Abstract
In the recently published 8th edition of the AJCC Cancer Staging Manual, new pathological elements are required for the N and T category determinations for oral cavity cancers. This includes determination of depth of tumor invasion and assessment of metastatic lymph nodes for extranodal extension. Although definitions and some guidance are provided for the interpretation of these elements, pathologists frequently encounter ambiguous situations that may result in interobserver and intraobserver variability. Pre-existing staging elements, such as assessment of bone invasion, can also be problematic to interpret. Difficulties in the interpretation of depth of invasion, bone invasion and extranodal invasion are discussed, with examples. Communication with the surgeon, proper specimen orientation, gross examination and sampling are crucial to assessment of these elements. Liberal use of deeper levels and submission of additional sections is suggested. Although general staging guidelines encourage clinicians and pathologists to choose the lower category when there is ambiguity, pathologists may choose to discuss difficulties in the interpretation of specific cases at interdisciplinary tumor boards, to allow a more informed choice of treatment on the part of treating physician and patient. More discussion is required among pathologists to develop specific guidelines for the interpretation of these staging elements.
Keywords: Neoplasm staging, Prognosis, Observer variation, Lymph nodes, Mouth neoplasms
Introduction
Publication of the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual introduced new requirements for the pathological interpretation of oral cancer resection specimens. Measurement of tumor depth of invasion (DOI) and pathological determination of extranodal extension (ENE) in metastatic lymph nodes were incorporated into the T and pN categories, respectively, thereby influencing the cancer stage groupings [1]. This direct impact on patient prognosis and treatment necessitates consistent interpretation by pathologists worldwide. Although definitions and guidelines for the interpretation of DOI and ENE exist, they can be difficult to apply across the diverse spectrum of oral cancer specimens. Other pathology reporting elements suggested in the AJCC manual are similarly subject to interobserver variability. This includes both staging elements (e.g. bone invasion) and prognostic factors that are not part of the staging system (e.g. worst pattern of invasion) [1].
Correct specimen designation and orientation are crucial to accurate pathological staging. This is best done intraoperatively in conjunction with margin assessment, to ensure complete resection (and avoid understaging). Similarly, the importance of accurate gross description, inking and dissection cannot be underestimated. Pathology assistants and residents require appropriate training and supervision, and it is beneficial for the pathologist reporting a case to have seen the gross specimen prior to submission of tissue sections. Gross photographs and diagrams are also helpful.
Discussion
Depth of Invasion
Tumor depth of invasion (DOI) is a predictor of disease-specific survival in oral cancer, warranting its incorporation into the staging system [2]. There are few useful precedents for oral DOI measurement. Few cancers from other sites require it for staging, the most notable being Breslow thickness for skin melanoma [3] and DOI for cutaneous carcinoma of the head and neck region [4], both of which are less complicated by virtue of the more uniform nature of skin specimens. Vulvar cancer pT categorization incorporates a DOI measurement, though involving small tumors only, for pT1 subcategorization [5].
DOI must be distinguished from tumor thickness. The latter is a more simplistic measurement from the mucosal surface of the tumor (which might be overlying normal epithelium, dysplastic epithelium, tumor surface or ulcer base) to the deepest point of invasion [1]. As such, no adjustment is made for exophytic tumors (with a large bulk of tumor above the adjacent mucosa) or ulcerated tumors (in which the ulcer base lies below the surrounding mucosa). The DOI measurement, however, attempts to more accurately determine the extent to which a tumor invades below the location of the pre-existing normal mucosa. Tumor thickness measurement would typically be an overestimate of the latter in an exophytic tumor, and an underestimate in an ulcerated one.
The AJCC staging manual provides written and illustrated guidelines for measuring depth of invasion. Briefly, it requires: (1) finding the “horizon” from the basement membrane of the adjacent squamous mucosa, (2) imagining a perpendicular “plumb” line from the horizon, and extending it to the deepest point of tumor invasion. This distance is recorded in millimeters [1]. Although seemingly straightforward and relatively easily applied to small tumors, sources of interobserver variability arise across the diverse spectrum of oral cancers, many of which cause considerable distortion of the original tissue architecture. Ultimately, one has to accept that in many cases the DOI is an estimate, and that only consistent interpretation of the “rules” will make it a useful prognostic marker.
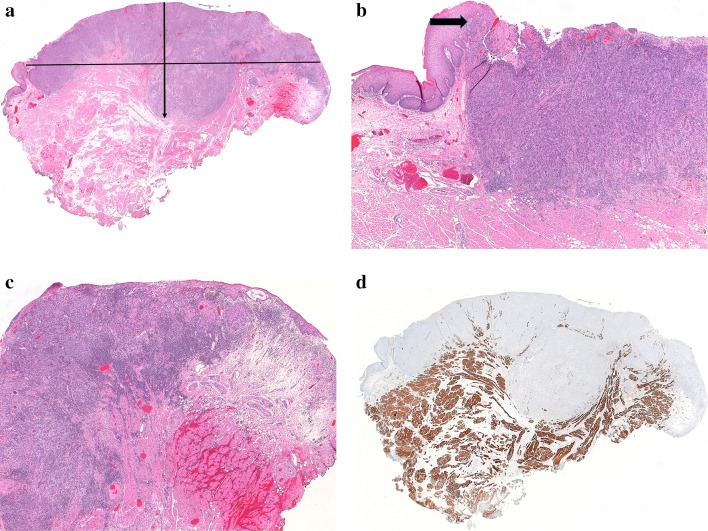
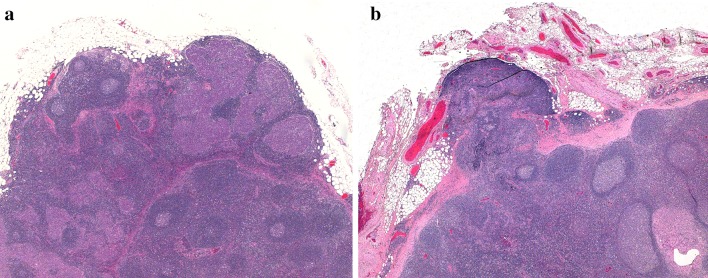
Step 1—drawing a “horizon” line across the surface of the tumor - in essence attempts to recreate the site of the original mucosa from which a tumor arose. In many cases, it is difficult to imagine a horizon line extending from only one side of a squamous cell carcinoma, as the epithelium is irregularly hyperplastic (+/- dysplastic) and/or obliquely oriented with respect to mucosa more remote from the tumor. The solution requires imagining a line joining the mucosa on both sides of the tumor. In ulcerated tumors, or those from relatively flat surfaces such as the buccal mucosa, this can be a straight line. In tumors from the lateral tongue, an arcuate (curved) line is more appropriate, to recreate the curved tongue surface (Fig. 1a) [6]. In large tumors, both sides of the tumor may not fit in a single cassette, further highlighting the benefit to the pathologist of examining the gross specimen and directing the sampling of the tumor with DOI measurement in mind. Correlation with a gross measurement of tumor thickness can also be helpful.
Fig. 1.
a Whole mount image of a lateral tongue squamous cell carcinoma. A horizontal line from adjacent benign epithelium on each side of this tumor would underestimate tumor depth of invasion (DOI). DOI was measured from the surface of the tumor to the deepest point of invasion (arrow). In effect, an “arcuate” line was created to account for the normal contour of the tongue. b Left side of this tumor as seen in 1a, with adjacent benign epithelium. Even though the tumor is not exophytic, the epithelium is drawn upwards, meeting an ulcer. The raised epithelium is a more appropriate choice as the origin for a “horizon” line (arrow). In this case, the line chosen was arcuate across the tumor surface rather than straight across to the opposite side of the tumor. c Right side of this tumor as seen in 1a. Tumor focally undermines adjacent benign epithelium. Choice of anything other than an arcuate line would be quite subjective and would underestimate DOI. d Desmin-stained slide of the same tumor. Skeletal muscle is drawn close to the surface of the mid-portion of the tumor, further justifying the method of DOI measurement described above
Step 2—drawing the “plumb” line perpendicular to the horizon—requires simply identifying the location of the deepest focus of tumor. However, if one recreates an arcuate line for a lateral tongue tumor, then an infinite number of plumb lines can be drawn from the deepest focus to the surface. Typically one would choose a line to the general epicenter of a tumor on the lateral tongue, or to an area of overlying severe dysplasia/carcinoma in situ. Some combined tongue and floor of mouth tumors can be architecturally complex, in which case several measurements of DOI from multiple sections may be required, choosing the deepest reasonable one.
Practically, most oral cancers are at least slightly exophytic (not including genuine papillary or verrucous carcinomas) and either raise the adjacent mucosa along their periphery or directly undermine it (Fig. 1b,c). Drawing a horizon line from flat mucosa surrounding the tumor may underestimate the DOI. This is emphasized in tongue tumors in which skeletal muscle is found within the tumor, above the surrounding, flat mucosa (Fig. 1d). The author would advocate for choosing the closest intact non-invasive epithelium (normal, dysplastic or carcinoma in situ) as the source of the horizon line, even if it is somewhat elevated by the tumor.
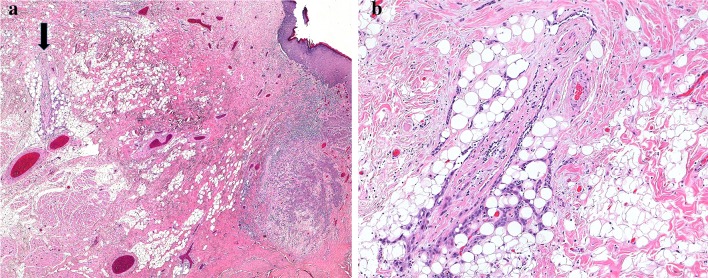
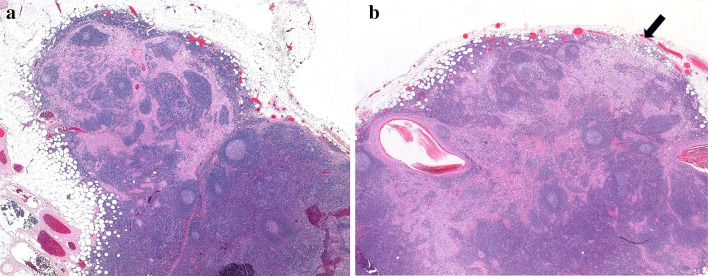
Worst pattern of invasion (WPOI) of an oral cancer is a factor recommended for clinical care in the AJCC manual and is an optional reporting component in the most recent College of American Pathologists (CAP) oral cavity protocol [7]. WPOI is a 5-category element of the Histologic Risk Model for oral cancer, proposed by Brandwein-Gensler [8]. The CAP protocol element requires distinguishing between pattern WPOI-5 and patterns 1–4. In WPOI-5, separate tumor foci are found more than 1 mm from the main tumor mass, with intervening normal tissue (Fig. 2a,b). These tumor foci may occur as a result of direct but apparently discontinuous tumor spread, perineural invasion (PNI) or lymphovascular invasion (LVI). The DOI measurement guidelines do not specifically exclude WPOI-5 tumor foci, although Berdugo et al. [6] found that extratumoral PNI or LVI rarely result in alteration of pT category, either because: (1) they are in a “peritumoral” (as opposed to deep) location (2) they do not bump a tumor beyond the 5 or 10 mm DOI measurements, or (3) tumors with these findings are often already pT3. As WPOI-5 tumor is an independent risk factor for locoregional recurrence in early stage oral cavity cancers [8], one could argue that it can be excluded from DOI measurement if consistently reported separately.
Fig. 2.
a Worst pattern of invasion type 5. A nest of tumor is present > 1 mm from the main bulk of the tumor (arrow), with intervening normal tissue. b High power image of separate tumor island. No perineural or lymphovascular origin is identified
DOI measurement may be complicated by the absence of residual carcinoma or a positive deep margin in the resection specimen. In Berdugo et al’s [6] study of early stage oral cancers, this was found in 14.2% and 8.8% of cases, respectively. They advocate for routine DOI measurement in diagnostic biopsies. This may also be of benefit to surgeons when they are debating the requirement for a neck dissection.
Bone Invasion
Assessment of bone invasion can be challenging in some cases. Invasion through the cortical bone is a criterion for increasing the T category of a tumor to T4a, whereas superficial erosion of cortical bone or a tooth socket does not [1]. In a site where tumors are closely applied to bone, such as the gingiva, edentulous alveolar ridge or hard palate, the presence of bone invasion can upstage a small, node-negative tumor to stage group IVA from group I or II, which usually prompts adjuvant treatment [1, 9, 10].
Bone invasion has been subclassified into erosive and infiltrative types. In the erosive type, the tumor has a broad, pushing front, with a sharp interface between tumor and bone, osteoclastic resorption and fibrosis along the tumor front, and an absence of bone islands within the tumor mass. In the infiltrative type, there are nests and projections of tumor cells along an irregular front, residual bone islands within the tumor, and haversian system penetration. Some tumors contain a mixed pattern. In a study of mandibular bone invasion by squamous cell carcinoma, Wong et al. [11] found a lower rate of positive bone margins, decreased risk of recurrence (local, regional and distant) and improved disease-free survival at 3 years (73% vs 30%) among the patients with erosive versus infiltrative invasion. Patients with infiltrative invasion had a fourfold increased risk of death. These authors recommended reporting the pattern of invasion in pathology reports. This opinion has been echoed by others, and some have questioned whether the presence of purely erosive invasion justifies the consequent stage IVA status [12].
The diagnosis of true bone invasion—as manifested by complete perforation of the cortical bone and involvement of the medullary space/cancellous bone—can be challenging, particularly in erosive- patterned tumors. In edentulous patients, the jaw bones lose both height and width, initially rapidly and then more slowly over a period of years following tooth extraction [13]. This can make it difficult to estimate how much bone loss is due to destruction by tumor versus simply recession [12].
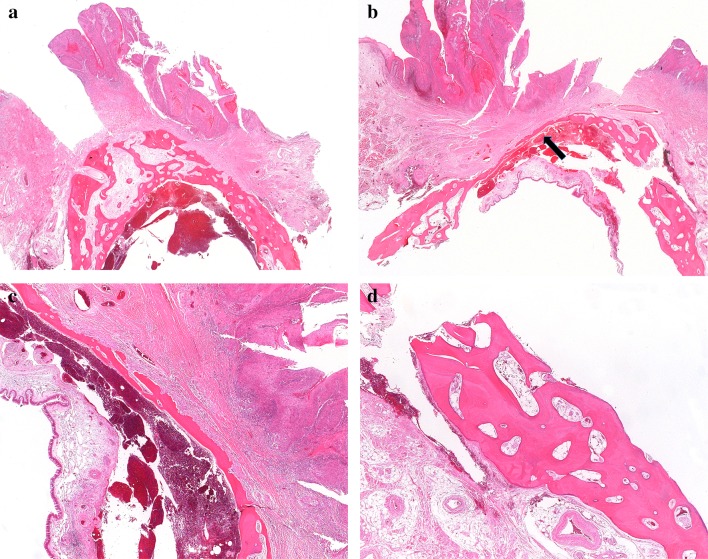
In tumors with a broad, cohesive pattern, the advancing tumor front often consists of inflamed fibrous stroma that precedes malignant cells (Fig. 3). Even in infiltrative tumors, there may be little direct contact between tumor and bone. Experimental evidence shows that this “tumor associated stroma” contains alpha smooth muscle actin-positive myofibroblasts (called cancer associated fibroblasts or CAF) that have a functional role in bone invasion and turnover. CAF secrete osteoclastogenic factors, such as RANKL (receptor activator of nuclear factor-kappa beta), that promote bone invasion. In fact, the in vitro effect of CAF on osteoclastogenesis is markedly greater than oral squamous cell carcinoma-derived tumor cells [14]. The presence of abundant tumor associated stroma at the leading edge of the tumor may make it questionable whether the malignant cells themselves have actually gained access to the medullary space.
Fig. 3.
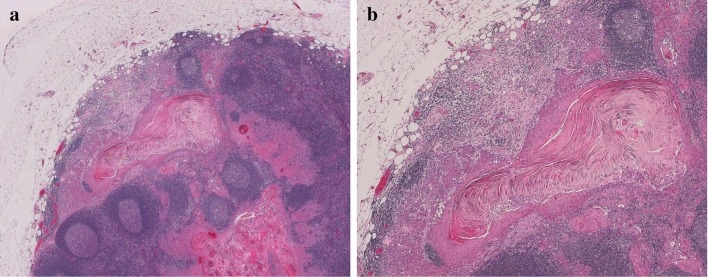
a An exophytic maxillary tumor with erosion of the cortical bone of the edentulous alveolar ridge. No bone invasion is identified at this location. b Same tumor at a different location. The leading edge of the tumor has eroded most of the bone, leaving only a thin layer of cortical bone under the sinus mucosa (arrow). Only a small amount of cancellous bone is visible, on the lower left. c The tumor has destroyed most of the bone, with a leading edge of fibroinflammatory tissue and no direct contact between tumor and bone. This was interpreted as bone invasion (category pT4a), but as only minimal cancellous bone is identified nearby, and there is no direct invasion of tumor into the medullary space, that diagnosis is debatable. d Bone of the maxilla from lower right of the field in 3b. In this edentulous patient, the bone is essentially cortical type
Finally, especially in the maxilla, thin portions of bone may be composed entirely of cortical-type bone, and thus despite the presence of extensive bone erosion, it may not be appropriate to consider these tumors category T4a unless invasion of the maxillary sinus is present.
In difficult cases, the assessment of bone invasion is facilitated in the following ways: (1) reviewing the pre-operative imaging (2) reviewing the gross specimen and sectioning it at close intervals prior to submission of tissue (3) liberal submission of bone sections from the tumor-bone interface and from the nearby bone (to assess the “native” state of the bone in the absence of adjacent tumor) [13].
Typically, there is a strong suspicion of bone invasion when a maxillectomy or mandibulectomy is performed, except in some gingival primaries in which a tumor cannot be adequately resected without also resecting bone. On occasion, one may find entrapped residual bone fragments within the advancing front and separate from the bone itself, which is further evidence of bone invasion.
Finally, caution is required in bone resections following radiotherapy. These are typically performed for osteoradionecrosis, but the surgeon may ask for assessment for residual/recurrent squamous cell carcinoma. Pseudoepitheliomatous hyperplasia is common in this circumstance, as mucosal defects permit ingrowth of benign surface epithelium which surrounds and “transmucosally eliminates” necrotic bone debris. To pathologists unfamiliar with this phenomenon, the appearance may raise the possibility of recurrent invasive cancer. The epithelium is bland, but often inflamed and reactive, and the bone fragments are necrotic, partially resorbed and admixed with dense fibrous tissue.
Extranodal Extension
Extranodal extension (ENE) is defined by the College of American Pathologists oral cavity cancer protocol “as extension of metastatic tumor, present within the confines of the lymph node, through the lymph node capsule into the surrounding connective tissue, with or without associated stromal reaction” [7]. ENE is measured from the external aspect of the lymph node capsule to the most distant tumor focus. It is also known as “extracapsular extension/spread”, but ENE is the term adopted in the AJCC Staging Manual [1]. ENE is a poor prognostic factor in cervical node-positive oral cavity cancer [15–18]. The presence of ENE correlates with the risk of regional recurrence, distant disease and overall survival. It is an important factor for oncologists when considering treatment with postoperative radiation or chemoradiation [19, 20].
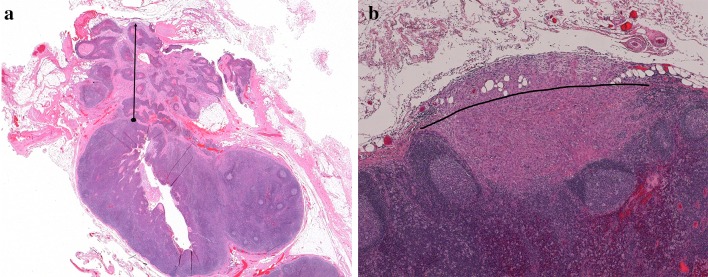
ENE can be determined both clinically and pathologically, and its assessment is incorporated into both the cN and pN nodal categories. Clinical ENE converts any nodal disease to the cN3 category, whereas pathological ENE converts pN1 nodal involvement to pN2, and pN2 to pN3. ENE is subcategorised pathologically as microscopic (ENEmi, less than or equal to 2 mm in extent) and major (ENEma, more than 2 mm in extent) (Fig. 4a,b). These subcategories are not required for pN categorisation, but are recommended by the AJCC for data collection and future analysis [1].
Fig. 4.
a Major extranodal extension (ENE), with tumor invading perinodal adipose tissue. The arrow from the capsule to the furthest point of invasion demonstrates how to measure extranodal extension. b Minor extranodal extension with tumor invading adipose tissue with a desmoplastic response. The node capsule is not preserved centrally, nor visualized on the right. An arcuate line has been created to approximate the contour of the node capsule/edge, from which ENE can be measured
Clinical ENE is clearly a more extensive process than most pathologically-determined ENE, particularly the microscopic subcategory. Clinical classification as ENE requires “unambiguous evidence of gross ENE on clinical examination supported by strong radiographic evidence”, which can include invasion of skin, infiltration of musculature, dense tethering to adjacent structures, and cranial nerve, brachial plexus, sympathetic trunk, or phrenic nerve invasion with dysfunction [1]. By comparison, some cases of pathological ENE are remarkably subtle extensions of tumor into adjacent adipose tissue.
Multiple studies have attempted to address the issue of the prognostic significance of microscopic versus macroscopic or extensive ENE, with contradictory results. Analysis is made more difficult by the addition of adjuvant therapy to those patients with ENE. For instance, Prabhu et al., in a study of 450 patients with oral cavity and laryngeal cancer, found that chemoradiation effectively mitigated the detrimental effect of most forms of ENE, including those cases with > 1 mm of ENE. However, patients with complete replacement of lymph nodes and no residual nodal architecture (soft tissue metastasis) retained a poorer prognosis [21]. In contrast, Wreesmann et al., in a 2016 study of 245 patients with oral cavity squamous cell carcinoma and positive neck lymph nodes, found that a cutoff of 1.7 mm of ENE was the optimal prognostic threshold for predicting disease specific survival (DSS). In their study, patients with > 1.7 mm of ENE had a DSS worse than those with ENE </= 1.7 mm. The group with minor ENE had similar DSS to those patients with no ENE [22].
Although general staging principals advise choosing the lower staging category when faced with an ambiguous finding [23], pathologists may be reluctant to “under-categorize” ENE for fear of a potentially life-saving adjuvant treatment being withheld. Other than the general definition of ENE given above, there are few guidelines for the definitive determination of ENE and for several reasons it can be subjective. At least two studies have demonstrated considerable interobserver and intraobserver variability in the assessment of ENE in metastatic head and neck cancer [24, 25].
Difficulty with ENE determination often falls into two situations: (1) cases with small volume nodal disease, an ill-defined capsule and questionable extension beyond the capsule, and (2) large volume nodal disease with expanded, tumor-replaced node(s) and thickened fibrous capsules that either merge with the surrounding adipose tissue or are fused to other nodes.
The first situation is exacerbated by the fact that benign neck nodes often have an incomplete capsule and/or have lymphoid tissue outside the capsule. Other architectural features of true lymph node parenchyma - such as cortical sinuses and high endothelial venules - may persist in this tissue. Lymphoid tissue directly abutting adipose tissue is common at the hilum of lymph nodes, but it also occurs peripherally (Fig. 5a, b). In some cases, tumor deposits are found partially or exclusively within this unencapsulated lymphoid tissue (Fig. 6a). There are no guidelines for the interpretation of this finding.
Fig. 5.
a Lymph node with an incomplete capsule and metastatic carcinoma. Although a subcapsular sinus is seen focally (upper left), in other areas the lymphoid tissue merges with perinodal fat. b A lymph node with a capsular defect and protruding lymphoid tissue. Metastatic carcinoma is seen on the lower right, confined to the node
Fig. 6.
a In this lymph node with metastatic carcinoma, there is no capsule surrounding the lymphoid tissue that contains tumor. The hilum of the node is towards the left. The edge of the lymphoid tissue with tumor was in smooth continuity with the rest of the node, and this was not interpreted as ENE. b Same case as 6a, but a different lymph node. Again the capsule is ill-defined and the lymphoid tissue in the upper part of the field could be interpreted as extranodal and reactive. The ill-defined periphery and desmoplastic response to tumor in the upper right was interpreted as microscopic extranodal extension. However, this determination and the site of the “external aspect of the capsule” for ENE measurement are both subjective
Since most tumors metastasize first to subcapsular sinuses, one approach is to regard any tumor in lymphoid tissue peripheral to an identifiable capsule as extranodal, regardless of the extent or appearance of that lymphoid tissue. This would be a low threshold for ENE. It also does not adequately address cases where tumor is found at the periphery of a lymph node in an area where the capsule is absent. A perhaps preferable approach is to assess ENE based on extension beyond the contour of the node, regardless of the presence of a complete capsule. The presence of a desmoplastic response at the periphery of the node or admixture of tumor cells with adipose tissue are both good indicators of ENE if this approach is used (Figs. 6b, 7a, b). Measuring extent of ENE presents a further problem in the absence of a capsule, but for now it does not affect pN category.
Fig. 7.
a Metastatic squamous cell carcinoma at the periphery of a lymph node, with a desmoplastic response and tumor admixed with fat, consistent with microscopic extranodal extension. Accurately measuring the extent of ENE is challenging, as the site from which to measure is open to interpretation. b Tumor islands clearly extend into adipose tissue
ENE can also be difficult to determine in bulky nodes, in which the native capsule is absent and is replaced by a thick rim of fibrous tissue that merges with adjacent adipose tissue. Tumor deposits or extensions into this “neo-capsule” often suggest ENE. The determination is subjective because the original, reticulin-rich nodal capsule has been destroyed. Features that are consistent with ENE include extension of tumor beyond the general contour of the lymph node (Fig. 8) or clear-cut tumor within fat or adjacent to structures incompatible with being inside a node, such as large vessels or nerves (Fig. 4a).
Fig. 8.
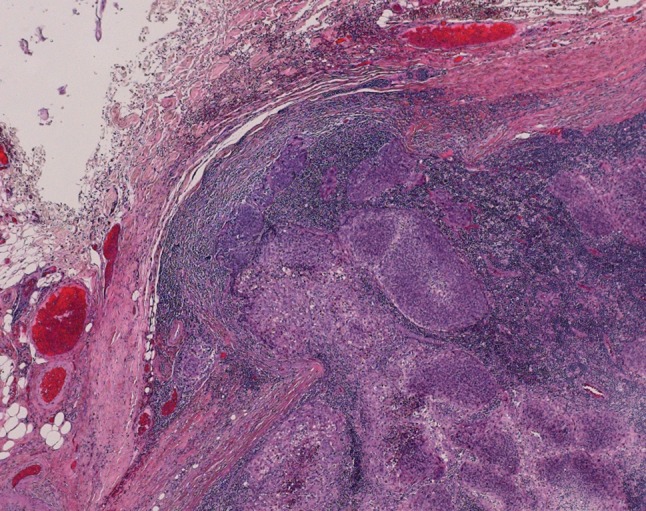
Metastatic squamous cell carcinoma with a thickened fibrous “neo-capsule”. This was interpreted as minor ENE as tumor clearly bulges beyond the contour of the periphery of the node
Measurement of the extent of ENE in bulky nodes is also subjective and it requires two determinations: (1) the site along the contour of the node from which measurement should occur, and (2) the site of the external aspect of the “neo-capsule”. Practically, one must be highly suspicious of extranodal extension in bulky, heavily fibrotic nodal disease, especially in matted nodes. Gross examination of the specimen, with submission of additional sections from suspicious areas, and liberal use of levels can often solve these dilemmas.
Finally, direct extension into lymph nodes from an oral cavity primary is regarded as pN-positive disease, if one follows general staging guidelines [22]. Assessment of extranodal extension in this circumstance is problematic, but it may not affect staging in many cases as these are typically advanced cancers. One approach is to assess ENE based only on the portion of the lymph node that is not contiguous with the main tumor mass.
Conclusion
As for many tumor sites, the staging of oral cancers requires increasingly detailed information from the surgical pathologist, with the results having a major impact on patient care. The measurement of depth of invasion, determination of extranodal extension and bone invasion are parameters for which definitions exist, but for which more detailed interpretive guidelines are lacking. As such, there may be a high degree of interobserver and perhaps intraobserver variability. This has the potential to obviate the central goals of cancer staging, which are to permit a system for cross-institutional comparisons of patient stages and outcomes, and for treatment planning.
Accurate staging of oral cancers requires input from surgeons with respect to correct specimen designation and orientation, and close attention from pathologists at the time of gross specimen submission. Discussion among head and neck and oral pathologists is required to develop best practices for the interpretation of difficult staging issues.
Acknowledgements
The author wishes to thank Patsy Morgan of the Division of Anatomical Pathology, Queen Elizabeth II Health Sciences Centre for secretarial assistance, Stephen Whitefield of the Faculty of Medicine, Dalhousie University for assistance with photomicrographs and Kelly Dakin-Hache, MD, FRCPC of the Department of Pathology, Dalhousie University for assistance with the revised submission.
Funding
No funding was received for this study.
Compliance with Ethical Standards
Conflict of interest
The author declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Ridge JA, Lydiatt WM, Patel SG, et al. Lip and oral cavity. In: Amin MB, et al., editors. AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 2.Ebrahimi A, Gil Z, Amit M. International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1138–1148. doi: 10.1001/jamaoto.2014.1548. [DOI] [PubMed] [Google Scholar]
- 3.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, et al., editors. AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 4.Califano JA, Lydiatt WM, Nehal KS, et al. Cutaneous squamous cell carcinoma of the head and neck. In: Amin MB, et al., editors. AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 5.Gibb RK, Olawaiye AB, Chen L, et al. Vulva. In: Amin MB, et al., editors. AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 6.Berdugo J, Thompson LDR, Purgina B, Sturgis CD, Tuluc M, Seethala R, Chiosea S. Measuring depth of invasion in early squamous cell carcinoma of the oral tongue: Positive deep margin, extratumoral perineural invasion, and other challenges. Head Neck Pathol. 2018 doi: 10.1007/s12105-018-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protocols for the. examination of specimens from patients with carcinomas of the lip and oral cavity. College of American Pathologists. http://www.cap.org/web/home/protocols-and-guidelines. Accessed 15 Nov 2018.
- 8.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Lin J, Men Y, et al. Does Medullary Versus Cortical Invasion of the Mandible Affect Prognosis in Patients With Oral Squamous Cell Carcinoma? J Oral Maxillofac Surg. 2017;75:403–415. doi: 10.1016/j.joms.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Fives C, Nae A, Roche P, et al. Impact of mandibular invasion on prognosis in oral squamous cell carcinoma four centimeters or less in size. Laryngoscope. 2017 Apr;127(4):849–854. 10.1002/lary.26211. [DOI] [PubMed]
- 11.Wong RJ, Keel SB, Glynn RJ, Varvares MA. Histological pattern of mandibular invasion by oral squamous cell carcinoma. The Laryngoscope. 2000;110:65–72. doi: 10.1097/00005537-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 2009;45:361–385. doi: 10.1016/j.oraloncology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Pagni G, Pellegrini G, Giannobile W, Rasperini G. Postextraction Alveolar Ridge Preservation: Biological Basis and Treatments. Int J Dentistry. 2012 doi: 10.1155/2012/151030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmusrati AA, Pilborough AE, Khurram SA, Lambert DW. Cancer-associated fibroblasts promote bone invasion in oral squamous cell carcinoma. Br J Cancer. 2017;117:867–875. doi: 10.1038/bjc.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JT, Barnes EL, Myers EN, Schramm VL, Jr, Borochovitz D, Sigler BA. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol. 1981;107(12):725–729. doi: 10.1001/archotol.1981.00790480001001. [DOI] [PubMed] [Google Scholar]
- 16.Dunne AA, Muller HH, Eisele DW, Kessel K, Moll R, Werner JA. Meta-analysis of the prognostic significance of perinodal spread in head and neck squamous cell carcinomas (HNSCC) patients. European journal of cancer. 2006;42(12):1863–1868. doi: 10.1016/j.ejca.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 17.Mernod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2016;62:60–71. doi: 10.1016/j.oraloncology.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 18.de Juan J, García J, López M, et al. Inclusion of Extracapsular Spread in the pTNM Classification System. A Proposal for Patients With Head and Neck Carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139(5):483–488. doi: 10.1001/jamaoto.2013.2666. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 20.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 21.Prabhu RS, Hanasoge S, Magliocca KR, Moeller BJ, Milas ZL, Hall WA, El-Deiry M, Wadsworth JT, Higgins KA, Beitler JJ. Extent of pathologic extracapsular extension and outcomes in patients with nonoropharyngeal head and neck cancer treated with initial surgical resection. Cancer. 2014;120:1499–1506. doi: 10.1002/cncr.28596. [DOI] [PubMed] [Google Scholar]
- 22.Wreesmann VB, Katabi N, Palmer FL, et al. Influence of extracapsular nodal spread on prognosis of oral squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1192–E1199. doi: 10.1002/hed.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gress DM, Edge SB, Greene FL, et al. Principles of cancer staging. In: Amin MB, et al., editors. AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 24.van den Brekel MWM, Lodder WL, Stel HV, Bloemena E, Leemans CR, van der Waal I. Observer variation in the histopathologic assessment of extranodal tumor spread in lymph node metastases in the neck, Wiley Periodicals, Inc. Head Neck. 2012;34:840–845. doi: 10.1002/hed.21823. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JS, Jr, Tarabishy Y, Luo J, Mani H, Bishop JA, Leon ME, Prasad ML, Xu H, Di Palma S. Inter- and intra-observer variability in the classification of extracapsular extension in p16 positive oropharyngeal squamous cell carcinoma nodal metastases. Oral Oncol. 2015;51(11):985–990. doi: 10.1016/j.oraloncology.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]