Abstract
Granulomatous lesions of the orofacial region are a heterogeneous group of disorders characterized by a granulomatous reaction to a variety of stimuli. Infectious agents, foreign material, systemic inflammation and metabolic disorders can all be associated with granulomatous inflammation. In the orofacial region primary causes of granulomatosis include foreign body reaction, delayed hypersensitivity to topical agents and idiopathic orofacial granulomatosis. Secondary causes of granulomas include infectious agents, sarcoid, and Crohn disease. For this review, infectious causes of orofacial granulomatosis (OFG) including bacteria, parasites and fungi will not be discussed.
Keywords: Orofacial granulomatosis, Sarcoid, Crohn disease, Cheilitis granulomatosis, Melkersson Rosenthal
Introduction
Sorting out the etiology and pathogenesis of granulomas in the orofacial region can be challenging as a wide range of disorders are associated with granulomatosis [1, 2]. Numerous etiologies have been proposed including genetic, immunologic, allergic, and infectious. Many cases of orofacial granulomatosis (OFG) are delayed type hypersensitivity, also termed type IV hypersensitivity reactions [1, 3, 4]. The granulomatous response represents a host defense mechanism to contain infections or foreign antigens, which are not always identified [3]. Local immune and host response promote the accumulation of macrophages and monocytes [5, 6]. The histologic appearance of the recruited cells, including the multinucleated giant cells within the tissue is called granulomatous inflammation. Granulomas are comprised chiefly of histiocytes whose main function is phagocytosis of the offending organism and/or foreign material and antigen presentation [7, 8]. Dendritic cells and T lymphocytes, predominately CD4+, are also present in granulomatous inflammation [5, 6]. Chemokines and cytokines are released, and these mediators recruit additional histiocytes [3, 5].
OFG may have both local and systemic etiologies (Fig. 1). Primary etiologies of orofacial granulomas include foreign material including amalgam, cosmetic fillers, and suture material [2]. Delayed hypersensitivity to flavoring agents and ingredients in oral hygiene products and dental restorations have been associated with granulomas [1, 9]. In some patients the etiology is unknown, and the process is termed idiopathic orofacial granulomatosis.
Fig. 1.
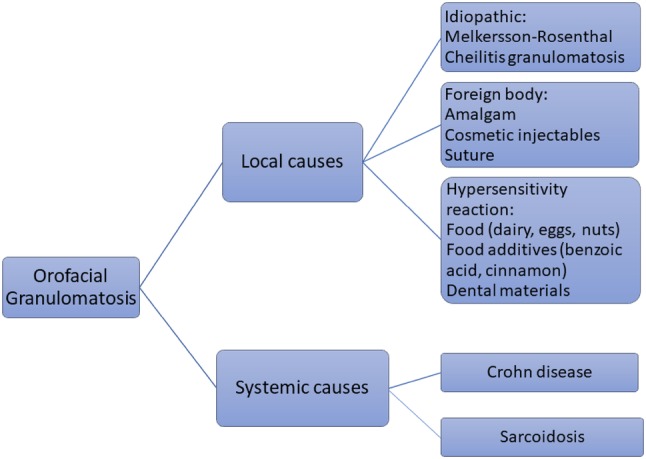
Local and systemic causes of non-infectious oral granulomas
Crohn disease (CD) and sarcoid are both systemic inflammatory diseases that can present as OFG [1–4, 10, 11]. Orofacial granulomas in children unlike in adults may presage the development of CD [12]. Granulomas may have an infectious etiology and appropriate work up including Periodic acid Schiff (PAS), Gomori methenamine–silver nitrate (GMS), and Ziehl–Neelsen (AFB) should be obtained as a matter of routine [7, 8]. Furthermore, if an infectious etiology is suspected and special stains are negative, culture and/or molecular studies are available to identify the causative agent.
The histology of non-infectious orofacial granulomas is non-necrobiotic/non-necrotizing but otherwise nonspecific and therefore the pathologist can only provide guidance to the clinician [3, 7, 8, 13]. The diagnosis, especially when no foreign material or infectious agent is identified requires additional testing to both rule out etiologies and to rule in a suspected etiology. This review will focus on the clinical and histologic features of local and systemic causes of non-infectious granulomas of orofacial soft tissue.
Local Causes of Noninfectious Orofacial Granulomatosis
Idiopathic Orofacial Granulomatosis
The term OFG was introduced by Wiesenfeld et al. in 1985 to describe a variety of clinical presentations that on biopsy show non-necrotizing granulomatous inflammation [14]. This term now encompasses Melkersson–Rosenthal syndrome and cheilitis granulomatosa of Miescher [3]. Melkersson–Rosenthal syndrome, first described in 1928 reported a patient with orofacial swelling, fissured tongue and facial palsy [15]. In 1945 cheilitis granulomatosa was described as granulomatous inflammation confined to the lips [16, 17]. The literature sometimes refers to cheilitis granulomatosa as a monosymptomatic form of Melkersson–Rosenthal syndrome [17].
The precise etiologies of idiopathic OFG are poorly understood. No specific genetic predisposition has been attributed to idiopathic OFG. Allergy to dental materials, toothpastes, food additives, dairy products, eggs, wheat and chocolate have been reported as possible inciting agents in idiopathic OFG [1–4]. Benzoates and cinnamic aldehyde are frequently reported as common triggers. Some studies report that patients with idiopathic OFG have a greater prevalence of atopy, however other studies do not show a clear association of food or contact sensitization [4]. Drug-related cheilitis has been reported, but often the temporal relationship of the offending medication and the onset of the lip swelling can occur months to years later. The diagnosis of idiopathic OFG is rendered when other known local and systemic causes of orofacial granulomas are ruled out.
The clinical presentation of idiopathic OFG is variable and can involve all facial tissues. The lip is the most common site of involvement, affecting more than 90% of patients, characterized by painless swelling which may be asymmetrical and involve one or both lips [18]. The swelling can last for a few days or weeks in the initial presentation and on palpation the lips are soft and doughy. With recurrence, the swelling becomes firm, indurated, and persists. Fissuring of the lips, exfoliation, and angular cheilitis can develop. Peri-oral dermatitis may be present. Facial swelling of the nonlabial tissues can occur, including the eyelids [18]. Granulomatous blepharitis is most likely a component of idiopathic OFG [19].
Neurologic manifestations of OFG (Melkersson–Rosenthal syndrome) involves the facial nerve and is typically unilateral. The exact prevalence of lower motor nerve facial palsy is unknown with a wide range reported in the literature of 8–30% [20]. The etiology of the facial nerve palsy is unknown. No documented biopsies of affected nerves have been reported although some authors propose inflammation or granuloma formation along the nerve pathway as a possible cause [1]. Often the triad of facial palsy, fissured tongue and orofacial edema is not seen, especially at time of presentation. As the symptoms can vary widely, various medical specialties including dermatology, oral pathology, otolaryngology and neurology are often consulted at different points in the disease [20, 21]. This can skew our understanding of idiopathic OFG as the lens in which the disease is viewed is colored by the clinician’s area of expertise.
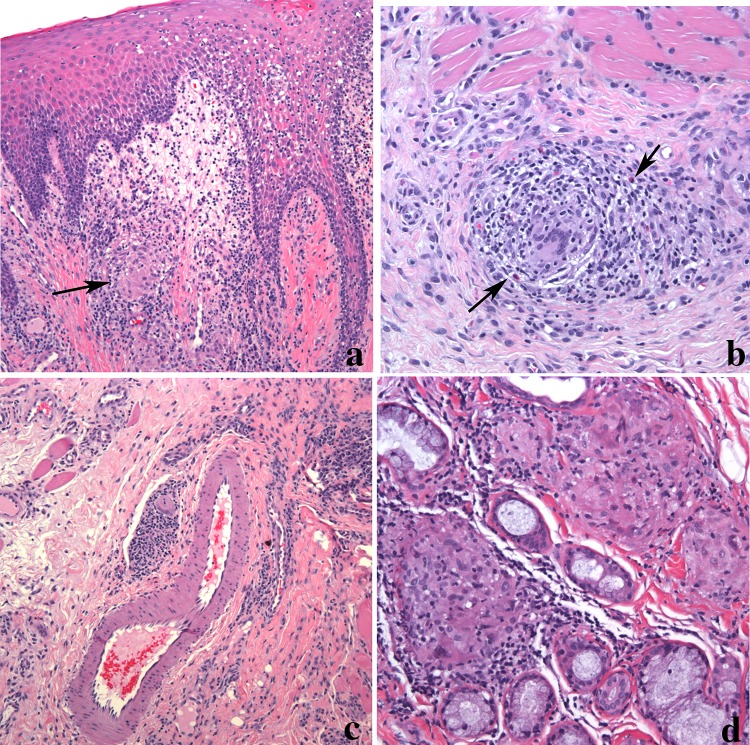
Corresponding to the clinical findings, the histologic features of OFG include edema of the superficial lamina propria with lymphatic and vascular ectasia (Fig. 2a). The overlying epithelium may show acanthosis but is uninvolved by the granulomatous inflammation. Transmigration of inflammatory cells can be seen and occasionally interface mucositis may be observed. The granulomas are composed of a loose collection of epithelioid histiocytes and lymphocytes. Multinucleated giant cells are not always evident. A mixed inflammatory cell infiltrate composed of plasma cells, lymphocytes and occasionally, eosinophils may be present adjacent to the granulomas unlike the typical “naked” granulomas associated with sarcoidosis (see below) (Fig. 2b). Granulomas are often present near blood vessels and can extend into the surrounding muscle and salivary glands (Fig. 2c, d) [7, 13]. At times, the granulomas may be present only in the deeper connective tissue and therefore a superficial biopsy may not demonstrate the granulomatous inflammation. Despite the name OFG, granulomas are not always detected. Depending on the series, granuloma identification ranges from 43 to 82% [13]. The presence of the intercellular edema coupled with the loose, noncompact nature of the granulomas may account for the absence of granulomas in some biopsies of OFG. As idiopathic OFG is a diagnosis of exclusion, appropriate stains to rule out an infectious origin is appropriate. PAS, GMS, and AFB stains should be negative. Attempts to identify foreign material, including polarization should be utilized.
Fig. 2.
Orofacial granulomatous in a lip biopsy. a Epithelial hyperplasia with inflammatory cell exocytosis overlying edematous connective tissue. Loosely arranged granulomas with histiocytic multinucleated giant cell (arrow) is seen (magnification × 100). b Granuloma composed of a mixture of inflammatory cells including eosinophils (arrow) extending into muscle (magnification × 200). c Granulomas are often present around dilated lymphovascular spaces (magnification × 100). d Granulomas can also extend into minor salivary glands. The granulomas in this image show a nodular collection of epithelioid histiocytes and giant cells surrounded by inflammation (magnification × 200)
The diagnosis of non-necrotizing OFG requires appropriate communication with the clinician to determine if there is an underlying cause. This is especially important when diagnosing OFG in a pediatric patient as early Crohn disease can present first with oral symptoms as highlighted below [22, 23]. However, despite appropriate clinical work-up for systemic causes and local causes, the inciting event is not often discovered.
Foreign Material
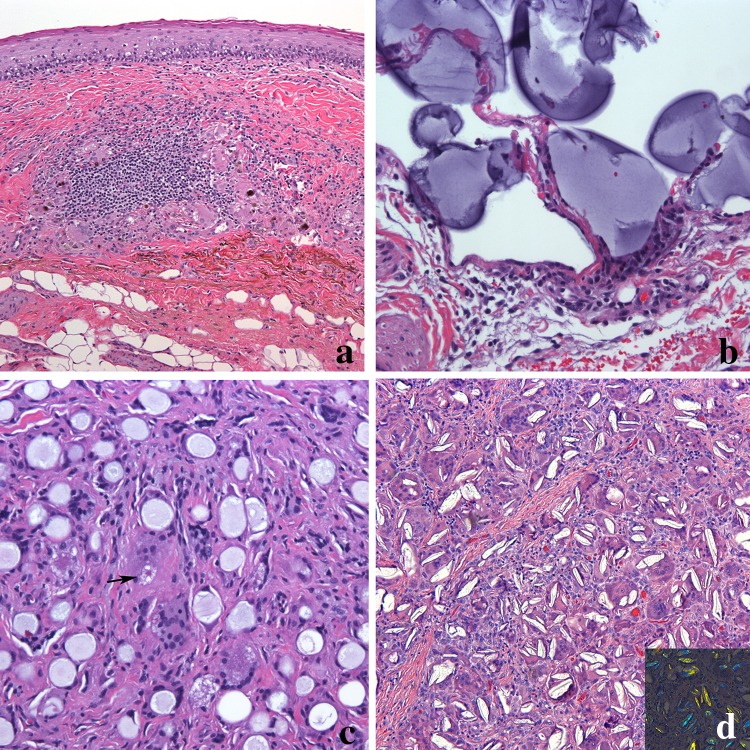
Non-necrotizing granulomas in the orofacial region can be caused by dental materials and cosmetic fillers. Amalgam is a common restorative material used in dentistry containing silver, copper, tin, zinc, mercury, palladium and indium [24]. At times there is iatrogenic implantation of the amalgam into the soft tissue termed amalgam tattoo. Generally, little to no inflammatory reaction is present in association with the implanted granules. However, on occasion, granulomas composed of macrophages, lymphocytes and giant cells are evident (Fig. 3a). If the patient does not have a history of amalgam restorations, other considerations are graphite implantation (pencil-related trauma). Cosmetic intraoral tattoos are uncommon but can also be associated with granulomatous inflammation [25]. Usually the lower labial mucosa is the anatomic site for intraoral cosmetic tattoos.
Fig. 3.
a Dental amalgam eliciting a foreign body giant cell reaction. Granulomas composed of histiocytes, giant cells and lymphocytes are present incorporating the black pigmented material (magnification × 100). b Hyaluronic acid foreign body reaction. Pools of homogeneous basophilic material forming cyst-like structures lined by epithelioid histiocytes (magnification × 200). c Calcium hydroxylapatite composed of uniformly sized spherules surrounded by histiocytes and giant cells. An asteroid body is seen (arrow) (magnification × 200). d Poly-l-lactic acid granuloma composed of fusiform, cholesterol cleft-like material within giant cells and surrounded by chronic inflammation. The material are refractile in polarized light (inset) (magnification × 200)
Adverse reactions to injectable cosmetic fillers including granulomatous inflammation have been reported [26]. Both temporary fillers (collagens and hyaluronic acid) and long-lasting fillers (poly-l-lactic acid, calcium hydroxyl apatite, silicone) can initiate a host response [26–28]. Granulomatous inflammation is a late complication of cosmetic fillers, developing months or even years after the injection [26, 27]. Shahrai-Farahani et al. presented a series of 25 patients whom received extraoral injections of cosmetic fillers but presented with intraoral nodules of the lip, maxillary and mandibular vestibule or buccal mucosa. Two patient developed nodules in the nasolabial or chin area. In the 20 patients with known injection dates, the lesions developed as early as 2 months and up to 36 months with a median of 6.5 months.
Some fillers have distinct histologic features allowing for a degree of confidence in identification of the offending material. Hyaluronic acid gel presents as deposits of basophilic amorphous material of variable shape and size surrounded by histiocytes and multinucleated giant cells (Fig. 3b) [27–29]. The presence of eosinophils and neutrophils have been reported associated with the foreign material.
Calcium hydroxyl apatite associated granulomas demonstrate non-polarizable, uniformly sized, 20 to 40 µm, mauve, gray or tan spherules surrounded by epithelioid histiocytes, foreign body giant cells and lymphocytes (Fig. 3c) [26–29]. The foreign material can be seen within some of the foreign body giant cells. Unlike granulomatous inflammation of idiopathic OFG, the granulomas are diffuse, not forming discrete granulomas. Extension into skeletal muscle and salivary glands can be seen, but the overlying epithelium is uninvolved.
Granulomas associated with poly-l-lactic acid have a distinct histology. Polarizable fusiform or ovoid foreign material similar to cholesterol clefts are identified (Fig. 3d). The particles are within and surrounded by multinucleated giant cells and associated with epithelioid histiocytes and a lymphocytic inflammation. Star-shaped spiculated structures termed Asteroid bodies are sometimes noted in the multinucleated giant cells [29]. Fibrosis can be present and inflammation can range from minimal to moderate.
Silicone is a permanent filler used for soft tissue augmentation. Silicone granulomas in early stages may show dispersed clear bubbly spaces of varying sizes that may be mistaken for lipoblasts [28–30]. However, this material is negative for S100 immunohistochemical and positive for CD68 histiocyte markers, ruling out an adipocyte origin. The silicone is surrounded by giant cells, foamy macrophages, and at times eosinophils [30]. Similar to granulomas associated with poly-l-lactic acid, asteroid bodies have been identified.
Systemic Causes of Noninfectious Orofacial Granulomatosis
Crohn Disease
Crohn Disease (CD) is a chronic idiopathic inflammatory disease that can involve any portion of the alimentary tract characterized by discontinuous skip lesions which can occur from the mouth to the anus. The exact etiology of CD is unknown but studies link alterations in gut microbiome, defects in intestinal epithelial barrier function and genetics [31]. The annual incidence of Crohn disease in North America ranges from 3 to 30 cases per 100,000 [32]. Differences in incidence and prevalence are seen based on geographic region, environment and ethnic groups. There is a bimodal age of onset with an average age of 30 years. The first peak is before the age of 30 and the second but smaller peak is around 50 years of age [30]. The Montreal classification of CD is based on the age at diagnosis (< 16, 17–40, > 40); location: ileal, colonic, ileocolonic, isolated upper GI; and disease behavior: nonstricturing/nonpenetrating, stricturing, penetrating [33].
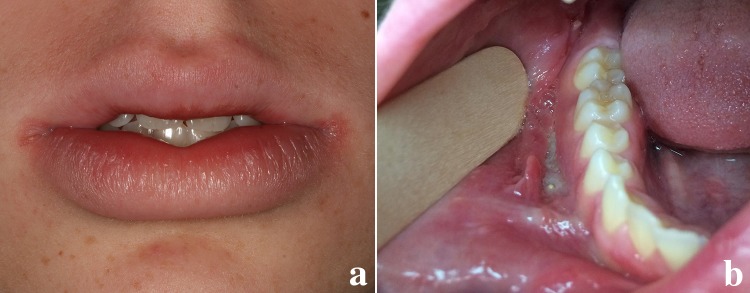
Oral involvement of CD has been reported in up to 80% of pediatric patients [34, 35]. In some studies more than 30–60% of pediatric patient’s oral manifestations precede gastrointestinal involvement [34]. The clinical features include intermittent swelling of the lips, swelling and erythema of the gingiva, and linear ulceration with hyperplastic margins often in the vestibules (Fig. 4a, b). Haaramo et al. reported upper lip swelling was statistically more common in patients who had CD than in patients with idiopathic OFG (p = 0.0265) [36]. Cobblestoning of the buccal mucosa and mucosal tags are often present. Patient symptoms range from complaints of tenderness on palpation to chronic pain.
Fig. 4.
Crohn disease in a 14-year-old White male. a The patient presented with lower lip swelling of a few weeks’ duration, angular cheilitis, and mild fissuring. b Intraoral presentation of oral Crohn disease with linear ulceration in the buccal vestibule with hyperplastic epithelial margins
Some authors propose that OFG is a subtype of CD as the presentation, histology and clinical course is similar [12, 22, 23, 36–39]. A systematic review of the literature of 173 pediatric cases of OFG showed age of onset ranged from 2 to 18 years with a mean age of 11.1 years [23]. The male to female ratio was 2:1 and 93.3% of children had lip swelling as the primary clinical finding. Other oral manifestations included ulcers, gingival swelling, and cobblestoning. GI signs and symptoms including abdominal pain and/or diarrhea were present in 26% of cases at time of OFG diagnosis. CD was diagnosed in 40% of the children at time of presentation or in the following months (range 3–36 months) [23]. Three children with OFG were diagnosed with tuberculosis, two with sarcoidosis and 19 with allergy/atopy.
Haaramo et al. in a study of 29 patients with pediatric onset of OFG, 72% of the patients developed GI CD within a median of 3.1 years [40]. Another study of 21 children with CD with oral features compared to 39 children with CD who lacked oral manifestations showed differences in disease phenotype. A significantly higher percentage of patients with oral manifestations of CD on endoscopic examination had more extensive disease including involvement of the upper GI tract and perianal region [12].
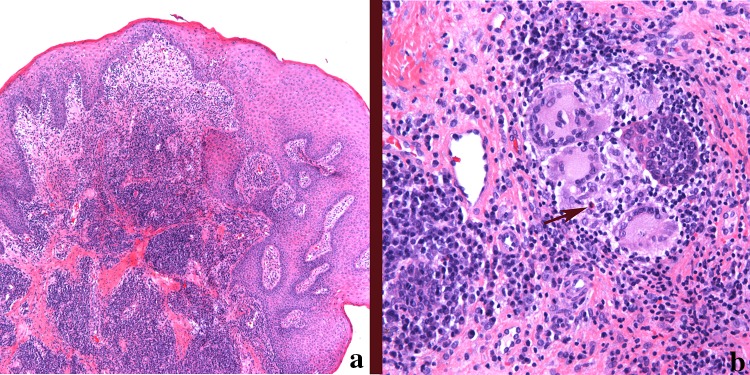
Histologically the granulomas in oral CD is indistinguishable from OFG (see above description). The granulomas are composed of loosely arranged epithelioid histiocytes, giant cells, plasma cells and lymphocytes (Fig. 5a, b). Mast cells and eosinophils may be present. No polarizable material should be seen and special stains for organisms are negative.
Fig. 5.
a Low power magnification of Crohn disease. There are numerous similarities to idiopathic orofacial granulomatosis including epithelial hyperplasia, stromal edema and loosely formed granulomas with mixed inflammation (magnification × 40). b A typical granuloma from the lip in a patient diagnosed with Crohn disease. The granuloma is composed of epithelioid histiocytes, multinucleated giant cells, plasma cells, lymphocytes, and occasionally eosinophils (arrow) (magnification × 200)
Sarcoidosis
Sarcoidosis is a granulomatous inflammatory disease of unknown etiology that can have multisystem involvement [41]. The lung is most commonly affected, but extrapulmonary disease is present in up to 50% of patients and nonpulmonary sarcoidosis has been reported [42]. James et al. reviewed 1,686 patients with sarcoidosis and 8.3% had nonpulmonary sarcoidosis. This cohort had a significantly higher prevalence of skin, salivary gland, bone marrow, and otorhinolaryngic lesions than patients with pulmonary sarcoidosis [43]. The soft tissues of the oral cavity are a rare site for sarcoidosis with an unknown prevalence and often present as nodules, multiple or single (Fig. 6a). Sarcoidosis can also involve the jaw bones and salivary glands. Less than 75 cases of sarcoidosis presenting as OFG have been reported in the English language literature [42]. Bozuaziz et al. reported 12 cases of sarcoidosis in the oral cavity in which 58% of the cases were the initial presentation [44]. The tongue was the most common site of occurrence, followed by lips. The lesions presented usually as solitary nodules or ulcers, but one patient had multiple lesions.
Fig. 6.
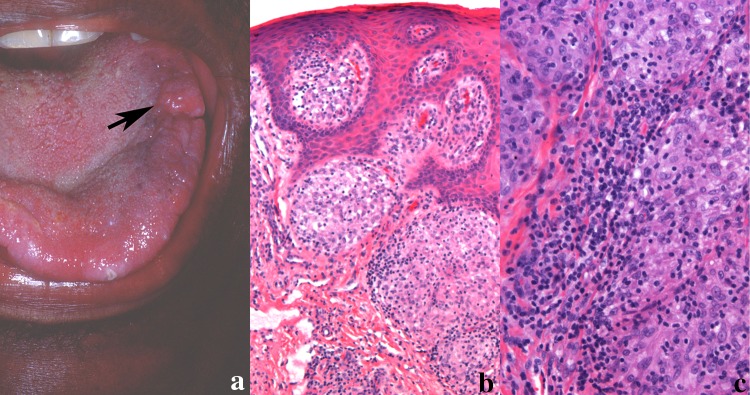
a Nodule on the tongue in a patient with a history of sarcoidosis. b Sarcoidal granuloma in the tongue. Non-necrotizing well-formed granulomas composed of epithelioid histiocytes and multinucleated giant cells with few admixed inflammatory cells often termed “naked” granulomas (magnification × 100). c Although not always present, sarcoid granulomas can have lymphocytes along the periphery and within the granuloma (magnification × 200)
Granulomas of sarcoidosis show a collection of epithelioid histiocytes, along with multinucleated cells [7, 8]. Typically, sarcoidal granulomas lack the associated lymphocytic infiltrate seen in OFG and are often call “naked” granulomas (Fig. 6b). This however is not always the case and lymphocytes may be prominent (Fig. 6c). Other histologic but nondiagnostic findings include the presence of asteroid bodies and concentric calcifications (Schaumann bodies) in the lesional multinucleated giant cells [7]. A mixed inflammation, including plasma cells and eosinophils are not usually identified. However, it must be stressed that there are histologic overlaps of sarcoidal granulomas with granulomas of idiopathic OFG and CD. No definitive histologic markers exist and therefore the pathology may be suggestive of the disease but requires clinical and radiologic studies for disease confirmation [45, 46].
Conclusion
When confronted with non-infectious granulomatous inflammation from the oral cavity, as pathologists it is important to relay information to the clinician to ensure appropriate testing is performed to rule out various causes of OFG. Crohn disease should be considered if abnormalities in hemoglobin, serum iron, transferrin or ferritin, Vitamin B12/folate are identified [1, 10, 11]. GI endoscopy to evaluate for bowel involvement may be considered. Abnormal erythrocyte sedimentation rate or C-reactive protein studies can be seen in CD, sarcoidosis and infectious diseases including tuberculosis [1, 11]. Elevated angiotensin-converting enzyme can be associated with sarcoidosis. Abnormal chest X-rays may indicate sarcoidosis or tuberculosis. Despite a negative AFB stain on the biopsy specimen, tuberculosis should be ruled out by tuberculin skin test or interferon γ release assays. Finally, if a local cause of OFG is a consideration, patch testing may identify the trigger.
Funding
No funding was required for this study.
Compliance with Ethical Standards
Conflict of interest
The author declares they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Al-Hamad A, Porter S, Fedele S. Orofacial granulomatosis. Dermatol Clin. 2015;33(3):433–446. doi: 10.1016/j.det.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Troiano G, Dioguardi M, Giannatempo G, et al. Orofacial granulomatosis: clinical signs of different pathologies. Med Princ Pract. 2015;24(2):117–122. doi: 10.1159/000369810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miest R, Bruce A, Rogers RS. Orofacial granulomatosis. Clin Dermatol. 2016;34(4):505–513. doi: 10.1016/j.clindermatol.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15(1):46–51. doi: 10.1111/j.1601-0825.2008.01500.x. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Connett JM, Kunkel SL, Matsukawa A. The linkage of innate and adaptive immune response during granulomatous development. Front Immunol. 2013;31:4–10. doi: 10.3389/fimmu.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, Laffitte E. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54(1):131–146. doi: 10.1007/s12016-017-8666-8. [DOI] [PubMed] [Google Scholar]
- 7.Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1–12. doi: 10.1016/j.jctube.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34(3):301–311. doi: 10.1053/j.semdp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick L, Healy CM, McCartan BE, et al. Patch testing for food-associated allergies in orofacial granulomatosis. J Oral Pathol Med. 2011;40(1):10–13. doi: 10.1111/j.1600-0714.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- 10.Miest RY, Bruce AJ, Comfere NI, et al. A diagnostic approach to recurrent orofacial swelling: a retrospective study of 104 patients. Mayo Clin Proc. 2017;92(7):1053–1060. doi: 10.1016/j.mayocp.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L. The multiform and variable patterns of onset of orofacial granulomatosis. J Oral Pathol Med. 2003;32(4):200–205. doi: 10.1034/j.1600-0714.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Gale G, Sigurdsson GV, Östman S, et al. Does Crohn’s disease with concomitant orofacial granulomatosis represent a distinctive disease subtype? Inflamm Bowel Dis. 2016;22(5):1071–1077. doi: 10.1097/MIB.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 13.Marcoval J, Penín RM. Histopathological features of orofacial granulomatosis. Am J Dermatopathol. 2016;38(3):194–200. doi: 10.1097/DAD.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 14.Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54(213):101–113. [PubMed] [Google Scholar]
- 15.Melkersson E. Ett fall av recidiverande facialispares i samband med ett angioneurotiskt dem. Stockholm: Hygiea; 1928. p. 737e41. [Google Scholar]
- 16.Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8(2):209–213. doi: 10.1007/s12105-013-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers RS. Granulomatous cheilitis, Melkersson–Rosenthal syndrome, and orofacial granulomatosis. Arch Dermatol. 2000;136(12):1557–1558. doi: 10.1001/archderm.136.12.1557. [DOI] [PubMed] [Google Scholar]
- 18.Al Johani K, Moles DR, Hodgson T, Porter SR, Fedele S. Onset and progression of clinical manifestations of orofacial granulomatosis. Oral Dis. 2009;15(3):214–219. doi: 10.1111/j.1601-0825.2009.01512.x. [DOI] [PubMed] [Google Scholar]
- 19.Cocuroccia B, Gubinelli E, Annessi G, Zambruno G, Girolomoni G. Persistent unilateral orbital and eyelid oedema as a manifestation of Melkersson–Rosenthal syndrome. J Eur Acad Dermatol Venereol. 2005;19(1):107–111. doi: 10.1111/j.1468-3083.2004.01084.x. [DOI] [PubMed] [Google Scholar]
- 20.Rivera-Serrano CM, Man LX, Klein S, Schaitkin BM. Melkersson–Rosenthal syndrome: a facial nerve center perspective. J Plast Reconstr Aesthet Surg. 2014;67(8):1050–1054. doi: 10.1016/j.bjps.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Kanerva M, Moilanen K, Virolainen S, Vaheri A, Pitkäranta A. Melkersson–Rosenthal syndrome. Otolaryngol Head Neck Surg. 2008;138(2):246–251. doi: 10.1016/j.otohns.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Lazzerini M, Martelossi S, Cont G, et al. Orofacial granulomatosis in children: think about Crohn’s disease. Dig Liver Dis. 2015;47(4):338–341. doi: 10.1016/j.dld.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Lazzerini M, Bramuzzo M, Ventura A. Association between orofacial granulomatosis and Crohn’s disease in children: systematic review. World J Gastroenterol. 2014;20(23):7497–7504. doi: 10.3748/wjg.v20.i23.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharti R, Wadhwani KK, Tikku AP, Chandra A. Dental amalgam: an update. J Conserv Dent. 2010;13(4):204–208. doi: 10.4103/0972-0707.73380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telang LA. Body art: intraoral tattoos. Br Dent J. 2015;218(4):212–213. doi: 10.1038/sj.bdj.2015.109. [DOI] [PubMed] [Google Scholar]
- 26.Haneke E. Adverse effects of fillers. Dermatol Ther. 2018;5:e12676. doi: 10.1111/dth.12676. [DOI] [PubMed] [Google Scholar]
- 27.Requena L, Requena C, Christensen L, et al. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;64(1):1–34. doi: 10.1016/j.jaad.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 28.Eversole R, Tran K, Hansen D, Campbell J. Lip augmentation dermal filler reactions, histopathologic features. Head Neck Pathol. 2013;7(3):241–249. doi: 10.1007/s12105-013-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahrabi-Farahani S, Lerman MA, Noonan V, Kabani S, Woo SB. Granulomatous foreign body reaction to dermal cosmetic fillers with intraoral migration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):105–110. doi: 10.1016/j.oooo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Requena C, Requena L, Alegre V, et al. Adverse reaction to silicone simulating orofacial granulomatosis. J Eur Acad Dermatol Venereol. 2015;29(5):998–1001. doi: 10.1111/jdv.12522. [DOI] [PubMed] [Google Scholar]
- 31.Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088–1103. doi: 10.1016/j.mayocp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon. 2018;64(2):20–57. doi: 10.1016/j.disamonth.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Eckel A, Lee D, Deutsch G, Maxin A, Oda D. Oral manifestations as the first presenting sign of Crohn’s disease in a pediatric patient. J Clin Exp Dent. 2017;9(7):e934–e938. doi: 10.4317/jced.53914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo VL. Oral manifestations of Crohn’s disease: a case report and review of the literature. Case Rep Dent. 2015;2015:830472. doi: 10.1155/2015/830472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanderson J, Nunes C, Escudier M, et al. Oro-facial granulomatosis: Crohn’s disease or a new inflammatory bowel disease? Inflamm Bowel Dis. 2005;11(9):840–846. doi: 10.1097/01.mib.0000178261.88356.67. [DOI] [PubMed] [Google Scholar]
- 37.Hoekman DR, Diederen K, Benninga MA. Crohn’s disease with orofacial granulomatosis is a distinct disease subtype, or is it? Inflamm Bowel Dis. 2016;22(7):E23. doi: 10.1097/MIB.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 38.Gale G, Östman S, Rekabdar E, et al. Characterisation of a Swedish cohort with orofacial granulomatosis with or without Crohn’s disease. Oral Dis. 2015;21(1):e98–e104. doi: 10.1111/odi.12236. [DOI] [PubMed] [Google Scholar]
- 39.Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17(10):2109–2115. doi: 10.1002/ibd.21599. [DOI] [PubMed] [Google Scholar]
- 40.Haaramo A, Alapulli H, Aine L, et al. Detailed Follow-up Study of Pediatric Orofacial Granulomatosis Patients. J Pediatr Gastroenterol Nutr. 2017;65(4):388–393. doi: 10.1097/MPG.0000000000001554. [DOI] [PubMed] [Google Scholar]
- 41.Arkema EV, Cozier YC. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis. 2018;9(11):227–240. doi: 10.1177/2040622318790197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nico MM, Guimarães AL, Correa PY, Lourenço SV. Oral mucosal lesions in sarcoidosis: comparison with cutaneous lesions. Acta Derm Venereol. 2016;96(3):392–393. doi: 10.2340/00015555-2262. [DOI] [PubMed] [Google Scholar]
- 43.James WE, Koutroumpakis E, Saha B, et al. Clinical features of extrapulmonary sarcoidosis without lung involvement. Chest. 2018;154(2):349–356. doi: 10.1016/j.chest.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105(8):755–767. doi: 10.1093/qjmed/hcs042. [DOI] [PubMed] [Google Scholar]
- 45.Bargagli E, Prasse A. Sarcoidosis: a review for the internist. Intern Emerg Med. 2018;13(3):325–331. doi: 10.1007/s11739-017-1778-6. [DOI] [PubMed] [Google Scholar]
- 46.Wessendorf TE, Bonella F, Costabel U. Diagnosis of sarcoidosis. Clin Rev Allergy Immunol. 2015;49(1):54–62. doi: 10.1007/s12016-015-8475-x. [DOI] [PubMed] [Google Scholar]