Abstract
Background and purpose: Favorable clinical outcomes of carbon-ion radiotherapy for pelvic recurrence of rectal cancer have been described by previous prospective phase I/II and II studies; however, these studies were performed at a single institution. Therefore, we conducted a prospective observational study aimed at exploring whether carbon-ion radiotherapy for post-operative pelvic recurrence of rectal cancer provides a less invasive local treatment strategy with higher cure rates than other anticancer treatments.
Materials and methods: Patients (1) with pelvic recurrence of rectal cancer, as confirmed by histology or diagnostic imaging; (2) without distant metastasis; (3) who had undergone curative resection of their primary disease and regional lymph nodes, without gross or microscopic residual disease; and (4) with radiographically measurable tumors were included in this study. The total carbon-ion radiotherapy dose for all patients was 73.6 Gy [relative biological effectiveness (RBE)] administered in 16 fractions once daily for 4 days a week (Tuesday to Friday).
Results: A total of 28 patients were enrolled between October 2011 and July 2017. The median follow-up duration was 38.9 months. The 3-year overall survival, local control, and progression-free survival rates were 92, 86, and 31%, respectively. At the time of the analysis, 4 patients had local recurrence, and 7 had died of rectal cancer. None of the patients developed grade 3 or higher acute toxicities. Late toxicities occurred in 2 and 7 patients who developed grade 3 pelvic infection and grade 2 peripheral neuropathy, respectively.
Conclusion: Carbon-ion radiotherapy for pelvic recurrence of rectal cancer showed favorable clinical outcomes and is a highly curative and less invasive local treatment.
Keywords: carbon ion radiotherapy, rectal cancer, pelvic recurrence, prospective observational study, curative treatment
Introduction
The global incidence of colorectal cancer in 2012 was an estimated 1.4 million, resulting in 693,900 deaths (1). Although surgery is the treatment of choice in patients with resectable rectal cancer, local recurrence occurs in 4–15% of patients after curative resection (2, 3). In pelvic recurrence of rectal cancer, while pelvic exenteration offers the highest potential of cure, it is highly invasive in terms of loss of function. Conventional X-ray radiotherapy (RT) for pelvic recurrence of rectal cancer is less invasive. However, the 5-year overall survival (OS) rate for treatment of pelvic recurrence of rectal cancer remains unsatisfactory at 5% to 6% after irradiation doses of 4.4–66.0 Gy (median = 30.0 Gy) (4–6).
At the Gunma University Heavy Ion Medical Center, carbon-ion (C-ion) RT was initiated in 2010 for treating various cancers, including head and neck cancer (7), non-small cell lung cancer (8), and cancers of the liver (9–11), prostate, and uterine cervix (12). Bone and soft tissue sarcomas have also been treated. C-ion RT shows higher dose localization and provides greater biological advantages than X-ray RT, including distal tail-off due to the Bragg peak, a sharp lateral penumbra, and high linear energy transfer in the Bragg peak (13–15). Compared to X-rays, C-ion beams with high linear energy transfer have a superior cell-killing effect against radioresistant tumor cells, which include hypoxic and cancer stem cells (14, 16).
Yamada et al. reported on the clinical outcomes of C-ion RT for pelvic recurrence of rectal cancer in prospective phase I/II and II studies at the National Institute of Radiological Sciences in Japan (17). They demonstrated favorable clinical outcomes compared to previous reports of X-ray RT and concluded that C-ion RT may constitute a promising alternative to surgical resection. However, these prospective clinical studies were performed at a single institution. Hence, we conducted a prospective observational study focusing on C-ion RT for treating pelvic recurrence of rectal cancer to confirm the reproducibility of clinical outcomes and to explore the benefits of this highly curative and less invasive local treatment strategy.
Materials and Methods
Patient Eligibility
Eligibility criteria for study participation were as follows: (1) the presence of pelvic recurrence of rectal cancer, as confirmed by histology or diagnostic imaging with computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography (FDG-PET); (2) the absence of distant metastasis; (3) patients had undergone curative resection for primary disease and regional lymph nodes, without gross or microscopic residual disease; (4) the presence of a radiographically measurable tumor; (5) Eastern Cooperative Oncology Group performance status ≤2; and (6) age 20–80 years. Patients were excluded if direct invasion of the bladder and/or intestinal tract was observed, if they had received chemotherapy and/or molecular targeted therapy within 4 weeks prior to the initiation of C-ion RT, if they had received prior RT to the target area, if they had intractable infections in the target area, if their tumor could not be covered by a field size of 15 × 15 cm, or if they had another active malignancy.
Pretreatment evaluation was performed before patient registration which included medical history, physical examinations, routine testing of blood cell counts, and chemistry, urine analysis, CT, MRI, and PET. Cystoscopy and/or proctoscopy were performed to exclude bladder or intestinal invasion when indicated. The treatment protocol for the current study was reviewed and approved by the Gunma University Institutional Review Board, and all patients signed an informed consent form before the initiation of C-ion RT. This study was registered at the University Hospital Medical Information Network in Japan (UMIN000009719, prospectively registered on January 8, 2013).
Carbon-Ion Radiotherapy
C-ion beams were generated by a synchrotron at Gunma University Heavy Ion Medical Center. The passive scattering technique was applied for the treatment of pelvic recurrence of rectal cancer, and the beam energy was selected according to tumor depth; the available energies were 290, 380, and 400 MeV/u. At our facility, the radiation dose is calculated using XiO-N, which is an XiO (Elekta)-based software incorporating a dose engine for ion-beam RT (K2dose) developed by the National Institute of Radiological Sciences, with interfaces from Mitsubishi Electric (18). The C-ion RT dose was expressed in Gy [relative biological effectiveness (RBE)] which was defined as the physical dose multiplied by the RBE of carbon ions (19). Before C-ion RT, patients were immobilized using tailor-made fixation cushions and thermoplastic shells to acquire treatment planning CT images; respiratory-gated and 4-dimensional CT images were then acquired. Images from the expiratory phase were used for treatment planning. Patients received C-ion RT once daily, 4 days a week (Tuesday to Friday).
Target Delineation and Treatment Planning
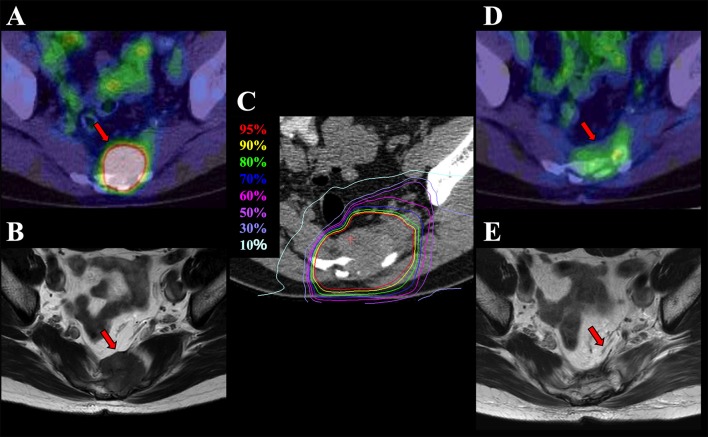
The treatment planning CT images were merged with the MRI and/or PET images to precisely delineate the gross tumor volume. The clinical target volume had a margin of least a 5-mm around the gross tumor volume to include microscopic disease. The internal margin was assessed with reference to 4-dimensional CT images for tumor movement. The planning target volume was defined as a summation of the clinical target volume, internal margin, and setup margin. Patient position was verified using digital orthogonal X-ray images and reference images that were digitally reconstructed based on the planning CT for daily patient position matching. C-ion RT was performed with 73.6 Gy (RBE) administered in 16 fractions over 4 weeks based on the schedule of the phase I/II and II studies at the National Institute of Radiological Sciences. The dose constraints were defined as the mean dose (Dmean) <50 Gy (RBE) and the maximum dose (Dmax) <60 Gy (RBE) to the intestinal tract and the dose delivered to a 1-cm3 volume of the bladder (D1cc) <60 Gy (RBE). Figure 1 shows a representative case of the dose distribution and diagnostic imaging in a case of pelvic recurrence of rectal cancer before and after C-ion RT.
Figure 1.
Pelvic recurrence of rectal cancer in a 58-year-old man treated with C-ion RT. (A) PET before treatment. (B) MRI before treatment. (C) Dose distribution on axial CT images. Highlighted are: 95% (red), 90% (yellow), 80% (green), 70% (blue), 60% (pink), 50% (purple), 30% (light purple), and 10% (light blue) isodose curves [100% = 73.6 Gy [RBE]]. (D) PET 3 months after treatment. (E) MRI 12 months after treatment demonstrating disappearance of the presacral mass. Arrows show the recurrent tumor. C-ion RT, carbon-ion radiotherapy; PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography; RBE, relative biological effectiveness.
Evaluation During Follow-Up
Patients were followed-up for 1 month after C-ion RT completion and every 3 months thereafter. The follow-up examinations comprised routine testing of blood cell counts and chemistry and diagnostic imaging using CT, MRI, or PET. Acute and late toxicities were graded by the Common Terminology Criteria for Adverse Events, version 4.0 of the National Cancer Institute (20). Acute and late toxicities were evaluated as the highest grade of toxicity that occurred within 3 months and after 3 months, respectively, from the initiation of C-ion RT. Local recurrence was defined as the presence of tumor regrowth on CT, MRI, or PET in the irradiated tumor bed, with or without a continuous elevation of blood levels of tumor markers which included carcinoembryonic antigen and carbohydrate antigen 19-9. Regional recurrence was defined as marginal recurrence in the C-ion RT field or recurrence in the site of the original tumor or the para-aortic or contralateral pelvic regions.
Statistical Analysis
All statistical analyses were performed using the JMP Pro 12.2.0 software package (SAS Institute, Inc., Cary, NC, USA). OS was measured from the date of initiation of C-ion RT to the date of death or most recent follow-up. Local control (LC) was defined as no evidence of local progression with no increase in tumor size on CT or MRI and no increase in FDG uptake on PET. Progression-free survival (PFS) was defined as no progression of both locoregional and distant metastases. PFS was measured from the date of initiation of C-ion RT to the date of observation of tumor progression or death from any cause. The probabilities of OS, LC, and PFS were calculated using the Kaplan-Meier method. The primary endpoint was the 3-year LC rate, and the secondary endpoints were the rates of OS, PFS, and acute and late toxicities. The variable risk was expressed as a hazard ratio with its corresponding 95% confidence interval (CI).
Results
Patient Characteristics
A total of 28 patients were enrolled between October 2011 and July 2017; the patient characteristics are summarized in Table 1. The median follow-up duration was 38.9 months (range: 7.6–74.1 months). The median age at the time of registration for C-ion RT was 63 years (range: 40–76 years). The median tumor size was 44 mm in diameter (range: 16–84 mm). A total of 24 and 4 patients were ineligible to undergo surgery and refused surgery, respectively. Direct invasion of the pelvic bone was observed in four patients. In terms of prior anticancer drug therapy, 16 patients had undergone adjuvant therapy after surgery, of whom 2 had undergone molecular targeted therapy combined with cytotoxic chemotherapy; 14 received cytotoxic chemotherapy alone. A total of 3 patients had received treatment after the recurrence of rectal cancer; 2 of them had received molecular targeted therapy combined with cytotoxic chemotherapy, and 1 had received cytotoxic chemotherapy alone. All patients received 73.6 Gy (RBE) in 16 fractions and completed C-ion RT as scheduled.
Table 1.
Patient characteristics (n = 28).
| Characteristics | |
|---|---|
| Age, years, median (range) | 63 (40–76) |
| PS, number | |
| 0 | 12 (42.9%) |
| 1 | 16 (57.1%) |
| Sex, number | |
| Male | 16 (57.1%) |
| Female | 12 (42.9%) |
| Primary tumor surgery, number | |
| Abdominoperineal excision | 16 (57.1%) |
| Low anterior resection | 9 (32.1%) |
| Hartmann's resection | 1 (3.6%) |
| Intersphincteric resection | 2 (7.2%) |
| Histology, number | |
| Well-differentiated adenocarcinoma | 10 (35.7%) |
| Moderately differentiated adenocarcinoma | 15 (53.6%) |
| Mucinous adenocarcinoma | 3 (10.7%) |
| Duration of surgery to C-ion RT, months, median (range) | 25.6 (2.6–117.6) |
| Tumor site, number | |
| Presacral | 7 (25.0%) |
| Side wall | 17 (60.7%) |
| Perineal | 4 (14.3%) |
| Tumor size, mm, median (range) | 44 (16–84) |
| Serum CEA level before C-ion RT, ng/mL, median (range) | 10.7 (0.3–617.3) |
CEA, carcinoembryonic antigen; C-ion RT, carbon ion radiotherapy; PS, performance status.
Overall Survival, Local Control, and Progression-Free Survival
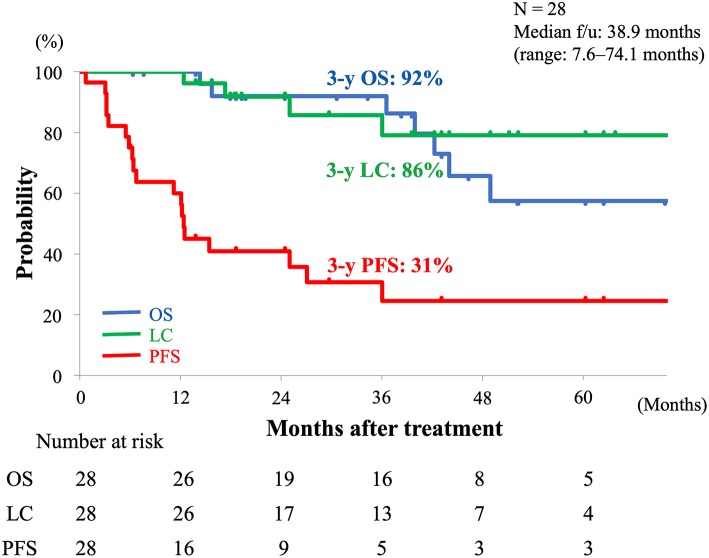
The OS, LC, and PFS curves of all the patients are shown in Figure 2. The 3-year estimated OS, LC, and PFS rates were 92% (95% CI, 73–98%), 86% (95% CI, 63–95%), and 31% (95% CI, 16–52%), respectively. At the time of the analysis, recurrence after C-ion RT was observed in 19 patients; 4 had local recurrence, and 9 each had regional recurrence and distant metastases. Among the 9 patients with distant metastases, 3 had both locoregional and distant metastases. A total of 7 patients died of rectal cancer.
Figure 2.
Kaplan-Meier curves of OS (blue line), LC (green line), and PFS (red line) of all patients. Number at risk is shown below the figure. OS, overall survival; LC, local control; PFS, progression-free survival.
Management of Post-carbon-Ion Radiotherapy Recurrences
Eighteen of 19 patients underwent salvage treatment for recurrence after C-ion RT. In terms of local recurrence, 3 patients received C-ion RT, and 1 received X-ray stereotactic body RT (SBRT). In the patients with regional recurrence alone, 2 received C-ion RT, 3 received molecular targeted therapy combined with cytotoxic chemotherapy, and 1 received cytotoxic chemotherapy alone. Among the patients with distant metastases with or without regional recurrence, 4 received molecular targeted therapy combined with cytotoxic chemotherapy, 1 received molecular targeted therapy alone, 2 received cytotoxic chemotherapy alone, and 1 underwent surgery.
Toxicity
All the observed acute and late toxicities are listed in Table 2. In terms of acute toxicity, none of the patients developed grade 3 or higher hematological toxicities, and only 1 patient developed grade 2 radiation dermatitis. As for late toxicities, 2 patients developed grade 3 pelvic infections. One of these patients was a 62-year-old man with presacral recurrence of rectal cancer with a history of pelvic abscess at the recurrent tumor site before C-ion RT. He developed a pelvic abscess in the irradiated area 6 months after the initiation of C-ion RT, requiring drainage and intravenous antibiotics. Another patient was a 69-year-old man who experienced a perineal recurrence of rectal cancer. He developed a pelvic abscess in the irradiated area 17 months after the initiation of C-ion RT. He had received molecular targeted therapy combined with cytotoxic chemotherapy for distant metastasis before he developed the pelvic abscess. A total of 7 patients developed grade 2 peripheral neuropathy, of whom 3 had peripheral neuropathy before the initiation of C-ion RT.
Table 2.
Acute and late toxicities graded by CTCAE, version 4.0 (n = 28).
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Acute hematological toxicities | |||||
| Leukopenia | 25 | 2 | 1 | 0 | 0 |
| Anemia | 25 | 3 | 0 | 0 | 0 |
| Thrombocytopenia | 23 | 5 | 0 | 0 | 0 |
| Acute non-hematological toxicities | |||||
| Dermatitis | 17 | 10 | 1 | 0 | 0 |
| GI tract | 27 | 1 | 0 | 0 | 0 |
| Urinary | 26 | 2 | 0 | 0 | 0 |
| Neuropathy | 27 | 1 | 0 | 0 | 0 |
| Infection | 28 | 0 | 0 | 0 | 0 |
| Late non-hematological toxicities | |||||
| Dermatitis | 22 | 6 | 0 | 0 | 0 |
| GI tract | 27 | 1 | 0 | 0 | 0 |
| Urinary | 28 | 0 | 0 | 0 | 0 |
| Neuropathy | 15 | 6 | 7 | 0 | 0 |
| Infection | 26 | 0 | 0 | 2 | 0 |
CTCAE, Common Terminology Criteria for Adverse Events; GI, gastrointestinal tract.
Discussion
The present prospective observational study was performed to confirm the reproducibility of C-ion RT and explore its efficacy as a highly curative and less invasive local treatment strategy for pelvic recurrences of rectal cancer. In our study, the 3-year OS, LC, and PFS rates were 92, 86, and 31%, respectively. In previous single-institution prospective and multi-institution retrospective studies using C-ion RT for pelvic recurrence of rectal cancer, the 3-year OS rates were 78 and 73%, respectively, and the 5-year LC rates were 88% for both (17, 21). Our study showed comparable clinical outcomes to the previous studies using similar therapeutic schedules in terms of dose fractionation and target volumes.
The most common curative treatment modality for pelvic recurrences of rectal cancer is surgical resection. Several researchers have reported that the 5-year OS rate after surgery in patients with pelvic recurrence of rectal cancer ranged from 15 to 60% (22–26). In terms of toxicities, however, Pereira et al. in their review reported that 7% to 50% of the patients developed pelvic abscesses, 5–10% developed intestinal obstructions, and 4–24% developed enterocutaneous or enteroperineal fistulas and secondary perineal wound dehiscence (26). Rahbari et al. reported that 42% of patients developed perioperative surgical morbidity with an in-hospital mortality rate of 3% (24). In contrast, our study demonstrated that complications after C-ion RT were relatively less, with 7% of the patients developing grade 3 pelvic infections and none developing intestinal obstruction. Although most of our participants were inoperable, the results, particularly in terms of OS after C-ion RT, were at least comparable to that of surgical resection. However, C-ion RT is less invasive than surgery.
X-ray RT was previously employed in treating inoperable patients with recurrence of rectal cancer, predominantly with palliative intent. However, combined with chemotherapy and recent advances in radiotherapeutic technology, it is being used to treat pelvic recurrence of rectal cancer with radical intent. Several researchers have reported the clinical outcomes of X-ray RT for recurrence of rectal cancer (27–33). Tanaka et al. reported the clinical outcomes after radical X-ray RT for pelvic recurrence of rectal cancer (33). In the study, the 1- and 3-year OS rates and the 1- and 3-year LC rates were 83 and 45%, and 52 and 20%, respectively. With respect to the relationship between dose and clinical efficacy, the 1-year OS and LC rates in the 75 Gy or higher biological effective dose (BED) group with α/β = 10 Gy (≥75 Gy10) vs. the 75 Gy or lower BED group (<75 Gy10) were 100 and 78% (p = 0.07) vs. 83 and 42% (p < 0.05). These results demonstrated that a higher BED may help in the achievement of favorable LC, potentially contributing to a better OS.
In recent years, X-ray SBRT, which can deliver a high dose to the target with high precision, has been widely used, and its clinical outcomes have been reported (28, 31). Franzese et al. demonstrated the clinical outcomes of X-ray SBRT for loco-regional recurrence of colorectal cancer with a median dose of 78.8 Gy10 (28). They showed that the 3-year OS and LC rates were 81 and 75%, respectively. Theoretically, C-ion RT can deliver a higher dose to the target volume than X-ray RT even if critical organs are nearby. In our study, the calculated C-ion RT BED was 107.5 Gy10, and regarding the clinical results, the OS and LC achieved by C-ion RT were more favorable than those achieved by X-ray RT and X-ray SBRT (Table 3). The present study showed that the use of a higher BED leads to favorable LC which may contribute to a better OS.
Table 3.
Comparison of the present study with previous studies on recurrence of rectal cancer.
| References | Year | n | Method of treatment | 3-year OS rate | 5-year OS rate | 3-year LC rate | 5-year LC rate | Late toxicities ≥grade 3 |
|---|---|---|---|---|---|---|---|---|
| Wong et al. (6) | 1998 | 214 | X-ray RT | NA | 5% | NA | 7% | Infection: none, GI: 5.7% |
| Tanaka et al. (33) | 2017 | 32 | X-ray RT | 45% | 23% | 19% | 13% | NA |
| Lee et al. (32) | 2011 | 22 | CCRT | NA | 41% | NA | 56% | NA |
| Cai et al. (27) | 2015 | 71 | CCRT | 37% | NA | 34% | NA | NA |
| Kim et al. (31) | 2008 | 23 | SBRT | NA | 23% | 74% (4-year) | NA | Infection: none, GI: none |
| Franzese et al. (28) | 2017 | 35 | SBRT | 81% | NA | 75% | NA | Infection: none, GI: none |
| Yamada et al. (17) | 2016 | 151 | C-ion RT | 78% | 59% | NA | 88% | Infection: none, GI: 0.7% |
| Shinoto et al. (21) | 2018 | 224 | C-ion RT | 73% | 51% | 93% | 88% | Infection: 3.1%, GI: 0.9% |
| Present study | 28 | C-ion RT | 92% | 58% | 86% | 79% | Infection: 7%, GI: none |
CCRT, concurrent chemoradiotherapy; C-ion RT, carbon ion radiotherapy; GI, gastrointestinal tract; LC, local control; NA, not available; OS, overall survival; RT, radiotherapy; SBRT, stereotactic body radiotherapy.
Systemic therapy has an important role in improving the prognosis of pelvic recurrences of rectal cancer. A previous study showed that OS was improved in the group undergoing X-ray RT with chemotherapy compared with that of the X-ray RT-alone group (29). Since regional recurrence and/or distant metastases occurred after C-ion RT in our study, the PFS rate was unsatisfactory. Concurrent or adjuvant use of chemotherapy may lead to improved PFS and OS with C-ion RT for pelvic recurrence of rectal cancer.
Recently, immunotherapy is an important topic in terms of anticancer treatment. Sato et al. reported an in vitro study in which DNA double-strand breaks as a result of X-ray irradiation upregulated PD-L1 expression in human osteosarcoma, lung cancer, and prostate cancer cell lines (34). Additionally, Oike et al. reported an in vitro study of human uterine cervical cancer in which radiotherapy-induced double-strand breaks were related to linear energy transfer, and double-strand breaks were observed more frequently with C-ion beam irradiation than with X-ray irradiation (35). Although there were no data of C-ion beam irradiation upregulating PD-L1 expression, the high linear energy transfer of C-ion RT has a potentially synergistic effect with immunotherapy using anti-PD-1 and anti-PD-L1 immune checkpoint antibodies, because C-ion RT could induce more double-strand breaks than X-ray RT and, C-ion RT combined with immunotherapy has potentially improved PFS and OS.
In the present study, grade 3 late toxicities were observed in 2 (7%) of the 28 patients; both developed grade 3 pelvic infections in the irradiated area. The presence of a past history of abscess and neutropenia due to chemotherapy may have contributed to the development of the pelvic abscesses. In previous studies on C-ion RT for pelvic recurrence of rectal cancer, 2 and 5% of patients developed grade 3 late toxicities (18, 22). The incidence of late toxicities in the present study was comparable to that observed in previous reports on C-ion RT.
Our study has some limitations. First, this prospective study was conducted at a single institution. A multi-institution prospective study is currently underway which will elucidate the efficacy and toxicity of C-ion RT in treating pelvic recurrence of rectal cancer. Second, patients with direct invasion of the bowel/bladder were excluded because of the high risk of severe toxicities such as perforation in this prospective study. Therefore, the toxicity might not be truly representative. Third, although this study showed favorable clinical outcomes, the follow-up duration was too short to determine the long-term safety of this treatment.
In conclusion, C-ion RT showed favorable OS and LC with low rates of toxicity in treating pelvic recurrence of rectal cancer. Our study demonstrated superior outcomes of C-ion RT for pelvic recurrence of rectal cancer, suggesting a potential role for C-ion RT in the radical treatment of patients who are unsuitable for surgery.
Data Availability
All datasets generated for this study are included in the manuscript.
Ethics Statement
The studies involving human participants were reviewed and approved by Gunma University Institutional Review Board. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
SS, MO, HKi, TO, and TN made substantial contributions to the conception and design of the study. SS, MO, HKi, TK, and TO treated and followed up the patients. SS, MO, and HKi collected the data. SS, MO, and TO drafted the manuscript and performed the statistical analysis. TO, TA, HO, KS, HKu, and TN were involved in critically revising the manuscript for important intellectual content. SS, MO, and TO participated in the acquisition and interpretation of the data. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank all the patients who were involved in this study and our colleagues at the Department of Radiation Oncology, Gunma University Graduate School of Medicine. We thank Editage (www.editage.jp) for English language editing.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. (2001) 345:638–46. 10.1056/NEJMoa010580 [DOI] [PubMed] [Google Scholar]
- 3.Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. (2004) 47:48–58. 10.1007/s10350-003-0012-y [DOI] [PubMed] [Google Scholar]
- 4.Frykholm GJ, Pahlman L, Glimelius B. Treatment of local recurrences of rectal carcinoma. Radiother Oncol. (1995) 34:185–94. 10.1016/0167-8140(95)01519-M [DOI] [PubMed] [Google Scholar]
- 5.Lybeert ML, Martijn H, de Neve W, Crommelin MA, Ribot JG. Radiotherapy for locoregional relapses of rectal carcinoma after initial radical surgery: definite but limited influence on relapse-free survival and survival. Int J Radiat Oncol Biol Phys. (1992) 24:241–6. 10.1016/0360-3016(92)90678-B [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Cummings BJ, Brierley JD, Catton CN, McLean M, Catton P, et al. Treatment of locally recurrent rectal carcinoma–results and prognostic factors. Int J Radiat Oncol Biol Phys. (1998) 40:427–35. 10.1016/S0360-3016(97)00737-2 [DOI] [PubMed] [Google Scholar]
- 7.Shirai K, Saitoh JI, Musha A, Abe T, Kobayashi D, Takahashi T, et al. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci. (2017) 108:2039–44. 10.1111/cas.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitoh JI, Shirai K, Abe T, Kubo N, Ebara T, Ohno T, et al. A phase I study of hypofractionated carbon-ion radiotherapy for stage III non-small cell lung cancer. Anticancer Res. (2018) 38:885–91. 10.21873/anticanres.12298 [DOI] [PubMed] [Google Scholar]
- 9.Shiba S, Abe T, Shibuya K, Katoh H, Koyama Y, Shimada H, et al. Carbon ion radiotherapy for 80 years or older patients with hepatocellular carcinoma. BMC Cancer. (2017) 17:721 10.1186/s12885-017-3724-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiba S, Shibuya K, Katoh H, Koyama Y, Okamoto M, Abe T, et al. No deterioration in clinical outcomes of carbon ion radiotherapy for sarcopenia patients with hepatocellular carcinoma. Anticancer Res. (2018) 38:3579–86. 10.21873/anticanres.12631 [DOI] [PubMed] [Google Scholar]
- 11.Shibuya K, Ohno T, Katoh H, Okamoto M, Shiba S, Koyama Y, et al. A feasibility study of high-dose hypofractionated carbon ion radiation therapy using four fractions for localized hepatocellular carcinoma measuring 3 cm or larger. Radiother Oncol. (2019) 132:230–5. 10.1016/j.radonc.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Ohno T, Noda SE, Murata K, Yoshimoto Y, Okonogi N, Ando K, et al. Phase I study of carbon ion radiotherapy and image-guided brachytherapy for locally advanced cervical cancer. Cancers. (2018) 10:338. 10.3390/cancers10090338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, et al. Biophysical characteristics of himac clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. (1999) 44:201–10. 10.1016/S0360-3016(98)00544-6 [DOI] [PubMed] [Google Scholar]
- 14.Nakano T, Suzuki Y, Ohno T, Kato S, Suzuki M, Morita S, et al. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res. (2006) 12:2185–90. 10.1158/1078-0432.CCR-05-1907 [DOI] [PubMed] [Google Scholar]
- 15.Tsujii H, Kamada T, Shirai T, Noda K, Tsuji H, Karasawa K. editors. Carbon- Ion Radiotherapy. Tokyo: Springer Science & Business Media; (2013). 10.1007/978-4-431-54457-9 [DOI] [Google Scholar]
- 16.Cui X, Oonishi K, Tsujii H, Yasuda T, Matsumoto Y, Furusawa Y, et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. (2011) 71:3676–87. 10.1158/0008-5472.CAN-10-2926 [DOI] [PubMed] [Google Scholar]
- 17.Yamada S, Kamada T, Ebner DK, Shinoto M, Terashima K, Isozaki Y, et al. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int J Radiat Oncol Biol Phys. (2016) 96:93–101. 10.1016/j.ijrobp.2016.04.022 [DOI] [PubMed] [Google Scholar]
- 18.Kanematsu N. Dose calculation algorithm of fast fine-heterogeneity correction for heavy charged particle radiotherapy. Phys Med. (2011) 27:97–102. 10.1016/j.ejmp.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Inaniwa T, Kanematsu N, Matsufuji N, Kanai T, Shirai T, Noda K, et al. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the national institute of radiological sciences, Japan. Phys Med Biol. (2015) 60:3271–86. 10.1088/0031-9155/60/8/3271 [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD: National Institutes of Health; National Cancer Institute; (2009). [Google Scholar]
- 21.Shinoto M, Yamada S, Okamoto M, Shioyama Y, Ohno T, Nakano T, et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan carbon-ion radiation oncology study group (J-CROS) study 1404 rectum. Radiother Oncol. (2019) 32:236–40. 10.1016/j.radonc.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 22.Heriot AG, Tekkis PP, Darzi A, Mackay J. Surgery for local recurrence of rectal cancer. Colorectal Dis. (2006) 8:733–47. 10.1111/j.1463-1318.2006.01018.x [DOI] [PubMed] [Google Scholar]
- 23.Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis. (2011) 13:732–42. 10.1111/j.1463-1318.2009.02167.x [DOI] [PubMed] [Google Scholar]
- 24.Rahbari NN, Ulrich AB, Bruckner T, Münter M, Nickles A, Contin P, et al. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: Is there still a chance for cure? Ann Surg. (2011) 253:522–33. 10.1097/SLA.0b013e3182096d4f [DOI] [PubMed] [Google Scholar]
- 25.Vermaas M, Ferenschild FT, Verhoef C, Nuyttens JJ, Marinelli AW, Wiggers T, et al. Total pelvic exenteration for primary locally advanced and locally recurrent rectal cancer. Eur J Surg Oncol. (2007) 33:452–8. 10.1016/j.ejso.2006.09.021 [DOI] [PubMed] [Google Scholar]
- 26.Pereira P, Ghouti L, Blanche J. Surgical treatment of extraluminal pelvic recurrence from rectal cancer: oncological management and resection techniques. J Visc Surg. (2013) 150:97–107. 10.1016/j.jviscsurg.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 27.Cai G, Zhu J, Palmer JD, Xu Y, Hu W, Gu W, et al. CAPIRI-IMRT: a phase II study of concurrent capecitabine and irinotecan with intensity-modulated radiation therapy for the treatment of recurrent rectal cancer. Radiat Oncol. (2015) 10:57. 10.1186/s13014-015-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franzese C, Fogliata A, Comito T, Tozzi A, Iftode C, Clerici E, et al. Stereotactic/hypofractionated body radiation therapy as an effective treatment for lymph node metastases from colorectal cancer: an institutional retrospective analysis. Br J Radiol. (2017) 90:20170422. 10.1259/bjr.20170422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu JB, Sun XN, Yang QC, Xu J, Wang Q, He C. Three-dimensional conformal radiotherapy combined with folfox4 chemotherapy for unresectable recurrent rectal cancer. World J Gastroenterol. (2006) 12:2610–4. 10.3748/wjg.v12.i16.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo S, Choi Y, Park SK, Kim JY, Kim HJ, Lee YH, et al. Efficacy of dose-escalated radiotherapy for recurrent colorectal cancer. Ann Coloproctol. (2016) 32:66–72. 10.3393/ac.2016.32.2.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MS, Choi C, Yoo S, Cho C, Seo Y, Ji Y, et al. Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol. (2008) 38:695–700. 10.1093/jjco/hyn083 [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Kim DY, Kim SY, Park JW, Choi HS, Oh JH, et al. Clinical outcomes of chemoradiotherapy for locally recurrent rectal cancer. Radiat Oncol. (2011) 6:51. 10.1186/1748-717X-6-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka H, Yamaguchi T, Hachiya K, Okada S, Kitahara M, Matsuyama K, et al. Radiotherapy for locally recurrent rectal cancer treated with surgery alone as the initial treatment. Radiat Oncol J. (2017) 35:71–7. 10.3857/roj.2016.02005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwra Y, Isono M, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. (2017) 8:1751. 10.1038/s41467-017-01883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oike T, Niimi A, Okonogi N, Murata K, Matsumura A, Noda SE, et al. Visualization of complex DNA double-strand breaks in a tumor treated with carbon ion radiotherapy. Sci Rep. (2016) 6:22275. 10.1038/srep22275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the manuscript.