Abstract
Background and Aim
Echocardiographic assessment of the inferior vena cava diameter (IVCD) and collapsibility index (IVCCI) is a noninvasive estimate of intravascular volume status (IVS) but requires validation for cirrhosis. We evaluated IVC dynamics in cirrhosis and correlated it with conventional tools such as central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP), and right atrial pressure (RAP).
Methods
A total of 673 consecutive cirrhotic patients were screened by echocardiography, and 125 patients underwent right heart catheterization with recording of hepatic venous pressure gradient (HVPG), RAP, pulmonary artery (PA) pressure, and PCWP. CVP data were available for 80 (64%) patients, and finally, 76 patients (84% male, 50% ethanol related, mean age 52.1 years, 57.8% with ascites) with complete data were enrolled.
Results
The mean CVP measured was 12.8 ± 4.8 mmHg, and IVCCI was 29.5 ± 10.9%. The IVCD ranged from 0.97 to 2.26 cm and from 0.76 to 1.84 cm during expiration and inspiration, respectively, with a mean of 1.8 ± 0.9 cm. The mean IVCD correlated with RAP (r = 0.633, P = 0.043) but not with HVPG (r = 0.344, P = 0.755), PCWP (r = 0.562, P = 0.072), or PA pressure (r = 0.563, P = 0.588). A negative linear correlation was observed between the CVP and the IVCCI (r = −0.827, P = 0.023) in all patients and substratified for those with (r = −0.748, P = 0.039) and without ascites (r = −0.761, P = 0.047). A positive correlation was observed between CVP and IVCDmax (r = 0.671, P = 0.037) and IVCDmin (r = 0.612, P = 0.040).
Conclusions
IVCD and collapsibility index provides noninvasive IVS assessment, independent of HVPG or ascites, with the potential for calculating fluid requirements in cirrhosis.
Keywords: central venous pressure, cirrhotic cardiomyopathy, echocardiography, inferior vena cava, inferior vena cava collapsibility, intravascular volume status
Introduction
Cirrhosis is associated with a hyperdynamic circulation characterized by increased cardiac output and reduced systemic vascular resistance (SVR), with a normal or even low mean arterial pressure (MAP). 1, 2 Optimizing intravascular volume is essential in managing patients with cirrhosis to avoid acute kidney injury induced by hypovolemia and reduce the risk of developing hepatorenal syndrome (HRS) in decompensated cirrhosis.3 However, the presence of cirrhotic cardiomyopathy and ascites and the poor correlation of central venous pressure (CVP) with volume status make clinical assessment difficult.4 CVP is one of the indices of intravascular volume status (IVS) and is an early goal of the goal‐directed therapy approach.5, 6 However, CVP and pulmonary capillary wedge pressure (PCWP), often used to measure static hemodynamics, are not reliable markers of circulatory volume.7, 8 In addition, patients with ascites and peripheral edema may still be relatively underfilled in terms of intravascular volume as some 40–50% of the extracellular fluid volume can be in the microcirculation.
Recently, noninvasive methods to assess IVS, such as ultrasonography,9 transthoracic echocardiography (TTE) with tissue Doppler imaging (TDI),10 and transesophageal echocardiography (TEE),11, 12 have been reported. They have reported a correlation between inferior vena cava diameter (IVCD) and CVP. Another study reported a correlation between internal jugular vein (IJV) sonographic diameter and CVP as a surrogate marker of IVS.13 In this study, we assessed the utility of point‐of‐care echocardiographic assessment of IVCD and collapsibility to assess the volume status in cirrhotic patients compared with conventional invasive parameters such as CVP and PCWP.
Methods
In this observational study, we reviewed the echocardiographic data of 673 consecutive individuals with cirrhosis who were screened between 1 August 2015 and 31 December 2016 at the Institute of Liver and Biliary Sciences, New Delhi. The inclusion criteria were as follows: patients with cirrhosis defined by clinical, biochemical, histological, or ultrasonographic criteria, aged between 18 and 65 years. The exclusion criteria were: patients >65 years, chronic renal disease, pregnancy and peripartum cardiomyopathy, hypertension, coronary artery disease, valvular heart disease, sick sinus syndrome/pacemaker, thyroid dysfunction, portal vein thrombosis, transjugular intrahepatic portosystemic shunt (TIPS) insertion, hepatocellular carcinoma, severe anemia, or declining consent to participate. The normal IVCD was measured both during inspiration and expiration using M‐mode echocardiography. Of the 673 patients who met the study criteria, 125 patients also underwent right heart catheterization to measure hepatic venous pressure gradient (HVPG) prior to potential liver transplant workup, prior to TIPS, or as a part of clinical assessment of beta‐blocker response, thus providing invasive assessment data of HVPG, right atrial (RA) pressure, and PCWP. The CVP data were available for 80 patients, and finally, 76 patients (73.6% male, 57.8% with ascites, 39.4% with prior variceal bleed) with complete data on all parameters were enrolled for the study with consent. The trial was approved by the Institutional Ethics Committee and was performed in accordance with the Declaration of Helsinki. All authors had access to the manuscript data and have approved the final manuscript.
Echocardiographic assessment
All the echocardiography tests were conducted by a cardiologist who was blinded to the patients’ CVP using a 3.5 MHz probe (ALOKA Medical Systems, Tokyo, Japan). The TTE evaluation was performed as per the American Society of Echocardiography (ASE) guidelines.14, 15, 16 The IVC was visualized, with individuals in the supine position, using a subcostal four‐chamber view (midline, inferior to the xyphoid, angling to the right) and turning the probe anticlockwise to 90°, with a slight tilt to the right to achieve subcostal IVC view. Once the 2D image of IVC entering the RA was acquired, the M‐mode line was placed through IVC, 1 cm caudal from its junction with the hepatic vein, and a tracing was obtained. IVC and aorta diameter were measured at end‐expiration and end‐inspiration in two‐dimensional long‐axis view. All evaluations were performed in the supine position. The IVC collapsibility index (IVCCI) was the difference between the maximum and minimum IVCDs divided by the maximum IVCD, expressed as a percentage ([IVCDmax − IVCDmin]/IVCDmax × 100%). The following parameters were recorded: heart rate (HR), MAP, left ventricular end systolic diameter (LVESD), left ventricular end diastolic diameter (LVEDD), left ventricular ejection fraction (LVEF), cardiac index, and SVR. SVR was calculated by (MAP − mean right atrial pressure [RAP]) × 80/cardiac output. LVESD, LVEDD, and left atrium (LA) diameter were assessed by M‐mode. LVEF was measured through the biplane two‐dimensional mode using Simpson's method.16 Cirrhotic cardiomyopathy was diagnosed if systolic or diastolic dysfunction, together with supporting criteria such as electrophysiological abnormalities or abnormal serum markers, was present.17 LVDD was defined and classified according to the ASE guidelines.15, 18 as given below:
Grade 1: e′ < 8 cm/s, E/e′ ratio < 8, E/A ratio < 0.8, and deceleration time (DT) >200 ms;
Grade 2: e′ < 8 cm/s, E/e′ ratio 9–15, E/A ratio 0.8–1.5, and DT 160–200 ms; and
Grade 3: e′ < 8 cm/s, E/e′ ratio > 15, E/A ratio > 2, and DT <160 ms.
All echocardiographic studies were performed by the same observer (Jelen S Khumuckham), and intraobserver coefficients of variability in the echocardiography laboratory were 5% for M‐mode and 10% for two‐dimensional and Doppler values.
Central venous pressure assessment
After central catheterization of the internal jugular vein (IJV) using the Seldinger technique, CVP was measured using an electronic transducer with the patient placed in a 15° Trendelenburg position.
Cardiac catheterization assessment
Hepatic venous catheterization was performed for the indications cited above from the right femoral route using a 7 Fr double‐lumen balloon‐tipped Swan‐Ganz catheter, and the parameters recorded included the free hepatic venous pressure (FHVP), wedged hepatic venous pressure (WHVP), IVC pressure, RV pressure, pulmonary artery (PA) pressure, and PCWP. The HVPG level was calculated as the difference between the WHVP and FHVP readings.19
Statistical analysis
Statistical analysis was conducted using student's t test for normally distributed data and Mann Whitney U test for nonparametric data. In addition, χ 2 analysis with correction for small numbers and anova were also carried out along with agreement of IVCD assessment with invasive monitoring parameters by Pearson correlation. Data are expressed as mean ± SD or percentages. Statistical significance was considered to be at or below the 5% level. Data were analyzed with SPSS software version 22 (IBM SPSS Statistics, Armonk, USA).
Ethical clearance was obtained from the Institutional Review Board, reference number F25/5/64/ILBS/AC2013/909.
NCT Identifier: NCT02294292.
Results
Demographic data
Of 76 individuals enrolled for the study, 64 (84.2%) were male, with the etiology of cirrhosis being ethanol related (50%), non‐alcoholic steatohepatitis (39.4%), and chronic viral hepatitis (10.5%). The demographics of individuals are outlined in Tables 1 and 2. The mean age of individuals was 52.1 years. The average corrected body mass index (BMI) was 22.9 kg/m2, which ranged between 14.1 and 29.8 kg/m2. Of the 76 patients, 36 (47.3%) met criteria for grade 1 LVDD, and 4 met criteria for grade 2 LVDD (5.2%). The criteria for cirrhotic cardiomyopathy were met in 22(28.9%) patients.
Table 1.
Measured parameters of IVC diameter and anthropometry indices in our patients as per gender
| Total (n = 76) (mean ± SD) | Female (n = 20) (mean ± SD) | Male (n = 56) (mean ± SD) | |
|---|---|---|---|
| IVCDmax (cm) | 1.9 ± 0.6 | 1.7 ± 0.6 | 2.1 ± 0.5 |
| IVCDmin (cm) | 1.6 ± 0.4 | 1.4 ± 0.7 | 1.6 ± 0.6 |
| Age (years) | 48.1 ± 5.5 | 52.2 ± 5.3 | 48.3 ± 5.5 |
| Height (m) | 1.65 ± 0.7 | 1.61 ± 0.6 | 1.67 ± 0.8 |
| Weight (kg) | 62.8 ± 13.3 | 59.3 ± 12.9 | 65.10 ± 12.7 |
| Body mass index (kg/m2) | 22.9 ± 3.6 | 22.3 ± 3.7 | 23.3 ± 3.4 |
IVCD, inferior vena cava diameter; IVCDmax, Maximum IVCD; IVCDmin, Minimum IVCD.
Table 2.
Comparison of demographic, clinical, biochemical, and hemodynamic parameters; echocardiographic parameters and IVC collapsibility index; mean CVP pressure; and maximum and minimum IVC diameter of patients without and with ascites
| Variables | Preascitic cirrhosis (n = 32) | Cirrhosis with ascites (n = 44) | P value |
|---|---|---|---|
| Age (years) | 46.8 ± 7.6 | 49.1 ± 9.8 | 0.048 |
| Hypertension | 5% | 7% | 0.265 |
| Smoker | 3% | 2% | 0.658 |
| Diabetes mellitus | 18% | 22% | 0.063 |
| Presence of esophageal varices | 22 | 34 | |
| Prior variceal bleeding | 13 | 17 | |
| Child‐Pugh score | 9.8 ± 1.0 | 10.3 ± 0.91 | 0 |
| MELD score | 14.2 ± 2.5 | 17.4 ± 2.3 | 0.001 |
| MELD Na | 16.4 ± 3.06 | 18.1 ± 3.06 | 0 |
| Serum creatinine (mg/dL) | 0.71 ± 0.24 | 0.84 ± 0.22 | 0.314 |
| INR | 1.5 ± 0.31 | 1.6 ± 0.31 | 0.259 |
| MAP (mmHg) | 83.7 ± 11.5 | 80.3 ± 12.9 | 0.378 |
| HR (bpm) | 78.1 ± 14.2 | 88.9 ± 11.2 | 0.056 |
| Central venous pressure (mmHg) | 10.3 ± 2.4 | 14.3 ± 2.8 | 0.045 |
| Echocardiographic parameters | |||
| IVC diameter | |||
| IVCDmax (cm) | 1.7 ± 0.31 | 2.5 ± 1.6 | 0.024 |
| IVCDmin (cm) | 1.25 ± 0.31 | 1.42 ± 0.24 | 0.364 |
| IVC collapsibility (%) | 32.2 ± 5.1 | 21.7 ± 2.9 | 0.036 |
| LVEF (%) | 55–60 | 55–60 | 0.9 |
| LVEDV (mL) | 75.0 ± 8.1 | 73.9 ± 5.7 | 0.342 |
| LVESV (mL) | 32.7 ± 4.2 | 28.7 ± 4.7 | 0.413 |
| LV end‐diastolic posterior wall thickness (cm) | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.452 |
| LV end‐diastolic septal thickness (cm) | 1.1 ± 0.3 | 1.0 ± 0.2 | 0.433 |
| LV end diastolic diameter (cm) | 4.6 ± 0.6 | 4.8 ± 0.5 | 0.056 |
| SVRI (dynes. s m2/cm5) | 2042 ± 416 | 1098 ± 566 | 0.004 |
| Hemodynamic assessment and cardiac catheterization | |||
| HVPG (mmHg) | 10.5 ± 2.9 | 14.8 ± 3.2 | 0.045 |
| RAP (mmHg) | 5.4 ± 2.3 | 6.9 ± 3.4 | 0.328 |
| PAP (mmHg) | 13.5 ± 4.4 | 14.5 ± 6.2 | 0.051 |
| PCWP (mmHg) | 12.6 ± 4.9 | 13.2 ± 7.5 | 0.060 |
| CO (L/min) | 5.4 ± 1.9 | 6.1 ± 1.7 | 0.041 |
| Therapy at the time of inclusion (%) | |||
| β‐blockers | 0 | 0 | |
| Furosemide | 5 | 38 | |
| Spironolactone | 10 | 38 | |
CO, cardiac output; CVP, central venous pressure; HR, heart rate; HVPG, hepatic venous pressure gradient; INR, XXX; IVCD, inferior vena cava diameter; LV, left ventricle; LVEDV, LV end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, LV end systolic pressure; LVSD, left ventricular systolic diameter; MAP, mean arterial pressure; MELD, model for end‐stage liver disease; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; SVI, stroke volume index; SVRI, systemic vascular resistance index.
CVP and IVC assessment
The mean CVP measured was 12.8 ± 4.8 mmHg, with an IVCCI of 29.5 ± 10.9%. The CVP was found to be less than 8 mmHg among 12 (15.7%) patients, while 42 (55.2%) patients had CVP between 8 and 12 mmHg, and 22 (28.9%) patients had CVP greater than 12 mmHg. During inspiration, the IVCD decreased in every individual to a variable degree. Expiration led to an increase in the IVCD in all individuals (Table 1). Pearson's correlation was performed, which demonstrated that IVCD was found to be unrelated to age, but strongly related to height, weight, and BMI in both males and females.
Table 2 shows the assessment of various baseline parameters in cirrhotic patients with and without ascites.
A one‐way anova test was used for comparisons between the three groups of patients with different IVS as per conventional CVP measurement. The patients were considered in three categories: CVP <8 mmHg, ≥8–12 mmHg, and ≥12 mmHg.20, 21
There was a statistically significant correlation in the mean CVP pressure and the IVCCI and the CVP with IVCDmin and IVCDmax between groups as determined by one‐way anova (P < 0.001). This is presented in Table 3.
Table 3.
Comparison of mean arterial pressure, heart rate, IVC collapsibility index, mean CVP pressure, and maximum and minimum IVC diameter of patients in the three groups of intravascular volume states
| Parameters | CVP < 8 mmHg (n = 12) | CVP 8–12 mmHg (n = 42) | CVP > 12 mmHg (n = 22) | anova (P‐value) |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 70.2 ± 14.2 | 75.0 ± 16.3 | 72.63 ± 12.4 | 0.625 |
| Heart rate (per minute) | 90.7 ± 12.9 | 89.6 ± 24.9 | 92.70 ± 21.5 | 0.065 |
| Mean CVP pressure (mmHg) | 6.4 ± 1.8 | 10.6 ± 2.4 | 15.7 ± 3.5 | 0.001 |
| IVCDmax (cm) | 1.6 ± 1.9 | 1.8 ± 2.56 | 2.3 ± 1.6 | 0.004 |
| IVCDmin (cm) | 1.0 ± 0.7 | 1.4 ± 0.51 | 2.1 ± 0.9 | 0.001 |
| IVCCI (%) | 51.5 ± 7.5 | 30.2 ± 10.1 | 20.7 ± 8.9 | 0.001 |
CVP, central venous pressure; IVC, inferior vena cava; IVCCI, IVC collapsibility index; IVCDmax, maximum IVC diameter; IVCDmin, minimum IVC diameter.
A Pearson correlation was conducted to determine the relationship between the CVP and the IVCCI (%) and the maximum and minimum IVCD. A strong negative linear correlation was observed between the CVP (10.3 ± 4.4 mmHg) and the IVCCI, which was statistically significant (r = −0.827, P = 0.023) in all patients and substratified for those with ascites (r = −0.748, P = 0.039) and without ascites (r = −0.761, P = 0.047) (Figs 1a–c). A strong positive correlation was demonstrated between the CVP and the IVCDmax (r = 0.671, P‐0.037) and IVCDmin (r = 0.612, P < 0.040). (Fig. 2a,b) The correlation coefficients for IVCDmax, IVCDmin, and CVP remained significant even in the presence of ascites.
Figure 1.
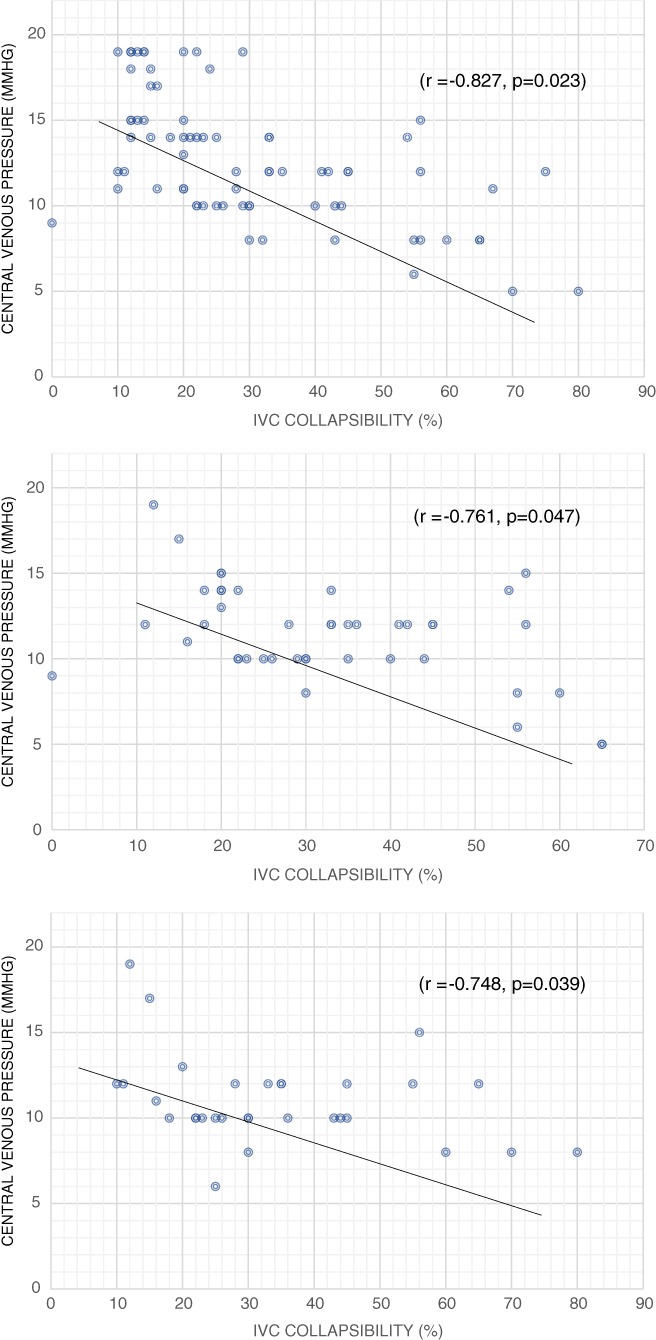
(a) Correlation between central venous pressure and inferior vena cava collapsibility index in all subjects (n = 76). (b) Correlation between central venous pressure and inferior vena cava (IVC) collapsibility index in patients with ascites.
Figure 2.
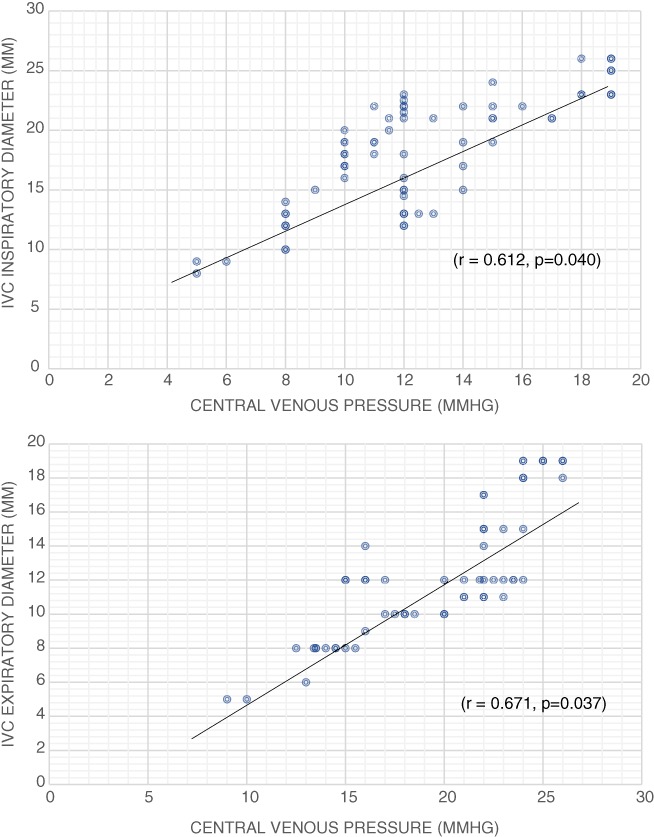
(a) Correlation between expiratory inferior vena cava (IVC) diameter and central venous pressure. (b) Correlation between inspiratory IVC diameter and central venous pressure.
Cardiac hemodynamic studies and IVC assessment
On right heart catheterization studies, a Pearson correlation coefficient was calculated between the mean IVCD and RAP (r = 0.633, P = 0.043) and PCWP (r = 0.562, P = 0.072), HVPG (r = 0.344, P = 0.755), or PA pressure (r = 0.563, P = 0.588). The correlation coefficients for IVCDmax and IVCDmin as a function of RA pressure were 0.686 and 0.767, respectively.
Determination of discriminant values in IVC measurement for predicting CVP
The area under receiver operating characteristic (ROC) curves for the predictors of volume status (CVP) using noninvasive IVC parameters are shown in Table 4. The collapsibility indices (maximum and minimum IVCCI) had the best area under the curve (AUC). The IVCDmax size cut‐off value with optimum predictive use for CVP above or below 10 mmHg was 2.0 cm, and the optimal IVCCImax cut‐off value was 40%. The IVCDmin <1.5 cm predicted CVP <10 mmHg and indicated the need for further volume resuscitation, with a sensitivity of 91%, specificity of 79%, and 96% negative predictive value. The most notable finding is that the IVC size measurements and the collapsibility indices had an excellent negative predictive value.
Table 4.
Area under receiver operating characteristic curve for prediction of volume status CVP above or below 10 mmHg
| Evaluation group (n = 76) | ||||||
|---|---|---|---|---|---|---|
| Parameter | AUC | Cut‐off | Sensitivity | Specificity | PPV | NPV |
| IVCDmax | 0.712 | 2.0 cm | 73 | 85 | 62 | 90 |
| IVCDmin | 0.678 | 1.5 cm | 86 | 79 | 59 | 96 |
| IVCCImin | 0.773 | 20% | 70 | 82 | 57 | 90 |
| IVCCImax | 0.791 | 40% | 78 | 84 | 62 | 90 |
AUC, area under receiver operating characteristic curve; cut‐off, parameter value for optimal performance; CVP, central venous pressure; ; IVCDmax, maximum inferior vena cava diameter; IVCDmin, minimum inferior vena cava diameter; NPV, negative predictive value; PPV, positive predictive value.
Determination of accuracy of IVC measurements to classify volume status
All patients were categorized by IVCDmax and IVCCI into one of nine subgroups as shown in Table 5. The mean CVP was calculated for each subgroup, and the percentages of patients falling within the traditional CVP ranges of 0–5, 5–10, 10–15, and >15 mmHg were determined. They were grouped according to whether their collapsibility was high (>55%), low (<35%), or normal (35–50%) and whether their IVC was small (<1.7 cm), normal (1.7–2.1 cm), or large (>2.1 cm). The specific subgroup's IVCCI and size were assigned according to current guidelines.14 Within each classification of size, there was an increase in mean CVP as collapsibility decreased. On the contrary, when grouped by IVCCI, there was no significant change in mean CVP between patients with small‐sized IVCDs.
Table 5.
Tabulation of patients as per IVC size (small [<1.7 cm], normal [1.7–2.1 cm], or large [>2.1 cm]) and collapsibility (high [>55%], low [<35%], or normal [35–50%]) with measured CVP as per 3 volume status subgroups
| Evaluation group (n = 76) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Volume status | CVP < 8 mmHg (decreased IVS) (n = 12) | CVP 8–12 mmHg (euvolemic) (n = 42) | CVP > 12 mmHg (increased IVS) (n = 22) | ||||||
| Collapsibility | (>55%) | 35–55% | <35% | ||||||
| Size (cm) | <1.7 | 1.7–2.1 | >2.1 | <1.7 | 1.7–2.1 | >2.1 | <1.7 | 1.7–2.1 | >2.1 |
| No. of patients | 6 | 4 | 2 | 3 | 27 | 10 | 6 | 4 | 12 |
| Mean CVP (mmHg) | 5 | 6 | 8 | 10 | 11.5 | 12 | 14 | 15.5 | 17 |
| 0–5 mmHg (%) | 72 | 80 | 50 | 67 | 15 | 0 | 0 | 0 | 0 |
| 5–10 mmHg (%) | 24 | 20 | 50 | 33 | 40 | 0 | 10 | 0 | 5 |
| 10–15 mmHg (%) | 4 | 0 | 0 | 0 | 45 | 80 | 55 | 50 | 50 |
| >15 mmHg (%) | 0 | 0 | 0 | 0 | 0 | 20 | 35 | 50 | 45 |
CVP, central venous pressure; IVC, inferior vena cava; IVS, intravascular volume status.
When collapsibility was high and IVCD was small or normal, CVP was between 0 and 5 mmHg in 72% and between 5 and 10 mmHg in 24% cases. Thus, this subgroup indicated hypovolemia and the potential need for volume expansion depending on the clinical scenario. When collapsibility was high with a large IVCD, or IVCCI was normal, and IVCD was small or normal, CVP was between 0 and 10 mmHg 87% of the time. If collapsibility was normal and the IVC was large, CVP was between 10 and 15 mmHg. On the other hand, if the IVC was large with low IVCCI, the CVP was between 10 and 20 mmHg.
Discussion
Overall, our findings support the use of point‐of‐care noninvasive echocardiographic measurements of IVCD or collapsibility to estimate CVP or RAP as a surrogate marker for IVS. Although CVP monitoring is a useful tool for guiding fluid management, it requires placement of a central venous catheter, which is an invasive procedure and is associated with complications. Bedside sonography/echocardiography has emerged as a potentially useful tool, optimizing hemodynamic measurements in cirrhosis with careful interpretation of right ventricular function integrated with cardiac output and perfusion pressure.20
Initially, IVS assessed noninvasively by the IVC ultrasound was focused on the correlation of the mean IVCD with the CVP.21 These results are comparable to our findings. There is an inverse relationship of the IVCCI to the CVP at extremes of intravascular fluid volume. Brennan et al. documented that the combination of both IVCD and collapsibility indices improve evaluations of the IVC with clinically important categories of RAP.22
Accuracy of noninvasive assessment of CVP
Several studies have evaluated the correlation between RA pressure and different IVC parameters with variable accuracy.23, 24 A good correlation between the IVCCI and RA pressure (0.57 < r ≤ 0.76) has been reported.25 Although there is a correlation between IVCD and RAP, because of the variability and overlap between patients with normal and elevated RAP, it can only be used as a surrogate marker for dynamic assessment rather than as an absolute index of RAP.26 An increase of RAP beyond a certain level may cause only minimal increases in IVCD and the degree of IVCCI. Thus, IVC dimensions and collapsibility can be used to detect elevated CVP, but they have limited utility in identifying the magnitude of CVP elevation. As per the ASE guidelines, IVCD ≤2.1 cm that collapses >50% with inspiration suggests normal RAP of 3 mmHg (range, 0–5 mmHg), whereas IVCD>2.1 cm that collapses <50% with inspiration suggests high RAP of 15 mmHg (range, 10–20 mmHg). In scenarios where IVCD and collapse have contradictory values, an intermediate value of 8 mmHg (range, 5–10 mmHg) may be used.27
Stawicki et al.28 demonstrated that the IVCCI strongly correlates with low (<20%) and high (>60%) CVP values and suggested that the closer the IVCCI is to 0 or 100%, the higher is the probability that the patient is either volume‐overloaded or volume‐depleted, respectively. The ability to predict CVP values precisely is of untested clinical gain, taking into account the poor performance of CVP as a marker of intravascular volume and fluid responsiveness. A very high IVCCI (often associated with a very low CVP) may serve as a rational sign that it is possible to give more fluid without precipitating volume overload and reduction in IVCCI, a marker of successful volume repletion.29
Previous studies have consistently demonstrated that patients with cirrhosis have diastolic dysfunction.30, 31, 32 The presence of cirrhotic cardiomyopathy may ultimately confound these readings, and the IVC and intracardiac pressures may not accurately reflect intravascular volume (IVV) any longer. In clinical practice, it is difficult to distinguish between portopulmonary hypertension from vascular overload using IVCD only without right heart catheterization.
In contrast, resting LV systolic functional impairment is not apparent when measured by conventional methods such as LV ejection fraction, partly because of reduced afterload due to a low SVR.33 Recently, 2D speckle‐tracking strain analysis has been proposed as a sensitive and accurate method to evaluate subclinical systolic dysfunction in various groups of disease.10 Our data serve to corroborate these findings in stable cirrhotic patients. In patients with decompensated liver cirrhosis and ascites, with creatinine >1.5, there was an impairment in IVCCI (IVC collapsibility <50%). This is further affected by the presence of cirrhotic cardiomyopathy and sepsis states, which alter volume status in critically ill cirrhotic patients.34 Sampaio et al. reported that IVCD and IVCCI are of value in the prediction of IVS in liver cirrhosis with renal dysfunction.10 On the other hand, the study of Kitamura and Kobayashi found that interpretation of caval indices is difficult due to factors that restrict the physiological variability of the IVC, such as cirrhosis, external compression of the IVC, and elevated intra‐abdominal pressure due to ascites.35
Fluid management strategy in cirrhosis
Incorporation of a goal‐directed sonographic protocol, including assessment of the IVC, has been shown to improve the accuracy of fluid assessment and resuscitation in critically ill noncirrhotic patients with shock.36 Point‐of‐care sonography evaluating cardiac contractility and IVC collapsibility in patients with suspected sepsis improves fluid management and, possibly, clinical decisions.37, 38 Our study determined that a small‐diameter IVC with high collapsibility correlates with low‐volume states. However, the treating clinician needs to gauge the need for fluid resuscitation in individual patients as a dynamic continuum. All patients with small IVC and high IVCCI need not be volume resuscitated unless there is a clinical indication with impaired hemodynamics. In clinically hypovolemic states, an anticipated change in IVCD can be used to predict a patient's response to volume expansion. The limitations of the study were the exclusion of ventilated patients, those on hemodialysis, or overt heart failure that limits the applicability of this approach in critically ill cirrhotic patients. In addition, we tested asymptomatic patients who were clinically stable. These data need further validation in scenarios like shock or sepsis, which will alter cardiac hemodynamics. In such situations, noninvasive IVC parameters need to be interpreted in terms of clinical status, such as tissue perfusion, renal function, and pulmonary fluid volume. Future prospective studies could be focused on finding a steadfast formula for calculating fluid requirements in cirrhosis patients who are septic, in shock, or mechanically ventilated using noninvasive point‐of‐care tests such as IVCD and collapsibility.
In conclusion, the dynamic IVC size and collapsibility index can provide a useful guide for noninvasive IVS assessment in cirrhosis. There is a positive relationship of CVP with minimum and maximum IVCDs but an inverse relation with IVCCI. Our findings corroborate the correlations of echocardiographic IVCD and BMI, RA pressure, and PCWP in cirrhosis with and without ascites, independent of HVPG.
Acknowledgment
Madhumita Premkumar was the recipient of the Young Scientist Grant, a research grant (SB/YS/LS‐189/2014) provided by the Science and Engineering Research Board, Department of Science and Technology, Govt. of India. We acknowledge the contribution of Mr Tony and Mr Jitender, our echocardiography technicians for their selfless support.
Declaration of conflict of interest: None of the authors have potential conflicts (financial, professional, or personal) which are relevant to this manuscript.
Author contribution: Madhumita Premkumar and Devaraja Rangegowda were involved in the diagnostic procedures and data collection. Jelen S Khumuckham performed all the cardiac assessments, including echocardiography. Kamal Kajal and Madhumita Premkumar performed the statistical analysis. All the authors have read and approved the manuscript.
References
- 1. Angeli P, Sanyal A, Moller S et al. Current limits and future challenges in the management of renal dysfunction in patients with cirrhosis: report from the International Club of Ascites. Liver Int. 2013; 33: 16–23. [DOI] [PubMed] [Google Scholar]
- 2. Abelmann WH. Hyperdynamic circulation in cirrhosis: a historical perspective. Hepatology. 1994; 20: 1356–8. [PubMed] [Google Scholar]
- 3. Salerno F, Cazzaniga M, Merli M et al. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J. Hepatol. 2011; 55: 1241–8. [DOI] [PubMed] [Google Scholar]
- 4. Brinch K, Moller S, Bendtsen F, Becker U, Henriksen J. Plasma volume expansion by albumin in cirrhosis. Relation to blood volume distribution, arterial compliance and severity of disease. J. Hepatol. 2003; 39: 24–31. [DOI] [PubMed] [Google Scholar]
- 5. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness A systematic review of the literature and the tale of seven mares. Chest. 2008; 134: 172–8. [DOI] [PubMed] [Google Scholar]
- 6. Mintz GS, Kotler MN, Parry WR, Iskandrian AS, Kane SA. Reat‐time inferior vena caval ultrasonography: normal and abnormal findings and its use in assessing right‐heart function. Circulation. 1981; 64: 1018–25. [DOI] [PubMed] [Google Scholar]
- 7. Gödje O, Peyerl M, Seebauer T, Lamm P, Mair H, Reichart B. Central venous pressure, pulmonary capillary wedge pressure and intrathoracic blood volumes as preload indicators in cardiac surgery patients. Eur. J. Cardiothorac. Surg. 1998; 13: 533–9. [DOI] [PubMed] [Google Scholar]
- 8. Kumar A, Anel R, Bunnell E et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit. Care Med. 2004; 32: 691–9. [DOI] [PubMed] [Google Scholar]
- 9. Davenport A, Argawal B, Wright G et al. Can non‐invasive measurements aid clinical assessment of volume in patients with cirrhosis? World J. Hepatol. 2013; 5: 433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sampaio F, Pimenta J, Bettencourt N et al. Systolic and diastolic dysfunction in cirrhosis: a tissue‐Doppler and speckle tracking echocardiography study. Liver Int. 2013; 33: 1158–65. [DOI] [PubMed] [Google Scholar]
- 11. Renner J, Gruenewald M, Brand P et al. Global end‐diastolic volume as a variable of fluid responsiveness during acute changing loading conditions. J. Cardiothorac. Vasc. Anesth. 2007; 21: 650–4. [DOI] [PubMed] [Google Scholar]
- 12. Arthur ME, Landolfo C, Wade M, Castresana MR. Inferior vena cava diameter (IVCD) measured with transesophageal echocardiography (TEE) can be used to derive the central venous pressure (CVP) in anesthetized mechanically ventilated patients. Echocardiography. 2009; 26: 140–9. [DOI] [PubMed] [Google Scholar]
- 13. Donahue SP, Wood JP, Patel BM, Quinn JV. Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am. J. Emerg. Med. 2009; 27: 851–5. [DOI] [PubMed] [Google Scholar]
- 14. Rudski LG, Lai WW, Afilalo J et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Appleton CP, Gillebert TC et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009; 10: 165–93. [DOI] [PubMed] [Google Scholar]
- 16. Gorcsan J 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J. Am. Coll. Cardiol. 2011; 58: 1401–13. [DOI] [PubMed] [Google Scholar]
- 17. Wong F. Cirrhotic cardiomyopathy. Hepatol. Int. 2009; 3: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruíz‐Del‐Árbol L, Achécar L, Serradilla R et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013; 58: 1732–41. [DOI] [PubMed] [Google Scholar]
- 19. Wadhawan M, Dubey S, Sharma BC, Sarin SK, Sarin SK. Hepatic venous pressure gradient in cirrhosis: correlation with the size of varices, bleeding, ascites, and child's status. Dig. Dis. Sci. 2006; 51: 2264–9. [DOI] [PubMed] [Google Scholar]
- 20. Magder S, Bafaqeeh F. The clinical role of central venous pressure measurements. J. Intensive Care Med. 2007; 22: 44–51. [DOI] [PubMed] [Google Scholar]
- 21. Dellinger RP, Carlet JM, Masur H et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004; 32: 858–73. [DOI] [PubMed] [Google Scholar]
- 22. Brennan JM, Blair JE, Goonewardena S et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J. Am. Soc. Echocardiogr. 2007; 20: 857–61. [DOI] [PubMed] [Google Scholar]
- 23. Ciozda W, Kedan I, Kehl DW, Zimmer R, Khandwalla R, Kimchi A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc. Ultrasound. 2016; 14: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno FL, Hagan AD, Holmen JR, Pryor TA, Strickland RD, Castle CH. Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function. Am. J. Cardiol. 1984; 53: 579–85. [DOI] [PubMed] [Google Scholar]
- 25. Simonson JS, Schiller NB. Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two‐dimensional echocardiographic measurements of the inferior vena cava during measured inspiration. J. Am. Coll. Cardiol. 1988; 11: 557–64. [DOI] [PubMed] [Google Scholar]
- 26. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right atrial pressure. J. Am. Soc. Echocardiogr. 2013; 26: 1033–42. [DOI] [PubMed] [Google Scholar]
- 27. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am. J. Cardiol. 1990; 66: 493–6. [DOI] [PubMed] [Google Scholar]
- 28. Stawicki SP, Braslow BM, Panebianco NL et al. Intensivist use of hand‐carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J. Am. Coll. Surg. 2009; 209: 55–61. [DOI] [PubMed] [Google Scholar]
- 29. Barbier C, Loubières Y, Schmit C. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004; 30: 1740–6. [DOI] [PubMed] [Google Scholar]
- 30. Valeriano V, Funaro S, Lionetti R et al. Modification of cardiac function in cirrhotic patients with and without ascites. Am. J. Gastroenterol. 2000; 95: 3200–5. [DOI] [PubMed] [Google Scholar]
- 31. Moreau R, Hadengue A, Soupison T et al. Septic shock in patients with cirrhosis: hemodynamic and metabolic characteristics and intensive care unit outcome. Crit. Care Med. 1992; 20: 746–50. [DOI] [PubMed] [Google Scholar]
- 32. Tiukinhoy‐Laing SD, Rossi JS, Bayram M et al. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am. J. Cardiol. 2006; 98: 178–81. [DOI] [PubMed] [Google Scholar]
- 33. Premkumar M, Devurgowda D, Vyas T et al. Left ventricular diastolic dysfunction is associated with renal dysfunction, poor survival and low health related quality of life in cirrhosis. J. Clin. Exp. Hepatol. 2018. 10.1016/j.jceh.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seif D, Mailhot T, Perera P, Mandavia D. Caval sonography in shock. A noninvasive method for evaluating intravascular volume in critically ill patients. J. Ultrasound Med. 2012; 31: 1885–90. [DOI] [PubMed] [Google Scholar]
- 35. Kitamura H, Kobayashi C. Impairment of change in diameter of the hepatic portion of the inferior vena cava: a sonographic sign of liver fibrosis or cirrhosis. J. Ultrasound Med. 2005; 24: 355–9. [DOI] [PubMed] [Google Scholar]
- 36. Haydar S, Moore ET, Higgins GLIII, Irish CB, Owens WB, Strout TD. Effect of bedside ultrasonography on the certainty of physician clinical decision making for septic patients in the emergency department. Ann. Emerg. Med. 2012; 60: 346–358.e4. [DOI] [PubMed] [Google Scholar]
- 37. Yanagawa Y, Sakamoto T, Okada Y. Hypovolemic shock evaluated by sonographic measurement of the inferior vena cava during resuscitation in trauma patients. J. Trauma. 2007; 63: 1245–8. [DOI] [PubMed] [Google Scholar]
- 38. Ferrada P, Anand RJ, Whelan J. Qualitative assessment of the inferior vena cava: useful tool for the valuation of fluid status in critically ill patients. Am. Surg. 2012; 78: 468–70. [PubMed] [Google Scholar]


