Abstract
Objectives:
To assess inter-platform reproducibility of ultrasonic attenuation coefficient (AC) and backscatter coefficient (BSC) estimates in adults with known/suspected nonalcoholic fatty liver disease (NAFLD).
Methods:
This HIPAA-compliant prospective study was approved by an institutional review board; informed consent was obtained. Participants with known/suspected NAFLD were recruited and underwent same-day liver examinations with clinical ultrasound scanner platforms from two manufacturers. Each participant was scanned by the same trained sonographer who performed multiple data acquisitions in the right liver lobe using a lateral intercostal approach. Each data acquisition recorded a B-mode image and the underlying radio-frequency (RF) data. AC and BSC were calculated using the reference phantom method. Inter-platform reproducibility was evaluated for AC and log-transformed BSC (logBSC = 10log10BSC) by intraclass correlation coefficient (ICC), Pearson correlation, Bland-Altman analysis with computation of limits of agreement (LOA), and within-subject coefficient of variation (wCV; applicable to AC).
Results:
Sixty-four participants were enrolled. Mean AC values measured using the two platforms were 0.90±0.13 and 0.94±0.15 dB/cm-MHz while mean logBSC values were −30.6±5.0 and −27.9±5.6 dB, respectively. Inter-platform ICC was 0.77 for AC and 0.70 for log-transformed BSC in terms of absolute agreement. Pearson correlation coefficient was 0.81 for AC and 0.80 for logBSC. 95% LOAs were −0.21 to 0.13 dB/cm-MHz for AC, and −9.48 to 3.98 dB for logBSC. The wCV was 7% for AC.
Conclusions:
Hepatic AC and BSC are reproducible across two different ultrasound platforms in adults with known or suspected NAFLD.
Keywords: Nonalcoholic fatty liver disease; Reproducibility of results; Ultrasonography; Prospective Studies; Phantoms, Imaging
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is emerging as the most common type of chronic liver disease in many parts of the world, affecting ~25% of the population globally [1]. NAFLD comprises a spectrum of liver pathologies ranging from simple steatosis to nonalcoholic steatohepatitis (NASH). Its mildest form, steatosis, is the accumulation of fat droplets within hepatocytes. The more severe form, NASH, is characterized by the presence of steatosis, ballooning degeneration and lobular inflammation, typically accompanied by pericellular fibrosis [2]. NASH may progress to cirrhosis, liver failure and hepatocellular carcinoma [1,2]. Early detection and treatment may halt or reverse NAFLD disease progression [2]. Liver biopsy, costly and invasive, remains the current clinical reference standard to diagnose and grade hepatic steatosis in NAFLD.
Several imaging modalities have been used to noninvasively diagnose and grade hepatic steatosis [3-6]. Conventional ultrasonography (CUS), the most commonly used imaging modality to diagnose and grade steatosis, is subjective, operator and machine dependent, and inaccurate [3]. Quantitative ultrasound (QUS) techniques, which quantify the ultrasound signals to remove the operator and machine dependence, show promise for objective and accurate hepatic fat quantification [7-9]. Two fundamental quantitative imaging biomarkers (QIBs) derived by QUS techniques, the attenuation coefficient (AC, dB/cm-MHz) and backscatter coefficient (BSC, 1/cm-sr), have been shown to be correlated to hepatic fat fraction [7-9]. AC is an objective measure of the spatial rate of ultrasound energy loss in tissue, which is sensitive to the tissue composition, and BSC is an objective measure of the fraction of ultrasound energy returned from tissue, which is sensitive to the tissue microstructure [10]. Other quantitative ultrasound parameters, such as Controlled Attenuation Parameter (CAP) [11,12] and sound speed [13], have also been used for hepatic fat quantification.
The accuracy of AC and BSC for hepatic fat content assessment has been demonstrated in the literature [8-9]. Lin et al. showed that BSC correlated with magnetic resonance imaging proton density fat fraction (MRI-PDFF) (Spearman ρ = 0.80) in a prospective, cross-sectional study of a cohort of 204 adults with and without NAFLD [8]. The BSC achieved an area under the receiver operating characteristic curve (AUC) of 0.98 for identifying patients with NALFD, using MRI-PDFF as a reference [8]. Paige et al. compared the diagnostic performance of CUS, QUS, and MRI-PDFF for predicting histology-confirmed steatosis grade in a cohort of 61 adults with NAFLD [9]. CUS achieved a grading accuracy of 51.7%, whereas AC, BSC, and MRI-PDFF had cross-validated grading accuracies of 55.0%, 68.3%, and 71.3%, respectively [9]. For differentiating between steatosis grade 1 versus ≥2, the AUCs were 0.79, 0.85, and 0.96 for AC, BSC, and MRI-PDFF, respectively [9].
However, no prior studies have evaluated the reproducibility of the QUS biomarkers measured from multiple platforms from different scanner vendors. Cross-platform reproducibility is essential for the generalization of the QUS method. If the same patient examined with multiple platforms yields reproducible QUS biomarker values, then the published accuracy evaluated on single machines will be generalizable. If not, the applicability of the published accuracy values will be more limited. Indeed, repeatability and reproducibility are important precision measures. Repeatability is “the measurement precision with conditions that remain unchanged between replicate measurements (repeatability conditions)” [14]. Reproducibility is “the measurement precision with conditions that vary between replicate measurements (reproducibility conditions)” [14]. Previous studies have assessed the repeatability, inter-sonographer reproducibility, and inter-transducer reproducibility for AC and BSC in phantoms [15] and in human subjects [16-17].
The purpose of this study was to assess inter-platform reproducibility of AC and BSC in a cohort of prospectively recruited adults with known or suspected NAFLD and with variable degrees of liver steatosis and fibrosis, scanned by the same set of trained expert sonographers using two different ultrasound imaging platforms.
MATERIALS AND METHODS
Study design and participants
An institutional review board approved this HIPAA (Health Insurance Portability and Accountability Act)-compliant study at the University of California at San Diego (UCSD). Written informed consent was obtained. Research participants were prospectively recruited from the UCSD NAFLD Research Center between March 2017 and June 2018 by the hepatologist (RL). Inclusion criteria were age ⩾ 18 years, known or suspected NAFLD, and willing and able to participate. Exclusion criteria were clinical, laboratory, or histology evidence of a liver disease other than NAFLD, excessive alcohol consumption [⩾ 14 (men) or ⩾ 7 (women) drinks/week], and steatogenic or hepatoxic medication use. Demographic, anthropometric, and biochemical data were recorded.
To help characterize the participant cohort, data from contemporaneous hepatic MRI research studies and/or from clinical-care liver biopsies were recorded if available. These data included the proton density fat fraction measured by confounder-corrected chemical-shift-encoded MRI (MRI-PDFF) [8] and the histological steatosis grade and fibrosis stage determined by an expert hepatopathologist according to the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) histological scoring system [18].
Ultrasound data acquisition
We used two clinical ultrasound systems, Siemens S3000 (Siemens Healthineers) with the 4C1 transducer (1-4 MHz nominal) and GE Logiq E9 (GE Healthcare) with the C1-6 transducer (1-6 MHz nominal), for direct post-beamformed radio-frequency (RF) data acquisition provided under research agreements. Three registered diagnostic medical sonographers (each with > 10 years of experience overall) were trained and had several months to 2 years’ experience performing the research protocol. Each participant was scanned by one of the three sonographers selected based on scheduling availability and underwent two same-day 15- to 20-minute exams. Each exam was performed using a different platform in random order. Between exams, participants took a 5- to 10-minute break and were repositioned on the gurney.
Each sonographer performed a standardized research protocol in the right liver lobe using a lateral intercostal approach. For each platform, the system settings were adjusted for each participant to optimize right hepatic lobe visualization prior to the first RF acquisition, but remained constant for all subsequent RF acquisitions. An acquisition was a single operator button press that recorded a B-mode image and the RF data corresponding to the B-mode image. Acquisitions were repeated during separate shallow expiration breath holds separated by about 15 seconds until at least 10 acquisitions were obtained. The skin-to-capsule distance was measured with the image caliper tool and recorded by the sonographer. Following completion of the repeated liver acquisitions on each platform, a calibrated reference phantom (CIRS, Inc.) with known AC and BSC was scanned to obtain RF without changing the system settings.
AC and BSC computation
AC and BSC frequency spectra were computed offline on a desktop personal computer using custom software programmed in MATLAB (The MathWorks) [15]. The software implemented established AC and BSC methodologies designed to remove instrumentation/setting dependencies by comparing the RF data from the liver and the calibrated phantom [19]. AC and BSC were computed within a freehand field of interest (FOI) outlining the liver boundary, drawn on a B-mode image reconstructed from the RF data (Figure 1). The FOIs were drawn under the supervision of an abdominal radiologist (CBS) by a research analyst (ASB) with 1-year experience in abdominal ultrasound research. A medical physicist (MPA) with career experience in medical ultrasound and MRI research and a research physician (YNZ) provided quality control checks on the work of the analyst by reviewing the FOIs and making corrections when necessary. To minimize analysis burden and in anticipation of possible future clinical applications of this technology, no effort was made to exclude hepatic vessels from the FOI. Five of the ten or more acquisitions were randomly chosen by the analyst for FOI drawing, excluding those that generate B-mode images showing blurring caused by participant breathing or artifacts from rib shadowing. The AC and BSC were computed automatically in the custom software by an investigator (AH, a biomedical engineer with 9 years’ experience in QUS research) independent from the group who provided the FOIs. The AC values and separately the BSC values at all frequency points within 2.6-3.0 MHz were averaged to yield a single AC and a single BSC measure per data acquisition. This frequency range was chosen to be consistent with previous repeatability and reproducibility studies of AC and BSC [16-17] at the center of the transducer bandwidth.
Figure 1.
Representative liver B-mode images reconstructed from the radio-frequency data acquired from a 51 years old male using (a) GE Logiq E9 and (b) Siemens S3000 clinical ultrasound scanners. The magenta field of interest lines were drawn on the reconstructed B-mode images to outline the liver boundary.
Statistical analysis
Statistical analysis was performed using R 3.4.2 (R Foundation for Statistical Computing). Participant characteristics were summarized descriptively. BSC was log-transformed (logBSC = 10log10BSC) to normalize the distribution.
The inter-platform reproducibility was assessed graphically using boxplots for AC and logBSC values obtained before intra-platform averaging of multiple acquisitions of the same participant. Scatter plots and Bland-Altman plots [20] were generated for AC and logBSC values obtained after intra-platform averaging of 5 acquisitions of the same participant. Pearson correlation and limits of agreements (LOAs) were calculated accordingly. Inter-platform reproducibility was also assessed by using intraclass correlation coefficient (ICC) [21] and within-subject coefficient of variation (wCV; not applicable for logBSC because of negative values) [22] for AC and/or logBSC values obtained by averaging rising numbers (1 to 5) of repeated acquisitions. ICC values were calculated based on a single-unit two-way mixed effects analysis of variance (ANOVA) model where the participant was treated as a random effect while the platform was treated as a fixed effect. The ICC for absolute agreement was reported. 95% confidence intervals (CIs) were computed when applicable. The following definitions were used to interpret the ICC estimates for absolute agreement: 0–0.39, poor; 0.40–0.59, fair; 0.60–0.74, good; and 0.75–1.0, excellent [23].
To test whether the inter-platform variability is affected by the participant body mass index (BMI) and subcutaneous fat, we calculate the Pearson’s correlation between the absolute between-platform difference in QUS biomarkers and the BMI, and separately the skin-to-capsule distance. For this analysis, the five repeated QUS measurements were averaged to yield a single measure for each platform.
The sample size was driven by feasibility and is typical for reproducibility studies [16, 17, 24, 25, 26].
RESULTS
Participants
Sixty-four participants (38 females) were enrolled; the Siemens data from 36 of the 64 participants were published in a previous study [17]. The demographic, physical, biochemical, imaging and histological characteristics of the study participants are summarized in Table 1. The mean age was 54 (F: 56; M: 50) years, and the age range was 26-74 (F: 26-74; M: 26-70) years. The mean BMI was 32.0 kg/m2, and the BMI range was 21.7-43.8 kg/m2. Fifty-three participants had MRI-PDFF within 0 to 110 days (mean: 15 days) of US; mean MRI-PDFF was 13.9%, and the MRI-PDFF range was 0.7-37.7%. Missing MRI scans were due to severe claustrophobia, exceeding bore diameter, refusing research MRI, or the scanner being nonoperational. Fifty-four participants had clinical-care liver biopsy within 0 to 302 days (mean: 47 days) of US, forty-three of whom also had MRI-PDFF. As indicated by the MRI-PDFF and histological data, the participant cohort of this reproducibility study covered a wide and relevant range of hepatic fat fractions and liver fibrosis ranges.
Table 1:
Demographic, Physical, Biochemical, Imaging and Histological Characteristics of the Study Participants.
| Summary Statistics |
Total Number of Participants with Data Available |
|
|---|---|---|
| Demographics | ||
| Male, no. (%) | 26 (40.6) | 64 |
| Age, ya | 54 ± 13 | 64 |
| Height, cma | 167.9 ± 11.0 | 63 |
| Weight, kga | 90.6 ± 17.8 | 63 |
| BMI, kg/m2a | 32.0 ± 4.7 | 63 |
| Ethnic origin | ||
| White, no. (%) | 34 (53.1) | 64 |
| Hispanic, no. (%) | 20 (31.3) | 64 |
| Asian, no. (%) | 9 (14.1) | 64 |
| Black, no. (%) | 1 (1.6) | 64 |
| Biochemical profilea | ||
| Hemoglobin, g/dL | 14.3 ± 1.4 | 63 |
| Hematocrit, % | 41.4 ± 6.2 | 63 |
| Platelet count, ×103/μL | 254 ± 78 | 63 |
| AST level, U/L | 43.7 ± 19.3 | 64 |
| ALT level, U/L | 59.1 ± 34.8 | 64 |
| Alkaline phosphatase level, U/L | 85.1 ± 23.5 | 63 |
| Total bilirubin level, mg/dL | 0.6 ± 0.3 | 64 |
| Albumin level, g/dL | 4.4 ± 0.6 | 64 |
| Hemoglobin A1c, % | 6.2 ± 1.2 | 62 |
| Triglycerides level, mg/dL | 153.2 ± 71.3 | 62 |
| Total cholesterol level, mg/dL | 176.3 ± 36.9 | 62 |
| HDL level, mg/dL | 46.3 ± 13.4 | 62 |
| LDL level, mg/dL | 99.7 ± 30.0 | 62 |
| INR | 1.04 ± 0.07 | 64 |
| Imaginga | ||
| MRI-PDFF 5-8, % | 13.9 ± 8.6 | 53 |
| AC from GE Logiq E9, dB/cm-MHz | 0.90 ± 0.13 | 64 |
| AC from Siemens S3000, dB/cm-MHz | 0.94 ± 0.15 | 64 |
| logBSC from GE Logiq E9, dB | −30.6 ± 5.0 | 64 |
| logBSC from Siemens S3000, dB | −27.9 ± 5.6 | 64 |
| Biopsy | ||
| Steatosis grade | ||
| S0, no. (%) | 5 (9.3) | 54 |
| S1, no. (%) | 24 (44.4) | 54 |
| S2, no. (%) | 21 (38.9) | 54 |
| S3, no. (%) | 4 (7.4) | 54 |
| Fibrosis stage | ||
| F0, no. (%) | 20 (37.0) | 54 |
| F1, no. (%) | 21 (38.9) | 54 |
| F2, no. (%) | 3 (5.6) | 54 |
| F3, no. (%) | 5 (9.3) | 54 |
| F4, no. (%) | 5 (9.3) | 54 |
NOTE. All fasting lipid labs were measured while patients were fasting.
AC, attenuation coefficient; AST, aspartate aminotransferase; ALT, alanine aminotransferase; logBSC, log-transformed backscatter coefficient; BMI, body mass index; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; INR, international normalized ratio; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; PDFF, proton-density-fat-fraction, mean calculated from segments 5 to 8.
Mean value provided with standard deviations.
AC and BSC results
Five AC and 5 logBSC values were yielded per participant per platform. The statistics of the AC and logBSC results are summarized in Tables 2 and 3, respectively, for AC and logBSC values obtained by averaging rising numbers of intra-platform acquisitions (N=1, 2, 3, 4 and 5). The inter-platform reproducibility was improved with increased number of acquisitions being averaged. When 5 acquisitions were used, the inter-platform ICC for AC (logBSC) was 0.77 (0.70) in terms of absolute agreement. The wCV was 7.3% for paired (GE vs Siemens) AC measurements. This metric is not applicable for logBSC, which can have negative measurement values.
Table 2.
Attenuation coefficient inter-platform reproducibility for measurements based on a single acquisition (N=1) and on the mean of multiple repeated intra-platform acquisitions (N=2, 3, 4, and 5).
| Number of acquisitions N |
GE mean ± SD (dB/cm-MHz) |
Siemens mean ± SD (dB/cm-MHz) |
ICC (95% CI) | wCV |
|---|---|---|---|---|
| 1 | 0.89 ± 0.13 | 0.94 ± 0.15 | 0.69 (0.50, 0.81) | 0.09 |
| 2 | 0.89 ± 0.13 | 0.94 ± 0.15 | 0.76 (0.58, 0.86) | 0.08 |
| 3 | 0.89 ± 0.13 | 0.94 ± 0.15 | 0.75 (0.57, 0.86) | 0.08 |
| 4 | 0.90 ± 0.13 | 0.94 ± 0.15 | 0.77 (0.58, 0.86) | 0.07 |
| 5 | 0.90 ± 0.13 | 0.94 ± 0.15 | 0.77 (0.59, 0.87) | 0.07 |
ICC was calculated based on a single-unit, absolute-agreement, two-way mixed effect analysis of variance (ANOVA) model.
CI confidence interval, ICC intraclass correlation coefficient, SD standard deviation, wCV within-subject coefficient of variation
Table 3.
Log-transformed backscatter coefficient inter-platform reproducibility for measurements based on a single acquisition (N=1) and on the mean of multiple repeated intra-platform acquisitions (N=2, 3, 4, and 5).
| Number of acquisitions N |
GE mean ± SD (dB) |
Siemens mean ± SD (dB) |
ICC (95% CI) |
|---|---|---|---|
| 1 | −30.8 ± 4.9 | −27.8 ± 5.7 | 0.67 (0.19, 0.85) |
| 2 | −30.7 ± 4.9 | −27.7 ± 5.7 | 0.68 (0.23, 0.85) |
| 3 | −30.7 ± 5.0 | −27.8 ± 5.7 | 0.69 (0.24, 0.85) |
| 4 | −30.6 ± 5.0 | −27.8 ± 5.7 | 0.70 (0.26, 0.86) |
| 5 | −30.6 ± 5.0 | −27.9 ± 5.6 | 0.70 (0.28, 0.86) |
ICC was calculated based on a single-unit, absolute-agreement, two-way mixed effect analysis of variance (ANOVA) model.
CI confidence interval, ICC intraclass correlation coefficient, SD standard deviation
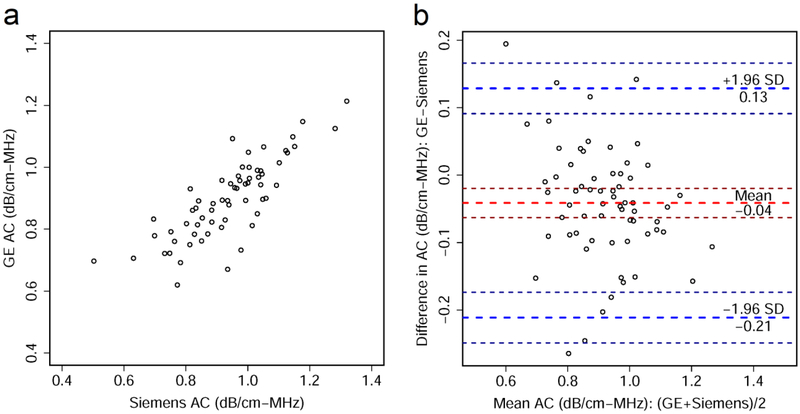
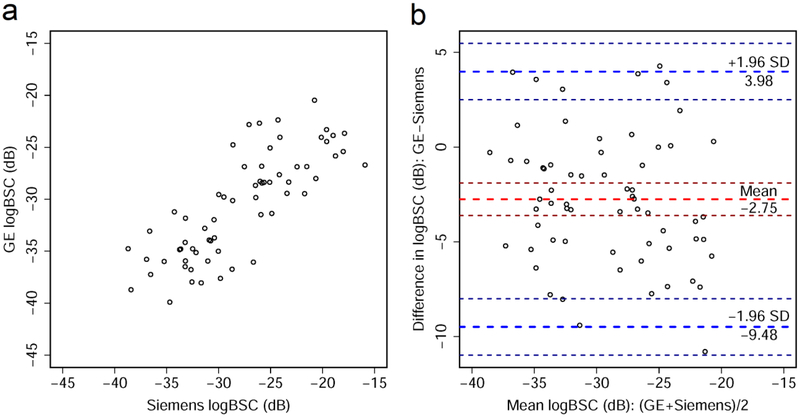
Scatter plots and Bland-Altman plots are shown for AC averaged from 5 repeated intra-platform acquisitions (Figure 2). Similar plots are also shown for logBSC (Figure 3). There was a significant correlation between the AC values measured from the two platforms with Pearson’s r = 0.81 (95% CI: 0.71-0.88, p = 0.29×10−16). Similar results were found for logBSC, with Pearson’s r = 0.80 (95% CI: 0.69-0.87, p = 0.29×10−15). The Bland-Altman plots show a slight bias between the two platforms, with a −0.04 dB/cm-MHz mean difference between GE and Siemens AC values, and −2.75 dB mean difference between logBSC values of the two platforms. The 95% limits of agreement range from −0.21 to 0.13 dB/cm-MHz for AC, and −9.48 to 3.98 dB for logBSC.
Figure 2.
(a) Scatter plot shows relationship between attenuation coefficient (AC) values measured from GE Logiq E9 and Siemens S3000. (b) Bland-Altman plot shows agreement between AC values measured using the two platforms. Thick red dashed line shows the mean difference in AC values between the two platforms and thick blue dashed lines demarcate ± 1.96 standard deviations (SD), with associated 95% confidence intervals indicated by thin dashed lines. The AC values in both plots were obtained by averaging five intra-platform repeated acquisitions per participant.
Figure 3.
(a) Scatter plot shows relationship between log-transformed backscatter coefficient (logBSC) values measured from GE Logiq E9 and Siemens S3000. (b) Bland-Altman plot shows agreement between logBSC values measured using the two platforms. Thick red dashed line shows the mean difference in logBSC values between the two platforms and thick blue dashed lines demarcate ± 1.96 standard deviations (SD), with associated 95% confidence intervals indicated by thin dashed lines. The logBSC values in both plots were obtained by averaging five intra-platform repeated acquisitions per participant.
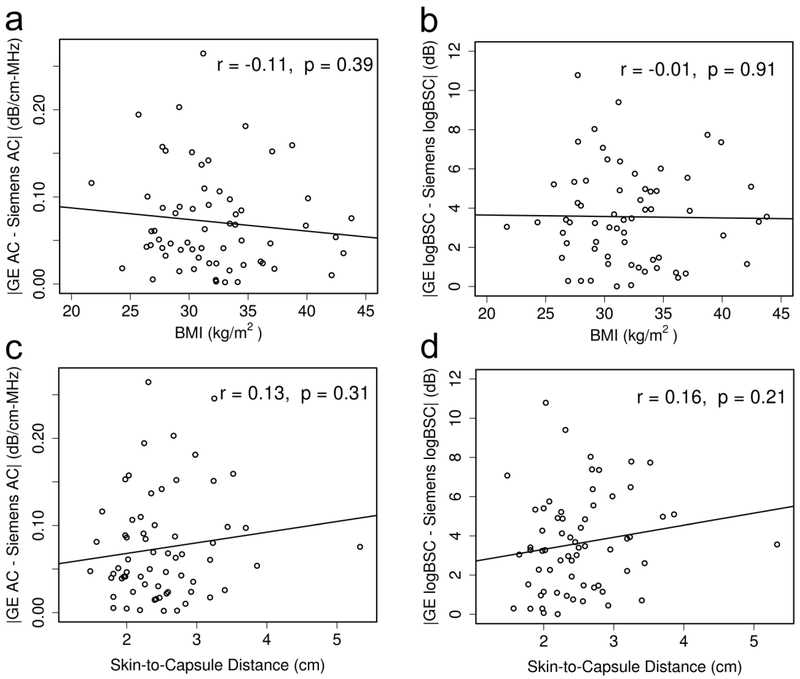
The absolute between-platform difference in the AC was not statistically significantly correlated with the BMI (Figure 4a; Pearson r = −0.11, p = 0.39) or the skin-to-capsule distance (Figure 4c; Pearson r = 0.13, p = 0.31). Similarly, the absolute between-platform difference in the logBSC was not statistically significantly correlated with the BMI (Figure 4b; Pearson r = −0.01, p = 0.91) or the skin-to-capsule distance (Figure 4d; Pearson r = 0.16, p = 0.21).
Figure 4.
Absolute between-platform difference in QUS biomarker versus participant condition plots (a: AC absolute difference versus BMI; b: logBSC absolute difference versus BMI; c: AC absolute difference versus skin-to-capsule distance; d. logBSC absolute difference versus skin-to-capsule distance) show that the QUS inter-platform variability is not significantly affected by the participant BMI or subcutaneous fat. The corresponding Pearson’s correlation and p value are displayed in each subfigure.
DISCUSSION
This study examines an essential aspect of QUS biomarker precision that was previously uninvestigated, the inter-platform reproducibility of AC and BSC. The inter-platform reproducibility is important if common cutoff values of AC and BSC are to be used for clinical diagnosis on different platforms. We demonstrated good to excellent absolute agreement (ICC: 0.77 for AC and 0.70 for logBSC) between two clinical ultrasound imaging platforms, GE Logiq E9 and Siemens S3000, in measuring the hepatic AC and BSC values in NAFLD participants with a wide range of hepatic fat fraction and fibrosis stages. We also demonstrated that the inter-platform reproducibility was not affected by the participant BMI or the skin-to-capsule distance, but it was affected by the number of acquisitions. The inter-platform reproducibility was improved with increasing number of acquisitions being used for AC. The improvement was less for BSC, likely because the AC measurement is noisier than the BSC measurement with the current implementation of QUS techniques. Furthermore, small inter-platform biases were found. These biases were smaller than or comparable to the overall measurement standard deviation due to repeatability and inter-transducer reproducibility reported in a previous human study on NALFD participants (AC: ~0.07 dB/cm-MHz; logBSC: 2.5-2.9 dB) [16].
This study complemented previous AC and BSC repeatability and reproducibility studies in phantoms and humans, and also enabled us to compare QUS with other imaging modalities in terms of inter-platform reproducibility. A phantom-based study [15] reported excellent overall repeatability and reproducibility in AC and BSC, as demonstrated by the small measurement standard deviation due to repeatability and reproducibility (AC: < 0.02 dB/cm-MHz; logBSC: ~0.5 dB). Another phantom study [27] assessed the repeatability and reproducibility of the phantom power spectra (although not AC and BSC) using 11 transducers of the same model (Siemens 6C1) and five clinical ultrasound systems of the same model (Siemens Acuson S3000). It was determined that the data acquired from those transducers and systems produced equivalent phantom power spectrum estimates. A human study [16] on NAFLD participants demonstrated repeatable and inter-transducer reproducible AC and logBSC measures, with small overall measurement standard deviation due to repeatability and inter-transducer reproducibility (AC: ~0.07 dB/cm-MHz; logBSC: 2.5-2.9 dB). Another human study on NAFLD participants [17] found AC and logBSC to be reproducible between sonographers, with a single-unit (measurement made by a single sonographer as the final result) absolute agreement inter-sonographer ICC of 0.78 for AC, and 0.79 for logBSC, and a double-unit (mean of measurements made by two sonographers as the final result) absolute agreement ICC of 0.88 for both AC and logBSC. Comparing the single-unit absolute agreement ICC values in [17] with those reported in the current study, we found similar ICC values for the AC (inter-sonographer: 0.78; inter-platform:0.77), but differing ICC values for the logBSC (inter-sonographer: 0.79; inter-platform: 0.70). The current paper did not report the double-unit ICC values because it was unlikely in future clinical practices that two platforms would be used to yield average AC and BSC measures as the final results for a patient. Comparing QUS with other modalities for liver assessment, the reproducibility of QUS biomarkers AC and BSC appeared to be better than ultrasound elastography, similar to MR elastography, and less than MRI PDFF. In ultrasound elastography, the shear wave speed or liver stiffness measures were not reproducible across manufacturers because the techniques vary between manufacturers, leading to manufacturer-dependent cutoff values for liver fibrosis assessment [28]. In terms of MR elastography, Trout et al. assessed the inter-manufacturer (GE and Philips) reproducibility in liver stiffness measures on 24 volunteer adult participants, reporting absolute agreement inter-manufacturer ICC values of 0.82, 0.71, 0.67, and 0.69, respectively, for four imaging conditions: 1.5-T 2D gradient-echo, 3.0-T 2D gradient-echo, 1.5-T 2D spin-echo echo-planar imaging and 3.0-T 2D spin-echo echo-planar imaging [24]. The inter-platform reproducibility of MRE in patients with liver disease is not yet well understood, however. For MRI-PDFF, Serai et al. reported absolute agreement inter-manufacturer ICC values ranging from 0.91 to 0.95 depending on the readers, derived from a study of 24 adult volunteers scanned with MR imagers from two vendors, GE and Philips [29]. Mashhood et al. reported ICC of 0.85-0.91 for reproducibility between 5 imaging centers using 3 different manufacturers and 3 different FF methods [30]. Kang et al. assessed the reproducibility of MRI-determined PDFF across two different MR scanner platforms, Siemens at 1.5T and GE at 3T, and reported that the MRI-determined PDFF differences at 1.5T compared to 3T ranged from −3.2 to +4.6%, while the limits for the interval defined by the mean difference ± 1.96 standard deviations were −1.9 and +3.7% [31]. In a multi-site, multi-vendor fat-water phantom study, Hernando et al. reported significant effect of vendor with a bias of −0.37% (Philips) and −1.22% (Siemens) relative to GE Healthcare [32]. Overall ICC across sites, vendors, protocols and field strengths was 0.999 in that phantom study [32].
There were a few limitations in the current study. This was a single-site study, and we only evaluated two vendor platforms. Also, inter-platform reproducibility was assessed only on the AC and BSC values, and not on the clinical diagnostic outcomes (e.g., predicted fat fraction and NAFLD diagnosis). Future studies may evaluate the reproducibility using more platforms at multiple sites and evaluate the reproducibility of clinical diagnostic outcomes. Furthermore, future efforts may be directed to develop QUS techniques for better repeatability and reproducibility.
In conclusion, our study showed good to excellent inter-platform reproducibility of AC and BSC in a cohort of adult participants spanning a wide and relevant range of hepatic steatosis and fibrosis and scanned by the same set of trained expert sonographers using the ultrasound scanners from two manufacturers. Additional research is needed to evaluate the impact of different imaging platforms on diagnostic performances.
KEY POINTS.
Ultrasonic attenuation coefficient and backscatter coefficient are reproducible between two different ultrasound platforms in adults with NAFLD.
This inter-platform reproducibility may qualify Quantitative Ultrasound Biomarkers for generalized clinical application in patients with suspected /known NAFLD.
Acknowledgements
The authors thank the research participants for making this study possible, the sonographers, Elise Housman, Susan Lynch, and Minaxi Trivedi, for the dedicated contributions and expertise, the clinical coordinator Vivian Montes for her outstanding organization of the many moving parts, and the pathologist Mark A. Valasek, MD, PhD, for reading the histology and determining the steatosis grade and fibrosis stage.
Funding
This study has received funding by the National Institutes of Health (R01DK106419), Siemens Healthineers USA, and GE Healthcare.
ABBREVIATIONS
- AC
Attenuation coefficient
- ANOVA
Analysis of variance
- AUC
Area under the receiver operating characteristic curve
- BMI
Body mass index
- BSC
Backscatter coefficient
- CAP
Controlled attenuation parameter
- CUS
Conventional ultrasound
- FDA
Food and Drug Administration
- FOI
Field of interest
- HIPAA
Health Insurance Portability and Accountability Act
- ICC
Intraclass correlation coefficient
- logBSC
log-transformed backscatter coefficient
- LOA
Limit of agreement
- MRI
Magnetic resonance imaging
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- PDFF
Proton density fat fraction
- QIB
Quantitative imaging biomarker
- QUS
Quantitative ultrasound
- RF
Radio-frequency
- wCV
within-subject coefficient of variation
Footnotes
Conflict of Interest:
The authors of this manuscript declare relationships with the following companies:
The work is supported in part by research grants from Siemens Healthineers USA and GE Healthcare. The use of the Siemens S3000 scanner was loaned to the University of California San Diego under a research agreement with Siemens Healthineers USA. The use of the GE Logiq E9 scanner was loaned to the University of California San Diego under a research agreement with GE Healthcare.
Guarantor:
The scientific guarantor of this publication is Claude B. Sirlin, MD (University of California at San Diego).
Statistics and Biometry:
One of the authors has significant statistical expertise.
Informed Consent:
Written informed consent was obtained from all subjects (patients) in this study.
Ethical Approval:
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap:
Some study subjects or cohorts have been previously reported in [17].
- prospective
- cross sectional study
- performed at one institution
REFERENCES
- 1.Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nature Reviews Gastroenterology & Hepatology 10: 686–690 [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ (2018) Mechanisms of NAFLD development and therapeutic strategies. Nature Medicine 24:908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado MV, Cortez-Pinto H (2013) Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 58:1007–1019 [DOI] [PubMed] [Google Scholar]
- 4.Park CC, Nguyen P, Hernandez C et al. (2017) Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeder SB, Cruite I, Hamilton G, Sirlin CB (2011) Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Im 34: 729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artz NS, Hines CD, Brunner ST et al. (2012) Quantification of hepatic steatosis with dual-energy computed tomography: comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Invest Radiol 47:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andre MP, Han A, Heba E, et al. (2014) Accurate diagnosis of nonalcoholic fatty liver disease in human participants via quantitative ultrasound. In: 2014 IEEE International Ultrasonics Symposium, pp 2375–2377 [Google Scholar]
- 8.Lin SC, Heba E, Wolfson T et al. (2015) Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol 13:1337–1345.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paige JS, Bernstein GS, Heba E et al. (2017) A pilot comparative study of quantitative ultrasound, conventional ultrasonography, and magnetic resonance imaging for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. Am J Roentgenol 208:W1–W10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oelze ML, Mamou J (2016) Review of quantitative ultrasound: Envelope statistics and backscatter coefficient imaging and contributions to diagnostic ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 63:336–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasso M, Beaugrand M, de Ledinghen V et al. (2010) Controlled Attenuation Parameter (CAP): A novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36:1825–1835 [DOI] [PubMed] [Google Scholar]
- 12.Caussy C, Alquiraish MH, Nguyen P et al. (2018) Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 67:1348–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imbault M, Faccinetto A, Osmanski BF et al. (2017) Robust sound speed estimation for ultrasound-based hepatic steatosis assessment. Physics Med Biol 62:3582–3598. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan DC, Obuchowski NA, Kessler LG et al. (2015) Metrology standards for quantitative imaging biomarkers. Radiology 277:813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han A, Andre MP, Erdman JW, Loomba R, Sirlin CB, O’Brien WD (2017) Repeatability and reproducibility of a clinically based QUS phantom study and methodologies. IEEE Trans Ultrason Ferroelectr Freq Control 64:218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han A, Andre MP, Deiranieh L et al. (2018) Repeatability and reproducibility of ultrasonic attenuation coefficient and backscatter coefficient measured in the right lobe of the liver in adults with known or suspected nonalcoholic fatty liver disease. J Ultrasound Med. 37:1913–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han A, Labyed Y, Sy EZ et al. (2018) Inter-sonographer reproducibility of quantitative ultrasound outcomes and shear wave speed measured in the right lobe of the liver in adults with known or suspected nonalcoholic fatty liver disease. Eur Radiol. 28:4992–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- 19.Yao LX, Zagzebski JA, Madsen EL (1990) Backscatter coefficient measurements using a reference phantom to extract depth-dependent instrumentation factors. Ultrason Imaging 12:58–70 [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG (1986). Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310 [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428 [DOI] [PubMed] [Google Scholar]
- 22.Raunig DL, McShane LM, Pennello G et al. (2015) Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res 24:27–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleiss JL: The Design and Analysis of Clinical Experiments. New York, John Wiley Sons, 1999, pp 1–32 [Google Scholar]
- 24.Trout AT, Serai S, Mahley AD et al. (2016) Liver stiffness measurements with MR elastography: agreement and repeatability across imaging systems, field strengths, and pulse sequences. Radiology 281: 793–804 [DOI] [PubMed] [Google Scholar]
- 25.Nobili V, Vizzutti F, Arena U et al. (2008) Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 48:442–448 [DOI] [PubMed] [Google Scholar]
- 26.Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D (2012) Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography-- preliminary results. Ultrasound Med Biol 38:1103–1108 [DOI] [PubMed] [Google Scholar]
- 27.Guerrero QW, Fan L, Brunke S, Milkowski A, Rosado-Mendez IM, Hall TJ (2018) Power Spectrum Consistency among Systems and Transducers. Ultrasound Med Biol, online. DOI: 10.1016/j.ultrasmedbio.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraioli G, Filice C, Castera L et al. (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol 41:1161–1179 [DOI] [PubMed] [Google Scholar]
- 29.Serai SD, Dillman JR, Trout AT (2017) Proton density fat fraction measurements at 1.5- and 3-T hepatic MR imaging: Same-day agreement among readers and across two imager manufacturers. Radiology 284:244–254 [DOI] [PubMed] [Google Scholar]
- 30.Mashhood A, Railkar R, Yokoo T et al. (2013) Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. J Magn Reson Imaging 37:1359–1370 [DOI] [PubMed] [Google Scholar]
- 31.Kang GH, Cruite I, Shiehmorteza M et al. (2011) Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 34:928–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernando D, Sharma SD, Aliyari M et al. (2017) Multi-site, multi-vendor validation of the accuracy and reproducibility of proton-density fat- fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med 77:1516–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]