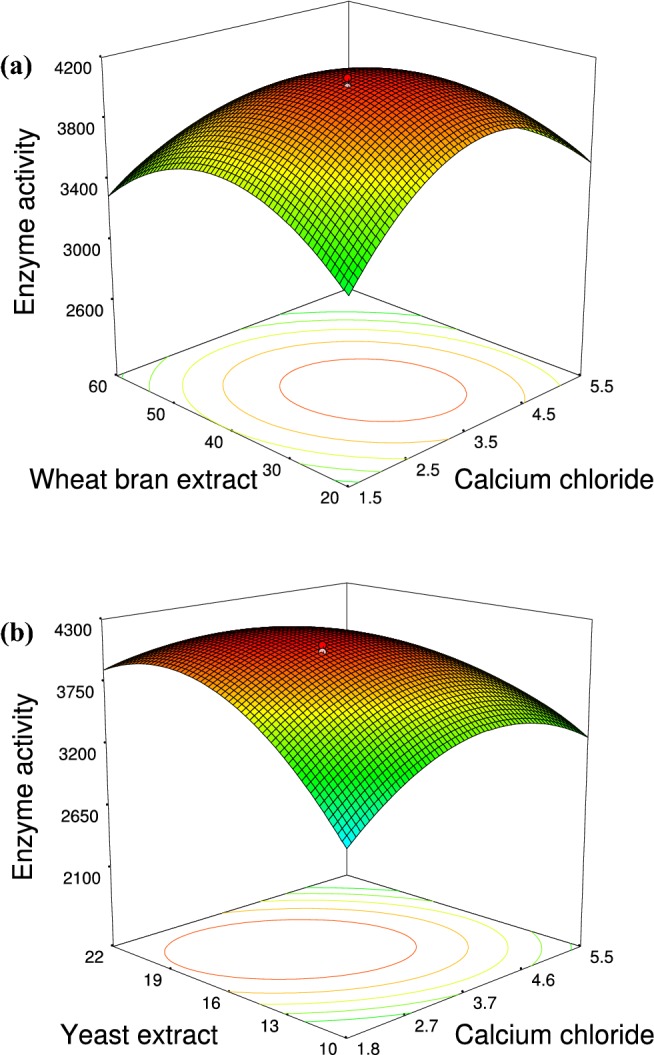
3D surface plots displaying the interactive effect of significant components optimized by response surface methodology (RSM). a WBE (wheat bran extract) vs. CaCl2 at fixed yeast extract level of 15 g/L. (b) Yeast extract vs. CaCl2 at fixed WBE level of 35 g/L
Figure 2b shows that an increase in yeast extract concentration enhances enzyme activity. However, a further increase led to a decrease in enzyme production. This aspect is similar to Bacillus species where extracellular protease production is a manifestation of nitrogen limitation at the onset of stationary phase (Gupta et al. 2002). The significant interactive effect of CaCl2 with both WBE and yeast extract shows the role of CaCl2 in stabilizing serine proteases and thereby increasing the enzyme production. Though RSM provided an excellent insight into the interactive effect of variables and the model parameters signified a favorable fit, the error in prediction was high and was, thus, compared to ANN model to validate its prediction capability.
Modeling and optimization using artificial neural network and linked genetic algorithm
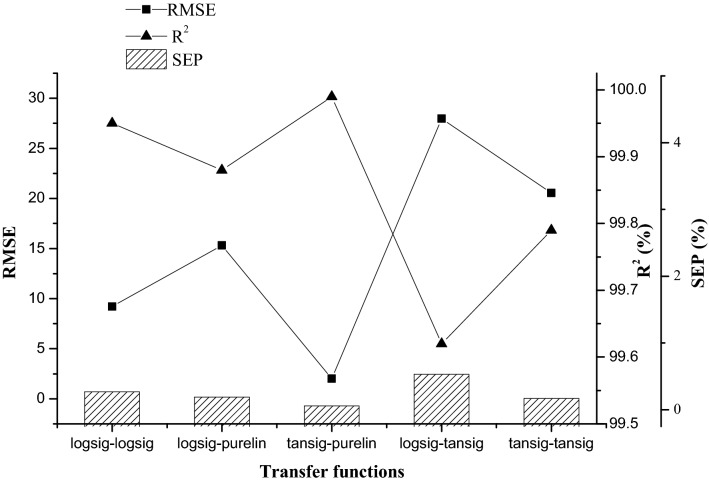
Levenberg–Marquardt’s back propagation algorithm was used for training a feedforward multilayer perceptron network to predict the fibrinolytic enzyme activity (U/ml) from input variables WBE, yeast extract, and CaCl2. Iteratively, the optimal number of hidden layer neurons was determined based on the simultaneous change in mean square error. That was a critical step since the increase in the number of neurons in hidden layer might show a better performance but may lead to overtraining of the network. Figure 3 shows the R2, RMSE, and SEP values obtained after repeated training with various combinations of transfer functions at hidden and output layer, respectively. Based on the results, network with transfer function tansig at hidden layer and purelin at output layer was chosen. Five neurons, which showed the least root mean square error, were chosen for the hidden layer and network topology of 3–5–1 was configured for the training of the neural network with the experimental data. The following Eq. 8 relates the input variables (X1, X2, and X3) to the output variable in terms of weights and biases.
| 8 |
where Y is the predicted fibrinolytic enzyme activity, f1 and f2 the transfer functions used in hidden and output layer, respectively, w1 and w2 the weights, b1 and b2 the biases whose values are reported in Table 4, and xt1 represents the transpose of the row vector of input variables (X1, X2, and X3) with a dimension of 3*1.
Fig. 3.
Root mean square error (RMSE), coefficient of determination (R2), and standard error of prediction (SEP) values obtained while training artificial neural network (ANN) with various transfer function combinations in the hidden-output layers
Table 4.
Optimal weights and bias values after artificial neural network (ANN) training
| Weights to hidden layer from input layer (w1) | |||||
|---|---|---|---|---|---|
| Wheat bran extract | − 2.3718 | 1.2944 | 0.3879 | 1.6876 | 1.9575 |
| Yeast extract | 2.3439 | 2.2344 | 2.3809 | – 0.74314 | 3.1364 |
| Calcium chloride | − 0.039955 | 1.5913 | 1.8403 | 1.0288 | − 0.2864 |
| Weights to output layer from hidden layer (w2) | 0.77462 | − 0.33388 | 0.49427 | 0.61962 | 0.46593 |
| Bias to hidden layer (b1) | 2.7377 | − 0.88533 | 1.5158 | 1.8552 | 1.8068 |
| Bias to output layer (b2) | − 1.4852 | ||||
For validating the trained ANN model, ten new trials were conducted which formed a different data set. It consisted of experiments with various combinations of input variables, which were not part of the training data. The actual and predicted values obtained for validation data from the regression equation of RSM and ANN simulation are shown in Table 5.
Table 5.
Validation data set for response surface methodology (RSM) and artificial neural network (ANN) model prediction
| Run | X 1 | X 2 | X 3 | Enzyme Activity (U/ml) | ||
|---|---|---|---|---|---|---|
| Experimental | RSM predicted | ANN predicted | ||||
| 1 | 60 | 13 | 1.5 | 3030.07 | 2996.93 | 3031.17 |
| 2 | 25 | 9 | 2.5 | 2838.23 | 2864.05 | 2837.36 |
| 3 | 55 | 11 | 2 | 2933.59 | 2986.92 | 2933.11 |
| 4 | 25 | 11 | 4.5 | 3609.92 | 3646.04 | 3610.80 |
| 5 | 45 | 15 | 3 | 4083.68 | 4058.40 | 4087.38 |
| 6 | 25 | 21 | 4 | 3751.23 | 3751.12 | 3753.33 |
| 7 | 5 | 13 | 3.5 | 3151.44 | 3062.13 | 3152.74 |
| 8 | 15 | 15 | 2.5 | 3486.69 | 3503.56 | 3484.43 |
| 9 | 30 | 13 | 4 | 3991.30 | 3968.32 | 3993.03 |
| 10 | 40 | 9 | 3 | 3156.06 | 3176.52 | 3155.82 |
The competency of the trained ANN model was tested against RSM by comparing the statistical parameters RMSE, SEP, AAD, and R2. The calculated values presented in Table 6 highlight the superiority of ANN over RSM. The coefficient of determination (R2) values of ANN (0.9999) and RSM (0.9898) for training data, ANN (0.999) and RSM (0.9912) for validation data indicate that they are powerful modeling tools since the experimental data could fit in perfectly. However, ANN showed a better prediction capability than RSM. RSM could generate a regression equation and explain the interactive effect of variables unlike ANN, but high RMSE (48.9) of RSM against low RMSE (2.0) of ANN questions its prediction ability. A similar trend was observed with SEP and AAD values (Table 6).
Table 6.
Comparison of prediction capability of response surface methodology (RSM) and artificial neural network (ANN)
| Statistical parameters | Design dataa | Validation datab | ||
|---|---|---|---|---|
| RSM | ANN | RSM | ANN | |
| R 2 | 0.9898 | 0.9999 | 0.9912 | 0.9999 |
| RMSE | 48.93 | 2 | 39.7 | 1.76 |
| SEP (%) | 1.34 | 0.06 | 1.17 | 0.05 |
| AAD (%) | 1.13 | 0.01 | 1 | 0.04 |
Despite having a good fit and R2 value, the inability of RSM model to be accurate in prediction may be because it could capture only quadratic approximations (Das et al. 2015) and its inferiority in predicting the values at the extremities of design points. ANN, in turn, had the capability of using all the given input data values to predict accordingly and could approximate diverse set of non-linear polynomials. In addition, ANN does not require a standard experimental design to build the model and unlike other statistical models, it can operate upon the empirical data without data transformations (Bingöl et al. 2012).
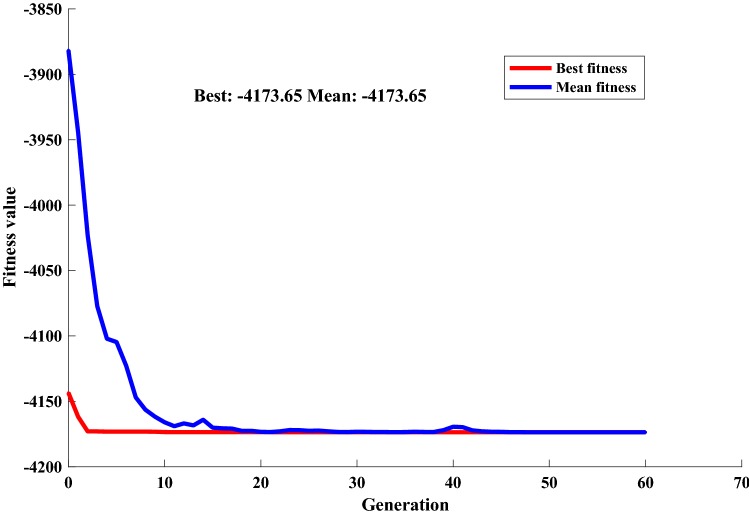
The fitness function used for the genetic algorithm was from the accurately trained ANN. The input variables were optimized using GA with the objective of finding the maximum response (fibrinolytic activity). The input space was repeatedly searched for the best global optimum. The major parameters used for optimization were population size 50, crossover rate 0.8, mutation probability 0.04, and a heuristic crossover function. Rank and roulette were used as the scaling and selection function, respectively. Figure 4 shows the best fitness and mean fitness generated by MATLAB R2017a after optimization. The ANN-GA model predicted a maximum activity of 4173.65 U/mL at 35.3 g/L WBE, 15.17 g/L yeast extract, and 2.87 g/L CaCl2. The predicted response was validated experimentally and the optimized medium gave a maximum enzyme production of 4175.40 U/mL, which was in good agreement with the predicted response.
Fig. 4.
Graphical representation of optimization by genetic algorithm (GA) showing best and average fitness values with successive generations and convergence to the optimum value after 60 generations
Research on ANN-GA for fibrinolytic enzyme production was restricted to limited comparisons. However, in the past, ANN was successfully used for the production of an extracellular protease from Pseudomonas sp. by applying radial basis function (RBF)-ANN (Dutta et al. 2004) and from Bacillus subtilis using back propagation ANN (Prasanthi et al. 2008). Also, a hybrid system of ANN-GA was used to optimize the fermentation conditions of alkaline protease production by B. circulans (Subba Rao et al. 2008). The results of all these studies demonstrated higher prediction accuracy of ANN compared to RSM.
Purification and molecular weight determination of fibrinolytic enzyme
Fibrinolytic enzyme purification from Fictibacillus sp. strain SKA27 was carried out in four consecutive steps described earlier. The crude enzyme had an initial specific activity of 1546.41 U/mg and a consistent specific activity of 125,107.85 U/mg was obtained after the final purification step. An overall 81-fold purification was thus achieved after purification (Table 7). The molecular weight and purity of the enzyme were analyzed on SDS-PAGE and a zymogram, which showed a single band with an apparent molecular weight of 28 kDa (Fig. 5). Fibrinolytic enzyme is a monomeric protein and its molecular weight generally ranges between 18 and 50 kDa in microorganisms. However, molecular weight higher than 90 kDa (Nascimento et al. 2016; Yogesh and Halami 2015) and lesser than 18 kDa (Kim et al. 2011) has also been reported. The purified fibrinolytic enzyme from Fictibacillus sp. strain SKA27 had a molecular weight similar to that of cocoonase from Antheraea pernyi (Geng et al. 2014), Bacillus subtilis ICTF-1 (Mahajan et al. 2012), and Cordyceps militaris (Liu et al. 2016). It was lower than fibrinolytic enzyme Bvsp from Bacillus sp. (34.4 kDa; Cheng et al. 2015), cyanobacterium Anabaena fertilissima (49 kDa; Banerjee et al. 2012) and higher than those from Pleurotus eryngii (14 kDa; Cha et al. 2010) and Streptomyces sp. XZNUM 00004 (20 kDa; Ju et al. 2012).
Table 7.
Purification summary of fibrinolytic enzyme from Fictibacillus sp. SKA27
| Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification fold | |
|---|---|---|---|---|
| Crude | 31,55,738 | 2040.7 | 1546.41 | |
| Ammonium sulfate precipitation | 30,57,377 | 325.0 | 9407.31 | 6.1 |
| DEAE A-50 | 1,960,656 | 22.0 | 89,120.72 | 57.6 |
| Ultracentrifugation | 885,246 | 8.0 | 110,655.74 | 71.6 |
| Sephacryl S-100 HR | 475,410 | 3.8 | 125,107.85 | 81 |
Fig. 5.
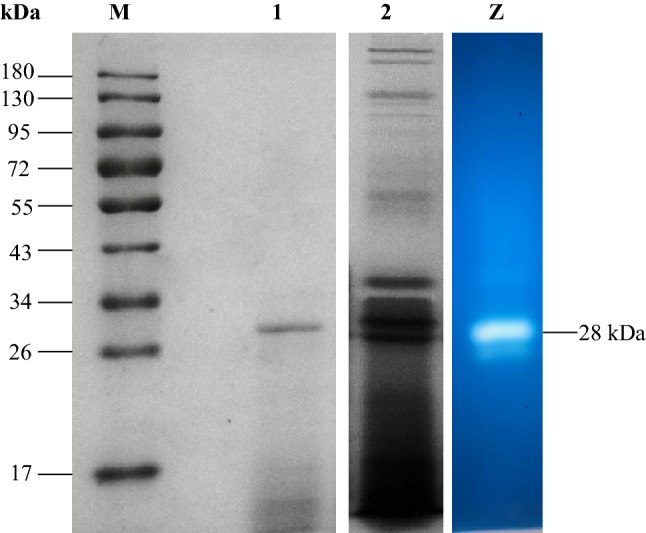
SDS-PAGE and zymogram of purified fibrinolytic enzyme from Fictibacillus sp. strain SKA27. Lane M: molecular weight marker proteins; lane 1: purified enzyme with a molecular weight of 28 kDa; lane 2: crude protein; lane Z: zymogram displaying a strong single band indicating high fibrinolytic activity
Evaluation of fibrinolytic activity in vitro
The effect of various doses (10 U, 50 U, and 100 U in PBS) of the purified enzyme on artificial blood clot was investigated by in vitro clot degradation method (Fig. 6). 100 U of the enzyme could completely dissolve the clot in 5 h (Fig. 6b). At the end of 18 h, 50 U enzyme also degraded the clot completely and released the RBC’s (red blood cells) from fibrin meshwork, while enzyme showed only a partial dissolution of the blood clot with 10 U (Fig. 6c). The normal saline used as control showed an intact blood clot.
Fig. 6.
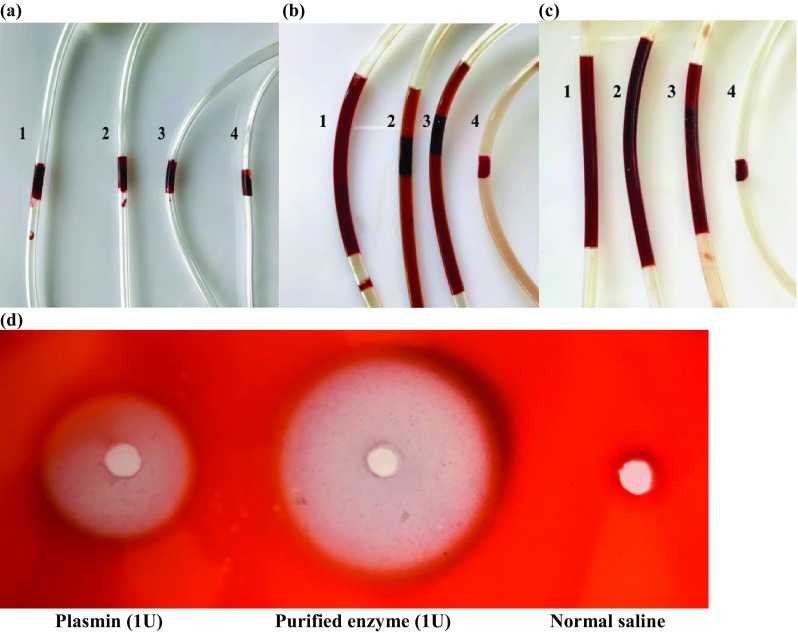
In vitro fibrinolytic activity of purified enzyme. a Spontaneous blood clot in four catheter tubes. b The effect of 100 U, 50 U, and 10 U of purified enzyme on blood clot in tubes 1, 2, and 3, respectively, after 5 h incubation. Normal saline was added to tube 4. Tube 1 shows complete dissolution of blood clot, while 2, 3 show partial degradation and 4 shows intact clot. (c) In vitro degradation after 18 h incubation. Tubes 1, 2 show complete clot lysis and 3 shows incomplete clot lysis while 4 shows intact clot. (d) Fibrinolytic activity of 1 U purified enzyme and plasmin on fibrin plate after Congo red staining. The diameters of the hydrolyzed clear zone were measured and compared
Qualitative detection of fibrinolysis on fibrin plates was done based on the ability of Congo red dye to bind to fibrin. The dye binds to the undigested fibrin in gel, leaving a clear area indicating fibrinolysis. Figure 6d shows a distinct zone surrounding the wells signifying fibrinolytic activity. The diameter of the clear zone of degradation around the purified enzyme measured 33% more than plasmin demonstrating its strong fibrinolytic activity and higher specificity for fibrin.
Conclusion
The present study reports the isolation of an efficient fibrinolytic enzyme producing strain from the marine source, identified as Fictibacillus sp. strain SKA27. This work critically examines RSM and a back propagation ANN model to predict the fibrinolytic enzyme production from three independent components (wheat bran extract, yeast extract, and CaCl2). Wheat bran extract showed immense prospect as a sole nutritional supplement, which may be applied in scale-up during industrial fibrinolytic enzyme production. The established superiority of ANN model supports its ability to supersede conventional optimization methods like RSM with high accuracy and low error, and ANN-GA optimized the input space for maximum enzyme production, which was validated experimentally. The purified 28 kDa monomeric enzyme showed a high specific activity of 125,107.85 U/mg, which may be the highest with fibrin as substrate. The simple and rapid in vitro detection of fibrinolytic activity using Congo red showed high specificity of the purified enzyme to fibrin and the blood clot degradation exhibited active fibrinolysis indicating its high potential as a thrombolytic agent.
Compliance with ethical standards
Conflict of interest
None.
References
- Anson ML. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol. 1939;22:79–89. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Prasanna R, Bagchi SN. Purification and characterization of a fibrino(geno)lytic protease from cultured natural isolate of a cyanobacterium, Anabaena fertilissima. J Appl Phycol. 2012;25:1111–1122. doi: 10.1007/s10811-012-9946-6. [DOI] [Google Scholar]
- Baweja M, Singh PK, Sadaf A, Tiwari R, Nain L, Khare SK, Shukla P. Cost effective characterization process and molecular dynamic simulation of detergent compatible alkaline protease from Bacillus pumilus strain MP27. Process Biochem. 2017;58:199–203. doi: 10.1016/j.procbio.2017.04.024. [DOI] [Google Scholar]
- Bi Q, Han B, Liu W, Feng Y, Jiang Z. UFEIII, a fibrinolytic protease from the marine invertebrate, Urechis unicinctus. Biotechnol Lett. 2013;35:1115–1120. doi: 10.1007/s10529-013-1187-5. [DOI] [PubMed] [Google Scholar]
- Bingöl D, Hercan M, Elevli S, Kiliç E. Comparison of the results of response surface methodology and artificial neural network for the biosorption of lead using black cumin. Bioresour Technol. 2012;112:111–115. doi: 10.1016/j.biortech.2012.02.084. [DOI] [PubMed] [Google Scholar]
- Cha W, Park S, Kim S, Choi D. Biochemical and enzymatic properties of a fibrinolytic enzyme from Pleurotus eryngii cultivated under solid-state conditions using corn cob. Bioresour Technol. 2010;101:6475–6481. doi: 10.1016/j.biortech.2010.02.048. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Xu F, Hu N, Liu X, Liu Z. A novel Ca2+-dependent alkaline serine-protease (Bvsp) from Bacillus sp. with high fibrinolytic activity. J Mol Catal B Enzym. 2015;117:69–74. doi: 10.1016/j.molcatb.2015.04.006. [DOI] [Google Scholar]
- Das S, Bhattacharya A, Haldar S, Ganguly A, Gu S, Ting YP, Chatterjee PK. Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: comparison between artificial neural network and response surface methodology. Sustain Mater Technol. 2015;3:17–28. doi: 10.1016/j.susmat.2015.01.001. [DOI] [Google Scholar]
- de Souza FASD, Sales AE, Costa e Silva PE, Bezerra RP, de Medeiros e Silva GM, de Araújo JM, de Campos GM, Porto TS, Teixeira JAC, Porto ALF. Optimization of production, biochemical characterization and in vitro evaluation of the therapeutic potential of fibrinolytic enzymes from a new Bacillus amyloliquefaciens. Macromol Res. 2016;24:587–595. doi: 10.1007/s13233-016-4089-2. [DOI] [Google Scholar]
- Dutta JR, Dutta PK, Banerjee R. Optimization of culture parameters for extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Process Biochem. 2004;39:2193–2198. doi: 10.1016/j.procbio.2003.11.009. [DOI] [Google Scholar]
- Erva RR, Venkateswarulu TC, Pagala B. Multi level statistical optimization of l-asparaginase from Bacillus subtilis VUVD001. 3 Biotech. 2018;8(24):1–8. doi: 10.1007/s13205-017-1020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng P, Lin L, Li Y, Fan Q, Wang N, Song L, Li W. A novel fibrin(ogen)olytic trypsin-like protease from Chinese oak silkworm (Antheraea pernyi): purification and characterization. Biochem Biophys Res Commun. 2014;445:64–70. doi: 10.1016/j.bbrc.2014.01.155. [DOI] [PubMed] [Google Scholar]
- Glaeser SP, Dott W, Busse HJ, Kämpfer P. Fictibacillus phosphorivorans gen. nov., sp. nov. and proposal to reclassify Bacillus arsenicus, Bacillus barbaricus, Bacillus macauensis, Bacillus nanhaiensis, Bacillus rigui, Bacillus solisalsi and Bacillus gelatini in the genus Fictibacillus. Int J Syst Evol Microbiol. 2013;63:2934–2944. doi: 10.1099/ijs.0.049171-0. [DOI] [PubMed] [Google Scholar]
- Gupta R, Beg QK, Khan S, Chauhan B. An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol. 2002;60:381–395. doi: 10.1007/s00253-002-1142-1. [DOI] [PubMed] [Google Scholar]
- Ju X, Cao X, Sun Y, Wang Z, Cao C, Liu J, Jiang J. Purification and characterization of a fibrinolytic enzyme from Streptomyces sp. XZNUM 00004. World J Microbiol Biotechnol. 2012;28:2479–2486. doi: 10.1007/s11274-012-1055-9. [DOI] [PubMed] [Google Scholar]
- Kim HC, Choi B, Sapkota K, Kim S, Lee HJ, Yoo JC, Kim S. Purification and characterization of a novel, highly potent fibrinolytic enzyme from Paecilomyces tenuipes. Process Biochem. 2011;46:1545–1553. doi: 10.1016/j.procbio.2011.04.005. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lima RN, Porto ALM. Recent advances in marine enzymes for biotechnological processes. In: Kim SK, Toldra F, editors. Marine enzymes biotechnology: production and industrial applications, part I—production of enzymes. Amsterdam: Academic Press Elsevier Inc.; 2016. pp. 153–192. [Google Scholar]
- Liu X, Kopparapu N, Li Y, Deng Y, Zheng PX. Biochemical characterization of a novel fibrinolytic enzyme from Cordyceps militaris. Int J Biol Macromol. 2016;94:793–801. doi: 10.1016/j.ijbiomac.2016.09.048. [DOI] [PubMed] [Google Scholar]
- Mahajan PM, Nayak S, Lele SS. Fibrinolytic enzyme from newly isolated marine bacterium Bacillus subtilis ICTF-1: media optimization, purification and characterization. J Biosci Bioeng. 2012;113:307–314. doi: 10.1016/j.jbiosc.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Moharam ME, El-Bendary MA, El-Beih F, Easa SMH, Elsoud MMA, Azzam MI, Elgamal NN. Optimization of fibrinolytic enzyme production by newly isolated Bacillus subtilis Egy using central composite design. Biocatal Agric Biotechnol. 2019;17:43–50. doi: 10.1016/j.bcab.2018.11.003. [DOI] [Google Scholar]
- Nascimento TP, Sales AE, Porto CS, Brandão RMP, de Campos-Takaki GM, Teixeira JAC, Porto TS, Porto ALF, Converti A. Purification of a fibrinolytic protease from Mucor subtilissimus UCP 1262 by aqueous two-phase systems (PEG/sulfate) J Chromatogr B. 2016;1025:16–24. doi: 10.1016/j.jchromb.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Nielsen SS. Phenol-sulfuric acid method for total carbohydrates. In: Heidelberg D, editor. Food analysis laboratory manual. 2. New York: Springer; 2010. pp. 47–53. [Google Scholar]
- Omura K, Hitosugi M, Zhu X, Ikeda M, Maeda H, Tokudome S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J Pharmacol Sci. 2005;99:247–251. doi: 10.1254/jphs.FP0050408. [DOI] [PubMed] [Google Scholar]
- Pan S, Chen G, Zeng J, Cao X, Zheng X, Zeng W, Liang Z. Fibrinolytic enzyme production from low-cost substrates by marine Bacillus subtilis: process optimization and kinetic modeling. Biochem Eng J. 2019;141:268–277. doi: 10.1016/j.bej.2018.11.002. [DOI] [Google Scholar]
- Prasanthi V, Nikku MY, Vuddaraju SP, Nalla KK, Raju CAI, Donthireddy SRR. Optimization of the fermentation media using statistical approach and artifical neural networks for the production of an alkaline protease from Bacillus subtilis. Int J Nat Eng Sci. 2008;2:51–56. [Google Scholar]
- Ramsby ML. Zymographic evaluation of plasminogen activators and plasminogen activator inhibitors. Adv Clin Chem. 2004;38:111–133. doi: 10.1016/S0065-2423(04)38004-2. [DOI] [PubMed] [Google Scholar]
- Rossello-Mora R, Amann R. The species concept for prokaryotes. FEMS Microbiol Rev. 2001;25:39–67. doi: 10.1111/j.1574-6976.2001.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Salim N, Santhiagu A, Joji K. Process modeling and optimization of high yielding l-methioninase from a newly isolated Trichoderma harzianum using response surface methodology and artificial neural network coupled genetic algorithm. Biocatal Agric Biotechnol. 2019;17:299–308. doi: 10.1016/j.bcab.2018.11.032. [DOI] [Google Scholar]
- Singh P, Shera SS, Banik J, Banik RM. Optimization of cultural conditions using response surface methodology versus artificial neural network and modeling of l-glutaminase production by Bacillus cereus MTCC 1305. Bioresour Technol. 2013;137:261–269. doi: 10.1016/j.biortech.2013.03.086. [DOI] [PubMed] [Google Scholar]
- Subba Rao C, Sathish T, Mahalaxmi M, Suvarna Laxmi G, Sreenivas Rao R, Prakasham RS. Modelling and optimization of fermentation factors for enhancement of alkaline protease production by isolated Bacillus circulans using feed-forward neural network and genetic algorithm. J Appl Microbiol. 2008;104:889–898. doi: 10.1111/j.1365-2672.2007.03605.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Liu Z, Qu Y, Li X. The effects of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Appl Biochem Biotechnol. 2008;146:119–128. doi: 10.1007/s12010-007-8049-3. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Ohara T, Yokomichi N, Nako T, Yahagi N, Igarashi S, Kobayashi Y, Yoshimatsu M, Takizawa K, Nakajima Y, Kiguchi K, Ishizuka B. Risk factors for perioperative venous thromboembolism: a retrospective study in Japanese women with gynecologic diseases. Thromb J. 2010;8:1–9. doi: 10.1186/1477-9560-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja K, Bajaj BK, Kumar S, Dilbaghi N. Production, purification and characterization of fibrinolytic enzyme from Serratia sp KG-2-1 using optimized media. 3 Biotech. 2017;7:184. doi: 10.1007/s13205-017-0808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trincone A. Marine biocatalysts: enzymatic features and applications. Mar Drugs. 2011;9:478–499. doi: 10.3390/md9040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hou Y, Xu Z, Miao J, Li G. Optimization of cold-active protease production by the psychrophilic bacterium Colwellia sp. NJ341 with response surface methodology. Bioresour Technol. 2008;99:1926–1931. doi: 10.1016/j.biortech.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Wang C, Du M, Zheng D, Kong F, Zu G, Feng Y. Purification and characterization of nattokinase from Bacillus subtilis Natto B-12. J Agric Food Chem. 2009;57:9722–9729. doi: 10.1021/jf901861v. [DOI] [PubMed] [Google Scholar]
- Waters AL, Hill RT, Place AR, Hamann MT. The expanding role of marine microbes in pharmaceutical development. Curr Opin Biotechnol. 2010;21:780–786. doi: 10.1016/j.copbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PG. Panning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotechnol. 2008;27:45–52. doi: 10.1016/j.tibtech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Yogesh D, Halami PM. A fibrin degrading serine metallo protease of Bacillus circulans with α-chain specificity. Food Biosci. 2015;11:72–78. doi: 10.1016/j.fbio.2015.04.007. [DOI] [Google Scholar]
- Yoon S, Ha S, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]