Abstract
Epstein–Barr Virus (EBV)-positive neuroendocrine carcinoma (NEC) of the nasopharynx is exceedingly rare, only two cases have been reported in the literature. While EBV infection is strongly associated with nasopharyngeal carcinoma, which is carcinoma with squamous differentiation, the link between EBV and NEC is not well known, and can be diagnostically challenging. In this study, we report the third case of EBV-positive large cell NEC of nasopharynx with neck lymph node metastasis. The patient was treated with combined radiation and chemotherapy and showed complete clinical and radiological response. Similar treatment response has been reported in another patient with high stage EBV-positive large cell NEC, suggesting that EBV status is an important prognostic factor. Recognition of this rare tumor is important for disease management and patient prognosis. We also review the literature about the clinical and pathologic presentation of neuroendocrine tumors of nasopharynx.
Keywords: Epstein–Barr virus, Nasopharynx, Neuroendocrine carcinoma, Nasopharyngeal carcinoma
Introduction
NEC of the head and neck is rare and can be seen in the sinonasal tract, larynx, salivary gland and the ear [1]. Head and neck NEC occurs predominantly in male patients in their sixth to seventh decades of age. Etiology includes heavy smoking for NEC arising from larynx, and human papilloma virus (HPV) for NEC arising from the sinonasal tract, while for NEC arising from other sites of head and neck, the causing factors are largely unknown. The current World Health Organization (WHO) classification of the tumors of the head and neck classifies neuroendocrine tumors into three categories based on cytomorphologic features and mitotic activity: well differentiated NEC, which are tumors with minimal nuclear atypia and < 2 mitoses/10 high power fields (HPF); moderately differentiated NEC, which are tumors with necrosis and/or 2–10 mitoses/10 HPF; and poorly differentiated NEC, which include large cell NEC and small cell NEC, are tumors with more than 10 mitoses/ 10 HPF. Poorly differentiated NECs of head and neck are highly aggressive malignancies that are associated with high rates of distal and regional metastasis, with 5-year survival rates of less than 20% according to some study [1].
Neuroendocrine tumor (NEC) of the nasopharynx is very rare. To the best of our knowledge, eleven cases have been reported in the literature, including two cases of well-differentiated NEC, four cases of large cell NEC, three cases of small cell NEC, and two cases with histologic type unspecified (Table 1). It occurred predominantly in male patients, with male to female ratio of 9:2. Patients’ age ranges from 9 to 74 years, with majority of patients in their 3rd and 4th decades of age [2–12].
Table 1.
Reported cases of nasopharyngeal neuroendocrine carcinoma
| Author | Histological type | Age | Gender | Follow-up | EBV status | |
|---|---|---|---|---|---|---|
| Case 1 | Weinreb [2] | Well diff NEC | 34 | Male | AWD 7 years | Unknown |
| Case 2 | Lee [3] | Small cell NEC | 41 | Male | AOD 9 months | Negative |
| Case 3 | Lin [4] | Small cell NEC | 43 | Male | DOD 38 months | Unknown |
| Case 4 | Vandist [5] | Well diff NEC | 48 | Male | Unknown | Unknown |
| Case 5 | Deviprasad [6] | Small cell NEC | 40 | Male | Unknown | Unknown |
| Case 6 | Elloumi [7] | Large cell NEC | 35 | Male | DOD 19 months | Unknown |
| Case 7 | Wasserman [8] | Large cell NEC | 40 | Male | AOD 3 years | Positive |
| Case 8 | Sturgis [9] | Large cell NEC | 38 | Male | Unknown | Positive |
| Case 9 | Dumars [10] | Large cell NEC | 9 | Female | DOD 9 months | Unknown |
| Case 10 | Guo [11] | Poorly differentiated NEC, not specified | 52 | Male | DOD 10 months | Unknown |
| Case 11 | Mohebbi [12] | NEC, not specified | 74 | Female | Unknown | Unknown |
| Case 12 | This study | Large cell NEC | 69 | Male | Unknown | Positive |
AWD alive with disease, AOD alive without disease, DOD died of disease
EBV is strongly associated with malignancies arising from nasopharynx, i.e., nasopharyngeal carcinoma, which is the most common epithelial malignancy of the nasopharynx, and NK/T-cell lymphoma [1]. EBV-positive nasopharyngeal carcinoma and NK/T-cell lymphoma show significantly improved response to treatment and better patient survival compared with EBV-negative malignancies [13]. Although rare, it seems that nasopharyngeal large cell NEC is another malignancy that is associated with EBV. Including the case in this study, at least three of five cases of large cell NEC of nasopharynx are positive for EBV, with unknown EBV status in the other two cases [7–10]. As already reported in the literature and also observed in the patient in this study, complete clinical and radiological response to combined radiotherapy and chemotherapy was seen in two of these three cases. One of these patients was reported to be disease free after three years of diagnosis [8]. The EBV status could also be an important prognostic indicator in nasopharyngeal large cell NEC.
Case Report
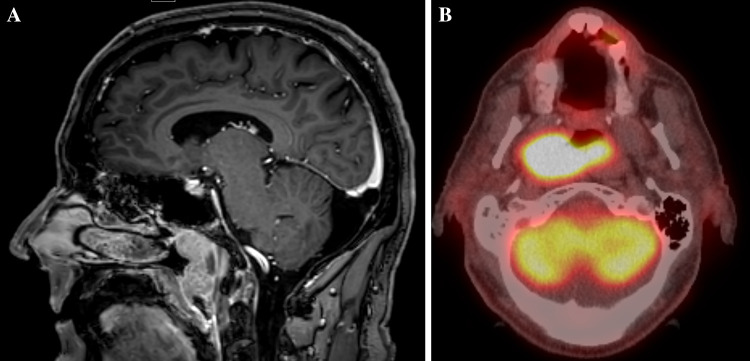
A 69-year-old man with past medical history of hypertension presented with progressive difficulty with breathing and multiple episodes of nose bleeding for 6 months. Pertinent history also included working as a farmer with occupational chemicals and crop dusters exposure. He was a former smoker with less than one pack of cigarette per day, but quitted 25 years ago. Computed tomography (CT) scan of the head and neck showed a large 5.5 × 4.5 × 2.4 cm mass arising from the right nasopharynx, and crossing the midline with left nasopharyngeal involvement. Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) scan showed a large FDG avid nasopharyngeal mass and two FDG avid lymph nodes at level IIA and IIB in the right neck that were suspicious for metastatic carcinoma (Fig. 1).
Fig. 1.
a CT showed a large nasopharyngeal mass arising from the right nasopharynx, crossing the midline and involving the left nasopharynx. b PET CT highlighted the large PDF avid nasopharyngeal mass
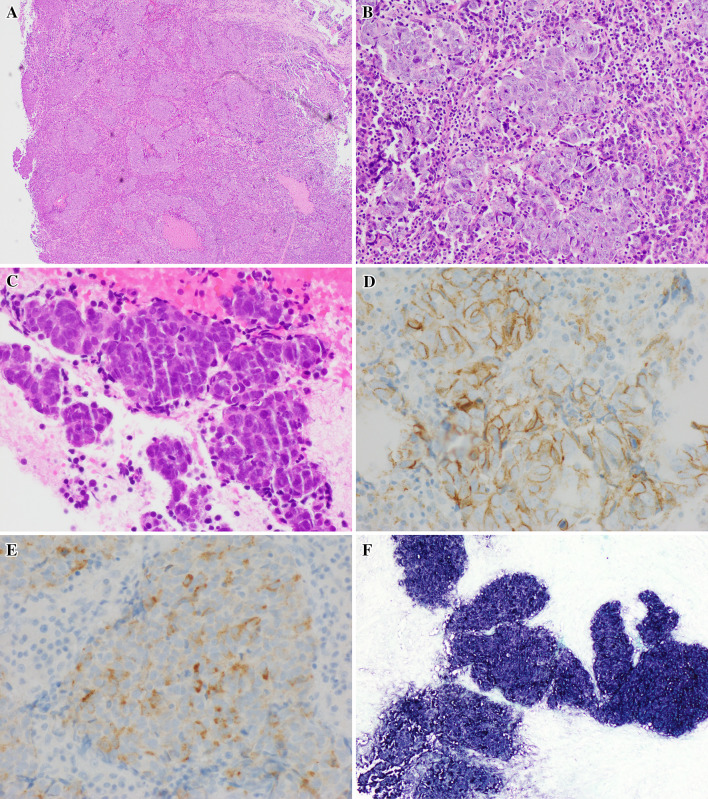
The patient underwent endoscopic excisional biopsy of the mass with the use of microdebrider and cutting instruments. Multiple fragments of the tumor were received and entirely submitted for microscopic examination. The neoplastic cells were large in size and showed predominantly nested growth pattern, with moderate amount of eosinophilic cytoplasm, and nuclei with significant pleomorphism, irregular nuclear borders, coarse chromatin and single prominent nucleoli. Areas of tumor showed more obvious neuroendocrine morphology with focal rosette formation, fine chromatin and inconspicuous nucleoli. Brisk mitotic and apoptotic figures were seen, with mitotic count of 21 mitoses/ 10 HPF. Focal necrosis was also present. The tumor cells were surrounded by abundant lymphocytes and plasma cells (Fig. 2).
Fig. 2.
a Tumor cells show nested growth pattern (a H&E ×40 and b H&E ×100), with moderate amount of cytoplasm and prominent nucleoli. c Focal rosette formation is seen. Subset of the tumor cells show fine chromatin and inconspicuous nucleoli (H&E ×200). Tumor cells are positive for CD56 (d, ×400), and synaptophysin (e, ×400), f In-situ hybridization for EBER (×100)
Ancillary immunohistochemical (IHC) studied were performed, including pancytokeratins AE1/3 and CAM5.2, synaptophysin, chromogranin, CD56, p40, p63, CK7 and CK5/6. The tumor cells were diffusely positive for AE1/3 and CAM5.2. Squamous cell markers p40, p63 and CK5/6 were nonreactive. In addition, the neoplastic cells were shown to be reactive to CD56 (membranous stain) and synaptophysin (cytoplasmic stain in about 30% of tumor cells). In-situ hybridization for EBV early RNAs (EBERs) was positive (Fig. 2). The morphologic and ancillary findings support a diagnosis of EBV-positive large cell NEC.
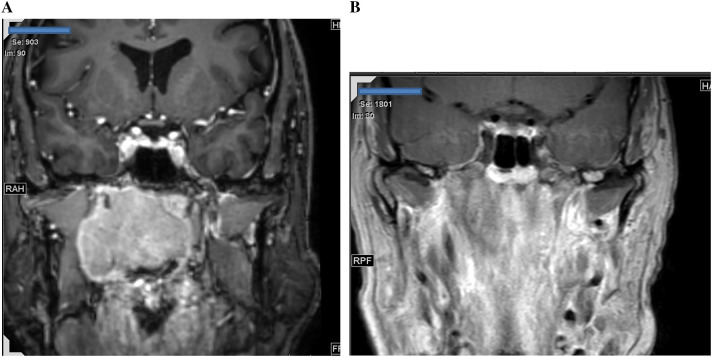
The patient received combined radiotherapy and Cisplatin chemotherapy. He tolerated the treatment well with excellent response to treatment. Magnetic resonance imaging (MRI) scan performed 3 months after initiation of chemoradiation showed complete response with resolution of the nasopharyngeal mass and neck lymph nodes (Fig. 3).
Fig. 3.
Magnetic resonance imaging (MRI) of the nasopharyngeal mass. a Pre-chemoradiation and b post-chemoradiation. Complete radiological response is seen in b with resolution of the large mass shown in a
Discussion
Carcinomas arising from nasopharynx are rare, including NPC, carcinomas of salivary gland origin, and nasopharyngeal papillary adenocarcinoma. Out of these, NPC is the most common carcinoma [1]. NPC is further classified into keratinizing, nonkeratinizing differentiated, nonkeratinizing undifferentiated, and basaloid subtypes. Nonkeratinizing differentiated and undifferentiated NPC are strongly associated with EBV, while basaloid and keratinizing NPC are generally not EBV-related in non-endemic regions. However, in endemic areas like Southern China and Hong Kong, EBV is associated with all subtypes of NPC. By definition, NPC is squamous cell carcinoma arising from nasopharynx, and stains strongly for squamous markers such as p40, p63, and CK5/6.
In this study, we present a case of high grade carcinoma arising from nasopharynx. The carcinoma showed nested growth pattern with high mitotic activity (> 20/10 HPF) and focal necrosis in a background of abundant lymphocytes and plasma cells. The tumor cells are large pleomorphic cells with moderate amount of eosinophilic cytoplasm and prominent nucleoli. Immunohistochemical stain showed that the tumor cells are negative for squamous differentiation by immunohistochemistry, including p40, p63, and CK5/6. These results argue against a diagnosis of NPC, nonkeratinizing undifferentiated type, which is strongly positive for squamous markers. Another differential diagnosis for this case is sinonasal undifferentiated carcinoma (SNUC). SNUC is a poorly differentiated carcinoma of the sinonasal tract that can extend to nasopharynx. By definition, SNUC is an undifferentiated carcinoma without squamous or glandular differentiation and not otherwise classifiable. Tumor cells of SNUC are positive for pancytokeratins including AE1/3, CAM5.2, and simple keratins such as CK7, CK8, and CK18. Neuroendocrine markers can be very focally present. However, SNUC is not associated with virus infection. If EBV or HPV is detected, the diagnosis of SNUC needs to be reconsidered. For the case in this study, imaging studies and intraoperative finding showed that the mass arose from the nasopharynx with focal maxillary sinus involvement. In addition, the tumor cells are positive for neuroendocrine markers including CD56 and synaptophysin in a diffuse pattern rather than patchy focal positivity seen in SNUC. More importantly, positivity for EBER essentially ruled out SNUC. Based on the nested growth pattern, cytomorphologic features, high mitotic count, and immunohistochemical results, the diagnosis of large cell NEC was made [14].
Nasopharyngeal NEC is very rare and not discussed in the current WHO classification of head and neck tumors. A literature search identified only 11 cases (Table 1), including two well differentiated NEC, eight poorly differentiated NEC, and one of unknown differentiation (not specified). Only three cases were tested for EBER. One case of small cell NEC was negative for EBER, while both cases of large cell NEC were positive [3, 8, 9]. Including the case in this study, all three large cell NEC arising from nasopharynx tested for EBER are all positive. The status of EBV in the other two cases is unknown. Thus it seems reasonable to conclude that EBV is associated with at least subset of nasopharyngeal large cell NEC (at least three of five cases).
The patient in this study showed excellent response to chemoradiotherapy. Three months after initiation of chemoradiotherapy, MRI showed essentially complete response with resolution of the large nasopharyngeal mass and neck lymph nodes. No residual mass or lymphadenopathy was seen (Fig. 3). Similar complete clinical and radiological response to chemoradiotherapy was also noted in the other case of EBV-positive LCNEC, and the patient was disease free after three years of diagnosis [8]. These observations suggest that EBV-positive LCNEC is sensitive to chemoradiation and may have better prognosis than EBV-negative LCNEC.
EBV infection is associated with a range of hematopoietic, epithelial, and soft tissue malignancies. Hematopoietic malignancies include Burkitt’s lymphoma, Hodgkin’s lymphoma, NK/T-cell lymphoma, post-transplant lymphoproliferative disorder (PTLD), and rare subtypes of diffuse large B-cell lymphoma (DLBCL). Epithelial malignancies include nasopharyngeal carcinoma, gastric adenocarcinoma, cholangiocarcinoma, and lymphoepithelial carcinoma of different organs. EBV-associated leiomyosarcoma is an uncommon smooth muscle tumor seen in immunocompromised patients. Possible mechanisms for EBV in tumorigenesis include multiple viral proteins and RNAs that have oncogenic properties, as well as chronic inflammation, host cell factors and genetic background that may contribute to oncogenesis [15]. Clinically, EBV-positive PTLD, NK/T-cell lymphoma, nasopharyngeal carcinoma, gastric adenocarcinoma and leiomyosarcoma are associated with a better prognosis than EBV negative counterparts. On the other hand, patients with EBV-positive Hodgkin’s lymphoma and DLBCL tend to have a poorer prognosis than EBV-negative patients. Treatment approaches remain similar for EBV-positive and EBV-negative malignancies [15]. However, clinical trials including immunotherapy and pathway specific therapy targeting EBV-associated malignancies are available and could provide potentially effective treatment. In addition, EBV vaccination seems promising in preventing EBV-infection in some trials and could potentially be effective in preventing EBV-related malignancies [13].
In summary, NEC of the nasopharynx is very rare, with only eleven cases reported in the literature and majority were poorly differentiated NEC. EBV seems to be associated with large cell NEC of the nasopharynx. Excellent response to chemoradiotherapy has been reported in two cases of EBV-associated large cell NEC. Proper recognition of this rare entity is important for patient to get proper treatment.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any conflicts of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.EL-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg P. WHO classification of head and neck tumours. 4. Lyon: IAPR Press; 2017. [Google Scholar]
- 2.Weinreb I, Perez-Ordoñez B. Non-small cell neuroendocrine carcinoma of the sinonasal tract and nasopharynx. Report of 2 cases and review of the literature. Head Neck Pathol. 2007;1(1):21–26. doi: 10.1007/s12105-007-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LY, Chang KP, Hsu CL, Chen TC, Kuo TT. Small-cell neuroendocrine carcinoma of the nasopharynx: report of a rare case lacking association with Epstein-Barr virus. Int J Surg Pathol. 2011;19(2):199–202. doi: 10.1177/1066896908316904. [DOI] [PubMed] [Google Scholar]
- 4.Lin IH, Hwang CF, Huang HY, Chien CY. Small cell carcinoma of the nasopharynx. Acta Otolaryngol. 2007;127(2):206–208. doi: 10.1080/00016480500401027. [DOI] [PubMed] [Google Scholar]
- 5.Vandist V, Deridder F, Waelput W, Parizel PM, Van de Heyning P, Van Laer C. A neuroendocrine tumour of the sphenoid sinus and nasopharynx: a case report. B ENT. 2010;6(2):147–151. [PubMed] [Google Scholar]
- 6.Deviprasad S, Rajeshwari A, Tahir M, Adarsha TV, Gangadhara S. Small-cell neuroendocrine carcinoma originating from the lateral nasopharyngeal wall. Ear Nose Throat J. 2008;87(11):E1–E3. [PubMed] [Google Scholar]
- 7.Elloumi F, Fourati N, Siala W, Ghorbell L, Jlidi R, Ghorbel A, Frikha M, Daoud J. Large cell neuroendocrine carcinoma of the nasopharynx: a case report. Cancer Radiother. 2014;18(3):208–210. doi: 10.1016/j.canrad.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman JK, Papp S, Hope AJ, Perez-Ordóñez B. Epstein-Barr virus-positive large cell neuroendocrine carcinoma of the nasopharynx: report of a case with complete clinical and radiological response after combined chemoradiotherapy. Head Neck Pathol. 2018 doi: 10.1007/s12105-017-0883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturgis CD, Burkey BB, Momin S, Hoschar AP. High grade (large cell) neuroendocrine carcinoma of the nasopharynx: novel case report with touch preparation cytology and positive EBV encoded early RNA. Case Rep Pathol. 2015;2015:231070. doi: 10.1155/2015/231070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumars C, Thebaud E, Joubert M, Renaudin K, Cariou-Patron G, Heymann MF. Large cell neuroendocrine carcinoma of the nasopharynx: a pediatric case. J Pediatr Hematol Oncol. 2015;37(6):474–476. doi: 10.1097/MPH.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 11.Guo C, Pan Q, Su M, Li R. Clinical immunophenotype of nasopharyngeal neuroendocrine carcinoma with metastatic liver cancer. Clin Chim Acta. 2017;471:283–285. doi: 10.1016/j.cca.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Mohebbi AR, Daneshi A, Emami AR. Nasopharyngeal neuroendocrine carcinoma: a case report. Ear Nose Throat J. 2008;87(12):E14. [PubMed] [Google Scholar]
- 13.Ozoya OO, Sokol L, Dalia S. EBV-related malignancies, outcomes and novel prevention strategies. Infect Disord Drug Targets. 2016;16(1):4–21. doi: 10.2174/1871526516666160407113536. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JS, Jr, Spence DC, Chiosea S, Barnes EL, Jr, Brandwein-Gensler M, El-Mofty SK. Large cell neuroendocrine carcinoma of the larynx: definition of an entity. Head Neck Pathol. 2010;4(3):198–207. doi: 10.1007/s12105-010-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooper LM, Bishop JA, Westra WH. INSM1 is a sensitive and specific marker of neuroendocrine differentiation in head and neck tumors. Am J Surg Pathol. 2018;42(5):665–671. doi: 10.1097/PAS.0000000000001037. [DOI] [PubMed] [Google Scholar]