Abstract
Background and Aim
The literature on medication adherence in patients with inflammatory bowel disease (IBD) is heterogeneous. The present study aimed to identify the rates and predictors of nonadherence to medications in IBD.
Methods
This cross‐sectional study included patients of IBD (ulcerative colitis [UC] and Crohn's disease [CD]) recruited between November 2016 and March 2017. Adherence was assessed with a questionnaire (interview based) that evaluated patients' sociodemographic and disease profile and rates and predictors of medication adherence.
Results
A total of 266 patients (204 UC, 62 CD) were included (mean age: 38.5 ± 12.7 years, males: 142 [53.4%], mean disease duration: 6.4 ± 5.2 years). The overall adherence rate was 82.3%, with the lowest for topical therapy (67.3%) and the highest for steroids (95.9%). Predominant reasons for nonadherence were forgetting dose (18.8%), unavailability of medications (13.2%), felt better (11.7%), adverse effects (6.8%), and cost of treatment (6.0%). Patients' education (P < 0.001), occupation (P = 0.097), and socioeconomic status (P = 0.021) had a negative association with adherence. Patients in upper socioeconomic strata with professional education/occupation were the least adherent (47%), whereas patients from lower socioeconomic strata who were illiterate and unemployed had the highest adherence (100%).
Conclusion
More than 80% of patients were adherent to their medications; adherence was the lowest for topical therapy. Higher education, occupation, and upper socioeconomic status were negatively associated with adherence.
Keywords: adherence, Crohn's disease, inflammatory bowel disease, mesalamine, ulcerative colitis
Introduction
Patients with inflammatory bowel disease (IBD) are characterized by periods of remission and relapse and require long‐term medications for control of disease activity.1, 2 The main classes of drugs used in the treatment of IBD include 5‐aminosalicyclic acid (5‐ASA) (e.g. sulphasalazine, and mesalamine), corticosteroids (e.g. hydrocortisone, prednisone, and prednisolone), immunomodulators (e.g. azathioprine, 6‐mercaptopurine, methotrexate, and cyclosporine), and biologics (e.g. infliximab, adalimumab, and vedolizumab).3, 4 The treatment is often lifelong, with multiple medications and frequent drug dosing, necessitating inconvenient routes of administration and producing multiple side effects. Many patients cite complaints with treatment regimen complexity, pill burden, and dose frequency as negative influencers of adherence.5 Research further suggests that nonadherence to treatment is emerging as an important determinant in the occurrence of relapse,6, 7, 8, 9 resulting in decreased quality of life and increased societal and personal costs.7 Some significant associations between demographic, clinical, and psychosocial factors and nonadherence in IBD have been identified.5, 7, 10, 11, 12, 13, 14, 15, 16 Research on the same from India is, however, sparse, with a single study that did not address the association of many factors with nonadherence to medication.9
Improving medication adherence in patients is a major challenge for physicians in the treatment of IBD. Understanding patients in terms of their sociodemographic profile, disease profile, personal habits, and medication‐taking behavior could be the first step toward improving medication adherence and thereby adopting patient‐tailored interventions to ensure that the patients receive the full benefits of their medication and achieve disease remission. Thus, via this study, we aimed to identify the rates, reasons, and predictors of nonadherence to prescribed treatment in patients with IBD.
Methods
This cross‐sectional observational study conducted at the IBD Clinic of Department of Gastroenterology at All India Institute of Medical Sciences, New Delhi from November 2016 to March 2017 included consecutive IBD patients aged ≥18 years. Patients who underwent bowel resection and those refusing consent were excluded. The study received Institute Ethics Committee approval prior to enrolment (IRB No.: IEC/477/7.10.2016, dated: 7th October 2016). Written informed consent was obtained from each patient, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Study design
From the previous studies available on the topic, an archetype9, 17, 18 questionnaire was drafted to obtain information on the patients' sociodemographic profile, smoking and drinking habits, disease profile, and rates and reasons for medication nonadherence. All interviews were conducted by the same researcher in person. The questionnaire extracted the following information.
Sociodemographic profile
This included age, gender, place of residence (categorized into North India, East India, and West/Central India), number of family members, education and occupation of head of household, total family income/month, patients' education level (professional/honors, graduate/postgraduate, intermediate/posthigh school diploma/high school certificate, middle school certificate/primary school certificate, and illiterate), patients' occupation (profession/semiprofession, clerical/ shop owner/farmer, skilled worker/semiskilled worker/unskilled worker, and unemployed), and patients' income/month. The Kuppuswamy Scale19, 20 was used to calculate the socioeconomic status.
Disease profile
This included IBD disease type, age at onset, age at diagnosis, comorbidities (diabetes mellitus, hypertension, thyroid disease, heart disease), and disease activity indices. The simple clinical colitis activity index21 was utilized for ulcerative colitis (UC) and Crohn's disease activity index22 for Crohn's disease (CD).
Personal habits
This included smoking (never smoke, exsmoker, or current smoker) and drinking habits (never drink, exdrinker, or current drinker).
Current treatment
All questions were answered entirely by the patients and reaffirmed by the patients' attendants. Current treatment included medication names, doses, and frequency of intake prescribed to the patient on the last visit as well as the time elapsed since the last visit to the IBD clinic. The medications included: corticosteroids, oral 5‐aminosalicylic acid (5‐ASA) compounds (mesalamine/sulfasalazine), 5‐ASA/steroid enemas and suppositories, immunomodulators (azathioprine and methotrexate), and biologicals. Thereafter, the total medication adherence rates were calculated by adding the individual medication adherence rates and dividing the total by the number of medications the patients were taking. The patients were then categorized according to adherence rates into five categories formed by dividing 0–100% at intervals of 20%. Nonadherence to treatment was defined as drug intake of <80% of the prescribed dose.2
Patients were asked to enlist the following reasons that apply to them: forgetting a dose, unavailability of medications, frequent drug dosing, no effect of medication, felt better, side/adverse effects, cost of treatment, and lifelong treatment. Patients were also encouraged to mention any other reasons for nonadherence relevant to them.
Statistical analysis
The data were analyzed using Stata software (version 11.0) (StataCorp; College Station, TX, USA). For quantitative variables, mean and standard deviation were calculated. Wherever possible, number and percentage are also given as appropriate. The student t‐test was used for comparison of differences in the means of two groups, and the χ 2 test was used for comparison of categorical variables. When fewer than 5 registers were expected, Fisher's exact test was used. A P‐value <0.05 was considered statistically significant. To find the factors predicting nonadherence, univariate, followed by multivariate (binary logistic regression), analysis was conducted, considering factors with P < 0.1 on univariate analysis.
Results
Clinical, sociodemographic, and personal characteristics
Of the 266 patients enrolled, 204 (76.7%) were diagnosed with UC and 62 (23.3%) with CD. There were 142 (53.4%) males and 124 (46.6%) females; mean age was 38.5 ± 12.7 years (Table 1). Mean disease duration was 6.4 ± 5.2 years. Patients with CD were older than UC (41.5 ± 14.5 vs 37.5 ± 12.0 years, P = 0.034), and CD patients had a longer disease duration (7.6 ± 4.7 vs 5.9 ± 5.4 years, P = 0.002). There was no statistically significant difference between patients with CD and UC in terms of gender, comorbidities, body mass index (BMI), education, occupation, socioeconomic status, and the total number of family members. There was, however, a statistically significant difference between the two subtypes in terms of per capita income (P = 0.02) (Table 2).
Table 1.
Clinical and personal profile of patients with inflammatory bowel disease
| Variables | Inflammatory bowel disease (n = 266) | Ulcerative colitis (n = 204) | Crohn's disease (n = 62) | P‐value |
|---|---|---|---|---|
| Current age (years) | 38.5 ± 12.7 | 37.5 ± 12.0 | 41.5 ± 14.5 | 0.034 |
| Gender (male) | 142 (53.4) | 109 (53.4) | 33 (53.2) | 0.977 |
| Duration of disease (years) | 6.4 ± 5.2 | 5.9 ± 5.4 | 7.6 ± 4.7 | 0.002 |
| 5 (0, 30) | 4 (0, 30) | 6.63 (1, 20) | ||
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 9 (3.4) | 6 (2.9) | 3 (4.5) | 0.439 |
| Hypertension | 3 (1.1) | 2 (0.9) | 1 (1.6) | 0.550 |
| Thyroid disease | 5 (1.9) | 3 (1.5) | 2 (3.3) | 0.331 |
| Heart disease | 1 (0.4) | 1 (0.5) | 0 (0.0) | 1.000 |
| Number of patients | ||||
| Remission | 155 (58.3) | SCCAI≤2: 123 (60.3) | CDAI<150: 32 (51.6) | |
| Active disease | 111 (41.7) | SCCAI>2: 81 (39.7) | CDAI (150–220):13 (20.9) CDAI (220–450): 17 (27.4) CDAI >450: – |
|
| BMI (kg/m2) | 21.5 ± 4.3 | 21.7 ± 4.3 | 21.0 ± 4.4 | 0.269 |
| Smoking, n (%) | ||||
| Never smoked | 238 (89.5) | 186 (91.2) | 52 (83.9) | 0.081 |
| Exsmoker | 25 (9.4) | 17 (8.3) | 8 (12.9) | |
| Currently smoking | 3 (1.1) | 1 (0.5) | 2 (3.2) | |
| Alcohol, n (%) | ||||
| Never/occasional | 250 (93.9) | 193 (94.6) | 57 (91.9) | 0.514 |
| Exdrinker | 14 (5.3) | 10 (4.9) | 4 (6.4) | |
| Current drinker | 2 (0.7) | 1 (0.5) | 1 (1.6) | |
BMI, body mass index; CDAI, Crohn's disease activity index; SCCAI, simple clinical colitis activity index.
Table 2.
Sociodemographic profile of patients with inflammatory bowel disease
| Variables | Inflammatory bowel disease (n = 266) | Ulcerative colitis (n = 204) | Crohn's disease (n = 62) | P‐value |
|---|---|---|---|---|
| Region of residence, n (%) | ||||
| North India | 207 (77.8) | 161 (78.9) | 46 (74.2) | 0.675 |
| East India | 51 (19.6) | 37 (18.1) | 14 (22.6) | |
| West/central India | 8 (3.01) | 6 (2.9) | 2 (3.2) | |
| Patient's education, n (%) | ||||
| Professional or honors | 30 (11.3) | 22 (10.8) | 8 (12.9) | 0.275 |
| Graduate or postgraduate | 77 (28.9) | 56 (27.4) | 21 (33.9) | |
| Intermediate or posthigh school diploma/high school certificate | 86 (32.3) | 70 (34.3) | 16 (25.8) | |
| Middle school certificate/primary school certificate | 47 (17.7) | 33 (16.2) | 14 (22.6) | |
| Illiterate | 26 (9.8) | 23 (11.3) | 3 (4.8) | |
| Patient's occupation, n (%) | ||||
| Profession/semiprofession | 38 (14.3) | 24 (11.7) | 14 (22.6) | 0.072 |
| Clerical, shop owner, farmer | 55 (20.7) | 40 (19.6) | 15 (24.2) | |
| Skilled worker/semiskilled worker/unskilled worker | 49 (18.4) | 42 (20.6) | 7 (11.3) | |
| Unemployed | 124 (46.6) | 98 (48.0) | 26 (41.9) | |
| Socioeconomic status, n (%) | ||||
| Upper | 26 (9.8) | 16 (7.8) | 10 (16.1) | 0.181 |
| Upper middle | 114 (42.9) | 86 (42.2) | 28 (45.2) | |
| Lower middle | 66 (24.8) | 54 (26.5) | 12 (19.3) | |
| Upper lower | 58 (21.8) | 47 (23.0) | 11 (17.7) | |
| Lower | 2 (0.7) | 1 (0.5) | 1 (1.6) | |
| Total number of family members | 5.5 ± 2.7 | 5.6 ± 2.9 | 5.2 ± 2.1 | 0.913 |
| 5 (1, 18) | 5 (1, 18) | 5 (1, 12) | ||
| Per capita income (Rs.) | 7967.4 ± 11 067.6 | 7371.2 ± 11 351 | 9929.2 ± 9912.8 | 0.023 |
| 4000 (0–100 000) | 3333 (25–100 000) | 6333.5 (0–40 000) | ||
Medication prescription patterns
Forty‐nine patients were taking steroids, 211 patients were taking mesalamine/sulfasalazine, 95 were taking enema/suppository, 106 patients were taking immunosuppressants, and 3 were taking biologics. A total of 108 patients were on a single medication, 124 on two, 28 on three, and 6 were on four medications (Table 3).
Table 3.
Medications prescribed to patients with inflammatory bowel disease
| Inflammatory bowel disease (n = 266) | Ulcerative colitis (n = 204) | Crohn's disease (n = 62) | P‐value | |
|---|---|---|---|---|
| Drugs prescribed, n (%)† | ||||
| Steroids | 49 (18.4) | 36 (17.6) | 13 (20.9) | 0.555 |
| Mesalamine/sulfasalazine | 211 (79.3) | 188 (92.2) | 23 (37.1) | <0.001 |
| Enema/suppository | 95 (35.7) | 93 (45.6) | 2 (3.2) | <0.001 |
| Immunosuppressant | 106 (39.9) | 62 (30.4) | 44 (70.8) | <0.001 |
| Monoclonal antibody | 3 (1.1) | 2 (0.9) | 1 (1.6) | 0.550 |
| Total no. of drugs taking, n (%) | ||||
| 1 | 108 (40.6) | 66 (32.3) | 42 (67.7) | <0.001 |
| 2 | 124 (46.6) | 105 (51.5) | 19 (30.6) | |
| 3 | 28 (10.5) | 27 (13.2) | 1 (1.6) | |
| 4 | 6 (2.3) | 6 (2.9) | 0 (0.0) | |
Total n of all drugs prescribed does not equal total number of patients, as some patients are on multiple drugs.
Medication adherence patterns
Individual medication adherence rates were the highest for steroids (95.9%) and biologicals (100%) and lowest for enema/suppository (67.3%). Adherence rates were similar for mesalamine/sulfasalazine (90.9%) and immunosuppressants (93.4%) (Table 4).
Table 4.
Adherence rates for individual drugs and overall adherence rates for patients with inflammatory bowel disease
| Inflammatory bowel disease (n = 266) | Ulcerative colitis (n = 204) | Crohn's disease (n = 62) | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Adherence rates for individual drugs | |||||||
| Drugs | n | N (%) | n | N (%) | n | N (%) | |
| Steroids | 49 | 47 (95.9) | 36 | 36 (100) | 13 | 11 (84.6) | 0.066 |
| Mesalamine/sulfasalazine | 211 | 192 (90.9) | 188 | 173 (92) | 23 | 19 (82.6) | 0.136 |
| Enema/suppository | 95 | 64 (67.3) | 93 | 62 (67.4) | 2 | 2 (100) | 1.000 |
| Immunosuppressant | 106 | 99 (93.4) | 62 | 60 (96.7) | 44 | 39 (88.6) | 0.124 |
| Monoclonal antibody | 3 | 3 (100) | 2 | 2 (100) | 1 | 1 (100) | — |
| Overall | 266 | 219 (82.3) | 204 | 168 (82.4) | 62 | 54 (87.1) | 0.379 |
| 1 drug | 108 | 97 | 66 | 61 (92.4) | 42 | 37 (88.1) | 0.449 |
| >1 drug | 158 | 122 | 138 | 107 (77.5) | 20 | 17 (85.0) | 0.570 |
| Overall adherence rates | |||||||
| 0–20% | 4 (1.5) | 3 (1.5) | 1 (1.6) | 0.958 | |||
| 21–40% | 4 (1.5) | 3 (1.5) | 1 (1.6) | ||||
| 41–60% | 27 (10.2) | 22 (10.8) | 5 (8.1) | ||||
| 61–80% | 12 (4.5) | 10 (4.9) | 2 (3.2) | ||||
| 81–100% | 219 (82.3) | 166 (81.4) | 53 (85.5) | ||||
A total of 219 (82.3%) patients reported >80% adherence to medication. Four (1.5%) patients had completely discontinued their medication, 12 (4.5%) reported 60–80%, 27 (10.2%) reported 40–60%, and 4 (1.5%) patients reported 20–40% adherence to medication. Therefore, 47 (17.7%) patients were nonadherent to medication (Table 4).
Reasons for non‐adherence
The reasons for nonadherence were forgetting dose in 50 (18.8%), unavailability of medications in 35 (13.2%), frequent drug dosing in 11 (4.1%), no effect of medication in 8 (3.0%), felt better in 31 (11.7%), side/adverse effects in 18 (6.8%), cost of treatment in 16 (6.0%), lifelong treatment in 9 (3.4%), and others in 49 (18.4%) patients (Table 5). Amongst the other reasons for nonadherence, the major ones reported were: inability to understand the prescription, not carrying the medications to work, fasting, inconvenient route of administration, difficulty in traveling to the hospital to renew the prescription, and discontinuity of medications when traveling away from home.
Table 5.
Reasons for nonadherence to medical therapy
| Reasons | Inflammatory bowel disease (n = 266) | Ulcerative colitis (n = 204) | Crohn's disease (n = 62) | P‐value |
|---|---|---|---|---|
| Forgetting dose | 50 (18.8) | 40 (19.6) | 10 (16.1) | 0.539 |
| Unavailability of medication | 35 (13.2) | 27 (13.2) | 8 (12.9) | 0.946 |
| Felt better | 31 (11.6) | 26 (12.7) | 5 (8.1) | 0.314 |
| Side/adverse effect of medication | 18 (6.8) | 10 (4.9) | 8 (12.9) | 0.029 |
| Cost of treatment | 16 (6.0) | 14 (6.9) | 2 (3.2) | 0.375 |
| Frequent drug dosing | 11 (4.1) | 10 (4.9) | 1 (1.6) | 0.466 |
| Lifelong treatment | 9 (3.4) | 7 (3.4) | 2 (3.2) | 1.000 |
| No effect of medications | 8 (3.0) | 7 (3.4) | 1 (1.6) | 0.686 |
| Others | 49 (18.4) | 33 (16.2) | 16 (25.8) | 0.087 |
Nonadherence was found to be significantly more likely to be secondary to frequent dosing (P = 0.044), felt better (P < 0.001), adverse effects of medication (P = 0.001), cost of treatment (P = 0.019), and lifelong treatment (P = 0.07). The other reasons for nonadherence showed no significant association with nonadherent behavior.
Predictors of non‐adherence
On univariate analysis, patients' education level (P < 0.001), socioeconomic status (P = 0.021), intake of steroids (P = 0.019), immunomodulators (P = 0.027), and topical therapy (P < 0.001) had a significant association with medication adherence rates (Table 6). Disease duration in nonadherent patients was significantly longer than adherent patients (8.48 + 5.3 vs 5.89 + 5.1 years, P = 0.001). Patients' occupation (P = 0.097) and 5‐ASA (P = 0.061) intake had a statistically insignificant association with adherence. We further subgrouped the patients into three categories based on their education, occupation, and socioeconomic status. Patients from upper socioeconomic strata with professional education and occupation (Group A) had the lowest adherence rate of 47%, whereas patients belonging to the lower socioeconomic strata who were illiterate and unemployed had the highest adherence rates (Group C) of 100% (Fig. 1). There was no effect of gender, age, place of residence, BMI, per capita income, patients' income, smoking, alcohol consumption, comorbidities, and disease activity on the medication adherence rates. On multivariate analysis, the predictors of adherence were the combined category of education, occupation and socioeconomic status, and disease duration (Table 6).
Table 6.
Predictors of nonadherence in patients with inflammatory bowel disease
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variables | Adherence rates | P value | Odds ratio | Odds ratio (95% CI) | P value |
| Gender | 0.348 | — | — | — | |
| Male (n = 142) | 114 (80.2) | ||||
| Female (n = 124) | 105 (84.6) | ||||
| Disease activity | 0.599 | — | — | — | |
| Remission (n = 155) | 126 (81.3) | ||||
| Active disease (n = 111) | 93 (83.8) | ||||
| Disease duration (years) | 8.5 ± 5.3 versus 5.9 ± 5.1 | 0.002 | 1.1 (1.02–1.14) | 1.2 (1.03–1.3) | 0.009 |
| Patient's education, n (%)† | <0.001 | — | — | — | |
| Professional or honors (n = 30) | 19 (63.3) | ||||
| Graduate or postgraduate (n = 77) | 66 (85.7) | ||||
| Intermediate or posthigh school diploma/high school certificate (n = 86) | 77 (89.5) | ||||
| Middle school certificate/primary school certificate (n = 47) | 32 (68.1) | ||||
| Illiterate (n = 26) | 25 (96.2) | ||||
| Patient's occupation, n (%)† | 0.097 | — | — | — | |
| Profession/semiprofession (n = 38) | 26 (68.4) | ||||
| Clerical, shop owner, farmer (n = 55) | 46 (83.6) | ||||
| Skilled worker/semiskilled worker/unskilled worker (n = 49) | 43 (87.8) | ||||
| Unemployed (n = 124) | 104 (83.9) | ||||
| Socioeconomic status, n (%)† | 0.021 | — | — | — | |
| Upper (n = 26) | 16 (61.5) | ||||
| Upper middle (n = 114) | 100 (87.7) | ||||
| Lower middle (n = 66) | 55 (83.3) | ||||
| Upper lower (n = 58) | 47 (81.0) | ||||
| Lower (n = 2) | 1 (50.0) | ||||
| Combined educational, occupational, and socioeconomic categories | <0.001 | 8.0 (2.6–25.1) | 17.6 (3.8–80.7) | <0.001 | |
| Group A (n = 17) | 8 (47) | ||||
| Group B (n = 69) | 60 (87) | ||||
| Group C (n = 8) | 8 (100) | ||||
| Steroids intake | 0.019 | 3.9 (1.2–13.2) | 4.03 (0.5–33.9) | 0.200 | |
| Yes (n = 49) | 46 (93.9) | ||||
| No (n = 217) | 173 (79.7) | ||||
| 5‐ASA intake | 0.061 | 2.5 (0.9–6.6) | 6.08 (0.5–70.6) | 0.149 | |
| Yes (n = 211) | 169 (80.1) | ||||
| No (n = 55) | 50 (90.9) | ||||
| Topical therapy | <0.001 | 3.8 (1.9–7.2) | 2.03 (0.5–8.2) | 0.320 | |
| Yes (n = 95) | 66 (69.5) | ||||
| No (n = 171) | 153 (89.5) | ||||
| Immunomodulators | 0.027 | 2.2 (1.1–4.4) | 5.3 (0.9–25.0) | 0.069 | |
| Yes (n = 106) | 94 (88.7) | ||||
| No (n = 160) | 125 (78.1) | ||||
Patient education, occupation, and socioeconomic status were not included in the multivariate analysis because they had collinearity with combined educational, occupational, and socioeconomic categories.
Group A: education: professional; occupation: professional; socioeconomic status: upper.
Group B: education: graduate‐postgraduate/intermediate or posthigh school diploma/high school certificate/middle school certificate/primary school certificate; occupation: clerical, shop owner, farmer/skilled worker/semiskilled worker/unskilled worker; socioeconomic status: upper middle and lower middle.
Group C: education: illiterate; occupation: unemployed; socioeconomic status: upper lower and lower.
5‐ASA, 5‐aminosalicylic acid; CI, confidence interval.
Figure 1.
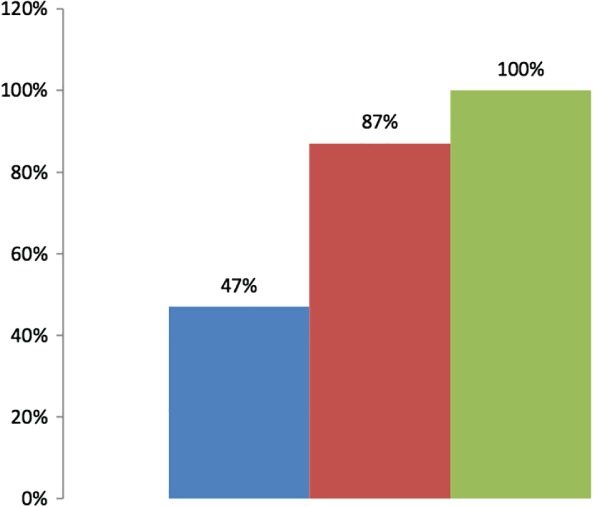
Adherence rates in patients with inflammatory bowel disease according to their professional, occupational, and socioeconomic categories. ( ), Group A (n = 17); (
), Group A (n = 17); ( ), Group B (n = 69); (
), Group B (n = 69); ( ), Group C (n = 8). Group A: education: professional; occupation: professional; socioeconomic status: upper. Group B: education: graduate‐postgraduate/intermediate or posthigh school diploma/high school certificate/middle school certificate/primary school certificate; occupation: clerical, shop owner, farmer/skilled worker/semiskilled worker/unskilled worker; socioeconomic status: upper middle and lower middle. Group C: education: illiterate; occupation: unemployed; socioeconomic status: upper lower and lower.
), Group C (n = 8). Group A: education: professional; occupation: professional; socioeconomic status: upper. Group B: education: graduate‐postgraduate/intermediate or posthigh school diploma/high school certificate/middle school certificate/primary school certificate; occupation: clerical, shop owner, farmer/skilled worker/semiskilled worker/unskilled worker; socioeconomic status: upper middle and lower middle. Group C: education: illiterate; occupation: unemployed; socioeconomic status: upper lower and lower.
Discussion
The efficacy of any treatment regimen depends on the patient's compliance or adherence, and the same applies to IBD. Various studies across the globe have explored this aspect; however, the data are limited from Asia, where there has been a recent increase in the disease burden of IBD.23, 24 The present study reports an adherence rate of 82%, which is quite high in comparison with other studies. The patients enrolled in this study were recruited from the IBD clinic, which only caters to patients with IBD. Patients following up at specialty clinics may be more motivated to follow the physician's advice, and this would account for relatively high adherence rates in this study. Furthermore, being a tertiary care center, we must acknowledge the fact that most of the patients that reach us have been referred by various primary and secondary health‐care centers from all over the country. Thus, it is likely that the patients under our care are those who were unable to achieve symptomatic remission or treatment satisfaction from their initial care provider. This might also explain the high rates of medication adherence seen in our study. A similar study conducted in a different part of the country reported opposite results, with 81% of the study population being nonadherent.9 Another study from Asia (Korea) reported adherence rates of 63.8%.25 In a systematic review of 17 studies (no Asian study),12 the nonadherence rates varied from 7 to 72%, and studies from Spain, Italy, Belgium, and Prague have reported adherence rates to IBD medications of 38, 64, 61, and 61%, respectively.17, 18, 26, 27 A study from the United States had reported good compliance with infliximab (75%) compared to the other medications, including steroids (69%), immunomodulators (72%), 5‐ASA (63%), and antibiotics (47%).28 The results of these studies, the systematic review, and the present study show a considerable heterogeneity in the adherence rates. This could be accounted for by differences in patient populations and different methods of assessing adherence to medications.
In the present study, the reasons for nonadherence as cited by the patients were forgetfulness, felt better, frequent drug dosing, no effect of medication, unavailability of medications, side/adverse effects, lifelong treatment, and cost of treatment. Unlike the previous studies, there was no relation of nonadherence to age, gender, and marital status, and similar findings were reported in the meta‐analysis of 17 studies.12 The disease duration in nonadherent patients was longer than adherent patients. In the other study from India, reasons for nonadherence were similar: forgetfulness (77%), felt better (14.2%), high frequency of doses (10.1%), no effect of medication (7.8%), nonavailability of medications (2.3%), side effects, and long‐term medications. However, the cost of medications and disease duration were not related to adherence in that study.9 Less than 10% of patients in the present study belonged to high socioeconomic status, whereas most of the patients in the other Indian study belonged to middle/upper socioeconomic status, which could explain this discrepancy. Another study from Asia (Korea) associated nonadherence with younger age, longer intervals between outpatient clinic visits, and limited knowledge of prescribed medication.25 In addition, nonadherent patients had a significantly greater risk of relapse of IBD than adherent patients. In a systematic review of 17 studies, psychological distress, patients' beliefs about medications, and doctor–patient discordance were associated with nonadherence, whereas demographic, clinical, and treatment variables were not.12 Amongst studies from the other parts of the globe, a Spanish study reported forgetting dose (60%), felt better (35%), felt worst after taking medication (25%), and careless about taking medication (38%) as reasons for nonadherence. The study also associated nonadherence with intestinal and social areas of the IBDQ‐32 (a tool for the measurement of quality of life), with long‐standing IBD, with patients who considered themselves to be inadequately informed about their treatment, those with high depression scores, and with high patient–physician discordance.17 Similar to the study above, many of our patients also mentioned the inability to understand the prescription or inadequate information about their treatment as a hindrance to medication adherence. Similar to our study, the Italian study also reported a negative association of adherence with disease duration and a significant association of nonadherence with forgetfulness, feeling better, feeling worse/side effects, frequent drug dosing, and topical therapy with enemas as opposed to oral therapy.18 In a study from Belgium, predictors of low adherence were age < 40 years, high education level, being single, and mesalamine use, whereas being self‐employed was a protective factor.26 Similar to our study, a study from Prague also associated nonadherence with a higher education level and side effects of medication. Contrary to our study, however, the nonadherence decreased with older age, and nonadherent patients were more likely to be chronically active or in relapse.27
The most surprising finding of the present study was the negative association of medication adherence with higher education, professional job profile, and high socioeconomic status. Interestingly, less than 50% of the most educated, professionally employed, and richest were adherent to their medications in contrast to the illiterate and unemployed hailing from the lower socioeconomic strata, who were all found to be adherent. In fact, the patients who had the highest education and occupation had the odds of being 17.6 times less adherent than the intermediate ones, who again were 17.6 times less adherent than the illiterate and unemployed group. Studies from Belgium and Prague have also related nonadherence to higher education level; however, no study, including the study from Mumbai, has related nonadherence with occupation or socioeconomic status. It could possibly be that the undereducated patients are more faithful toward the treatment prescribed by their doctors, whereas the more educated patients had a second opinion and decided to alter their treatment. Those with more demanding job profiles could possibly be so engaged in work that they become negligent toward their health, such as seeing the doctor regularly and taking their medications. Fear could also play a role; the undereducated and those with low‐profile jobs might be acting out of fear as a relapse could mean having to take time away from work and could jeopardize their earnings. Of course, the aptitude and attitude toward the disease vary largely amongst the two populations, and that could be dictating the variations seen in our study. It could also be that patients belonging to higher socioeconomic status visit the hospital earlier with mild disease activity than those hailing from the lower socioeconomic status, and subsequently, these patients tend to forget to take medications prescribed to them. Furthermore, the patients who were uneducated come to the doctor with almost no background knowledge about the illness or the medication. It is also probable that the only source of information about the illness for these patients would be their care provider; hence, they were able to trust more and follow advice blindly. The educated patients, on the other hand, would most likely already have tried researching about the illness, whether over the internet, through books, or even another care provider. Thus, a situation may arise for these patients where the knowledge they have gathered from the various other resources conflicts with that provided by the specialists at our institute. There is a possibility that these patients researched the side effects of long‐term use of steroids and immunosuppressants and chose to seek alternative treatments. It may also be that these patients have learned to control their symptoms with dietary modifications and lifestyle changes such that they do not feel the need to take medications to control their symptoms.
Our study, although conducted to the best of our capability, has some limitations. First, rates of medication adherence would vary at different points on a patients' disease timeline. We, however, calculated the rate of medication adherence from the previous visit to the IBD clinic to the current and, based on that, classified the patients as adherent or nonadherent. However, as explained above, many previous studies have used other methods to measure adherence. So, as yet, there is no set gold standard for measuring medication adherence. Second, although the study was centered around an interview‐based questionnaire administered by a single researcher in order to eliminate interobserver bias, it is hard to predict the underestimation of nonadherence to medication. Patients, out of fear or respect for their treating physicians, might have overreported their adherence rates, thus leading to the higher adherence rates within our subjects in comparison to the other studies that have been carried out in the past.9, 17, 18 The lower alcohol consumption and smoking habits observed within our subjects could also be because of this reason. The other explanation for this, however, could just be distinct demographic characteristics.
In conclusion, 82.3% of the IBD patients were adherent to their prescribed medications. Adherence rates are much lower for topical preparations in comparison to oral medications. Predictors for low adherence rates include high education level, high job profile, upper socioeconomic status, and longer disease duration. This study emphasizes a need to educate all the patients with IBD and warn them of the consequences of nonadherent behavior.
Declaration of conflict of interest: None to declare.
REFERENCES
- 1. Magro F, Rodrigues A, Vieira AI et al. Review of the disease course among adult ulcerative colitis population‐based longitudinal cohorts. Inflamm. Bowel Dis. 2012; 18: 573–83. [DOI] [PubMed] [Google Scholar]
- 2. Cosnes J, Cattan S, Blain A et al. Long‐term evolution of disease behavior of Crohn's disease. Inflamm. Bowel Dis. 2002; 8: 244–50. [DOI] [PubMed] [Google Scholar]
- 3. Magro F, Gionchetti P, Eliakim R et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra‐intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo‐anal pouch disorders. J. Crohns Colitis. 2017; 11: 649–70. [DOI] [PubMed] [Google Scholar]
- 4. Gomollón F, Dignass A, Annese V et al. 3rd European evidence‐based consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management. J. Crohns Colitis. 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 5. Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 2006; 23: 577–85. [DOI] [PubMed] [Google Scholar]
- 6. Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am. J. Med. 2003; 114: 39–43. [DOI] [PubMed] [Google Scholar]
- 7. Robinson A. Review article: improving adherence to medication in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2008; 27 (Suppl. 1): 9–14. [DOI] [PubMed] [Google Scholar]
- 8. Higgins PDR, Rubin DT, Kaulback K, Schoenfield PS, Kane SV. Systematic review: impact of non‐adherence to 5‐aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment. Pharmacol. Ther. 2009; 29: 247–57. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt J, Patil S, Joshi A, Abraham P, Desai D. Self‐reported treatment adherence in inflammatory bowel disease in Indian patients. Indian J. Gastroenterol. 2009; 28: 143–6. [DOI] [PubMed] [Google Scholar]
- 10. Lenti MV, Selinger CP. Medication non‐adherence in adult patients affected by inflammatory bowel disease: a critical review and update of the determining factors, consequences and possible interventions. Expert Rev. Gastroenterol. Hepatol. 2017; 11: 215–26. [DOI] [PubMed] [Google Scholar]
- 11. Herman ML, Kane SV. Treatment nonadherence in inflammatory bowel disease: identification, scope, and management strategies. Inflamm. Bowel Dis. 2015; 21: 2979–84. [DOI] [PubMed] [Google Scholar]
- 12. Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non‐adherence to oral medication for inflammatory bowel disease: a systematic review. Am. J. Gastroenterol. 2010; 105: 525–39. [DOI] [PubMed] [Google Scholar]
- 13. Hawthorne AB, Rubin G, Ghosh S. Review article: medication non‐adherence in ulcerative colitis–strategies to improve adherence with mesalazine and other maintenance therapies. Aliment. Pharmacol. Ther. 2008; 27: 1157–66. [DOI] [PubMed] [Google Scholar]
- 14. Kane SV, Brixner D, Rubin DT, Sewitch MJ. The challenge of compliance and persistence: focus on ulcerative colitis. J. Manag. Care Pharm. 2008; 14 (1 Suppl. A): s2‐12‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kane SV. Overcoming adherence issues in ulcerative colitis. Gastroenterol. Hepatol. 2007; 3: 795–9. [PMC free article] [PubMed] [Google Scholar]
- 16. López‐Sanromán A, Bermejo F. Review article: how to control and improve adherence to therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006; 24 (Suppl. 3): 45–9. [DOI] [PubMed] [Google Scholar]
- 17. López San Román A, Bermejo F, Carrera E, Pérez‐Abad M, Boixeda D. Adherence to treatment in inflammatory bowel disease. Rev. Esp. Enferm. Dig. 2005; 97: 249–57. [DOI] [PubMed] [Google Scholar]
- 18. D'Incà R, Bertomoro P, Mazzocco K, Vettorato MG, Rumiati R, Sturniolo GC. Risk factors for non‐adherence to medication in inflammatory bowel disease patients. Aliment. Pharmacol. Ther. 2008; 27: 166–72. [DOI] [PubMed] [Google Scholar]
- 19. Gadhave S, Nagarkar A. Kuppuswamy scale for measuring socio‐economic status: revised monthly income figures for 2015. Indian J. Pediatr. 2015; 82: 1175–6. [DOI] [PubMed] [Google Scholar]
- 20. Sharma R. Kuppuswamy's socioeconomic status scale–revision for 2011 and formula for real‐time updating. Indian J. Pediatr. 2012; 79: 961–2. [DOI] [PubMed] [Google Scholar]
- 21. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998; 43: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976; 70: 439–44. [PubMed] [Google Scholar]
- 23. Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia‐Pacific area: a comparison with developed countries and regional differences. J. Dig. Dis. 2010; 11: 134–47. [DOI] [PubMed] [Google Scholar]
- 24. Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest. Res. 2017; 15: 138–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tae CH, Jung S‐A, Moon HS et al. Importance of Patients’ knowledge of their prescribed medication in improving treatment adherence in inflammatory bowel disease. J. Clin. Gastroenterol. 2016; 50: 157–62. [DOI] [PubMed] [Google Scholar]
- 26. Coenen S, Weyts E, Ballet V et al. Identifying predictors of low adherence in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016; 28: 503–7. [DOI] [PubMed] [Google Scholar]
- 27. Cervený P, Bortlík M, Kubena A, Vlcek J, Lakatos PL, Lukás M. Nonadherence in inflammatory bowel disease: results of factor analysis. Inflamm. Bowel Dis. 2007; 13: 1244–9. [DOI] [PubMed] [Google Scholar]
- 28. Waters H, Annunziata K, Naim A, Freedman D, Piech C. Inflammatory Bowel Disease patients’ adherence to and satisfaction with treatment: P‐0087. Inflamm. Bowel Dis. 2008; 14: S37.18816717 [Google Scholar]


