Abstract
Francisella tularensis (Ft), the causative agent of lethal tularemia, is classified as a category A biological warfare threat agent. While Ft infection is treatable by antibiotics, many failed antibiotic treatments were reported, highlighting the need for effective new treatments. It has been demonstrated that binding of antibody-coated bacteria to the Fc receptor located on phagocytic cells is a key process needed for efficient protection against Ft. Yet, Ft utilizes the same receptor to enter the phagocytic cells in order to escape the immune system. To address the question whether an anti-Ft LPS antibody lacking the ability to bind the Fc receptor may inhibit the entry of Ft into host cells, a soluble scFv (TL1-scFv) was constructed from an anti Ft-LPS antibody (TL1) that was isolated from an immune single-chain (scFv) phage-display library. Bacterial uptake was assessed upon infection of macrophages with Ft live attenuated strain (LVS) in the presence of either TL1 or TL1-scFv. While incubation of LVS in the presence of TL1 greatly enhanced bacterial uptake, LVS uptake was significantly inhibited in the presence of TL1-scFv. These results prompt further experiments probing the therapeutic efficacy of TL1-scFv, alone or in combination with antibiotic treatment.
Subject terms: Bacterial infection, Pathogens
Introduction
Francisella tularensis (Ft), the causative agent of lethal tularemia, is a virulent Gram-negative, facultative intracellular bacterium. Due to its high infectivity and mortality rates, Ft is classified as a category A biological warfare threat agent by the CDC1. Tularemia is usually treatable by antibiotics, however, only few are recommended as the treatment of choice2. Moreover, there are natural strains of Ft that acquired antibiotic resistance (for example to beta-lactams and colistin)3,4. In addition, many therapeutic failures and relapses of infected patients were reported5,6 with about 2% mortality rates of antibiotic-treated patients7. Thus, several approaches were made to develop novel and effective treatments for tularemia8.
The role of antibody-mediated protection against intracellular pathogens in general and Ft in particular has long been controversial. For example, several studies have shown that antibodies directed against the LPS of Ft can be used for the treatment of mice infected with Ft attenuated strain (LVS) and not for those infected with the virulent strain (type A SchuS4)9–11. While Ft utilizes several receptors, including Fc receptor (FcγR) to enter the cytosol and escape from the immune system12–15, binding of antibody-coated bacteria to the same receptor is a key process needed for efficient protection against LVS9,16. Interestingly, this exact uptake mechanism is also being investigated as a way to enhance the uptake of inactivated Ft in order to provoke efficient immune response and as a mean to create a platform for vaccination17.
It was previously suggested that the failure of anti-Ft antibodies to provide efficient protection against the virulent strain, although they can bind it very efficiently, is due to a complete shutdown of the inflammatory response needed for efficient antibody-mediated clearance of the bacteria10. Yet, others have shown that opsonization of the SchuS4 strain using antibodies changed the intracellular fate of the bacteria and limited its ability to replicate in the cytosol18. As the recognition of Ft at the host cell membrane is a key step in the infection process, we asked whether the creation of an anti-Ft LPS antibody that lacks the ability to bind to the FcγR will inhibit the entry of Ft into the host cell. In order to create a specific and high-affinity antibody, it was decided to incorporate an immunization methodology that promotes high affinity antibodies in vivo, together with efficient screening methods using phage-display libraries. This practice was successfully applied previously, resulting in the isolation of potent and high-affinity antibodies against ricin and abrin from immunized non-human primates and rabbits, respectively19,20. In this work we report the isolation of an anti-Ft LPS antibody that reduced bacterial uptake by cultured macrophages.
Results and Discussion
Immunization and characterization of the antibody response
Previous studies have demonstrated that the combination of an efficient immunization protocol with proficient screening methods, results in the isolation of potent antibody clones19,20. Accordingly, we hypothesized that in order to isolate highly specific antibodies toward the Ft LPS moiety, the immunization process should be carried out with live bacteria. Since rabbits are a vector of tularemia and are naturally susceptible to this pathogen, the immune reaction following infection was studied previously by evaluating anti-bacteria antibody titers, changes in clinical and hematological parameters and more21–23. Others have also further analyzed the antibody responses towards specific proteins and towards the LPS moieties24,25. Here, we took advantage of the fact that rabbits can tolerate infection with live LVS26 in order to generate an immunization protocol that involved repeated exposures of rabbits to this strain, in order to elicit a strong immune response. To this end, a female rabbit was infected with three successive injections of 1 × 108 CFU LVS and the elicited titer against the whole bacteria was evaluated and presented as the half dilution value (Dil50) corresponding to 50% of the maximal binding of the animal serum towards the coated antigen (Fig. 1A). Interestingly, the antibody titer continued to increase within the following 40 days post injection, thus raising the possibility that LVS was still present at that time point. To increase the anti-LVS titer, the animal was further exposed to two successive high doses of LVS (1 × 109 CFU) until a plateau was reached.
Figure 1.
Characterization of the elicited polyclonal anti-Ft antibodies. (A) Monitoring of anti-Ft polyclonal antibodies development during rabbit immunization. The rabbit was injected with sub-cutanic injections of 1 × 108 CFU (red arrows) or 1 × 109 CFU (blue arrows). Antibody titer was determined by ELISA using LVS as the coated layer. (B) Western blot analysis of the elicited antibodies. M - Protein size marker; 1 - LVS lysate; 2 - LVS-S lysate; 3 - SchuS4 lysate; 4 - purified LPS of LVS.
To evaluate the pattern of the elicited antibodies toward Ft, a Western Blot analysis was performed using bacterial lysates (Fig. 1B). We first evaluated the reaction of the elicited antibodies against a purified LPS fraction, extracted from LVS bacteria (lane 4). The resulting binding-pattern (characterized by the ladder pattern) indicated that at least part of the response was raised against the LPS moiety. An examination of the response against whole LVS bacterial lysate (lane 1) indicated that the elicited antibodies recognized also other, non LPS components of the bacteria. To further confirm these findings, the antibodies were reacted with LVS-S lysate (lane 2), a phase variation of Ft that has impaired O-antigen synthesis (which resembles the LVS gray variant27,28). As expected, the antibody binding pattern lacks the characteristic LPS ladder while demonstrating a strong binding to other bacterial proteins. Importantly, a similar binding-pattern was observed toward SchuS4 extracts.
Isolation of anti-LPS high affinity antibody
A final injection of LVS (1 × 109 CFU) at day 165 was administered to the immunized animal and a scFv phage-display library was constructed from cDNA templates derived from RNA isolated from its spleen, bone marrow and peripheral blood taken at day 172. Following three rounds of panning using plate-coated LVS bacteria, individual clones were screened by direct phage-ELISA for their ability to bind LVS. It was found that 80% of the colonies reacted with LVS, and were all found to possess the same VH-VL sequence.
The isolated scFv-displayed antibody was reformatted and expressed as a chimeric antibody20,29 composed of rabbit variable chains and human constant regions (IgG1/κ). The novel antibody, termed TL1 was expressed in cultured cells and then further characterized for its ability to bind the target bacteria. Using ELISA, it was found that TL1 binds both LVS and SchuS4 with high affinity, while it does not bind the LVS-S strain (Fig. 2A), suggesting that it is specific to the Ft O-antigen. Western blot analysis also confirmed that observation, where TL1 reacted solely with LVS, SchuS4 or purified LPS (Fig. 2B). According to a previous report that characterized the binding pattern of several anti-Ft LPS mAbs30, it can be assumed that TL1 binds the longer chains of the LPS ladder and more specifically the four-sugar repeats in the LPS O-antigen chains.
Figure 2.
Binding characterization of TL1. (A) The reactivity profile of TL1 was determined by ELISA using either LVS (circles), SchuS4 (triangles) or LVS-S (squares) as the adsorbed layer. Points are the mean ± STD of quadruplicates. (B) Western blot analysis of TL1 reacted with: 1 - LVS lysate; 2 - LVS-S lysate; 3 - SchuS4 lysate; 4 - purified LPS of LVS. M - Protein size marker; (C) Western blot analysis of anti-Ft polyclonal antibodies or TL1 reacted with: 1 – LVS lysate; 2 – Fp strain 25015 lysate; 3 – Fp strain 25017 lysate. (D) Immunofluorescence assay of LVS using Alexa 488-conjugated TL1.
The specificity of this antibody toward Ft was further confirmed using two strains of the closely related bacteria Francisella Philomiragia (Fp). Indeed, while control anti-Ft polyclonal antibodies recognize Fp, TL1 does not recognizes this species (Fig. 2C). In addition, in ELISA binding assays where other gram-negative bacteria (including Y. pestis and S. typhimurium) were used, TL1 did not recognize the above species, thus further indicating its high specificity (data not shown).
The binding of TL1 to LVS was also analyzed by an immunofluorescence assay (IFA), where it exhibited the LPS characteristic staining as would be expected from an anti-LPS antibody (Fig. 2D).
To further characterize the binding of TL1 to Ft, we used the Octet Red biolayer interferometry system. The binding profile of TL1 with different concentrations of LVS revealed a positive dose response where at higher LVS concentrations a faster association and saturation was achieved (Fig. 3A). Accurate determination of antibody affinity requires the interaction of the antibody with several concentrations of the antigen. Here, due to the repetitive nature of the target antigen of TL1, it is impossible to calculate its concentration and therefore the association constant (kon) could not be determined. On the other hand, the dissociation constant (koff) does not require prior knowledge of the antigen concentrations and therefore can be calculated. It was found that the dissociation rate was extremely slow, below 1 × 10−7 s−1 which is the Octet Red detection limit, indicating that TL1 exhibits high affinity that is probably in the sub-pM range. Moreover, no dissociation between TL1 and LVS was observed even at a highly acidic environment (pH 2.7).
Figure 3.
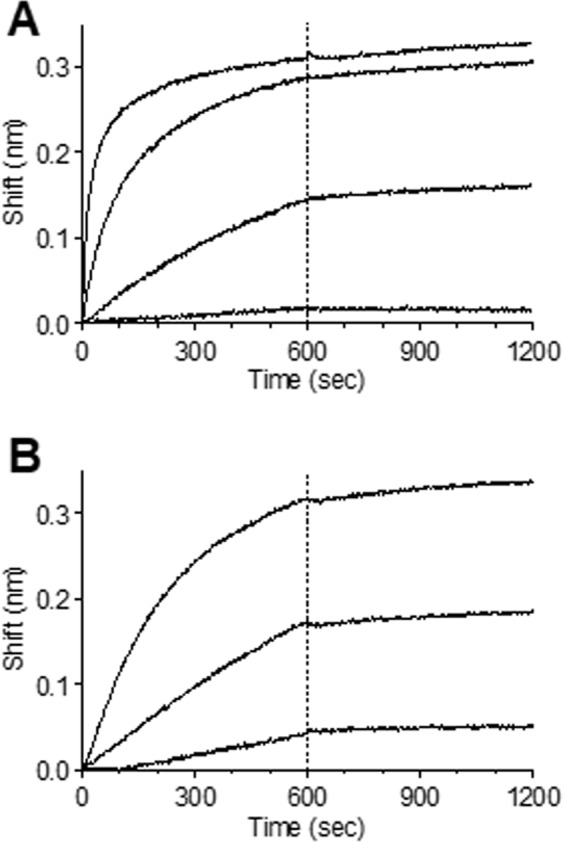
Affinity measurements. The binding kinetics of TL1 were measured using the Octet Red system. Biotinylated TL1 was immobilized on the sensor and reacted for 600 s with increasing concentrations of (A) LVS (from bottom up:1 × 106, 1 × 107, 1 × 108 and 1 × 109 CFU/ml) or (B) purified LPS (from bottom up: 0.2, 1 and 5 μg/ml). The sensors were then immersed in buffer for another 600 s (marked by dashed line).
To further strengthen this observation, the binding assay was repeated using several concentrations of purified LPS (the exact molarity cannot be determined due to the high variability of the LPS chains length within the sample) and indeed, a similar binding pattern was observed and no dissociation could be detected (Fig. 3B). This phenomena (antibodies exhibiting sub-pM affinities) was previously reported by our group for several antibodies against ricin, that were isolated from phage-display libraries originated from immunized non-human primates20.
Sensitive detection of F. tularensis
Early and sensitive detection of Ft is of high importance in order to initiate prompt life-saving antibiotic treatment31, several assays were introduced aiming for sensitive and specific detection of this agent32–34. The findings that TL1 exhibits high affinity and specificity toward Ft prompted us to examine its activity in a detection assay of the virulent SchuS4 (work was carried out under BSL-3 Class microbiological safety conditions). Thus, TL1 was immobilized on an ELISA plate to serve as the capture moiety, incubated with increasing concentrations (102–109 CFU/ml) of live SchuS4 and the IgG fraction of anti-Ft hyper-immune rabbit sera (termed T5) served as the detection component. Indeed, a sigmoidal dose response curve was generated with an estimated limit of detection (LOD) of 1 × 104 CFU/ml (Fig. 4). We have previously demonstrated that using a novel assay that was based on biolayer interferometry (BLI) biosensor and the T5 antibody preparation, an LOD of 1 × 104 CFU/ml was reached35. In comparison, the LOD values of commercially available detection kits for Ft are in the range of 1 × 105 to 1 × 107 CFU/ml36. It should be noted that by combining ELISA and gold-nanoparticles-linked oligonucleotide, an even lower sensitivity (1 × 103 CFU/ml) could be reached34. Thus, it can be suggested that by incorporating TL1 in other assay formats that are more sensitive than the classical ELISA, further improvements in the detection of Ft may be achieved.
Figure 4.
Detection of Ft by ELISA. SchuS4 at increasing concentrations was incubated in TL1-coated wells for one hour. The plate was then washed and HRP conjugated anti-Ft IgG antibodies were added. Points are average ± STD fitted by non-linear regression.
Binding of TL1-scFv inhibits F. tularensis uptake by macrophages
Having such a potent anti Ft-LPS monoclonal antibody prompted us to ask whether the binding of this antibody will affect the uptake of Ft by macrophages. The experimental setup included cultured J774A.1 murine macrophages that were incubated with LVS-pXB173-lux that constitutively express luciferase. The cells were lysed 24 hours later and the intracellular luminescence levels were determined. In preliminary experiments it was found that there is a direct correlation between the bacterial LVS-pXB173-lux CFU (ranging from 1.4 × 104 to 1 × 107 CFU) and the luminescence levels (see Supplementary Fig. S1). Here, the cultured J774A.1 were incubated with LVS-pXB173-lux at MOI = 1 (1.23 × 105 CFU), in the absence (control) or in the presence of TL1 and the intracellular luminescence levels were determined 24 hours later. As expected, incubation of LVS in the presence of TL1 (0.2 and 2 nM) significantly enhanced their uptake by 13–16 fold (Fig. 5A). Interestingly, at higher concentrations of TL1, the bacterial uptake level dramatically declined in an antibody-dose dependent manner (to 6 and 4-fold over control at 20 and 200 nM of TL1, respectively). These results are in line with previous observations that binding of antibody-coated bacteria to FcγR enhance bacterial-uptake by macrophages and neutrophils9,18. However, the fact that at higher concentrations the binding of the antibodies to Ft-LPS interferes with the phagocytosis process may suggest that two mechanisms co-exist (uptake-enhancement versus uptake-inhibition). It was therefore of interest to test the direct effect of antibody binding to Ft-LPS on bacterial uptake, while eliminating the FcγR mediated uptake.
Figure 5.
The effect of TL1 on Ft uptake by macrophages. Cultured J774A.1 murine macrophages were incubated with LVS-pXB173-lux in the absence (control) or in the presence of the indicated concentrations of either (A) TL1 or (B) TL1-scFv. The cells were lysed 24 hours later and the intracellular luminescence levels were determined. Bars are mean ± SEM of three independent experiments; *p < 0.05. (C–E) Macrophages were incubated with LVS (MOI = 1) in the absence (C) or in the presence of TL1 (200 nM) (D) or TL1-scFv (200 nM) (E) for two hours. The cells were then washed, and LVS bacterial cells were stained using an Alexa 488-conjugated rabbit anti-Ft antibodies. Cell nuclei were stained with DAPI.
We therefore created a soluble single-chain fragment (scFv) of TL1 (TL1-scFv) that comprises of the VH-VL regions of the antibody and lack the Fc region. Binding studies using octet revealed that the TL1-scFv retained its affinity toward Ft (exhibiting the same binding pattern as the IgG format; data not shown). Next, cultured macrophages were incubated with LVS-pXB173-lux in the presence of increasing concentrations of TL1-scFv and bacterial uptake was measured 24 hours later. Here, it was found that in the presence of 0.2 and 2 nM of TL1-scFv, there is no significant change in the amount of bacterial uptake when compared to control (Fig. 5B). However, increasing the TL1-scFv concentrations to 20 and 200 nM dramatically affected the bacterial uptake, where at 200 nM this process was inhibited by 70%.
Using qualitative confocal microscopy we also verified that the observed inhibitory effect of TL1-scFv is due to its effect on the direct interactions of LVS with the macrophages and not due to the bacteria’s inability to multiply intracellularly, To this end, murine macrophages were incubated with LVS and either TL1 or TL1-scFv for a short incubation period (2 hours) to limit the bacteria’s ability to multiply intracellularly, followed by fixation and imaging using polyclonal anti-LVS antibodies. Indeed, only in the presence of TL1 an increase of macrophages-containing bacteria was observed (Fig. 5C–E).
The role of antibodies in protection against Ft is not well understood. Several studies have demonstrated that passive administration of anti-Ft antibodies, and especially these that target the LPS can provide protection against LVS but not against the virulent SchuS4 type9,11,37,38. It was hypothesized that the main function of these antibodies is to enhance the bacterial clearance upon binding of the antibody-coated bacteria to FcγR on macrophages and neutrophils9. This route of bacterial clearance was also found to be complement-independent, and it was suggested that Ft is protected against complement-mediated killing through the expression of the O-antigen9,39. Moreover, the Fc-receptor targeting was also used to enhance the immune response toward Ft by administrating monoclonal antibody-inactivated Ft immune complexes40. On the other hand, both macrophages and neutrophils are the cell types that support Ft growth in vivo, therefore posing a possible paradox for the role of antibody-mediated opsonization of Ft.
In light of our recent findings, it is possible that the antibodies used as passive immunization to Ft infections in the literature vary in their dose, affinity and ability to bind the LPS moiety thereby providing conflicting results. For example, at lower doses the antibodies may enhance the bacterial uptake and thus hinder effective therapy, while in the cases where higher doses are implemented, the antibodies interfere with the ability of the bacteria to interact with macrophages and neutrophils thereby providing advantageous treatment. The results of this study may suggest that anti Ft-LPS that lack the ability to bind FcγR might provide a more uniformed outcome and inhibit bacterial uptake.
The applicability of this approach is not straight forward as it requires the production of engineered antibodies that contain a mutated Fc-region that lack the ability to bind FcγR while retaining their pharmacokinetics parameters41. Alternatively, one can use the scFv molecule as a passive therapy for Ft infection. However, since scFv’s have very short half-life in the circulation42, it should be taken into account when conceiving a study to test its effectiveness in vivo.
To conclude, we have isolated a specific anti Ft-LPS antibody that exhibits high affinity, thus making it an attractive candidate for incorporation in various detection systems for sensitive detection of the bacteria. Moreover, we have demonstrated that by eliminating its ability to bind the FcγR, binding of the antibody-derivative to the bacterial surface inhibits it uptake by macrophages in vitro. These results may promote further experiments to test whether this effect will provide better therapeutic efficacy in vivo, alone or in combination with antibiotic treatment.
Materials and Methods
Bacterial and cell cultures
Francisella tularensis subsp. tularensis (SchuS4) strain and Francisella tularensis subsp. holarctica strain LVS were grown as described before43. Work with SchuS4 was carried out under BSL-3 Class microbiological safety conditions. Francisella fillomeragia strains (ATCC25015, ATCC25017) were grown as described earlier for the LVS stain43. The bioluminescence reporter plasmid pXB173-lux was generously obtained from James E. Bina44 and introduced into wild-type F. tularensis LVS, resulting in constitutive bioluminescence production. In order to increase the bioluminescent signal, the original gro promoter was replaced with the bfr promoter, which has been found to be more potent45. The resulting LVS-pXB173-lux was grown in TSBC broth (0.1% L-cysteine, 3% tryptic soy broth) or CHA agar (1% hemoglobin, 5.1% Cysteine heart agar) supplemented with 2 µg/ml chloramphenicol (Cm).
J774A.1 murine macrophage like cells were obtained from the American Type Culture Collection (ATCC, BALB/C macrophage). The cells were grown in flasks in Dulbecco’s Modified Eagle Medium (DMEM, Biological Industries, Beit Haemek, Israel) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and 1 mM sodium pyruvate and maintained at 37 °C in a humidified 5% CO2 incubator.
LPS was purified from LVS bacteria as described before46. LVS was inactivated by exposure of 5 × 109 CFU/ml to 3 doses of 75,000 µj/cm3 UV radiation. SchuS4 was inactivated by boiling approximately 8.5 × 1010 CFU/ml in 2 × Laemmli sample buffer (Bio-Rad, USA) for 30 min.
Rabbit immunization
Animals were treated according to the regulations outlined in the U.S. Department of Agriculture (USDA) Animal Welfare Act and the conditions specified in the Guide for Care and Use of Laboratory Animals (National Institute of Health, 2011). The immunization study (RB-15-2013) was approved by the “Israel Institute for Biological Research Institutional Ethical Committee on Animal Experiments”. Female New Zealand White (NZW) Oryctolagus cuniculus (rabbit) was immunized by live LVS strain injected at 6 consecutive sub-cutanic injections over a period of 24 weeks. The first four injections consisted of 1 ml of 1 × 108 CFU that were given monthly, with the exception of the 4th injection that was given two months after the 3rd injection. The next two monthly booster injections consisted of 1 × 109 CFU. Seven days after the last boost, the rabbit was sacrificed and samples were taken from its blood and lymphatic nodes for library construction, as described before19.
Antibodies
Anti F. tularensis polyclonal IgG fraction (designated T5) was obtained by HiTrap Protein A chromatography (GE Healthcare, Uppsala, Sweden) of the hyper-immune rabbit serum immunized as described in the previous section.
scFv Library construction and screening
RNA extracted from spleen, bone marrow and blood samples was used as a template for first-strand cDNA synthesis and a set of degenerate primers was used to amplify all known sequences of Oryctolagus cuniculus VH and Vk immunoglobulin families19. All other methods for the construction and screening of the library were essentially as described before19 with the following changes: Inactivated LVS were used (1 × 108 cfu/mL in carbonate-bicarbonate buffer) to coat a polystyrene immuno-tube (Nunc, Denmark). The bacterial solution was then removed, followed by the tube baing blocked (2% SM + 0.05% Tween 20 in PBS) and used for the next panning steps. Single colonies were randomly picked from the third panning output, and phages were rescued and tested for their binding to LVS bacteria.
ELISA
Direct ELISA: All steps were performed essentially as described before19 with the following changes: Plates were coated with 2 × 108 CFU/mL of inactivated LVS in Carbonate bicarbonate buffer (Sigma-Aldrich, St. Louis, MO, USA). Individual phage clones, antibodies or rabbit sera were added to the plates for a one-hour incubation; the plates were then washed with PBST and incubated with the detecting antibody: horseradish peroxidase (HRP)-conjugated anti-M13 antibody (GE healthcare, Little Chalfont, UK) for phage clones, anti-human IgG conjugated to alkaline phosphatase (Jackson immunoresearch, West Grove, PA, USA) for full antibodies or anti-rabbit conjugated to alkaline phosphatase (Sigma-Aldrich, St. Louis, MO, USA) for serum ELISA. Detection of HRP conjugates was achieved with 3,3′,5,5′-tetramethybenzidine (TMB/E, Millipore, Billerica, MA, USA) while detection of alkaline phosphatase conjugates was achieved with SIGMAFAST p-nitrophenyl phosphate tablets (Sigma-Aldrich, St. Louis, MO, USA).
Capture ELISA: Plates were coated overnight with 2 µg/ml TL1 antibody in Carbonate bicarbonate buffer. Live SchuS4 bacteria (work was carried out under BSL-3 Class microbiological safety conditions) were diluted in PBS and added at different concentrations to the plates for a one-hour incubation; the plates were then washed with PBST and incubated with HRP conjugated T535. The rest of the steps were as described for direct ELISA.
Production of Full-Length antibodies
VH and VL sequences of the TL1 antibody clone were cloned into a mammalian full-length immunoglobulin expression vector29 resulting in IgG1/κ chimeric rabbit-human antibody expression. The vector was expressed in FreeStyle Max 293 cells (Thermo Scientific, Waltham, MA, USA) by transient transfection and the antibody was purified on a HiTrap Protein-A column. Purified TL1 antibody is now available from Sigma-Aldrich, USA (#SAB4200833).
Antibody labeling
Biotinylation of the purified IgG antibody was carried out using sulfo-NHS-SS-biotin (sulfosuccinimidyl-2-(biotinamido) ethyl-1,3-dithiopropionate; Pierce; USA) according to the manufacturer’s instructions. Alkaline-phosphatase labeling of antibodies was carried out using the Lightning-link alkaline phosphatase conjugation kit (Innova Biosciences, UK). Conjugation of TL1 to Alexa488 was carried out using a commercial kit (Thermo fisher Scientific, USA) according to the manufacturer’s instruction.
Construction and purification of soluble scFv TL1
The pET SUMO plasmid, part of the ChampionTM pET SUMO protein expression system (Invitrogen, USA), was used for cloning of TL1-scFv antibody for soluble expression. The scFv was amplified from phagemid DNA and cloned into linearized pET SUMO using A/T ligation. The plasmid was freshly transformed to E. coli BL21 (DE3) (Novagen, USA) and expression was carried out in Terrific Broth medium supplemented with 1% glucose and 50 µg/mL Kanamycin at 37 °C, 250 rpm. When the suspension reached an OD600 of 0.7–0.9, IPTG was added to a final concentration of 0.5 mM and the temperature was lowered to 25 °C. After an O.N growth the cells were harvested, re-suspended in 20 mM phosphate buffer pH 7.4 (supplemented with 0.5 M NaCl, 20 mM Imidazole and Protease inhibitors; Sigma-Aldrich, USA) and sonicated under ice. After sonication, the suspension was precipitated (9500 g, 20 minutes, 4 °C) and Benzonase nuclease (Sigma-Aldrich, USA) was added to a final concentration of 50 units/mL to the supernatant. The supernatant was then filtered (45 µm) and the SUMO-scFv was purified on a HisTrap column (GE, USA) according to the manufacturer’s instructions. The buffer of the purified antibody was exchanged to PBS using a 10 Kd Amicon ultra (Millipore, USA).
Binding studies
Binding studies were carried out using the Octet Red system (ForteBio, USA) essentially as described before19 using LVS bacteria or LPS extract46. The Octet data analysis software 8.1 (Fortebio, USA) was applied for sensorgrams fitting with a 1:1 binding model. The presented values are an average of several repeated measurements.
Western blot
To obtain bacteria lysate, inactivated bacteria was boiled for 10 min with 4xLaemmli sample buffer (Bio-Rad, USA). Bacterial lysates, LPS and protein markers (Precision Plus protein standards dual color; Bio-Rad, USA) were resolved on NuPAGE 4–12% Bis-Tris gel 1.5 mm × 10well (Invitrogen, USA). Gels were blotted on a nitrocellulose filter (iBlot NC gel transfer stacks, Mini; Invitrogen, USA) and blocked for 1 hour in Odyssey blocking buffer (Li-Cor, USA). The nitrocellulose filters were then washed 3 times in wash solution (1% 10 mM Tris 1 M pH 8, 3% NaCl 5 M, 0.05% Tween20 in 1 liter dH2O) and then probed (4 °C, O.N) with T5 or TL1 that were diluted in incubation buffer (5% nonfat dry milk; Bio-Rad, USA). The nitrocellulose filters were then washed 3 times in wash solution. T5 was detected with Goat anti rabbit IRDye 800 CW (Li-Cor, USA) diluted 1:20,000 in incubation buffer. TL1 was detected with Goat anti human IRDye 800 CW (Li-Cor, USA) diluted 1:20,000 in incubation buffer. After another extensive wash step the filters were developed in ODYSSEY CLx (Li-Cor, USA).
IFA
IFA was carried out with LVS bacteria (1 × 108 cfu/ml) air dried on a multispot slide. The slide was incubated for 30 min (37 °C, humid incubator) with Alexa 488 conjugated TL1, diluted to a final concentration of 1 µg/ml in assay buffer (PBS supplemented with 2% BSA and 0.05% tween20). Following incubation the slide was rinsed with water and dried. The slide was than examined under fluorescent illumination with a Nikon phase microscope (Nikon eclipse E400).
Macrophage infection assay
J774A.1 macrophages were seeded at 2 × 104 cells/well in white 96 well plates (Corning, Corning, NY), and allowed to adhere overnight. On the next day, logarithmic phase LVS-pXB173-lux bacteria were washed twice with PBS and incubated with 0.2, 2, 20 or 200 nM of TL1 or scFv-TL1 for 1 hour at room temperature. Bacteria were added to the macrophages at an MOI (multiplicity of infection) of 1, and the plate was then centrifuged at 1000 rpm for 5 minutes. After 1 hour incubation at 37 °C in a humidified 5% CO2 incubator, cells were washed twice with PBS and gentamicin (2 µg/ml) was added to the growth medium for 24 hours, after which the luminescence level was evaluated using the Victor3 (Perkin Elmer) luminometer. Statistical analyses (p value) were performed using Prism software (Version 5.01, GraphPad Software Inc., La Jolla, CA, USA, 2007) in an unpaired two-tailed t-test.
For the confocal microscopy, J774A.1 cells were seeded on 8-well chamber slides (ibidi, Martinsried, Germany) at 1 × 105 cells/well, and allowed to adhere overnight. Cells were then infected as described above for 2 hours, washed three times with PBS and fixed in ice-cold 100% methanol for 2 minutes. Cells were blocked in PBS + 2% BSA + 2% naïve rabbit serum for 20 minutes in 37 °C. Bacteria were stained using an Alexa 488-conjugated rabbit anti-F. tularensis serum (1:200). Cell nuclei were stained with DAPI (1μg/ml, Sigma-Aldrich, St. Louis, MO, USA). Samples were viewed using a Zeiss LSM710 confocal microscope (Zeiss, Oberkochen, Germany).
-No datasets were generated or analyzed during the current study.
Supplementary information
Acknowledgements
This study was supported by the Israel Institute for Biological Research.
Author Contributions
A.M., O.C. and O.M. conceived of and designed the experiments. A.M., U.E., R.A., H.C., E.A., O.C. and O.M. performed the experiments and analyzed the data. A.M., O.C. and O.M. wrote the paper.
Competing Interests
A.M., O.C. and O.M. are listed as inventors on a patent application (IL260412) filed by the Israel Institute for Biological Research for using antibody TL1 in detection and treatment of Ft infection. TL1 antibody is now available for purchase from Sigma-Aldrich. All other authors declare no competing interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ofer Cohen, Email: oferc@iibr.gov.il.
Ohad Mazor, Email: ohadm@iibr.gov.il.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47931-w.
References
- 1.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 2.Dennis DT, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 3.Caspar Y, Maurin M. Francisella tularensis Susceptibility to Antibiotics: A Comprehensive Review of the Data Obtained In vitro and in Animal Models. Front Cell Infect Microbiol. 2017;7:122. doi: 10.3389/fcimb.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llewellyn AC, et al. NaxD is a deacetylase required for lipid A modification and Francisella pathogenesis. Mol Microbiol. 2012;86:611–627. doi: 10.1111/mmi.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosker M, et al. A case of oculoglandular tularemia resistant to medical treatment. Scand J Infect Dis. 2013;45:725–727. doi: 10.3109/00365548.2013.796089. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Castrillon JL, Bachiller-Luque P, Martin-Luquero M, Mena-Martin FJ, Herreros V. Tularemia epidemic in northwestern Spain: clinical description and therapeutic response. Clin Infect Dis. 2001;33:573–576. doi: 10.1086/322601. [DOI] [PubMed] [Google Scholar]
- 7.Evans ME, Gregory DW, Schaffner W, McGee ZA. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 1985;64:251–269. doi: 10.1097/00005792-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Boisset S, Caspar Y, Sutera V, Maurin M. New therapeutic approaches for treatment of tularaemia: a review. Front Cell Infect Microbiol. 2014;4:40. doi: 10.3389/fcimb.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 10.Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol Rev. 2008;225:244–255. doi: 10.1111/j.1600-065X.2008.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savitt AG, Mena-Taboada P, Monsalve G, Benach JL. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin Vaccine Immunol. 2009;16:414–422. doi: 10.1128/CVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balagopal A, et al. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens DL, Lee BY, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krocova Z, Macela A, Kubelkova K. Innate Immune Recognition: Implications for the Interaction of Francisella tularensis with the Host Immune System. Front Cell Infect Microbiol. 2017;7:446. doi: 10.3389/fcimb.2017.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierini LM. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 2006;8:1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 16.Duffy EB, Periasamy S, Hunt D, Drake JR, Harton JA. FcgammaR mediates TLR2- and Syk-dependent NLRP3 inflammasome activation by inactivated Francisella tularensis LVS immune complexes. J Leukoc Biol. 2016;100:1335–1347. doi: 10.1189/jlb.2A1215-555RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitsaktsis C, Babadjanova Z, Gosselin EJ. In vivo mechanisms involved in enhanced protection utilizing an Fc receptor-targeted mucosal vaccine platform in a bacterial vaccine and challenge model. Infect Immun. 2015;83:77–89. doi: 10.1128/IAI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geier H, Celli J. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect Immun. 2011;79:2204–2214. doi: 10.1128/IAI.01382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mechaly Adva, Alcalay Ron, Noy-Porat Tal, Epstein Eyal, Gal Yoav, Mazor Ohad. Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication. Toxins. 2018;10(2):80. doi: 10.3390/toxins10020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noy-Porat Tal, Rosenfeld Ronit, Ariel Naomi, Epstein Eyal, Alcalay Ron, Zvi Anat, Kronman Chanoch, Ordentlich Arie, Mazor Ohad. Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized Non-Human Primates. Toxins. 2016;8(3):64. doi: 10.3390/toxins8030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed DS, et al. Pneumonic tularemia in rabbits resembles the human disease as illustrated by radiographic and hematological changes after infection. PLoS One. 2011;6:e24654. doi: 10.1371/journal.pone.0024654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed DS, et al. Live attenuated mutants of Francisella tularensis protect rabbits against aerosol challenge with a virulent type A strain. Infect Immun. 2014;82:2098–2105. doi: 10.1128/IAI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stinson Elizabeth, Smith Le'Kneitah P., Cole Kelly Stefano, Barry Eileen M., Reed Douglas S. Respiratory and oral vaccination improves protection conferred by the live vaccine strain against pneumonic tularemia in the rabbit model. Pathogens and Disease. 2016;74(7):ftw079. doi: 10.1093/femspd/ftw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaur R, Alam SI, Kamboj DV. Immunoproteomic Analysis of Antibody Response of Rabbit Host Against Heat-Killed Francisella tularensis Live Vaccine Strain. Curr Microbiol. 2017;74:499–507. doi: 10.1007/s00284-017-1217-y. [DOI] [PubMed] [Google Scholar]
- 25.O’Malley KJ, et al. Aerosol prime-boost vaccination provides strong protection in outbred rabbits against virulent type A Francisella tularensis. PLoS One. 2018;13:e0205928. doi: 10.1371/journal.pone.0205928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasetti MF, et al. An improved Francisella tularensis live vaccine strain (LVS) is well tolerated and highly immunogenic when administered to rabbits in escalating doses using various immunization routes. Vaccine. 2008;26:1773–1785. doi: 10.1016/j.vaccine.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowley SC, Myltseva SV, Nano FE. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity and nitric oxide production. Mol Microbiol. 1996;20:867–874. doi: 10.1111/j.1365-2958.1996.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 28.Soni S, et al. Francisella tularensis blue-gray phase variation involves structural modifications of lipopolysaccharide o-antigen, core and lipid a and affects intramacrophage survival and vaccine efficacy. Front Microbiol. 2010;1:129. doi: 10.3389/fmicb.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld R, et al. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal Bacillus anthracis infection. PLoS One. 2009;4:e6351. doi: 10.1371/journal.pone.0006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche MI, Lu Z, Hui JH, Sharon J. Characterization of monoclonal antibodies to terminal and internal O-antigen epitopes of Francisella tularensis lipopolysaccharide. Hybridoma (Larchmt) 2011;30:19–28. doi: 10.1089/hyb.2010.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotem S, et al. Consequences of delayed ciprofloxacin and doxycycline treatment regimens against Francisella tularensis airway infection. Antimicrob Agents Chemother. 2012;56:5406–5408. doi: 10.1128/AAC.01104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durighello E, Bellanger L, Ezan E, Armengaud J. Proteogenomic biomarkers for identification of Francisella species and subspecies by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Anal Chem. 2014;86:9394–9398. doi: 10.1021/ac501840g. [DOI] [PubMed] [Google Scholar]
- 33.Lamont EA, et al. A combined enrichment and aptamer pulldown assay for Francisella tularensis detection in food and environmental matrices. PLoS One. 2014;9:e114622. doi: 10.1371/journal.pone.0114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo SH, et al. Highly sensitive detection of a bio-threat pathogen by gold nanoparticle-based oligonucleotide-linked immunosorbent assay. Biosens Bioelectron. 2015;64:69–73. doi: 10.1016/j.bios.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Mechaly A, Cohen H, Cohen O, Mazor O. A biolayer interferometry-based assay for rapid and highly sensitive detection of biowarfare agents. Anal Biochem. 2016;506:22–27. doi: 10.1016/j.ab.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Zasada AA, Forminska K, Zacharczuk K, Jacob D, Grunow R. Comparison of eleven commercially available rapid tests for detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis. Lett Appl Microbiol. 2015;60:409–413. doi: 10.1111/lam.12392. [DOI] [PubMed] [Google Scholar]
- 37.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/S0264-410X(01)00189-X. [DOI] [PubMed] [Google Scholar]
- 38.Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Immun. 2011;79:1770–1778. doi: 10.1128/IAI.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raynaud C, et al. Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect Immun. 2007;75:536–541. doi: 10.1128/IAI.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham GH, Iglesias BV, Gosselin EJ. Fc receptor-targeting of immunogen as a strategy for enhanced antigen loading, vaccination, and protection using intranasally administered antigen-pulsed dendritic cells. Vaccine. 2014;32:5212–5220. doi: 10.1016/j.vaccine.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes JM, Wormald MR, Rudd PM, Davey GP. Fc gamma receptors: glycobiology and therapeutic prospects. J Inflamm Res. 2016;9:209–219. doi: 10.2147/JIR.S121233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, et al. Influence of molecular size on tissue distribution of antibody fragments. MAbs. 2016;8:113–119. doi: 10.1080/19420862.2015.1111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar-On L, et al. Protection of vaccinated mice against pneumonic tularemia is associated with an early memory sentinel-response in the lung. Vaccine. 2017;35:7001–7009. doi: 10.1016/j.vaccine.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 44.Bina XR, Miller MA, Bina JE. Construction of a bioluminescence reporter plasmid for Francisella tularensis. Plasmid. 2010;64:156–161. doi: 10.1016/j.plasmid.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaide G, et al. Identification and characterization of novel and potent transcription promoters of Francisella tularensis. Appl Environ Microbiol. 2011;77:1608–1618. doi: 10.1128/AEM.01862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. Novel modification of lipid A of Francisella tularensis. Infect Immun. 2004;72:5340–5348. doi: 10.1128/IAI.72.9.5340-5348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.