Abstract
Background and Aim
Acute kidney injury (AKI) commonly occurs in patients with chronic liver disease (CLD). As per the International Club of Ascites, AKI is classified into three stages; stage 1 has recently been divided into subgroups 1A and 1B. We performed a prospective study to validate the association between subgrouping and outcome.
Methods
This study was conducted using decompensated cirrhosis (DC) patients hospitalized in the Gastroenterology ward between August 2016 and May 2018. Demographic, clinical, and laboratory parameters were compared between AKI 1A and AKI 1B patients. The duration of hospitalization and outcome were compared.
Results
A total of 528 subjects were enrolled; 296 (56.1%) had AKI, and of them, 61.48% (n = 182) had stage 1, 20.95% (n = 62) had stage 2, and 17.57% (n = 52) had stage 3 AKI. Of the enrolled patients, 100 (54.94%) had early (AKI 1A) and 82 (45.06%) had late stage 1 AKI (AKI 1B). Patients with AKI 1B had higher total leucocyte count, total bilirubin, serum urea, serum creatinine (SCr), model for end‐stage liver disease (MELD), MELD‐Na+, and child‐turcotte‐pugh (CTP) score and decreased serum albumin than AKI 1A. The prevalence of hepatorenal syndrome (HRS), acute on chronic liver failure (ACLF) were higher in AKI 1B patients, and they had a prolonged hospital stay compared to AKI 1A patients. Furthermore, AKI 1B patients had significantly lower survival both at 28 days and 90 days.
Conclusion
Our study validates the subclassification of stage 1 AKI. Patients with AKI 1B more often progress to higher AKI stages with significantly lower 28‐day and 90‐day survival rates. Results justify subclassification and suggest the need for early intervention. The small increase in SCr should be viewed with caution in AKI stage 1A.
Keywords: acute kidney injury, chronic liver disease, serum creatinine
Introduction
Cirrhosis of the liver with portal hypertension is associated with severe arterial vasodilation as a result of the release of vasodilators in the splanchnic circulation.1 This mechanism leads to a reduction of effective circulating volume and a compensatory activation of endogenous vasoconstrictor systems (sympathetic nervous system, renin angiotensin aldosterone system, and nonosmotic release of vasopressin), which are responsible for hyperdynamic circulation and sodium and water retention, resulting in the appearance of ascites and/or dilutional hyponatremia. In the advanced stages, the maximal activation of vasoconstrictor systems may cause severe renal vasoconstriction, leading to hepatorenal syndrome (HRS), which is characterized by a functional renal failure occurring in patients with advanced liver disease (cirrhosis or acute alcoholic hepatitis) and ascites.2 HRS‐acute kidney injury (AKI) has a special status for two reasons: (i) it is a specific type of AKI in patients with cirrhosis, and (ii) it is associated with the worst survival in patients with cirrhosis.3 Two other factors have also been identified as being responsible for the hemodynamic alterations in patients with cirrhosis, namely, reduction in cardiac output and systemic inflammation.4 Furthermore, systemic inflammation may also cause damage in organs other than the kidneys, such as the brain, the heart, the lungs, or the liver itself, causing a multiorgan failure syndrome, such as acute on chronic liver failure (ACLF),5 which is an ominous entity in patients with cirrhosis of liver. ACLF has been defined differently by various learned hepatology societies. As per the Asian Pacific Association for the Study of the Liver (APASL) consensus, “ACLF is an acute hepatic insult manifesting as jaundice (serum bilirubin ≥ 5 mg/dL [85 μmol/L]) and coagulopathy (International Normalized Ratio [INR] ≥1.5 or prothrombin activity <40%) complicated within 4 weeks by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease (CLD) or cirrhosis, and is associated with a high 28‐day mortality.”6 However, the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) working group define ACLF as “Acute deterioration of pre‐existing CLD usually related to a precipitating event and associated with increased mortality at 3 months due to multi‐system organ failure, and using the criteria of the Consortium Acute‐on‐Chronic Liver Failure in Cirrhosis (CANONIC) study ACLF has been classified into ACLF I, II, or III.”7
As a result of the multifactorial insults described above, patients with cirrhosis have a high prevalence of renal dysfunction.8 About 50% of patients admitted to the hospital for acute decompensation of cirrhosis have AKI during hospitalization, and one‐third develops AKI after admission during the course of treatment.9, 10, 11, 12 Furthermore, even stable outpatients with cirrhosis may frequently develop AKI during follow‐up.13 This is of great importance as kidney dysfunction is associated with poor outcomes in patients with cirrhosis. In patients with cirrhosis, AKI has been defined as (i) an increase of serum creatinine (SCr) by 0.3 mg/dL (26.5 μmol/L) within 48 h or (ii) a percentage increase of SCr by 50% from baseline that is known, or presumed, to have occurred within the prior 7 days.14 Furthermore, AKI has been staged, and the stage of AKI has been seen to be of utmost important in clinical practice. There is a stepwise increase in the 90‐day mortality rate with AKI stage.9, 10, 11, 15 AKI has been classified into three stages (1–3) depending on the intensity of changes in SCr; this staging classification correlates well with prognosis in patients with cirrhosis,7, 16, 17with stages 2 and 3 having a worse prognosis compared to stage 1.4, 9, 10, 11, 16 However, recently, stage 1 has been further subdivided into two subgroups on the basis of serum levels of creatinine (SCr): stage 1A (SCr < 1.5 mg/dL) and stage 1B (SCr ≥ 1.5 mg/dL), and this subclassification is supported by differential outcomes.12 However, there is a lack of external validation of this subclassification of stage 1 AKI into 1A and 1B.
Methods
This study was conducted in decompensated cirrhosis (DC) patients; data were collected prospectively from inpatients admitted to the Gastroenterology Department, SCB Medical College, Cuttack, between August 2016 and May 2018. Exclusion criteria included the presence of chronic kidney disease, obstructive uropathy, hepatocellular carcinoma, cardiopulmonary diseases, and other malignancies. In DC patients, AKI was defined at admission based on levels of SCr at diagnosis, and patients were followed up until death or for 90 days. Patients with AKI stage 1 were subgrouped into stage 1A (SCr < 1.5 mg/dL) and stage 1B (SCr ≥ 1.5 mg/dL) using a cut‐off value of SCr level of 1.5 mg/dL. The best cut‐off point of 1.5 mg/dL admission SCr for determining 3‐month prognosis in patients with AKI stage 1 was adopted from a previous study by Huelin et al. 12, 18
Patients were screened for HRS‐AKI per International Club of Ascites (ICA) criteria and ACLF using both APASL and the CANONIC study diagnostic criteria.6, 7, 10 All patients with AKI were managed according to the standard of care. Patients were assessed for risk factors of AKI at admission, that is, nephrotoxic drugs (diuretics, nonsteroidal anti‐inflammatory drugs [NSAIDs], angiotensin‐converting enzyme [ACE]) inhibiters, angiotensin II receptor blockers [ARB], vasodilators, and aminoglycoside antibiotics), and risk factors were removed. Dehydration was corrected with intravenous saline, and variceal bleeding was treated with blood transfusion and intravenous terlipressin till endoscopic variceal ligation or sclerotherapy was performed. Intravenous albumin was used for initial volume expansion for 48 h in patients with SCr ≥ 1.5 mg/dL; patients with volume‐nonresponsive AKI fulfilling the criteria for HRS were treated with intravenous albumin and terlipressin or noradrenaline, and hemodialysis was performed when indicated.14, 19, 20 Patients with suspected or proven bacterial infection received intravenous antibiotics and albumin, and later on, antibiotics were changed according to the culture and sensitivity report. In septic shock patients, vasopressor noradrenaline infusion was used.14, 21, 22
We examined and compared differences in outcomes—for example, survival both at 28 days and 90 days, progression of AKI to higher stages, duration of hospital stay, and difference in the prevalence of HRS and ACLF (using both APASL and CANONIC study criteria)—between stages 1A and 1B AKI patients.
Primary and secondary outcomes
Survival at 28 days and 90 days were defined as the primary end‐points for our survival analysis, while progression of AKI, prevalence of HRS, and prevalence of ACLF served as the secondary end‐points. Duration of hospital stay was the other secondary end‐point when comparing stage 1A and stage 1B AKI patients.
Statistical methods
Demographic, clinical, and laboratory parameters and outcomes were compared between patients with stage 1A and stage 1B AKI. Normally distributed continuous variables were reported as mean and standard deviation and compared using the Student t‐test. Nonnormally distributed continuous variables were reported as median and interquartile range and compared using the Mann–Whitney U test. Categorical variables were reported as proportions and compared using the χ 2 test or Fisher's exact test, as appropriate. The 28‐day and 90‐day survival rates were estimated by the Kaplan–Meier method and compared using the log‐rank test. Cox regression analysis was performed to calculate the hazards ratio (HR) of mortality and its 95% confidence interval (CI) between stages 1A and 1B AKI. All tests were two‐tailed, and P values <0.05 were considered significant. The statistical analysis was performed using SPSS statistical package, version 20.0 (IBM Corp, Armonk, NY, USA).
Ethical clearance has been obtained from Institutional Ethics Committee, S.C.B. Medical College, Cuttack‐753007, Odisha, Regd. No. ECR/84/Inst/OR/2013.
Results
We screened 528 DC patients, (440 males; 83.33% and 88 females; 16.67%) for the study. Of these, 296 (56.1%) patients had AKI as per ICA criteria. On evaluation of the 296 AKI patients, 182 (61.48%) had stage 1 AKI, 62 (20.95%) had stage 2 AKI, and 52 (17.57%) had stage 3 AKI. These 182 patients with stage 1 AKI were included in the study and categorized into two groups according to the admission SCr cut‐off value of 1.5 mg/dL, and a comparison was conducted between patients with admission levels of SCr < 1.5 mg/dL (AKI 1A) and ≥ 1.5 mg/dL (AKI 1B). Of them, 100 (54.94%) had AKI 1A, and 82 (45.06%) had AKI 1B (Fig. 1).
Figure 1.
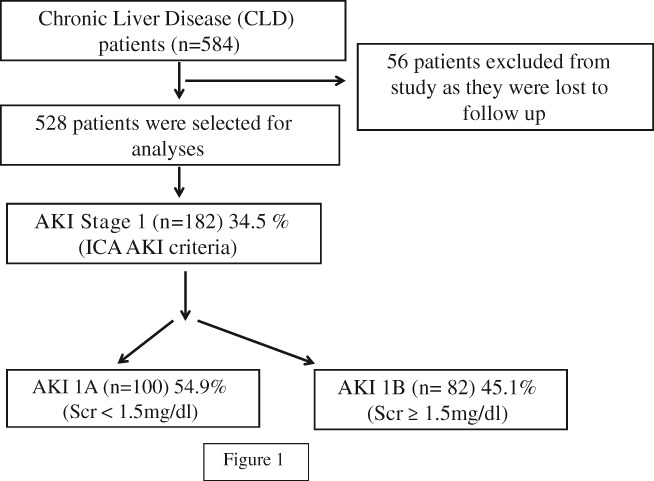
Schematic flow diagram for the study. AKI, acute kidney injury; ICA, International Club of Ascites.
Table 1 shows the comparison of the baseline characteristics of patients with stage 1 AKI between patients of stages 1A and 1B. Patients with AKI 1B had a higher total leucocyte count, total bilirubin, SCr, serum urea, model for end‐stage liver disease (MELD) The United Network for Organ Sharing (UNOS), MELD (Na+), and child‐turcotte‐pugh (CTP) score; associated bacterial infection except serum albumin; and prevalence of variceal bleeding, which was more commonly associated with stage 1A AKI. However, age, gender, body mass index (BMI), mean arterial pressure (MAP), serum protein, serum sodium, serum potassium, serum‐ascites albumin gradient (SAAG), INR, prevalence of ascites, and encephalopathy were comparable between the two groups (Table 1).
Table 1.
Comparison of baseline characteristics and outcome between in patients with AKI stages 1A and 1B, categorized according to level of serum creatinine at admission
| Sl No | Parameters | AKI 1A (n = 100) | AKI 1B (n = 82) | P value | |
|---|---|---|---|---|---|
| 1 | Age (mean ± SD) | 50.05 ± 11.96 | 51.04 ± 13.09 | 0.593 | |
| 2 | Gender (male) (male, [%]) | 92 (92) | 75 (91.5) | 0.896 | |
| 3 | BMI (kg/m2) (mean ± SD) | 22.04 ± 3.76 | 22.29 ± 4.11 | 0.670 | |
| 4 | MAP (mmHg) (mean ± SD) | 86.37 ± 9.69 | 83.63 ± 11.55 | 0.084 | |
| 5 | Etiology of cirrhosis (alcohol [%]) | 63 (63) | 52 (63.4) | 0.877 | |
| 6 | Total leucocyte count (103 cells/dL) (median [IQR]) | 8200 (6425–10 350) | 9400 (7400–12 600) | 0.048 | |
| 7 | Serum bilirubin (total) (mg/dL) (median [IQR]) | 8.60 (5.79–13.95) | 10.60 (6.90–22.40) | 0.001 | |
| 8 | Serum creatinine (mg/dL) (mean ± SD) | 1.26 ± 0.09 | 1.70 ± 0.15 | <0.001 | |
| 9 | Urea (mg/dL) (median [IQR]) | 32.50 (25–42.75) | 46.50 (37–63.50) | <0.001 | |
| 10 | Serum protein (g/dL) (median [IQR]) | 6.30 (5.83–7.08) | 6.40 (5.78–7.30) | 0.279 | |
| 11 | Serum albumin (g/dL) (mean ± SD) | 2.77 ± 0.51 | 2.61 ± 0.52 | 0.035 | |
| 12 | Serum sodium (mEq/L) (mean ± SD) | 135.34 ± 7.27 | 131.77 ± 16.11 | 0.066 | |
| 13 | Serum potassium (mEq/L) (median [IQR]) | 4.20 (3.90–4.70) | 4.20 (3.50–4.90) | 0.324 | |
| 14 | SAAG (mean ± SD) | 2.31 ± 0.56 | 2.19 ± 0.56 | 0.149 | |
| 15 | INR (mean ± SD) | 1.79 ± 0.49 | 1.96 ± 0.81 | 0.101 | |
| 16 | MELD (UNOS) (mean ± SD) | 18.51 ± 5.25 | 23.95 ± 7.22 | <0.001 | |
| 17 | MELD (Na+) (mean ± SD) | 21.07 ± 5.83 | 26.56 ± 7.14 | <0.001 | |
| 18 | CTP score (mean ± SD) | 10.55 ± 2.37 | 11.55 ± 2.25 | 0.004 | |
| 19 | CTP (%) | A | 3 (3) | 2 (2.4) | 0.342 |
| B | 26 (26) | 15 (18.3) | |||
| C | 71 (71) | 65 (79.3) | |||
| 20 | Variceal bleeding (%) | 58 (58) | 33 (40.2) | 0.017 | |
| 21 | Infection (%) | 39 (39) | 47 (57.3) | 0.014 | |
| 22 | Ascites (%) | 94 (94) | 79 (96.3) | 0.469 | |
| 23 | Encephalopathy (%) | 84 (84) | 65 (79.3) | 0.410 | |
AKI, acute kidney injury; BMI, body mass index; CTP, Child‐Turcotte‐Pugh; INR, International Normalized Ratio; IQR, interquartile range; MAP, mean arterial pressure; MELD, model for end‐stage liver disease; SAAG, serum‐ascites albumin gradient; SD, standard deviation; UNOS, The United Network for Organ Sharing.
Table 2 shows the comparison of outcomes between AKI 1A and AKI 1B patients. Significant differences were found in the following parameters: prevalence of HRS and ACLF defined as per criteria of APASL and the CANONIC study. Higher‐grade ACLF as per European Association for the Study of the Liver‐Chronic Liver Failure (EASL‐CLIF) Consortium criteria was observed more frequently in AKI 1B patients. Stage AKI 1B patients had decreased the proportions of reversal of AKI. However, patients with stage AKI 1B had decreased survival both at 28 days and 90 days and had prolonged hospital stay (Table 2).
Table 2.
Comparison of outcome between in patients with AKI stages 1A and 1B, categorized according to level of serum creatinine at admission
| Sl. no | Parameters | AKI 1A (n = 100) | AKI 1B (n = 82) | P value | |
|---|---|---|---|---|---|
| 1 | ACLF (APASL) (%) | 24 (24) | 32 (39.02) | 0.029 | |
| 2 | ACLF (CANONIC) (%) | 24 (24) | 43 (52.4) | <0.001 | |
| 3 | Grade of ACLF (%) | ACLF 0 | 76 (76) | 39 (47.6) | <0.001 |
| ACLF 1 | 19 (19) | 21 (25.6) | |||
| ACLF 2 | 5 (5) | 15 (18.3) | |||
| ACLF 3 | 0 (0) | 7 (8.5) | |||
| 4 | HRS (%) | 6 (6) | 25 (30.5) | <0.001 | |
| 5 | Reversal of AKI (%) | 86 (86) | 53 (64.6) | 0.001 | |
| 6 | Duration of hospital stay (days) (Median [IQR]) | 4 (3–5) | 6 (4–7) | 0.012 | |
| 7 | 28 days survival (%) | 82 (82) | 54 (65.9) | 0.013 | |
| 8 | 90 days survival (%) | 61 (61) | 33 (40.2) | 0.005 | |
ACLF, acute on chronic liver failure; AKI, acute kidney injury; APASL, Asian Pacific Association for the Study of the Liver; CANONIC, Consortium Acute‐on‐Chronic Liver Failure in Cirrhosis; HRS, hepatorenal syndrome; IQR, interquartile range.
Furthermore, Kaplan–Meier survival curves (Figs 2, 3) also showed significant differences in survival between AKI 1A and AKI 1B patients both at 28 days (log rank P value 0.017) and 90 days (log rank P value 0.005) at the SCr cut‐off level of 1.5 mg/dL.
Figure 2.
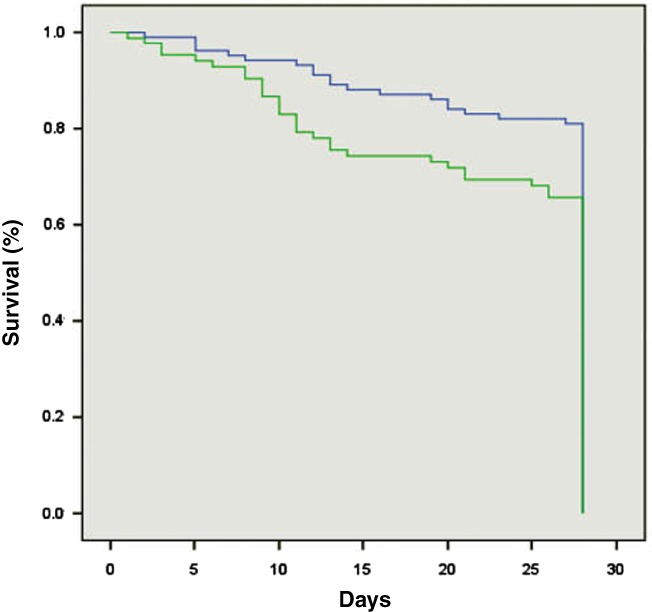
Kaplan–Meier survival curves showed significant differences in survival between acute kidney injury (AKI) 1A and AKI 1B patients at 28 days (log rank P value 0.017). ( ), AKI 1A; (
), AKI 1A; ( ), AKI 1B.
), AKI 1B.
Figure 3.
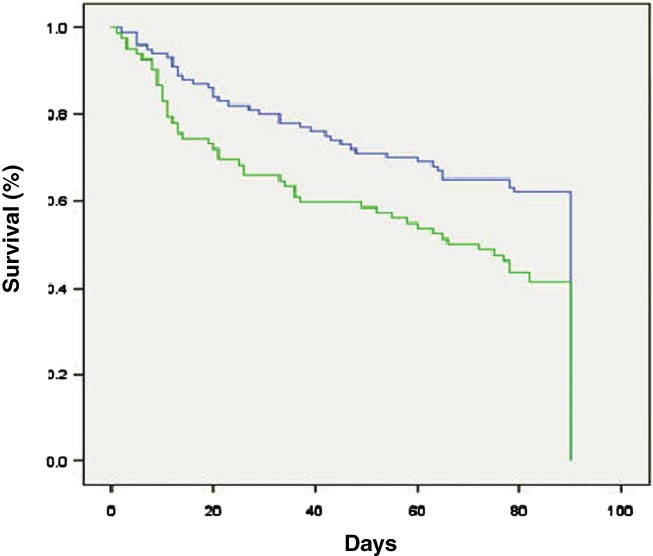
Kaplan–Meier survival curves showed significant differences in survival between acute kidney injury (AKI) 1A and AKI 1B patients at 90 days (log rank P value 0.005). ( ), AKI 1A; (
), AKI 1A; ( ), AKI 1B.
), AKI 1B.
Discussion
The assessment of renal function is crucial in the management of patients with cirrhosis as it is important to diagnose and guide the treatment of AKI from the outset and to provide the prognostic stratification. Patients with cirrhosis and AKI represent a population at high risk of mortality, who need timely treatment and appropriate intervention and withdrawal of precipitants of AKI. Nephrotoxic drugs such as NSAIDs and vasodilators should be discontinued, and doses of diuretics should be tapered or discontinued according to the severity of AKI. In patients on treatment with β‐blockers, the decision to taper and/or discontinue β‐blockers should be taken on a case‐by‐case basis, but the discontinuation of β‐blockers should be encouraged in hypotensive patients as well as in all patients with HRS.18
We had set out to see whether there are significant differences between stages 1A and 1B patients as per subclassification by Huelin et al. and to justify the subclassification. Our study showed significant differences between stages 1A and 1B patients in (i) severity of liver cirrhosis; (ii) prevalence of HRS and ACLF (as per criteria of both APASL and CANONIC study); (iii) AKI resolution; (iv) duration of hospital stay; and (v) survival both at 28 days and 90 days (Tables 1, 2). Most importantly, those with AKI stage 1B had HRS more frequently than those with stage 1A (30.5 vs 6%; P < 0.001). Variceal bleeding was more associated with AKI stage 1A than AKI 1B, indicating increased prerenal/hypovolemic AKI associated with AKI 1A. Furthermore, AKI 1A resolved in a higher proportion of these patients in comparison to stage 1B disease (86 vs 64.6%; P < 0.001). Another important difference between stages 1A and 1B was the prevalence of ACLF. Patients with AKI stage 1B were more commonly associated with ACLF, both as per APASL criteria (39.02 vs 24%; P = 0.029) and CANONIC study criteria (52.4 vs 24.6%; P < 0.001). To start with, we used the APASL criteria because, at our center, we always use the APASL criteria for diagnosis; however, in our study, we wanted to compare which AKI stage (stage IA and/or stage 1B) had higher grades of ACLF, and for this, CANONIC classification was used to screen our patients. Besides, the prevalence of bacterial infection was also higher in AKI 1B (57.3 vs 39%; P = 0.014) (Table 2), which may account for the poor outcomes of patients with stage 1B disease. These findings are consistent with the results of Huelin et al. 12 However, in contrast to the study by Huelin et al., in our study, a greater proportion of patients had stage 1A (59.94 vs 29.44%).12 Another important difference between these two groups (1A and 1B) was the decreased survival in patients with AKI stage 1B in comparison to AKI stage 1A both at 28 days (65.9 vs 82%; P = 0.013) and 90 days (40.2 vs 61%; P = 0.005). The differences in severity and outcome observed in our study between the substages validates the need for the subclassification of AKI stage 1 into stages 1A and 1B.
In conclusion, prognosis in DC patients is relatively better in the absence of renal impairment, which is common and found in about half of these patients at the time of hospitalization.4, 5, 6 AKI is a life‐threatening complication that requires timely management at the early stages of AKI. Our study validates the subclassification of stage 1 AKI into stages 1A and 1B. Patients with AKI 1B more often progress to higher AKI stages and HRS and also have greater proportions for developing ACLF (as per both APASL and EASL‐CLIF Consortium criteria). Besides, in comparison to AKI 1A, patients with AKI 1B have prolonged hospitalization and decreased survival both at 28 days and 90 days. The results clearly justify the subclassification of AKI stage 1 and advocate the need for early intervention in AKI stage 1B. Therefore, a small increase in SCr in any patient with AKI is very important prognostically and should be viewed with caution.
Declaration of conflict of interest: None.
Author contribution: Authors have contributed to study design, data collection, analysis and interpretation of data, statistical analysis, and manuscript writing.
References
- 1. Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988; 8: 1151–7. [DOI] [PubMed] [Google Scholar]
- 2. Arroyo V, Ginès P, Gerbes AL et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996; 23: 164–76. [DOI] [PubMed] [Google Scholar]
- 3. Martín‐Llahí M, Guevara M, Torre A et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011; 140: 488.e4–96.e4. [DOI] [PubMed] [Google Scholar]
- 4. Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 2015; 63: 1272–84. [DOI] [PubMed] [Google Scholar]
- 5. Clària J, Stauber RE, Coenraad MJ et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute‐onchronic liver failure. Hepatology. 2016; 64: 1249–64. [DOI] [PubMed] [Google Scholar]
- 6. Sarin SK, Kumar A, Kedarisetty CK et al. Acute‐on‐chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 2014; 8: 453–71. [DOI] [PubMed] [Google Scholar]
- 7. Moreau R, Jalan R, Gines P et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144: 1426–37, 1437.e1–1437.e9. [DOI] [PubMed] [Google Scholar]
- 8. Piano S, Romano A, Di Pascoli M, Angeli P. Why and how to measure renal function in patients with liver disease. Liver Int. 2017; 37 (Suppl. 1): 116–22. [DOI] [PubMed] [Google Scholar]
- 9. Fagundes C, Barreto R, Guevara M et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J. Hepatol. 2013; 59: 474–81. [DOI] [PubMed] [Google Scholar]
- 10. Piano S, Rosi S, Maresio G et al. Evaluation of the acute kidney injury network criteria in hospitalized patients with cirrhosis and ascites. J. Hepatol. 2013; 59: 482–9. [DOI] [PubMed] [Google Scholar]
- 11. Belcher JM, Garcia‐Tsao G, Sanyal AJ et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013; 57: 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huelin P, Piano S, Solà E et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute‐on‐chronic liver failure. Clin. Gastroenterol. Hepatol. 2017; 15: 438.e5–45.e5. [DOI] [PubMed] [Google Scholar]
- 13. Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013; 62: 131–7. [DOI] [PubMed] [Google Scholar]
- 14. Angeli P, Ginès P, Wong F et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015; 62: 968–74. [DOI] [PubMed] [Google Scholar]
- 15. Angeli P, Rodríguez E, Piano S et al. Acute kidney injury and acute‐onchronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015; 64: 1616–22. [DOI] [PubMed] [Google Scholar]
- 16. Fede G, D'Amico G, Arvaniti V et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J. Hepatol. 2012; 56: 810–18. [DOI] [PubMed] [Google Scholar]
- 17. de Carvalho JR, Villela‐Nogueira CA, Luiz RR et al. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J. Clin. Gastroenterol. 2012; 46: e21–6. [DOI] [PubMed] [Google Scholar]
- 18. Piano S, Brocca A, Angeli P. Renal function in cirrhosis: a critical review of available tools. Semin. Liver Dis. 2018; 38: 230–41. [DOI] [PubMed] [Google Scholar]
- 19. Ghosh S, Choudhary NS, Sharma AK et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int. 2013; 33: 1187–93. [DOI] [PubMed] [Google Scholar]
- 20. Nassar Junior AP, Farias AQ, D’ Albuquerque LA, Carrilho FJ, Malbouisson LM. Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta‐analysis. PLoS One. 2014; 9: e107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J. Hepatol. 2017; 66: 849–59. [DOI] [PubMed] [Google Scholar]
- 22. Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018; 38 (Suppl. 1): 126–33. [DOI] [PubMed] [Google Scholar]


