Abstract
Evaluation of minor salivary gland biopsy can be fraught with a wide range of problems, including technical limitations due to the small size and distorted nature of tissue received and interpretive difficulties navigating the considerable morphologic and immunohistochemical overlap between widely disparate entities. As such, common pathologic findings can evoke a perplexing differential diagnosis that encompasses malignant, benign, and non-neoplastic processes. This review will present the diagnostic considerations that arise from four histologic patterns that are frequently encountered on minor salivary gland biopsies: squamous differentiation, tubular and cribriform growth, mucin production, and myxoid stroma. The discussion herein will emphasize practical strategies and priorities for navigating these differential diagnoses in a clinically-relevant and cost-effective manner.
Keywords: Minor salivary glands, Mucoepidermoid carcinoma, Adenoid cystic carcinoma, Polymorphous adenocarcinoma, Pleomorphic adenoma, Clear cell carcinoma
Introduction
Although salivary gland lesions comprise only a small fraction of oral cavity pathology, biopsies from minor salivary glands can pose a broad spectrum of diagnostic challenges. Some of these difficulties are factors inherent in interpreting any small biopsy: obscuring tissue distortion, scant material for ancillary testing, and inability to evaluate the interface with normal tissue. Other issues arise in the evaluation of any salivary gland specimen: morphologic similarity between different tumor types, overlapping immunohistochemical profiles, and limited experience with salivary entities among general surgical pathologists. These diverse problems can combine to generate frustrating differential diagnoses that encompass malignant neoplasms, benign tumors, and non-neoplastic processes and span both salivary and non-salivary lesions. Several excellent recent reviews have provided a detailed overview of common salivary tumor types and the ancillary testing available to aid in their diagnosis [1–4], and a comprehensive characterization of these neoplasms will not be provided here. Instead, this review will focus on four morphologic challenges commonly encountered in minor salivary biopsies: squamous differentiation, tubular and cribriform growth, mucin production, and myxoid stroma. Discussion will emphasize practical strategies and priorities for resolving the differential diagnoses for each of these patterns in a clinically-relevant and cost-effective manner.
General Considerations
Before delving into specific diagnostic quandaries, it is worthwhile to first consider a few principles to guide the approach to minor salivary biopsies. One important question when formulating a differential diagnosis is the likelihood of encountering any given lesion in a minor salivary gland. Although pleomorphic adenoma (PA) is the most common benign tumor and mucoepidermoid carcinoma (MEC) is the most common malignant tumor regardless of anatomic site, the frequency of other neoplasms varies widely between major and minor glands [5–7]. Polymorphous adenocarcinoma (PAC), cribriform adenocarcinoma of minor salivary glands (CAMSG), clear cell carcinoma (CCC), and canalicular adenoma (CA) almost exclusively affect minor salivary glands, whereas acinic cell carcinoma, salivary duct carcinoma, epithelial-myoepithelial carcinoma, basal cell adenocarcinoma, and basal cell adenoma are rare outside major glands. Meanwhile, MEC, adenoid cystic carcinoma (ACC), secretory carcinoma (SC), PA, and myoepithelioma occur in either site. As such, even though tumors that preferentially involve major glands cannot be ruled out de-facto in minor salivary sites, entities that arise more commonly in these locations should be the first considerations in the differential diagnosis.
Another key issue in the evaluation of minor salivary lesions is the availability of relevant ancillary testing and the necessity of employing it in any given case. Recurrent genetic events have been identified in the vast majority of salivary gland tumors, and fluorescence in-situ hybridization (FISH) assays that reliably detect many of them are readily available for the diagnostic laboratory. However, given the high cost of molecular assays and limited tissue available, a more relevant question is when these tests need to be performed. Specific classification of most salivary gland neoplasms usually is possible using H&E alone or with the aid of a few key immunohistochemical stains (IHC); if a definitive diagnosis cannot be reached on small biopsy material, it frequently becomes obvious on the subsequent resection specimen. Additionally, even though defining molecular findings are present in the vast majority of certain salivary tumors, such as ETV6-NTRK3 in SC [8] and EWSR1-ATF1 in CCC [9], genetic abnormalities such as CRTC1-MAML2 in MEC [10] and MYB-NFIB in ACC [11, 12] are only identified in 50–80% of these lesions, meaning that a negative result may not rule out a given diagnosis. Furthermore, while translocation-positive tumors have a controversial association with better outcomes in MEC and ACC [4, 13], and targeted TRK inhibitors show great promise in rare patients with advanced SC [14], identification of specific gene fusions currently carries limited clinical utility for most patients in the biopsy setting. Consequently, it is reasonable to reserve ancillary testing on minor salivary gland biopsies for situations where confirming a specific diagnosis would determine the extent of subsequent operative management.
Challenge #1: Squamous Differentiation
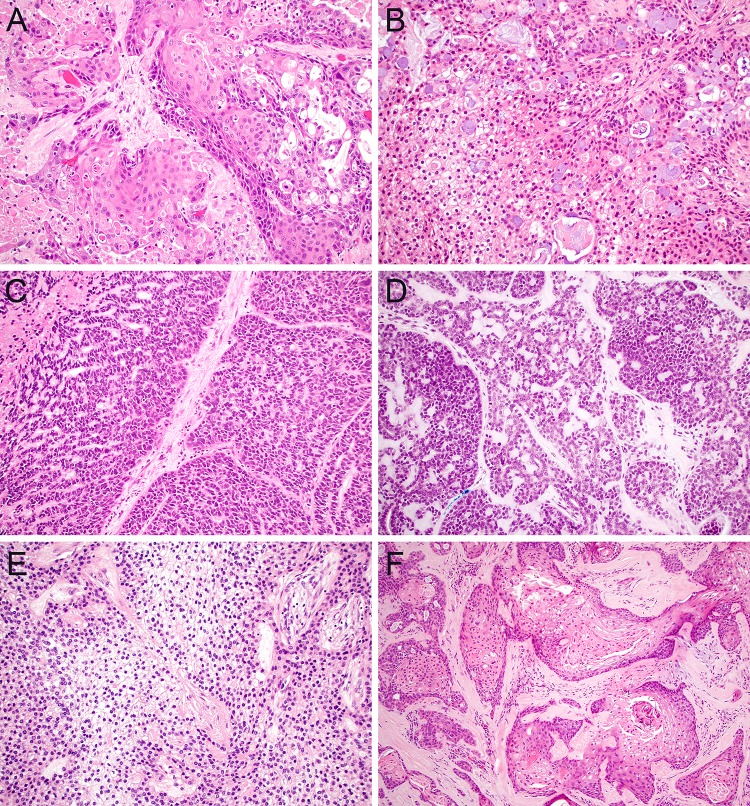
One of the most important considerations in evaluating minor salivary gland biopsies is differentiating salivary gland lesions that demonstrate squamous differentiation from squamous cell carcinoma (SCC). This is one distinction that is essential to make at the time of biopsy if possible, since salivary carcinomas and SCC can require different surgical management. There are two variants of SCC that are potent mimickers of minor salivary gland neoplasms. Adenosquamous carcinoma is a rare subtype of SCC that can be mistaken for MEC because it shows both squamous features and glandular components [15]. Morphologically, the presence of overt surface dysplasia and biphasic architecture with restriction of glandular elements to the deeper aspect of the tumor favors adenosquamous carcinoma (Fig. 1a), whereas true goblet cell formation and the absence of significant overt keratinization favors MEC (Fig. 1b). IHC is not particularly helpful in this differential diagnosis as both adenosquamous carcinoma and MEC show diffuse positivity for p63 and p40. Although FISH for MAML2 rearrangement is specific for MEC within this differential diagnosis [16], this finding has been reported to be less common in high-grade MEC [17, 18]. As such, it may not be possible to distinguish these lesions on biopsy in all cases. Similarly, the basaloid variant of SCC shows overlap with high-grade ACC due to pseudo-glandular architecture, myxoid matrix deposition, and production of abundant basement membrane material [19]. The presence of surface dysplasia, well-developed keratinization, marked nuclear pleomorphism, and comedo-pattern necrosis all support classification as basaloid SCC (Fig. 1c) while identification of more monotonous nuclei and well-formed cribriform spaces is consistent with a diagnosis of ACC (Fig. 1d). IHC can also facilitate this distinction, with only focal expression of myoepithelial markers such as S100 and SMA in basaloid SCC [20, 21]; however, frequent reactivity for c-kit [22] and SOX10 [23] in basaloid SCC can pose a pitfall. FISH for MYB rearrangements can confirm a classification as ACC, but a negative result does not exclude this diagnosis [11, 12].
Fig. 1.
Squamous differentiation. Adenosquamous carcinoma demonstrates overt squamous differentiation with glandular features that are generally restricted to the deeper aspect of the tumor (a, × 20), while MEC lacks true keratinization with an intimate admixture of epidermoid and goblet cells throughout the tumor (b, × 20). Basaloid SCC can show adenoidal growth with myxoid to hyaline matrix deposition but also has marked nuclear pleomorphism, focal keratinization, and comedo-pattern necrosis (c, × 20) whereas ACC usually shows at least focal cribriform architecture and greater cytological monotony (d, × 20). CCC also demonstrates a squamoid appearance but consistently has low-grade cytology and lacks overt keratinization (e, × 20). Prominent squamous metaplasia can frequently be seen in PA, but residual ductal and myoepithelial elements are generally evident (f, × 10)
Conversely, several minor salivary gland lesions, including malignant, benign, and non-neoplastic processes, can also mimic SCC. CCC is a low grade malignancy that poses a notorious pitfall in the diagnosis of SCC due to its clear to eosinophilic cytoplasm, infiltrative growth pattern, and diffuse positivity for squamous markers such as p63 and p40 (Fig. 1e). CCC has also recently been reported to show frequent p16 positivity [24]- an especially acute peril when it arises in the base of tongue. The cytologic monotony, lack of significant keratinization, and distinctive hyalinized stroma in CCC should point away from SCC. However, no IHC can reliably aid this distinction, and FISH for EWSR1 rearrangement, which is highly sensitive for this diagnosis, may be necessary to confirm classification as CCC in limited material [9]. PA also poses a significant risk of being misdiagnosed as SCC when it undergoes squamous metaplasia (Fig. 1f), which can be diffuse and dramatic in the setting of fine needle aspiration or local trauma [25]. Squamous metaplasia in PA can be especially challenging in minor salivary sites, where fibrous or hyaline stroma and an incomplete capsule can make the underlying adenoma difficult to recognize [26]. A clinical impression of a well-circumscribed submucosal mass and histologic appreciation of the circumscribed growth, bland cytology, and residual ductal and myoepithelial elements are the most helpful discriminatory features. Finally, necrotizing sialometaplasia is a non-neoplastic process that can also be mistaken for SCC. Necrotizing sialometaplasia occurs when minor salivary glands undergo ischemic changes, resulting in replacement of ducts and acini with squamous epithelium [27]. The deep location, prominent reactive atypia, and ischemic necrosis seen in necrotizing sialometaplasia can mimic SCC. Nevertheless, appreciation of the lobulated nature of the squamous nests and close association with intact minor salivary glands can avoid this pitfall.
Challenge #2: Cribriform and Tubular Growth
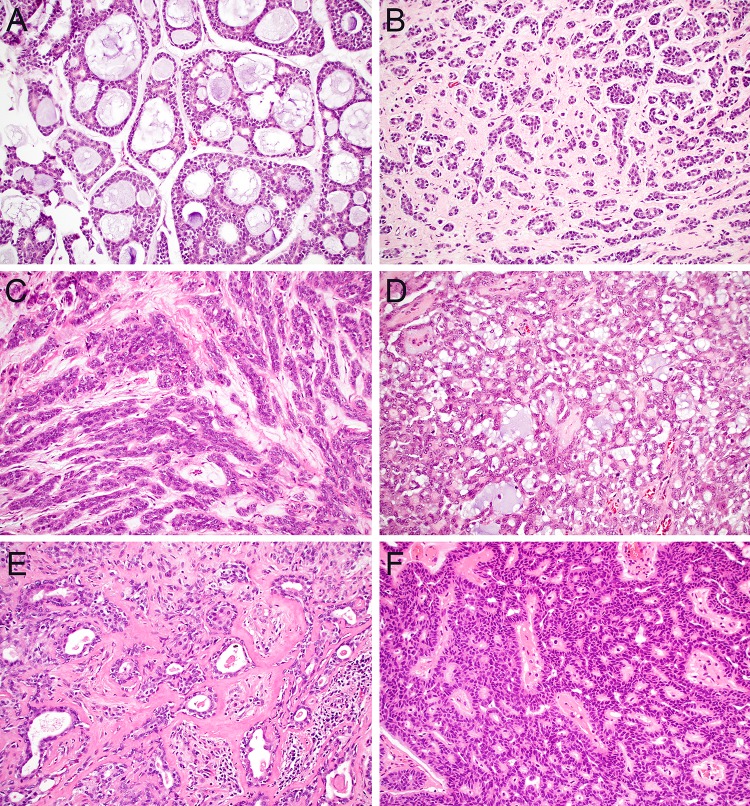
Another perennial challenge in salivary gland pathology is the distinction between the various salivary tumors that show cribriform and tubular growth. In minor salivary glands, malignant tumors such as ACC, PAC, and CAMSG as well as benign tumors such as PA and CA can all demonstrate these overlapping patterns. At the time of biopsy, the most critical tumor to differentiate from this spectrum is ACC, which is consistently managed more aggressively than other salivary carcinomas. Fortunately, despite broadly overlapping architecture, all of the tumors within this differential diagnosis have other characteristic histologic features that usually make it possible to classify them based on morphology alone, even in limited biopsy material. ACC generally shows at least focal classic cribriform growth with sharply punched out spaces containing myxoid or hyaline matrix (Fig. 2a); its tubules are biphasic with clear inner ductal and outer myoepithelial elements that demonstrate hyperchromatic and angulated nuclei (Fig. 2b). In contrast, one of the most distinctive features of PAC is monotonous oval nuclei with open, ground-glass chromatin that can mimic papillary thyroid carcinoma; it contains tubules and cords of monophasic cells with peripheral streaming (Fig. 2c) and a unique targetoid pattern of perineural invasion. Despite its name, CAMSG demonstrates more poorly-formed cribriform spaces than ACC with accompanying glomeruloid, solid, and tubular elements and nuclei similar to PAC (Fig. 2d). PA contains a characteristically rich variety of architectural patterns and stromal elements with nondescript round to oval nuclei (Fig. 2e). Finally, CA is defined by prominent anastomosing cords and ribbons of basaloid cells with a beading pattern at the points where the strands connect (Fig. 2f).
Fig. 2.
Cribriform and tubular growth. ACC contains cells with hyperchromatic angulated nuclei that generally form at least focal well-formed cribriform spaces (a, × 20); tubular growth in ACC tends to be biphasic with distinct ductal and myoepithelial elements (b, × 20). Classic PAC is comprised of monophasic strands and tubules that show a streaming appearance (c, × 20) while CAMSG has mostly poorly-formed cribriform spaces with additional glomeruloid and tubular patterns (d, × 20). PA demonstrates a broad range of architectural patterns and stromal appearances with both ductal and myoepithelial components (e, × 20). CA consists of anastomosing cords of basaloid cells with a beaded appearance (f, × 20)
If morphology alone does not allow for a specific diagnosis in tumors with cribriform and tubular architecture, ancillary testing can provide a helpful yet imperfect aid. As discussed above, although MYB-NFIB is the most common fusion in ACC, a significant subset of tumors with alternate or undefined translocations will be negative for MYB FISH [11, 12, 28, 29]. MYB IHC provides a sensitive but not entirely specific correlate for these molecular findings [30]. PA frequently harbors PLAG1 or HMGA2 fusions with various partners [31]. Although rarely necessary for diagnosis, these changes can reliably be identified by FISH or corresponding IHC [32, 33]. A diverse and overlapping range of molecular findings are seen in PAC and CAMSG, including hotspot activating PRKD1 mutations in PAC [34] and PRKD1, PRKD2, and PRKD3 fusions or rearrangements in CAMSG [35]. However, testing for these alterations is not readily available in most clinical laboratories. Recurrent molecular alterations have not been identified in CA. Recently, IHC has emerged as a helpful aid for differentiating cribriform and tubular salivary gland tumors as well. Even though many stains historically attempted in this differential diagnosis show significant overlap between tumor types, S100, p63, and p40 carry considerable discriminatory utility. While both ACC and PA show only patchy S100 expression, PAC, CAMSG, and CA consistently demonstrate diffuse, strong positivity [36–38]. Additionally, PAC and CAMSG have a unique discordant p63-positive, p40-negative immunophenotype while ACC and PA are generally positive for both p63 and p40 in an abluminal/myoepithelial distribution [39, 40]; CA are largely p63-negative [41].
Challenge #3: Mucin Production
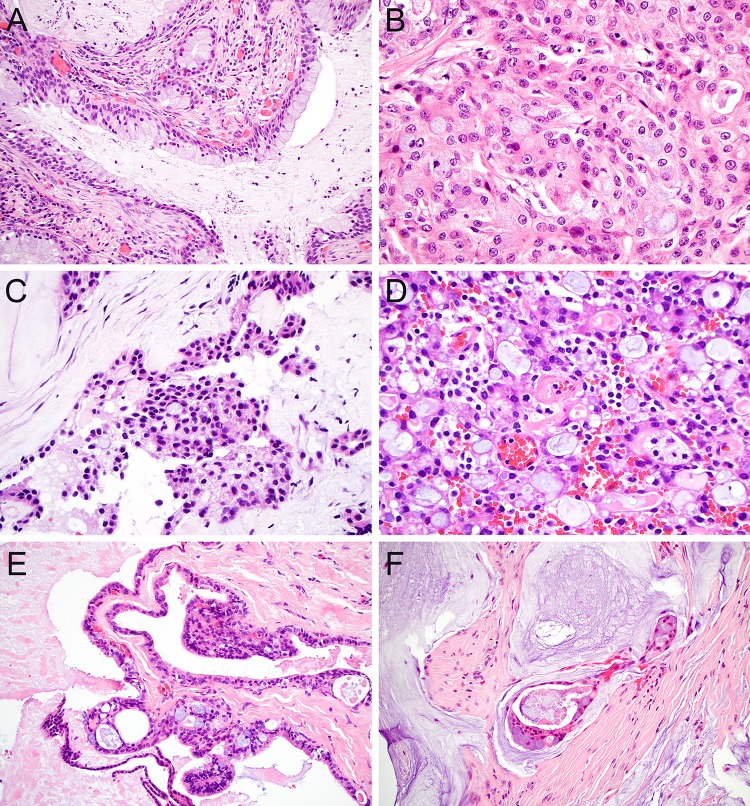
Another common differential dilemma in minor salivary biopsies is distinguishing the multiple lesions that produce mucinous secretions. Of course, MEC is the archetypal mucin-producing salivary gland neoplasm. In its most classic form, low-grade MEC demonstrates abundant goblet cells and frequent cyst formation with prominent luminal mucin (Fig. 3a); cyst rupture with mucin extravasation into surrounding stroma is also common. However, mucin production can be somewhat scant in high-grade tumors as well as rare subtypes such as oncocytic variant (Fig. 3b), making this diagnosis considerably more challenging. In cases with less classic morphology, identification of MAML2 translocations by FISH can help confirm classification as MEC [42, 43], although this finding is not entirely sensitive for the diagnosis. It is also essential to remember that mucin production is also seen in other salivary malignancies that should not be mistaken for MEC. Perhaps the most challenging differential diagnosis is CCC, which can produce mucin in almost 50% of cases [9]; these tumors also show diffuse p63 and p40 positivity and squamoid features that overlap with MEC (Fig. 3c). A recent evaluation of translocation-negative MEC revealed the presence of several CCC [44], highlighting this issue as a significant diagnostic pitfall. While the distinctive corded growth pattern, hyalinized stroma, and a lack of true goblet cells can favor a diagnosis of CCC over MEC, EWSR1 FISH may be necessary in cases with unusual morphology. SC is another important consideration for a mucin-producing minor salivary gland neoplasms. Although widely recognized for its foamy eosinophilic secretions, luminal mucin is also commonly seen in SC (Fig. 3d). Fortunately, the characteristic microcystic and papillary-cystic architecture, abundant vacuolated cytoplasm, and vesicular nuclei of SC can help distinguish it from MEC. By IHC, positivity for S100 and mammaglobin is also highly specific for this diagnosis [45]; demonstration of consistent ETV6 rearrangements by FISH can confirm classification as SC [8].
Fig. 3.
Mucin production. Low-grade MEC classically contains prominent goblet cells and cyst formation with abundant luminal mucin (a; × 20); goblet cells can be much more sparse in the oncocytic variant of MEC (b, × 40). CCC frequently demonstrate at least focal mucin production (c, × 40). SC has characteristic luminal secretions that can be both eosinophilic and mucinous (d, × 40). Although salivary duct cysts can demonstrate mucinous metaplasia, the presence of papillary and cribriform elements confirms the diagnosis of MEC (e, × 20). While mucoceles can demonstrate prominent extravasated mucin, floating epithelium with complex architecture favors MEC (d, × 20)
Mucin production is commonly seen in a broad spectrum of non-neoplastic salivary entities that can also mimic salivary carcinomas, particularly MEC. As these benign reactive processes do not usually require additional resection, they should be carefully differentiated from true neoplasms at the time of biopsy. The non-neoplastic lesions that most closely overlap with true tumors are salivary duct cysts, also known as mucous retention cysts. These cysts, which are thought to represent dilation of the salivary duct system secondary to obstruction, can demonstrate a wide range of metaplastic changes, most notably including mucinous metaplasia with well-developed goblet cell formation [46]. Importantly, even though a significant subset of salivary duct cysts can show irregularities and undulations of their walls, the absence of complex glandular architecture or infiltrative growth can help distinguish them from MEC (Fig. 3e). Necrotizing sialometaplasia can pose a pitfall in the mucinous differential diagnosis as well. Minor salivary glands undergoing necrotizing sialometaplasia display a spectrum of histologic appearances as ischemic changes evolve [27]. Although complete replacement of lobules with squamous metaplasia raises concern for SCC as discussed above, partial replacement with residual mucinous material may show more overlap with MEC. The lobulated nature of the proliferation and association with intact glands are the most useful features to resolve this differential diagnosis. Finally, extravasational mucoceles also pose a small risk of being mistaken for salivary tumors when large pools of extravasated mucin are present in association with damaged minor salivary epithelium [47]. However, an absence of abnormal epithelial proliferation in mucoceles should help differentiate them from MEC (Fig. 3f).
Challenge #4: Myxoid Stroma
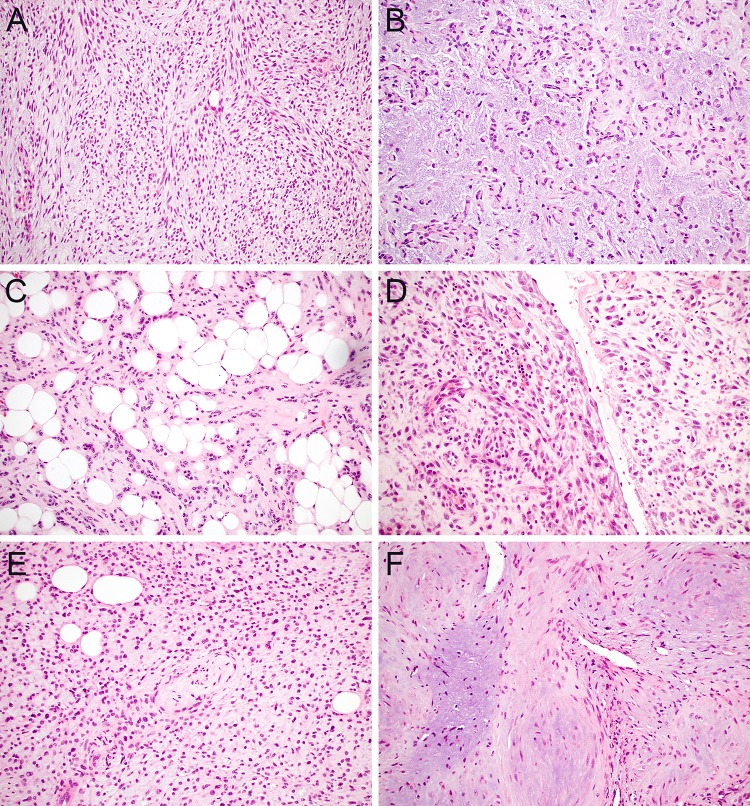
Finally, tumors that demonstrate prominent myxoid stroma also pose a unique spectrum of diagnostic challenges in minor salivary biopsy. PA is by far the most common salivary gland neoplasm that demonstrates myxoid to chondromyxoid stroma; its monophasic counterpart myoepithelioma also can show prominent myxoid stromal change. Although these tumors demonstrate a wide range of cellularity and histologic patterns, the myxoid stroma is generally more prominent in areas with embedded spindled (Fig. 4a), stellate, or plasmacytoid (Fig. 4b) myoepithelial cells. However, it should not be assumed that the presence of myxoid stroma is a prerequisite for the diagnosis of PA or myoepithelioma. Indeed, approximately half of PAs in minor salivary glands have stroma that is predominantly fibrous or hyalinized [26]. Fatty stromal metaplasia can also rarely be seen in PA and is important to recognize so associated epithelial or myoepithelial cells are not mistaken for invasive growth (Fig. 4c). Furthermore, PA and myoepithelioma are not the only salivary gland lesions that demonstrate myxoid stromal changes. Extravasational mucocele often shows extensive infiltration of mucin into oral stroma that can masquerade as a myxoid neoplasm [47]. In these lesions, prominent muciphages, reactive fibroblasts and synovial metaplasia can also mimic neoplastic cells (Fig. 4d). Additionally, myxoid stroma can be at least focally seen in a wide range of other salivary neoplasms ranging from CA to PAC, but all of these tumors have a predominance of cellular elements that aids classification.
Fig. 4.
Myxoid stroma. A defining feature of PA is myxoid to chondromyxoid stroma embedded with myoepithelial cells that demonstrate a spindled to stellate morphology (a, × 20); myoepitheliomas also can have myxoid stroma and often show plasmacytoid myoepithelial morphology (b, × 20). PA frequently lacks myxoid stroma in minor salivary glands, and the presence of fatty stromal metaplasia should not be mistaken for invasive growth (c, × 20). Extravasational mucoceles can mimic a myxoid neoplasm due to extensive stromal mucin deposition, prominent muciphages, and synovial metaplasia (d, × 20). ECMT contains a net-like array of ovoid, stellate, and spindled cells in a myxoid stroma (e, × 20). Myofibromas have myxoid stroma with hypocellular myoid nodules and more hypercellular peripheral aggregates of spindled to epithelioid cells (f, × 20)
But myxoid stroma is not restricted to primary salivary lesions in the oral cavity; mesenchymal neoplasms that also have myxoid features can mimic salivary lesions on small biopsy material. The main overlap between myoepithelial-rich and mesenchymal neoplasms occurs with ectomesenchymal chondromyxoid tumor (ECMT), a unique benign tumor that is defined by net-like cords and trabeculae of oval, stellate, and spindled cells embedded in myxoid stroma (Fig. 4e). Interestingly, anatomic site is extremely helpful in this differential diagnosis, as ECMT are essentially restricted to the anterior tongue [48] and salivary tumors rarely occur in this location. Positivity for both S100 and GFAP with minimal cytokeratin expression also can help distinguish ECMT from salivary tumors [49]. Although not usually needed for diagnosis, up to 90% of ECMTs have recently been shown to harbor recurrent RREB1-MRTFB fusions [50]. Beyond ECMT, a wide range of other mesenchymal neoplasms with myxoid stroma and spindled to epithelioid cells can also raise consideration of salivary neoplasms in limited material. Myofibroma is perhaps the most common of these lesions to occur in the oral cavity [51]; it is characterized by hypocellular myoid nodules with myxoid stroma interspersed with more dense proliferations of spindled to ovoid cells with hemangiopericytoma-like vessels (Fig. 4f). While myofibroma is positive for SMA, negativity for cytokeratin and S100 can help confirm its mesenchymal nature. Other tumors that less frequently occur in the oral cavity but can fall within this diagnostic spectrum include ossifying fibromyxoid tumor and myxoid nerve sheath tumors.
Conclusion
A vast spectrum of malignant, benign, and non-neoplastic entities that demonstrate overlapping histologic and immunohistochemical features can be seen in minor salivary biopsy. This review details the diagnostic considerations that arise from four common patterns encountered in these specimens: squamous differentiation, tubular and cribriform growth, mucin production, and myxoid stroma. While these findings each evoke a broad differential diagnosis, careful attention to morphologic details, parsimonious application of key IHC, and strategic use of molecular testing can allow for specific classification in a clinically-relevant and cost-effective fashion.
Compliance with Ethical Standards
Conflict of interest
The author has no conflicts of interest to declare. This work is exempt from IRB review.
References
- 1.Griffith CC, Schmitt AC, Little JL, Magliocca KR. New developments in salivary gland pathology: clinically useful ancillary testing and new potentially targetable molecular alterations. Arch Pathol Lab Med. 2017;141(3):381–395. doi: 10.5858/arpa.2016-0259-SA. [DOI] [PubMed] [Google Scholar]
- 2.Jo VY, Krane JF. Ancillary testing in salivary gland cytology: a practical guide. Cancer Cytopathol. 2018;126(Suppl 8):627–642. doi: 10.1002/cncy.22010. [DOI] [PubMed] [Google Scholar]
- 3.Seethala RR. Salivary gland tumors: current concepts and controversies. Surg Pathol Clin. 2017;10(1):155–176. doi: 10.1016/j.path.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Skalova A, Stenman G, Simpson RHW, Hellquist H, Slouka D, Svoboda T, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42(2):e11–e27. doi: 10.1097/PAS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 5.Pires FR, Pringle GA, de Almeida OP, Chen SY. Intra-oral minor salivary gland tumors: a clinicopathological study of 546 cases. Oral Oncol. 2007;43(5):463–470. doi: 10.1016/j.oraloncology.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Venkata V, Irulandy P. The frequency and distribution pattern of minor salivary gland tumors in a government dental teaching hospital, Chennai, India. Oral Surg, Oral Medicine, Oral Pathol, Oral Radiol, Endod. 2011;111(1):e329. doi: 10.1016/j.tripleo.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66(3):323–333. doi: 10.1016/0030-4220(88)90240-x. [DOI] [PubMed] [Google Scholar]
- 8.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 9.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 10.Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 11.Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17(22):7003–7014. doi: 10.1158/1078-0432.CCR-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson M, Andren Y, Moskaluk CA, Frierson HF, Jr, Cooke SL, Futreal PA, et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer. 2012;51(8):805–817. doi: 10.1002/gcc.21965. [DOI] [PubMed] [Google Scholar]
- 13.Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012;122(8):1690–1694. doi: 10.1002/lary.22419. [DOI] [PubMed] [Google Scholar]
- 14.Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7(4):400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad ML, Cardesa A, Helliwell TR, Hille J, Nadal A. Adenosquamous carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. p. 89. [Google Scholar]
- 16.Kass JI, Lee SC, Abberbock S, Seethala RR, Duvvuri U. Adenosquamous carcinoma of the head and neck: Molecular analysis using CRTC-MAML FISH and survival comparison with paired conventional squamous cell carcinoma. Laryngoscope. 2015;125(11):E371-6. doi: 10.1002/lary.25519. [DOI] [PubMed] [Google Scholar]
- 17.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 18.Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46(7):708–715. doi: 10.1002/gcc.20458. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JS, Gillison ML, Westra WH, Zidar N. Basaloid squamous cell carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. pp. 85–86. [Google Scholar]
- 20.Banks ER, Frierson HF, Jr, Mills SE, George E, Zarbo RJ, Swanson PE. Basaloid squamous cell carcinoma of the head and neck. A clinicopathologic and immunohistochemical study of 40 cases. Am J Surg Pathol. 1992;16(10):939–946. doi: 10.1097/00000478-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Emanuel P, Wang B, Wu M, Burstein DE. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18(5):645–650. doi: 10.1038/modpathol.3800329. [DOI] [PubMed] [Google Scholar]
- 22.Mino M, Pilch BZ, Faquin WC. Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma. Mod Pathol. 2003;16(12):1224–1231. doi: 10.1097/01.MP.0000096046.42833.C7. [DOI] [PubMed] [Google Scholar]
- 23.Rooper LM, McCuiston AM, Westra WH, Bishop JA. SOX10 immunoexpression in basaloid squamous cell carcinomas: a diagnostic pitfall for ruling out salivary differentiation. Head Neck Pathol. 2018;42:665–671. doi: 10.1007/s12105-018-0990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop JA, Rooper LM, Chiosea SI, Westra WH. Clear cell carcinoma of salivary glands is frequently p16 positive: a pitfall in the interpretation of oropharyngeal biopsies. Am J Surg Pathol. 2018;42(3):367–371. doi: 10.1097/PAS.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boecker W, Stenman G, Loening T, Andersson MK, Berg T, Lange A, et al. Squamous/epidermoid differentiation in normal breast and salivary gland tissues and their corresponding tumors originate from p63/K5/14-positive progenitor cells. Virchows Arch. 2015;466(1):21–36. doi: 10.1007/s00428-014-1671-x. [DOI] [PubMed] [Google Scholar]
- 26.Lopes M, Barroso KMA, Henriques ACG, Dos Santos JN, Martins MD, de Souza LB. Pleomorphic adenomas of the salivary glands: retrospective multicentric study of 130 cases with emphasis on histopathological features. Eur Arch Otorhinolaryngol. 2017;274(1):543–551. doi: 10.1007/s00405-016-4253-5. [DOI] [PubMed] [Google Scholar]
- 27.Carlson DL. Necrotizing sialometaplasia: a practical approach to the diagnosis. Arch Pathol Lab Med. 2009;133(5):692–698. doi: 10.5858/133.5.692. [DOI] [PubMed] [Google Scholar]
- 28.Mitani Y, Liu B, Rao PH, Borra VJ, Zafereo M, Weber RS, et al. Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res. 2016;22(3):725–733. doi: 10.1158/1078-0432.CCR-15-2867-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rettig EM, Talbot CC, Jr, Sausen M, Jones S, Bishop JA, Wood LD, et al. Whole-genome sequencing of salivary gland adenoid cystic carcinoma. Cancer Prev Res (Phila) 2016;9(4):265–274. doi: 10.1158/1940-6207.CAPR-15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brill LB, 2nd, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 31.Stenman G. Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol. 2013;7(Suppl 1):12-9. doi: 10.1007/s12105-013-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katabi N, Xu B, Jungbluth AA, Zhang L, Shao SY, Lane J, et al. PLAG1 immunohistochemistry is a sensitive marker for pleomorphic adenoma: a comparative study with PLAG1 genetic abnormalities. Histopathology. 2018;72(2):285–293. doi: 10.1111/his.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mito JK, Jo VY, Chiosea SI, Dal Cin P, Krane JF. HMGA2 is a specific immunohistochemical marker for pleomorphic adenoma and carcinoma ex-pleomorphic adenoma. Histopathology. 2017;71(4):511–521. doi: 10.1111/his.13246. [DOI] [PubMed] [Google Scholar]
- 34.Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. doi: 10.1038/ng.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–856. doi: 10.1002/gcc.22195. [DOI] [PubMed] [Google Scholar]
- 36.Darling MR, Schneider JW, Phillips VM. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: a review and comparison of immunohistochemical markers. Oral Oncol. 2002;38(7):641–645. doi: 10.1016/s1368-8375(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 37.Ferreiro JA. Immunohistochemical analysis of salivary gland canalicular adenoma. Oral Surg Oral Med Oral Pathol. 1994;78(6):761–765. doi: 10.1016/0030-4220(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32(6):521–529. doi: 10.1046/j.1365-2559.1998.t01-2-00410.x. [DOI] [PubMed] [Google Scholar]
- 39.Owosho AA, Aguilar CE, Seethala RR. Comparison of p63 and p40 (DeltaNp63) as basal, squamoid, and myoepithelial markers in salivary gland tumors. Appl Immunohistochem Mol Morphol. 2016;24(7):501–508. doi: 10.1097/PAI.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 40.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40- immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015;9(1):79–84. doi: 10.1007/s12105-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson LD, Bauer JL, Chiosea S, McHugh JB, Seethala RR, Miettinen M, et al. Canalicular adenoma: a clinicopathologic and immunohistochemical analysis of 67 cases with a review of the literature. Head Neck Pathol. 2015;9(2):181–195. doi: 10.1007/s12105-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia JJ, Hunt JL, Weinreb I, McHugh JB, Barnes EL, Cieply K, et al. Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: utility as a diagnostic test. Hum Pathol. 2011;42(12):2001–2009. doi: 10.1016/j.humpath.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Bishop JA, Cowan ML, Shum CH, Westra WH. MAML2 rearrangements in variant forms of mucoepidermoid carcinoma: ancillary diagnostic testing for the ciliated and warthin-like variants. Am J Surg Pathol. 2018;42(1):130–136. doi: 10.1097/PAS.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsieh MS, Wang H, Lee YH, Ko JY, Chang YL. Reevaluation of MAML2 fusion-negative mucoepidermoid carcinoma: a subgroup being actually hyalinizing clear cell carcinoma of the salivary gland with EWSR1 translocation. Hum Pathol. 2017;61:9–18. doi: 10.1016/j.humpath.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013;44(10):1982–1988. doi: 10.1016/j.humpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stojanov IJ, Malik UA, Woo SB. Intraoral salivary duct cyst: clinical and histopathologic features of 177 cases. Head Neck Pathol. 2017;11(4):469–476. doi: 10.1007/s12105-017-0810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chi AC, Lambert PR, 3rd, Richardson MS, Neville BW. Oral mucoceles: a clinicopathologic review of 1,824 cases, including unusual variants. J Oral Maxillofac Surg. 2011;69(4):1086–1093. doi: 10.1016/j.joms.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 48.Smith BC, Ellis GL, Meis-Kindblom JM, Williams SB. Ectomesenchymal chondromyxoid tumor of the anterior tongue. Nineteen cases of a new clinicopathologic entity. Am J Surg Pathol. 1995;19(5):519–530. doi: 10.1097/00000478-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Aldojain A, Jaradat J, Summersgill K, Bilodeau EA. Ectomesenchymal chondromyxoid tumor: a series of seven cases and review of the literature. Head Neck Pathol. 2015;9(3):315–322. doi: 10.1007/s12105-014-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickson BC, Antonescu CR, Argyris PP, Bilodeau EA, Bullock MJ, Freedman PD, et al. Ectomesenchymal chondromyxoid tumor: a neoplasm characterized by recurrent RREB1-MKL2 fusions. Am J Surg Pathol. 2018;42(10):1297–1305. doi: 10.1097/PAS.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montgomery E, Speight PM, Fisher C. Myofibromas presenting in the oral cavity: a series of 9 cases. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endod. 2000;89(3):343–348. doi: 10.1016/s1079-2104(00)70100-4. [DOI] [PubMed] [Google Scholar]