Abstract
Formation of the vasculature is an essential developmental process, delivering oxygen and nutrients to support cellular processes needed for tissue growth and maturation. Retinoic acid (RA) and its downstream signaling pathway is vital for normal pre- and post-natal development, playing key roles in the specification and formation of many organs and tissues. Here we review the role of RA in blood and lymph vascular development, beginning with embryonic yolk sac vasculogenesis and remodeling and discussing RA’s organ-specific roles in angiogenesis and vessel maturation. In particular, we highlight the multi-faceted role of RA signaling in CNS vascular development and acquisition of blood-brain barrier properties.
Introduction
From the initial specification of the vascular endothelium to formation and refinement of the vascular plexus via vasculo- and angiogenesis, development of a stable vascular system is essential to the forming embryo. As organs and tissues emerge in the embryo, development of the vasculature, which includes both blood and lymph vessels, is driven by signals from the surrounding cells and tissues. For example, in the central nervous system (CNS) neural-derived signals stimulate the formation of a high density vascular plexus and development of a blood-brain barrier (BBB) (R Daneman et al., 2009; Haigh et al., 2003; Stenman et al., 2008). Many of the key factors that orchestrate embryonic vascular development such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), Wnt-β-catenin and Notch, have been identified and their elucidation has facilitated therapies for several diseases, most notably anti-tumor angiogenesis therapies (reviewed in (Adams & Alitalo, 2007; Caporarello et al., 2017; Giacomini et al., 2016; Reis & Liebner, 2013).
Retinoic acid (RA), a lipid soluble hormone derived from Vitamin A, has numerous well documented functions in embryonic development. RA’s intracellular signaling is mediated through binding to nuclear hormone receptors retinoic acid receptors (RARα, RARβ, RARγ) and retinoid X receptors (RXRα, RXRβ, RXRγ). The all-trans-RA isomer has the highest affinity for RARs whereas RXRs binds with high affinity to the 9-cis-RA isomer (Heyman et al., 1992). All-trans-RA, but not 9-cis-RA, is detected in the mouse embryo (Mic, Molotkov, Benbrook, & Duester, 2003), thus all-trans-RA is likely the main ligand activating RA receptor signaling during development. That said, small, local amounts of 9-cis RA may be present in the embryo but not detectable with current methods (Kane, 2012). RAR homodimers and RAR/RXR heterodimers bind to DNA at specific sequences and act as transcriptional activators upon RA binding to the receptor. RARs, in particular RARα, have RA-activated functions outside the nucleus separate from their function as transcriptional activators (reviewed in (Al Tanoury, Piskunov, & Rochette-Egly, 2013). The essential role of RA in development was first recognized by investigations of Vitamin A deficiency and have been confirmed by genetic manipulation of RA production and RA receptors in mice; for reviews see (Cunningham & Duester, 2015; Mark, Ghyselinck, & Chambon, 2009). This review will focus on the role of RA in vascular development, discussing RA in early blood and lymph vessel formation, highlighting RA’s role in brain vasculature development and outlining RA roles in the formation of other organ specific vasculatures such as heart, lung and the eye.
Retinoic acid signaling in vasculogenesis
Genetic mouse mutants of the enzymes that synthesize RA have been critical for revealing its functions in embryonic development, including early roles in vascular development. Vitamin A metabolite retinol is oxidized to form retinal by retinol dehydrogenases (Rdh) and retinal is further oxidized to form retinoic acid via retinal dehydrogenases (Raldh). There are three Raldh proteins expressed in the embryo (Raldh1, 2 and 3) with Raldh2 being the most widespread (Niederreither, Fraulob, Garnier, Chambon, & Dollé, 2002). Global mouse mutants of Raldh2 (Raldh2−/−) have embryo-wide deficiency in RA synthesis and die in utero at E10.5 with substantial defects in heart (Niederreither et al., 2001), CNS, craniofacial (Ribes, Wang, Dolle, & Niederreither, 2006), and limb development (Mic, Sirbu, & Duester, 2004).
Studies of Raldh2−/− mutant embryos revealed a role for retinoic acid in vasculogenesis in the yolk sac and the embryo proper (Lai, Bohnsack, Niederreither, & Hirschi, 2003). Vasculogenesis is the de novo formation of blood vessels via the fusion of individual endothelial cells. Soon after the formation of the germ layers (endo-, meso- and ectoderm) at embryonic day (E)6.5 in mouse, blood cell and endothelial precursors (angioblasts) are specified and migrate from the mesoderm to form blood islands in the extraembryonic yolk sac by E7.5. A primitive yolk sac vascular plexus emerges at E8.5 as angioblasts proliferate and differentiate into endothelial cells and fuse to form small, similar sized vessels arranged in a pattern reminiscent of a honeycomb. By E9.5, this primitive yolk sac plexus has undergone extensive remodeling, including vessel fusion and pruning, and sprouting of new vessels from existing vessels. The result is a well-organized, hierarchically branched yolk sac vasculature (reviewed in (Garcia & Larina, 2014). Examination of E8.5 Raldh2−/− embryos showed the presence of a primitive yolk sac vasculature however the vasculature failed to remodel by E9.5, including impaired recruitment of vascular smooth muscle cell to the large vessels of the yolk sac (Lai et al., 2003). The intraembryonic vasculature also showed defects in Raldh2−/− mutants; plexuses in the head and trunk regions appeared mis-patterned and the vessels were dilated. Further analysis showed that yolk sac and intraembryonic endothelial cell proliferation was elevated in the absence of RA synthesis and that RA treatment of cultured endothelial cells suppressed cell proliferation and elevated expression of cyclin-dependent kinase inhibitors p21 and p27 (Lai et al., 2003). Impairing RA synthesis through deletion of Raldh2 causes altered vasculogenesis but RA is not required for the initial specification of angioblasts from the mesoderm.
Early lethality of Raldh2−/− mutant mice can be overcome by maternal supplementation of RA in the diet and controlling the duration of RA exposure can often separate complex phenotypes in these mutants. Using this approach, investigators provided data that RA may control yolk sac vascular remodeling and yolk sac endothelial cell proliferation through separate mechanisms (Bohnsack, Lai, Dolle, & Hirschi, 2004). They showed RA synthesized by the yolk sac visceral endoderm stimulates transforming growth factor-β1 (TGFβ1) expression by the adjacent yolk sac mesoderm. TGFβ1 ligand and its downstream signaling is required for yolk sac vascular development (Dickson et al., 1995), in part via regulation of extracellular matrix protein fibronectin (Goumans et al., 1999). Fibronectin promotes cell survival signaling in the visceral endoderm, a major source of proteins required for yolk sac vascular development including VEGFA, indian hedgehog (Ihh) and fibroblast growth factor (FGF). Raldh2−/− mutants displayed significant visceral endoderm cell death and ex vivo treatment with visceral endotherm-derived factors VEGFA, Ihh and FGF rescued vascular remodeling in ex vivo Raldh2−/− but not endothelial cell proliferation (Bohnsack et al., 2004). Fibronectin, downstream of RA-TGFβ1, appears to regulate yolk sac endothelial cell proliferation via integrin receptors α5 (fibronectin receptor) and αv (Bohnsack et al., 2004). It is not clear, however, if endothelial integrin α5 and αv transduces this signal as endothelial conditional deletion of αv and α5 does not result in yolk sac vascular defects (van der Flier et al., 2010). Further, this study focused on the yolk sac vasculature and did not determine if a similar downstream mechanism (TGFβ1-fibronectin) was responsible for intraembryonic vascular mis-patterning and elevated endothelial cell proliferation in Raldh2−/− mutants. TGFβ1 is well known to inhibit endothelial cell proliferation (Jakobsson & van Meeteren, 2013) and elevated endothelial cell proliferation is seen in the development CNS vasculature of mutants with endothelial conditional deletion of TGFβr2 (Arnold et al., 2014). Yet, studies of other developmental defects in Raldh2−/− mutants show RA is an inhibitor in of TGFβ signaling, like primary lung bud induction (F. Chen et al., 2007). Thus, RA plays an important role in earliest stages of embryonic vascular development but more studies are needed to fully elucidate underlying mechanisms.
Overabundance of RA in the early embryo also appears to be disruptive to extra- and intraembryonic vascular development. P450 cytochromes Cyp26a1 and Cyp26b1 metabolize retinoic acid in the developing embryo, often in select areas, where they degrade RA to tightly control RA levels and locally regulate signaling (Ross & Zolfaghari, 2011). Cyp26a1−/− mouse embryos are much more sensitive to exposure to low doses of RA in maternal diet, causing increased RA signaling as compared to wildtype embryos exposed to the same dose. Cyp261a−/− embryos exposed to low doses of retinoic acid form a primitive yolk sac vasculature however it fails to remodel by E9.5 (Ribes, Fraulob, Petkovich, & Dollé, 2007). Further, the intraembryonic vascular plexus appeared poorly developed. Deletion of cytochrome P450 reductase (Cpr or Por), an essential electron donor to cytochrome P450 proteins like Cy26a1 and Cyp26b1, causes a severe early intraembryonic and yolk sac remodeling defect which can be rescued by decreasing RA synthesis (Otto et al., 2003; Ribes, Otto, et al., 2007). These phenotypes are strikingly similar to that observed in Raldh2−/− embryos, demonstrating that lack or excess of RA is detrimental to early embryonic vascular development. Currently, no studies have yet identified how excessive RA embryonic levels might impair yolk sac remodeling and intraembryonic vascular development.
Retinoic acid in development of the embryonic lymphatic vasculature
The lymphatic vasculature facilitates the drainage of interstitial fluids to maintain fluid homeostasis and acts as a passage way for certain immune cell populations, though the lymphatic vasculature has other important organ and tissue specific functions (reviewed in (Petrova & Koh, 2018). Lymphatic vessels are made up of lymphatic endothelial cells (LECs) and differ greatly from the blood vasculature, lacking perivascular cells like pericytes or vascular smooth muscle cells (vSMCs), are surrounded by an intermittent basement membrane and have specialized, discontinuous cell junctions that allow fluid and immune cells to enter the vessel lumen. Lymphatic vascular development in the embryo first begins with the appearance of LEC progenitor cells in the cardinal and intersomitic veins at ~E9.5, identified by their upregulation of LEC markers Prox1 and LYVE-1, which subsequently dissociate from the veins, migrate away and coalesce to form the early lymphatic vascular plexuses and lymph sacs. These further grow via lymphangiogenesis in which new lymphatic vessels grow from the existing plexus (reviewed in (Ulvmar & Mäkinen, 2016).
Several studies point to an important role for RA in embryonic lymphatic vascular development. A study mapping the different components of retinoic acid synthesis, signaling and metabolism near the cardinal vein, the site of initial LEC specification, showed that LEC progenitors in the dorsal-lateral cardinal vein express Cyp26b1 (Fig. 1A), a RA degrading enzyme whereas Raldh2 was expressed ventrally, opposite to LEC progenitors (Bowles et al., 2014). This suggested that RA levels may need to be low near the LEC progenitors during their initial development. In support of this, retinoic acid signaling, as measured by the reporter mouse line RARE-hsp68-LacZ, was polarized in the cardinal vein with much lower signaling activity associated with LEC progenitors (Fig. 1A). Analysis of Cyb26b1−/− mutants with elevated RA levels revealed substantial expansion of Prox1 and LYVE-1 expressing LEC progenitors beyond their normal region within the cardinal vein (Fig. 1A). Embryonic lymphatic vascular development was also severely disrupted, evidenced by decreased branching and increased vessel diameter, as was development of the lymph sacs that are made up of LECs. In line with studies of the Cyb26b1−/− mutants, exogenous retinoic acid is sufficient to increase LEC progenitors in the cardinal vein of exposed mouse embryos (Marino, Dabouras, Brändli, & Detmar, 2011). However, pharmacological inhibition of RARα, the RA receptor expressed by LECs, did not significantly impair LEC progenitor specification or numbers in the cardinal vein (Marino et al., 2011). From these studies emerges a model in which RA levels must be kept low near LEC progenitors to prevent inappropriate expansion but RA signaling in LEC progenitors may be dispensable for LEC progenitor specification in the cardinal vein. More studies are needed to test the latter idea, for example conditional disruption of RA signaling during LEC specification.
Figure 1. RA regulation of lymphatic vascular development.

(A) Schematic based on data presented in Bowles et al., 2014 of the cardinal vein at E11.5 in mouse depicting the location of specifying lymphatic endothelial cell (LEC) progenitors (red line) in relation to expression of RA degrading enzyme Cyp26b1 (high near LECs) and RA signaling as measured by RARE-hsp26-lacZ mouse reporter (low near LECs). Global Cy26b1−/− mutants have ectopic LECs in the cardinal vein, providing support for a model that RA levels are suppressed by Cyb26b1 to prevent inappropriate expansion of LECs in the cardinal vein. DA=dorsal aorta.
(B) Based on culture studies using human LECs, RA may promote lymphatic vascular development (proliferation, tube formation) in the developing embryo via upregulation of FGF receptors and, as a result, FGF signaling in LECs that is required for lymphangiogenesis.
Culture studies indicate that the expanded numbers of LEC progenitors in the Cyb26b1−/− mutants and RA exposed embryos are potentially a result of excess RA acting directly on LECs (Fig. 1B). In embryoid body vascular differentiation assays, RA is sufficient to promote expression of LEC markers like Prox1 and LYVE-1 (Marino et al., 2011). Further, RA treatment of primary human LECs increases proliferation and promotes tube formation (Choi et al., 2012). Culture studies have provided some insight into pathways downstream of RA signaling in LECs. RA treatment of primary human LECs transiently increased expression of fibroblast growth factor receptors (FGFR)-3 and FGFR4 and signaling through these receptors mediated RA’s pro-proliferative effect on LECs (Choi et al., 2012). FGF signaling, along with VEGFC-VEGFR3, is a potent stimulator of LEC proliferation in culture and is required for lymph vascular development (Yu et al., 2017). Studies are needed in the developing embryo to determine if RA regulation of FGFR expression and downstream signaling underlies the expansion of LEC progenitors observed with excess RA levels.
RA may also have a role slightly later in embryonic lymphatic vascular development with formation of the lymphatic sacs. Following specification in the cardinal vein, LECs migrate as streams away from the cardinal vein to form the jugular lymphatic sacs from which lymphatic vessels will sprout via lymph-angiogenesis. Raldh2−/− mutants and mouse embryos treated in the pan-RAR inhibitor BMS493 had impaired development of the jugular lymphatic sacs (Burger et al., 2014). Further, Prox1 and LYVE-1 cells were present but the sacs were poorly formed and the embryos displayed edema indicative of impaired lymphatic vascular development. A similar, though slightly less severe, lymphatic sac phenotype was observed in mouse embryos over-expressing Cyb26b1 as a mechanism to lower RA levels (Bowles et al., 2014).
Retinoic acid signaling in regulation of angiogenesis
Angiogenesis is the formation of new vessels from existing vasculature and is an essential process in the growth of the embryonic blood vasculature. The formation of a new blood vessel via angiogenesis is a multi-step process that begins with the degradation of the ECM surrounding the vessel and activation of an endothelial cell within an existing vessel to become a tip cell, extension of the sprout via tip cell migration and endothelial proliferation in the ‘stalk’ of the sprout, fusion with another nascent sprout and, finally lumen formation to permit blood flow. Vessel maturation occurs next with the recruitment of perivascular vSMCS and pericytes to the new vessels and re-establishment of the ECM and help ensure vessel stabilization (reviewed in (Potente & Mäkinen, 2017). Several signaling pathways regulate angiogenesis in the developing embryo, perhaps most important of these is the pro-angiogenic ligand VEGFA that binds to and activates signaling via the Vegfr2 receptor that is expressed by endothelial cells. VEGFA signaling via VEGFR2 promotes ECM degradation, tip cell migration, endothelial proliferation and cell survival and is required for developmental angiogenesis in many different parts of the embryo (reviewed in (Olsson, Dimberg, Kreuger, & Claesson-Welsh, 2006)). FGF ligands, in particular the classical ligands FGF1 and FGF2, are equally potent pro-angiogenic ligands and signal via binding to their receptors FGF receptor 1 (FGFr1) and FGFr2 expressed by endothelial cells. Unlike VEGFA, the role of FGF signaling in developmental angiogenesis has conflicting findings (Lee, Schloss, & Swain, 2000; Oladipupo et al., 2014). More recently, however, a role for FGF in developmental angiogenesis has been supported by a report that embryos with endothelial deletion of FGFr1 coupled with global deletion of FGFr3 were found to have defects in blood vessel development in the skin and retina (Yu et al., 2017).
Animal, explant and culture-based studies have been used extensively to explore the role of RA signaling in angiogenesis. RA is often described as an anti-angiogenic factor, first demonstrated with studies using a classic model of angiogenesis, the chorioallantoic membrane of the chick embryo, which showed RA administration inhibited angiogenic growth in this assay (Oikawa et al., 1989). Several studies report RA exposure inhibits tumor angiogenesis in a variety of cancers (Gee et al., 2005; Hoffmann et al., 2007; Kini, Peterson, Tallman, & Lingen, 2001; Lingen, Polverini, & Bouck, 1998; Majewski, Szmurlo, Marczak, Jablonska, & Bollag, 1993). That said, pro-angiogenic properties of RA or RAR-agonists have also been documented (Lansink, Koolwijk, van Hinsbergh, & Kooistra, 1998), often via upregulation of VEGFA (Al Haj Zen et al., 2016; Saito et al., 2007; L. Wang et al., 2014) or FGF ligands (Gaetano et al., 2001). This apparent dual nature of RA with regard to angiogenesis may reflect the cell-type specific nature of RA signaling pathways (ex: differential expression of RA receptors and signaling partners), made more complex in that RA may not act directly on endothelial cells to exert its effects but on adjacent cells or tissues that shape the pro- or anti-angiogenic response to RA.
Retinoic acid in organ-specific vascular development
Vascularization of organs in the embryo is needed to support their growth and maturation. Some organs, like the brain and spinal cord, are vascularized via angiogenic sprouting from a primitive, intraembryonic vascular plexus that forms during vasculogenesis. In other organs like the heart, endothelial cells arise from multiple sources and form via a combination of angiogenic sprouting and vasculogenesis. Growth and maturation of the vasculature is frequently controlled by signals released from cells within an organ, often in response to tissue hypoxia (ex: VEGFA) or to promote specialization of the vasculature (ex: BBB development in the CNS endothelium). RA and its downstream signaling have been implicated in development of the vasculature in several organs, in particular the heart, CNS, lung, and eye.
Coronary vascular development
Development of the coronary vasculature begins with the appearance of a vascular plexus in the epicardium that expands via angiogenesis to eventually cover the dorsal (E14.5 in mouse) and ventral (E15.5 in mouse) surface. Co-coincident with expansion over the surface, the superficial epicardial plexus sprouts into the myocardium, forming a parallel plexus in this layer. The coronary arteries and veins form via vascular remodeling and, following the establishment of blood flow with attachment of the coronary artery to the aorta (~E15.5 in mouse), the coronary plexus undergoes additional growth and remodeling (reviewed in (Sharma, Chang, & Red-Horse, 2017). The coronary vascular plexus has three different origins, the main source is the sinus venosus with endothelial cells derived from the endocardium forming the vasculature in the rest of the heart in a complimentary pattern (H. I. Chen et al., 2014; Red-Horse, Ueno, Weissman, & Krasnow, 2010; Wu et al., 2012). A third, albeit much smaller source, is proepicardium that give rise to endothelial cells that contribute to vasculature throughout the coronary plexus (Katz et al., 2012).
Retinoic acid signaling in the epicardium appears to play a key role in the development of the coronary vasculature. Conditional deletion of retinoid receptor Rxra specifically in the epicardium causes impaired remodeling of the coronary vasculature, specifically abnormal arterial branching (Merki et al., 2005). Raldh2−/− embryos have significant impairment in coronary vascular growth and patterning when permitted to develop further following short term rescue with exogenous RA (Lin et al., 2010). Exposure of embryos to a Raldh2 inhibitor (WIN 18,446) to globally suppress RA synthesis from E9.5-E13.5 resulted in a similar phenotype, diminished growth of the coronary vasculature and reduced branching (S. Wang et al., 2018). This group also studied mouse embryos that lack Dhrs3, an enzyme that reduces retinal to retinol to prevent RA synthesis, to test if excess embryonic RA levels effect coronary vascular development. The coronary vasculature in Dhrs3−/− embryos had impaired growth of the primary plexus in the epicardium and decreased vessel density of the myocardial vasculature. In addition to defects in coronary vascular growth, Dhrs3−/− embryos had impaired recruitment of vascular smooth muscle cells (vSMCs), which are primarily derived from the epicardium, to the coronary vasculature (S. Wang et al., 2018).
The epicardium is a major source of pro-angiogenic signaling molecules required for coronary vascular development, including FGFs which act on the myocardium to stimulate release of pro-angiogenic VEGF ligands and Ang2 via activation of sonic hedgehog signaling (Lavine et al., 2006). FGF2 expression is diminished in the hearts of epicardial-specific Rxra KO embryos and Raldh2−/− embryos (Lin et al., 2010; Merki et al., 2005), suggesting RA may regulate coronary vascular development by promoting epicardially-derived FGF2. Most studies implicating RA in coronary vascular development preceded the discovery of multiple sources of the coronary vasculature. The epicardium derived pro-angiogenic factors are particularly important for sinus venosus sprouting, factors like VEGF-C, whereas myocardium appears to play the major role in promoting angiogenesis from the endocardium (Sharma et al., 2017). With this in mind, it would be interesting to test if RA is involved in coronary vascular development from all three sources or is more limited in its role.
CNS vascular development
The CNS vasculature is highly specialized to support neuronal function, meet high energy demands and provide protection from damaging blood contents. Formation of the vascular plexus and acquisition of CNS specific vascular properties, like the BBB, occur essentially simultaneously with CNS development. Blood vessels grow into the brain and spinal cord via angiogenesis with new vessels sprouting from the perineural vascular vascular plexus (PNVP), a vascular net that forms during vasculogenesis and surrounds the brain and spinal cord. Neural stem cells and young neurons release several signals, mostly notably VEGFA and Wnt ligands, which act on endothelial cells to stimulate angiogenic growth from the PNVP into the neural tissue, establish vessel patterning within the brain and spinal cord and acquisition of BBB properties. VEGF-A is expressed by neural stem and progenitor cell (NSPC) populations and, to a lesser extent, by post-mitotic neurons (Breier, Albrecht, Sterrer, & Risau, 1992; Breier, Clauss, & Risau, 1995; Miquerol, Gertsenstein, Harpal, Rossant, & Nagy, 1999; Ogunshola et al., 2002). Wnt ligands and receptors are uniquely required for CNS vascular development. CNS endothelial cells express Wnt receptor Frizzled6 and show evidence of high Wnt transcriptional activity and high gene expression of Wnt-β-catenin pathway proteins (R. Daneman et al., 2010; Hupe et al., 2017; Liebner et al., 2008; Stenman et al., 2008). Wnt7a and Wnt7b are the key Wnt ligands in CNS vascular development and are expressed, to varying degrees, by NSPCs and neurons (R Daneman et al., 2009; Osumi et al., 1997). Wnt-driven vascularization of the CNS is largely mediated by canonical Wnt signaling via the Lrp5/6 receptors and β-catenin transcriptional activity (Zhou et al., 2014). Transduction of Wnt7a/7b binding to the Frizzled/LRP receptors is achieved through the G-coupled protein receptor, Gpr124 and its co-factor, Reck (C. Cho, Smallwood, & Nathans, 2017; Zhou & Nathans, 2014).
RA is an important regulator of development of the vasculature in the cerebral cortex (cerebrovasculature) upstream of Wnt-β-catenin and VEGFA (Fig. 2A). Foxc1 mutant mice locally lack RA in the cerebral cortex due to absence of the cerebral meninges, a fibroblast rich structure that surrounds brain that is a major producer of RA for this brain region (Siegenthaler et al., 2009). The PNVP is contained within the meninges, intermingling with fibroblasts of the pial layer. The PNVP overlaying the cerebral cortex and the cerebrovasculature do not develop normally in Foxc1 mutants but these vascular phenotypes are rescued with maternal retinoic acid supplementation, pointing to RA as a key regulator of this process (Mishra, Choe, Pleasure, & Siegenthaler, 2016). Rdh10 mutant embryos have globally reduced RA levels due to reduced activity of Rdh10 needed for the first step in RA synthesis and exhibit a similar, though more severe, cerebrovascular phenotype (Bonney et al., 2016). In the PNVP of Foxc1 and Rdh10 mutants, vessels are hyperplastic and endothelial cell proliferation was elevated (Bonney et al., 2016; Mishra et al., 2016). The PNVP phenotype in Rdh10 and Foxc1 mutants correlated with reduced endothelial Wnt-β-catenin signaling in the PNVP; in Rdh10 mutants, vessels within the cerebral cortex also had reduced endothelial Wnt-β-catenin signaling (Bonney et al., 2016; Mishra et al., 2016). Wnt inhibitors Dkk1 and soluble frizzled receptor protein (Sfrp)-1, 2, 4 and 5 were substantially elevated in the meningeal/PNVP of Foxc1 mutants and throughout the cerebral cortex in Rdh10 mutants. From this emerges a model in which RA from the meninges acts locally in the PNVP and within the cerebral cortex to promote endothelial Wnt signaling through repression of Wnt inhibitors (Fig. 2A). In support of this, RA can suppress Dkk1 and Sfrp5 gene expression in cultured neocortical progenitors (Bonney et al., 2016) and maternal RA supplementation suppresses elevated Dkk1 and Sfrp1 in the PNVP/meninges of Foxc1 mutants (Mishra et al., 2016). RA also appears to regulate cerebrovascular development by stimulating expression of NSPC-derived VEGFA. Foxc1 mutants have reduced cerebral cortical VEGFA protein expression and this is rescued with maternal RA supplementation (Mishra et al., 2016). RA-exposed Foxc1 mutants and controls had higher VEGFA expression, indicating RA promotes VEGFA. In support of this, RA treatment robustly increases VEGFA gene expression and protein release by cultured NSPCs (Mishra et al., 2016). In all, this work demonstrates RA functions non-cell autonomously to promote cerebrovascular development, acting on cells within the meninges and the cerebral cortex to create a permissive environment for vascular growth in this brain region (Fig. 2A).
Figure 2. Multi-faceted role for RA in regulation of CNS vascular development.
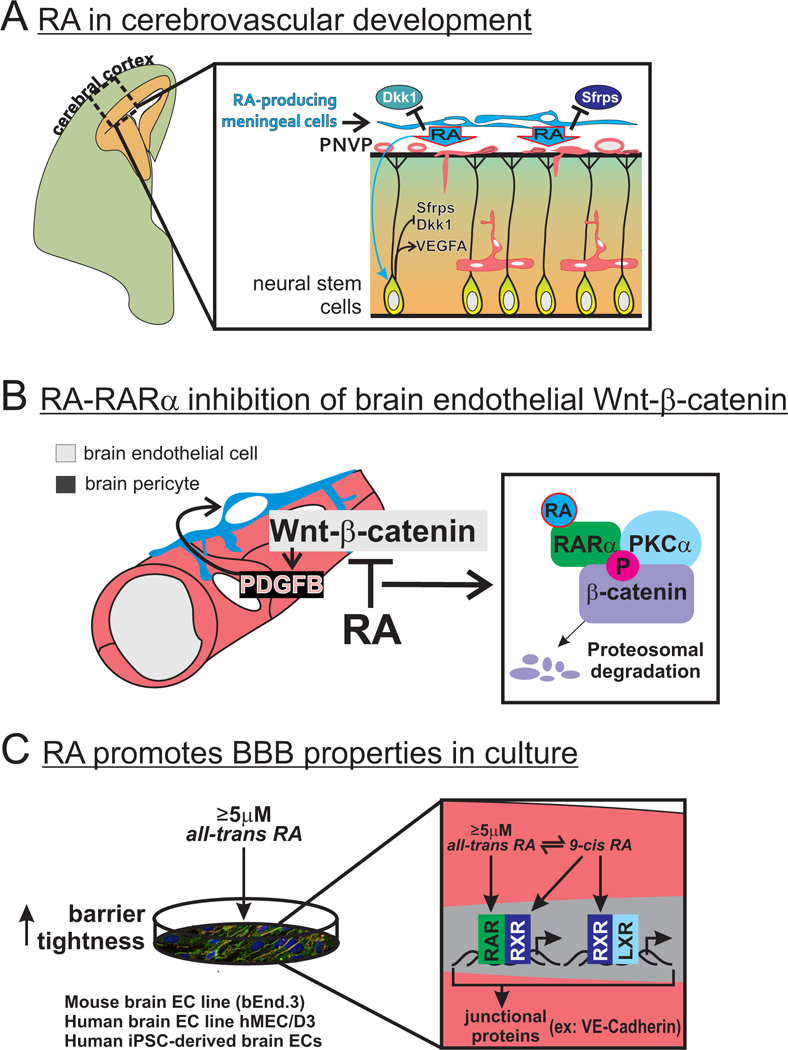
(A) RA from the meninges acts locally in the perineural vascular plexus (PNVP) and on neural stem cells in the cerebral cortex to suppress expression of Wnt inhibitors Sfrps and Dkk1 and promote expression of VEGFA. Collectively, these actions of RA create a permissive environment for endothelial Wnt-β-catenin signaling and VEGFA signaling required for cerebrovascular development.
(B) Cell autonomous RA signaling in brain endothelial cells acts to inhibit Wnt-β-catenin signaling by decreasing β-catenin protein expression, potentially to prevent over-stimulation of PDGFB-PDGFrβ signaling that might impair important cell-cell communication between brain pericytes and endothelial cells needed for brain vascular development. Mechanistically, RA binding to its receptor RARα results in interaction with β-catenin, phosyphorylation of β-catenin by protein kinase C (PKC)-α and increased targeting of β-catenin for degradation.
(C) Supra-physiological concentrations (≥5μM) of all-trans RA can promote barrier properties in a mouse and human brain endothelial cells in culture. Activation of RAR-RXR by all-trans RA and 9-cis RA (isomerized from all-trans RA) and RXR-LXR by 9-cis RA leads to increased barrier tightness via upregulation of tight junctional proteins, most consistently observed is increased expression of adherens junction protein VE-cadherin.
Conditional disruption of RA signaling in endothelial cells has revealed a separate, cell autonomous role for RA, acting to limit Wnt-β-catenin signaling in brain endothelial cells (Fig. 2B). Unlike the cerebrovascular defects observed in Foxc1 and Rdh10 mutants, conditional disruption of RA signaling in endothelial cells did not overtly alter brain vascular growth but instead resulted in hyperplastic vessels and micro-hemorrhages (Bonney et al., 2016). Endothelial Wnt-β-catenin signaling was significant elevated in the brain vasculature of mice with conditional disruption of endothelial RA signaling (Bonney et al., 2016), providing evidence that RA acts cell autonomously in endothelial cells to inhibit Wnt-β-catenin. More recently, animal and cell culture studies provide evidence that RA, through binding to its receptor RARα, inhibits Wnt signaling by targeting β-catenin for degradation and shutting down its signaling (Bonney, Dennison, Wendlandt, & Siegenthaler, 2018). In the absence of Wnt ligand and downstream signaling, β-catenin is phosphorylated on resides Ser33/Ser37 to target it for proteasomal degradation, thus preventing its translocation to the nucleus to activate Wnt-mediated transcriptional activity. RA treatment increases Ser33/Ser37 phosphorylation via protein kinase C-α (PKCα), likely by fostering a complex between RARα, PKCα and β-catenin (Bonney et al., 2018). Possibly, RA suppresses Wnt-β-catenin signaling to regulate endothelial-pericyte interactions in the brain vasculature (Fig. 2B). Pericytes are perivascular cells involved in vascular development through direct contact with the endothelium, regulating vascular stability and help to establish and maintain the BBB (reviewed in (Armulik, Genove, & Betsholtz, 2011). In response to endothelial cell-derived platelet derived growth factor-B (PDGFB), PDGFrβ-expressing pericytes proliferate and migrate to cover newly formed blood vessels (Bjarnegard et al., 2004). Wnt-β-catenin signaling stimulates PDGFB expression in brain vasculature (Bonney et al., 2018; Reis et al., 2012) and over-activation of Wnt-β-catenin or loss of endothelial RA signaling increases brain pericyte numbers (Bonney et al., 2018). Potentially, RA inhibition of Wnt-β-catenin sets the appropriate PDGF-B-PDGFrβ signaling level in the developing brain endothelium, allowing pericytes to form interactions with and secrete factors onto brain endothelial cells required for vessel stability.
RA has emerged as potent inducer of BBB properties in brain endothelial cell culture models. RA treatment in culture promotes BBB ‘tightness’ by increasing expression of adherens and tight junctional proteins that prevent the paracellular movement of molecules between brain endothelial cells (Fig. 2C). Culture studies in murine endothelial cells (Bonney & Siegenthaler, 2017), human brain endothelial cells (Mizee et al., 2013), and induced human induced pluripotent stem cell (iPSC)-derived brain endothelial cells (Katt, Xu, Gerecht, & Searson, 2016; Lippmann, Al-Ahmad, Azarin, Palecek, & Shusta, 2014; Stebbins et al., 2018) demonstrate that treatment with supra-physiological concentrations of all-trans-RA (≥5μm) increases protein expression of tight junctional protein occludin and adherens junction protein VE-cadherin, with the latter the most consistently observed effect. These high concentrations of all-trans-RA may activate multiple RA receptors to stimulate junctional protein expression (Fig. 2C). Mouse endothelial cells cultured in 5μM RA showed elevated liver X receptor (LXR) signaling (Bonney & Siegenthaler, 2017). This is potentially mediated by heterodimerization of LXRs with RXRs that can be activated by high concentrations of all-trans-RA in culture due to isomerization of all-trans to 9-cis-RA (Urbach & Rando, 1994). In human iPSCs-derived endothelial cells, RARα, RARγ or RXRα activation via specific agonists was sufficient to increase barrier tightness and VE-cadherin expression with agonist combinations resulting in more potent effects (Stebbins et al., 2018). In addition to numerous cell culture studies, animal studies have shown that RA treatment can improve BBB integrity following brain ischemia (Kong et al., 2015) and spinal cord injury (Zhou et al., 2016). RA is valuable tool to improve barrier properties in brain endothelial culture models and has the potential to be used therapeutically to ameliorate BBB breakdown in injury and disease.
The role of RA in inducing barrier properties during CNS vascular development is less clear. Concentrations of RA needed to induce barrier properties in culture are substantially higher than what has been detected in the embryo (~25nM in mouse embryos) (Mic et al., 2003). Exposure to comparable concentrations (50nM) in cultured mouse brain endothelial cells suppresses expression of junctional genes (Bonney & Siegenthaler, 2017) and inhibits Wnt-β-catenin signaling, a potent inducer of tight junctional gene expression (Bonney et al., 2018). Further, Rdh10 mutants with globally reduced RA levels and embryonic mice with conditional disruption in endothelial RA signaling do not have overt barrier leakage (Bonney et al., 2018; Bonney & Siegenthaler, 2017), suggesting that RA signaling is not required for BBB development. In contrast, exposure to a pan-RAR inhibitor BMS493 during embryonic mouse development led to BBB leakage in the embryonic brain (Mizee et al., 2013) though it cannot be ruled out that BBB defects were due to inhibition of RA signaling in non-endothelial cell types. Possibly, RA has a CNS region specific role in BBB development that depends on the concentration of RA the vasculature is exposed to. Blood-retinal barrier (BRB) integrity in zebrafish is dependent on RA. Inhibition of RA synthesis or signaling with pharmacological antagonists resulted in BRB leakage and this was rescued with addition of exogenous RA (Pollock, Xie, Bell, & Anand-Apte, 2018). Studies in mice show that concentrations of RA are much higher in the embryonic retina as compared to other CNS regions (McCaffery & Dräger, 1993; McCaffery, Lee, Wagner, Sladek, & Dräger, 1992), setting up the possibility that high levels of RA present in the retina are needed to maintain BRB properties. The vasculature in the hypothalamo-neurohypophyseal system (HNS) of the brain is ‘leaky’, necessary to allow hormones and other blood-borne molecules to enter the brain. Glial-like pituicytes in the HNS that surround the vessels in this area were recently shown in zebrafish to express high levels of RA synthesis enzymes as well as RA degradation enzyme cyp26b (Anbalagan et al., 2018). Cyp26b inhibition in zebrafish led to barrier tightening and increased expression of tight junctional protein cldn5, indicating Cyp26b may degrade RA locally to promote barrier leakiness in the HNS vasculature. Naturally, more work is needed to probe the physiologically role of RA in BBB development.
Lung vascular development
The lungs facilitate gas exchange through alveoli sacs, which are a single layer of epithelium, encapsulated by capillaries (Hsia, Hyde, & Weibel, 2016). Lung vascular development and alveoli formation occur simultaneously and are co-dependent (Hines & Sun, 2014; Woik & Kroll, 2015). For example, if lung epithelial development is disrupted by conditional deletion of β-catenin, the pulmonary vascular also fails to form (Peng et al., 2013). The lung epithelium coordinates vascular formation by secreting factors, in particular VEGFA, to promote endothelial cell growth and vessel branching (Gebb & Shannon, 2000; Healy, Morgenthau, Zhu, Farber, & Cardoso, 2000; Ng, Rohan, Sunday, Demello, & D’Amore, 2001; Woik & Kroll, 2015). Signals from endothelial cells also drive lung epithelial development and alveologenesis. For example, studies where endothelial cell function is impaired via expression of a dominate negative VEGF receptor or deletion of endothelial adhesion molecule PECAM, lung vascular development is blocked and epithelial branching is severely compromised (DeLisser et al., 2006; Lazarus et al., 2011). Endothelial cells are the main source of RA in the developing lung (Yun, Lorizio, Seedorf, Abman, & Vu, 2016) and RA has an important role in lung development. Studies of Vitamin A deficiency and genetic deletion of RA receptors both show impaired alveolar and lung vascular development (Biesalski & Nohr, 2003; Maden & Hind, 2004; McGowan et al., 2000). VEGFA produced by the lung epithelium stimulates endothelial cells to produce RA, and RA acts to drive lung endothelial cell proliferation and tube formation (S. J. Cho, George, Snyder, & Acarregui, 2005; Yun et al., 2016). Evidence suggests RA can regulate lung endothelial cell behavior in both a VEGFA dependent and independent manner (S. J. Cho et al., 2005; Yun et al., 2016). RA production by endothelial cells also facilitates alveoli maturation by stimulating fibroblasts to make elastin and FGF18 (Yun et al., 2016). Thus RA is plays a critical role in both lung vascular and lung alveoli development.
Eye vascular development.
The mammalian retinal vasculature consists of the choroidal vessels, located just outside the retina, and the intra-retinal vessels which are organized in distinct plexi of varying depths called the superficial, intermediate, and deep in mouse (Stahl et al., 2010). The mouse retinal vasculature forms postnatally, where at birth the retinal nourishment is provided by hyaloid and choroidal vessels that form embryonically (Connolly, Hores, Smith, & D’Amore, 1988; Fruttiger, 2007; Selvam, Kumar, & Fruttiger, 2018; Stahl et al., 2010). Retinal vascular formation starts with the superficial plexus, which forms from P0 to P7 as blood vessels expand radially from the optic nerve to the retinal periphery. Starting around P7, the superficial vessels sprout inwards, extending to the deeper layers of the retina, to form the deep and intermediate vascular plexi. By P21 the superficial, intermediate, and deep vascular layers are fully interconnected and mature.
RA is produced by multiple cells of the retina starting early in development, but not by retinal pigment epithelial cells (RPE) or cells of the choroid (Goto et al., 2018; Matt et al., 2005; McCaffery, Tempst, Lara, & Dräger, 1991). RA is essential for proper embryonic eye development as genetic deletion of either the enzymes that make RA or RA receptors lead to severe eye formation defects (Dupé et al., 2003; Fan et al., 2003; Kastner et al., 1994; Kastner et al., 1997; Lohnes et al., 1994; Matt et al., 2005). Surprisingly, the role of RA in postnatal retinal blood vessel formation has not been extensively investigated but it likely has an important function. If RA is delivered systemically to mice in a model of retinopathy of prematurity (ROP), RA treatment can prevent the loss of VEGFA and improves vascular growth (L. Wang et al., 2014). Whole body knockouts of Raldh1, the RA synthesizing enzyme expressed in the postnatal retinal, do not have retinal defects but do show impaired choroidal vascular development (Goto et al., 2018). Analysis of the Raldh1−/− knockout mice shows that RA produced by cells in the retina stimulate RPE cells to produce VEGFA and in the absence of Raldh1, RPE cells make less VEGF (Goto et al., 2018). Development of the choroidal vascular is dependent on VEGFA produced by the RPE cells (Saint-Geniez, Kurihara, Sekiyama, Maldonado, & D’Amore, 2009) and Vitamin A deficient mice also have same choroidal vascular defects (Goto et al., 2018). Thus, these initial studies are consistent with a role for RA in retinal vascular development.
Concluding remarks
Over the last 20 years, RA and its downstream signaling pathways have emerged as vital for the development of blood and lymphatic vasculature, both in the early embryo and later in organ-specific vascular development. Our comprehensive survey reveals unifying themes with regard to RA in embryonic vascular development. First, RA and its downstream signaling pathways often regulate vascular development by controlling the production of pro- and anti-vasculogenic or angiogenic signals. VEGFA is frequent target of regulation by RA, underlying RA’s pro-angiogenic effects on vascular growth in the choroid, cerebral cortex, and lung. Tissue hypoxia and subsequent stabilization of hypoxia inducible factor-1α (HIF1α) is a major stimulant of VEGFA production during embryonic development (Lange et al., 2016; Liu et al., 2009; Stone et al., 1995). RA signaling can stimulate VEGFA expression via transcriptional upregulation of Hif1a (Liang, Guo, & Yang, 2013, 2014; Mishra, Kelly, Rumian, & Siegenthaler, 2018). Possibly, RA acts in parallel to tissue hypoxia in certain organs to promote VEGFA-driven vascular development. Second, the appropriate concentration of RA ligand is crucial for normal vascular development. RA synthesis deficiency and overabundance of RA due to deletion of RA degradation enzymes often show similar vascular phenotypes, as is the case in remodeling of the yolk sac vasculature and coronary vascular development. In lymphatic vascular development, RA levels and signaling in LEC progenitors much be kept in check by expression of RA degrading enzymes.
So far, almost all studies of RA in vascular development have relied on global knockout mutants or pharmacological inhibition of RA synthesis enzymes, degradation enzymes, RA receptors, or nutritional deficiency in Vitamin A. Though important, it is difficult to ascertain from these animal models in which cell type(s) RA signaling is needed to support vascular development. More developmental studies using temporal and cell type specific disruption of RA synthesis, degradation or signaling are needed to create a complete picture of RA and its downstream signaling components in embryonic vascular development.
Acknowledgments
Funding: This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke [R01 NS098273 to J.A.S].
REFERENCES
- Adams RH, & Alitalo K (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol, 8(6), 464–478. doi: 10.1038/nrm2183 [DOI] [PubMed] [Google Scholar]
- Al Haj Zen A., Nawrot DA, Howarth A, Caporali A, Ebner D, Vernet A, … Bhattacharya S (2016). The Retinoid Agonist Tazarotene Promotes Angiogenesis and Wound Healing. Mol Ther, 24(10), 1745–1759. doi: 10.1038/mt.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Tanoury Z., Piskunov A, & Rochette-Egly C (2013). Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res, 54(7), 1761–1775. doi: 10.1194/jlr.R030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbalagan S, Gordon L, Blechman J, Matsuoka RL, Rajamannar P, Wircer E, … Levkowitz G (2018). Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces. Dev Cell, 47(6), 711–726.e715. doi: 10.1016/j.devcel.2018.10.017 [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, & Betsholtz C (2011). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell, 21(2), 193–215. doi: 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, … Reichardt LF (2014). Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development, 141(23), 4489–4499. doi: 10.1242/dev.107193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesalski HK, & Nohr D (2003). Importance of vitamin-A for lung function and development. Molecular Aspects of Medicine, 24(6), 431–440. [DOI] [PubMed] [Google Scholar]
- Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, … Betsholtz C (2004). Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development, 131(8), 1847–1857. doi: 10.1242/dev.01080131/8/1847[pii] [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Lai L, Dolle P, & Hirschi KK (2004). Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev, 18(11), 1345–1358. doi: 10.1101/gad.118490418/11/1345[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney S, Dennison BJC, Wendlandt M, & Siegenthaler JA (2018). Retinoic Acid Regulates Endothelial β-catenin Expression and Pericyte Numbers in the Developing Brain Vasculature. Front Cell Neurosci, 12, 476. doi: 10.3389/fncel.2018.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney S, Harrison-Uy S, Mishra S, MacPherson AM, Choe Y, Li D, … Siegenthaler JA (2016). Diverse Functions of Retinoic Acid in Brain Vascular Development. J Neurosci, 36(29), 7786–7801. doi: 10.1523/JNEUROSCI.3952-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney S, & Siegenthaler JA (2017). Differential Effects of Retinoic Acid Concentrations in Regulating Blood-Brain Barrier Properties. eNeuro, 4(3). doi: 10.1523/ENEURO.0378-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Secker G, Nguyen C, Kazenwadel J, Truong V, Frampton E, … François M (2014). Control of retinoid levels by CYP26B1 is important for lymphatic vascular development in the mouse embryo. Dev Biol, 386(1), 25–33. doi: 10.1016/j.ydbio.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, & Risau W (1992). Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development, 114(2), 521–532. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, & Risau W (1995). Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn, 204(3), 228–239. doi: 10.1002/aja.1002040303 [DOI] [PubMed] [Google Scholar]
- Burger NB, Stuurman KE, Kok E, Konijn T, Schooneman D, Niederreither K, … Bekker MN (2014). Involvement of neurons and retinoic acid in lymphatic development: new insights in increased nuchal translucency. Prenat Diagn, 34(13), 1312–1319. doi: 10.1002/pd.4473 [DOI] [PubMed] [Google Scholar]
- Caporarello N, Lupo G, Olivieri M, Cristaldi M, Cambria MT, Salmeri M, & Anfuso CD (2017). Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions. Mol Med Rep, 16(4), 4393–4402. doi: 10.3892/mmr.2017.7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lü J, & Cardoso WV (2007). Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development, 134(16), 2969–2979. doi: 10.1242/dev.006221 [DOI] [PubMed] [Google Scholar]
- Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivelä R, Saharinen P, … Red-Horse K (2014). The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development, 141(23), 4500–4512. doi: 10.1242/dev.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Smallwood PM, & Nathans J (2017). Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron, 95(5), 1056–1073 e1055. doi: 10.1016/j.neuron.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SJ, George CLS, Snyder JM, & Acarregui MJ (2005). Retinoic acid and erythropoietin maintain alveolar development in mice treated with an angiogenesis inhibitor. American Journal of Respiratory Cell and Molecular Biology, 33(6), 622–628. doi: 10.1165/rcmb.2005-0050OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Lee S, Kyoung Chung H., Suk Lee Y., Eui Kim K., Choi D, … Hong YK (2012). 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation, 125(7), 872–882. doi: 10.1161/CIRCULATIONAHA.111.030296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SE, Hores TA, Smith LEH, & D’Amore PA (1988). Characterization of vascular development in the mouse retina. Microvascular Research, 36(3), 275–290. doi: 10.1016/0026-2862(88)90028-3 [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, & Duester G (2015). Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Reviews. Molecular Cell Biology, 16(2), 110–123. doi: 10.1038/nrm3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo C, & Barres B (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A, 106(2), 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, & Barres BA (2010). The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One, 5(10), e13741. doi: 10.1371/journal.pone.0013741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, … Savani RC (2006). Loss of PECAM-1 function impairs alveolarization. The Journal of Biological Chemistry, 281(13), 8724–8731. doi: 10.1074/jbc.M511798200 [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, & Akhurst RJ (1995). Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development, 121(6), 1845–1854. [DOI] [PubMed] [Google Scholar]
- Dupé V, Matt N, Garnier J-M, Chambon P, Mark M, & Ghyselinck NB (2003). A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 14036–14041. doi: 10.1073/pnas.2336223100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, … Duester G (2003). Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol, 23(13), 4637–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M (2007). Development of the retinal vasculature. Angiogenesis, 10(2), 77–88. doi: 10.1007/s10456-007-9065-1 [DOI] [PubMed] [Google Scholar]
- Gaetano C, Catalano A, Illi B, Felici A, Minucci S, Palumbo R, … Capogrossi MC (2001). Retinoids induce fibroblast growth factor-2 production in endothelial cells via retinoic acid receptor alpha activation and stimulate angiogenesis in vitro and in vivo. Circ Res, 88(4), E38–47. [DOI] [PubMed] [Google Scholar]
- Garcia MD, & Larina IV (2014). Vascular development and hemodynamic force in the mouse yolk sac. Front Physiol, 5, 308. doi: 10.3389/fphys.2014.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebb SA, & Shannon JM (2000). Tissue interactions mediate early events in pulmonary vasculogenesis. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 217(2), 159–169. doi: [DOI] [PubMed] [Google Scholar]
- Gee MF, Tsuchida R, Eichler-Jonsson C, Das B, Baruchel S, & Malkin D (2005). Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene, 24(54), 8025–8037. doi: 10.1038/sj.onc.1208939 [DOI] [PubMed] [Google Scholar]
- Giacomini A, Chiodelli P, Matarazzo S, Rusnati M, Presta M, & Ronca R (2016). Blocking the FGF/FGFR system as a “two-compartment” antiangiogenic/antitumor approach in cancer therapy. Pharmacol Res, 107, 172–185. doi: 10.1016/j.phrs.2016.03.024 [DOI] [PubMed] [Google Scholar]
- Goto S, Onishi A, Misaki K, Yonemura S, Sugita S, Ito H, … Takahashi M (2018). Neural retina-specific Aldh1a1 controls dorsal choroidal vascular development via Sox9 expression in retinal pigment epithelial cells. Elife, 7. doi: 10.7554/eLife.32358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Zwijsen A, van Rooijen MA, Huylebroeck D, Roelen BA, & Mummery CL (1999). Transforming growth factor-beta signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development, 126(16), 3473–3483. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, … Nagy A (2003). Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Developmental Biology, 262(2), 225–241. doi: 10.1016/S0012-1606(03)00356-7 [DOI] [PubMed] [Google Scholar]
- Healy AM, Morgenthau L, Zhu X, Farber HW, & Cardoso WV (2000). VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 219(3), 341–352. doi: [DOI] [PubMed] [Google Scholar]
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, & Thaller C (1992). 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell, 68(2), 397–406. [DOI] [PubMed] [Google Scholar]
- Hines EA, & Sun X (2014). Tissue crosstalk in lung development. Journal of Cellular Biochemistry, 115(9), 1469–1477. doi: 10.1002/jcb.24811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Rockenstein A, Ramaswamy A, Celik I, Wunderlich A, Lingelbach S, … Zielke A (2007). Retinoic acid inhibits angiogenesis and tumor growth of thyroid cancer cells. Mol Cell Endocrinol, 264(1–2), 74–81. doi: 10.1016/j.mce.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Hsia CCW, Hyde DM, & Weibel ER (2016). Lung Structure and the Intrinsic Challenges of Gas Exchange In Comprehensive Physiology (pp. 827–895): American Cancer Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe M, Li MX, Kneitz S, Davydova D, Yokota C, Kele-Olovsson J, … Gessler M (2017). Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci Signal, 10(487). doi: 10.1126/scisignal.aag2476 [DOI] [PubMed] [Google Scholar]
- Jakobsson L, & van Meeteren LA (2013). Transforming growth factor β family members in regulation of vascular function: in the light of vascular conditional knockouts. Exp Cell Res, 319(9), 1264–1270. doi: 10.1016/j.yexcr.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Kane MA (2012). Analysis, occurrence, and function of 9-cis-retinoic acid. Biochim Biophys Acta, 1821(1), 10–20. doi: 10.1016/j.bbalip.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, … Chambon P (1994). Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell, 78(6), 987–1003. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupé V, Grondona JM, & Chambon P (1997). Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development (Cambridge, England), 124(2), 313–326. [DOI] [PubMed] [Google Scholar]
- Katt ME, Xu ZS, Gerecht S, & Searson PC (2016). Human Brain Microvascular Endothelial Cells Derived from the BC1 iPS Cell Line Exhibit a Blood-Brain Barrier Phenotype. PLoS One, 11(4), e0152105. doi: 10.1371/journal.pone.0152105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, & Tabin CJ (2012). Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell, 22(3), 639–650. doi: 10.1016/j.devcel.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini AR, Peterson LA, Tallman MS, & Lingen MW (2001). Angiogenesis in acute promyelocytic leukemia: induction by vascular endothelial growth factor and inhibition by all-trans retinoic acid. Blood, 97(12), 3919–3924. [DOI] [PubMed] [Google Scholar]
- Kong L, Wang Y, Wang XJ, Wang XT, Zhao Y, Wang LM, & Chen ZY (2015). Retinoic acid ameliorates blood-brain barrier disruption following ischemic stroke in rats. Pharmacol Res, 99, 125–136. doi: 10.1016/j.phrs.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Lai L, Bohnsack BL, Niederreither K, & Hirschi KK (2003). Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development, 130(26), 6465–6474. doi: 10.1242/dev.00887dev.00887[pii] [DOI] [PubMed] [Google Scholar]
- Lange C, Turrero Garcia M., Decimo I, Bifari F, Eelen G, Quaegebeur A, … Carmeliet P (2016). Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. doi: 10.15252/embj.201592372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink M, Koolwijk P, van Hinsbergh V, & Kooistra T (1998). Effect of steroid hormones and retinoids on the formation of capillary-like tubular structures of human microvascular endothelial cells in fibrin matrices is related to urokinase expression. Blood, 92(3), 927–938. [PubMed] [Google Scholar]
- Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, … Ornitz DM (2006). Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev, 20(12), 1651–1666. doi: 10.1101/gad.1411406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, & Keshet E (2011). A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development (Cambridge, England), 138(11), 2359–2368. doi: 10.1242/dev.060723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Schloss DJ, & Swain JL (2000). Maintenance of vascular integrity in the embryo requires signaling through the fibroblast growth factor receptor. J Biol Chem, 275(43), 33679–33687. doi: 10.1074/jbc.M004994200 [DOI] [PubMed] [Google Scholar]
- Liang C, Guo S, & Yang L (2013). All-trans retinoic acid upregulates VEGF expression in glioma cells in vitro. J Biomed Res, 27(1), 51–55. doi: 10.7555/JBR.27.20120048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Guo S, & Yang L (2014). Effects of all‑trans retinoic acid on VEGF and HIF‑1α expression in glioma cells under normoxia and hypoxia and its anti‑angiogenic effect in an intracerebral glioma model. Mol Med Rep, 10(5), 2713–2719. doi: 10.3892/mmr.2014.2543 [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla C, … Dejana E (2008). Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol, 183(3), 409–417. doi:jcb.200806024 [pii] 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Dollé P, Ryckebüsch L, Noseda M, Zaffran S, Schneider MD, & Niederreither K (2010). Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc Natl Acad Sci U S A, 107(20), 9234–9239. doi: 10.1073/pnas.0910430107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingen MW, Polverini PJ, & Bouck NP (1998). Retinoic acid and interferon alpha act synergistically as antiangiogenic and antitumor agents against human head and neck squamous cell carcinoma. Cancer Res, 58(23), 5551–5558. [PubMed] [Google Scholar]
- Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, & Shusta EV (2014). A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep, 4, 4160. doi: 10.1038/srep04160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang Q, Radhakrishnan K, Whitfield DE, Everhart CL, Parsons-Wingerter P, & Fisher SA (2009). Role of VEGF and tissue hypoxia in patterning of neural and vascular cells recruited to the embryonic heart. Dev Dyn, 238(11), 2760–2769. doi: 10.1002/dvdy.22103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, … Chambon P (1994). Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development, 120(10), 2723–2748. [DOI] [PubMed] [Google Scholar]
- Maden M, & Hind M (2004). Retinoic acid in alveolar development, maintenance and regeneration. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 359(1445), 799–808. doi: 10.1098/rstb.2004.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S, Szmurlo A, Marczak M, Jablonska S, & Bollag W (1993). Inhibition of tumor cell-induced angiogenesis by retinoids, 1,25-dihydroxyvitamin D3 and their combination. Cancer Lett, 75(1), 35–39. [DOI] [PubMed] [Google Scholar]
- Marino D, Dabouras V, Brändli AW, & Detmar M (2011). A role for all-trans-retinoic acid in the early steps of lymphatic vasculature development. J Vasc Res, 48(3), 236–251. doi: 10.1159/000320620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, & Chambon P (2009). Function of retinoic acid receptors during embryonic development. Nuclear Receptor Signaling, 7, e002. doi: 10.1621/nrs.07002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, & Ghyselinck NB (2005). Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development, 132(21), 4789–4800. [DOI] [PubMed] [Google Scholar]
- McCaffery P, & Dräger UC (1993). Retinoic acid synthesis in the developing retina. Adv Exp Med Biol, 328, 181–190. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Lee MO, Wagner MA, Sladek NE, & Dräger UC (1992). Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development, 115(2), 371–382. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Tempst P, Lara G, & Dräger UC (1991). Aldehyde dehydrogenase is a positional marker in the retina. Development (Cambridge, England), 112(3), 693–702. [DOI] [PubMed] [Google Scholar]
- McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, & Snyder JM (2000). Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. American Journal of Respiratory Cell and Molecular Biology, 23(2), 162–167. doi: 10.1165/ajrcmb.23.2.3904 [DOI] [PubMed] [Google Scholar]
- Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, … Ruiz-Lozano P (2005). Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A, 102(51), 18455–18460. doi: 10.1073/pnas.0504343102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, & Duester G (2003). Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci U S A, 100(12), 7135–7140. doi: 10.1073/pnas.1231422100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Sirbu IO, & Duester G (2004). Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation. J Biol Chem, 279(25), 26698–26706. doi: 10.1074/jbc.M401920200 [DOI] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, Rossant J, & Nagy A (1999). Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol, 212(2), 307–322. doi:S0012–1606(99)99355–7 [pii] 10.1006/dbio.1999.9355 [DOI] [PubMed] [Google Scholar]
- Mishra S, Choe Y, Pleasure SJ, & Siegenthaler JA (2016). Cerebrovascular defects in Foxc1 mutants correlate with aberrant WNT and VEGF-A pathways downstream of retinoic acid from the meninges. Dev Biol, 420(1), 148–165. doi: 10.1016/j.ydbio.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Kelly KK, Rumian NL, & Siegenthaler JA (2018). Retinoic Acid Is Required for Neural Stem and Progenitor Cell Proliferation in the Adult Hippocampus. Stem Cell Reports, 10(6), 1705–1720. doi: 10.1016/j.stemcr.2018.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizee MR, Wooldrik D, Lakeman KA, van Het Hof B., Drexhage JA, Geerts D, … Reijerkerk A (2013). Retinoic Acid Induces Blood-Brain Barrier Development. J Neurosci, 33(4), 1660–1671. doi: 10.1523/JNEUROSCI.1338-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, Demello DE, & D’Amore PA (2001). Differential expression of VEGF isoforms in mouse during development and in the adult. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 220(2), 112–121. doi: [DOI] [PubMed] [Google Scholar]
- Niederreither K, Fraulob V, Garnier JM, Chambon P, & Dollé P (2002). Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev, 110(1–2), 165–171. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, & Dolle P (2001). Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development, 128(7), 1019–1031. [DOI] [PubMed] [Google Scholar]
- Ogunshola O, Antic A, Donoghue M, Fan S, Kim H, Stewart W, … Ment L (2002). Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem, 277(13), 11410–11415. doi:M111085200 [pii] 10.1074/jbc.M111085200 [DOI] [PubMed] [Google Scholar]
- Oikawa T, Hirotani K, Nakamura O, Shudo K, Hiragun A, & Iwaguchi T (1989). A highly potent antiangiogenic activity of retinoids. Cancer Lett, 48(2), 157–162. [DOI] [PubMed] [Google Scholar]
- Oladipupo SS, Smith C, Santeford A, Park C, Sene A, Wiley LA, … Ornitz DM (2014). Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci U S A, 111(37), 13379–13384. doi: 10.1073/pnas.1324235111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, & Claesson-Welsh L (2006). VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol, 7(5), 359–371. doi: 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- Osumi N, Hirota A, Ohuchi H, Nakafuku M, Iimura T, Kuratani S, … Eto K (1997). Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development, 124(15), 2961–2972. [DOI] [PubMed] [Google Scholar]
- Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, … Wolf CR (2003). Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic Acid homeostasis. Mol Cell Biol, 23(17), 6103–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, … Morrisey EE (2013). Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature, 500(7464), 589–592. doi: 10.1038/nature12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, & Koh GY (2018). Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med, 215(1), 35–49. doi: 10.1084/jem.20171868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock LM, Xie J, Bell BA, & Anand-Apte B (2018). Retinoic acid signaling is essential for maintenance of the blood-retinal barrier. FASEB J, 32(10), 5674–5684. doi: 10.1096/fj.201701469R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, & Mäkinen T (2017). Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol, 18(8), 477–494. doi: 10.1038/nrm.2017.36 [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, & Krasnow MA (2010). Coronary arteries form by developmental reprogramming of venous cells. Nature, 464(7288), 549–553. doi: 10.1038/nature08873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, … Liebner S (2012). Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med, 209(9), 1611–1627. doi:jem.20111580 [pii] 10.1084/jem.20111580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, & Liebner S (2013). Wnt signaling in the vasculature. Exp Cell Res, 319(9), 1317–1323. doi: 10.1016/j.yexcr.2012.12.023 [DOI] [PubMed] [Google Scholar]
- Ribes V, Fraulob V, Petkovich M, & Dollé P (2007). The oxidizing enzyme CYP26a1 tightly regulates the availability of retinoic acid in the gastrulating mouse embryo to ensure proper head development and vasculogenesis. Dev Dyn, 236(3), 644–653. doi: 10.1002/dvdy.21057 [DOI] [PubMed] [Google Scholar]
- Ribes V, Otto DM, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, … Dollé P (2007). Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol, 303(1), 66–81. doi: 10.1016/j.ydbio.2006.10.032 [DOI] [PubMed] [Google Scholar]
- Ribes V, Wang Z, Dolle P, & Niederreither K (2006). Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development, 133(2), 351–361. doi:133/2/351 [pii] 10.1242/dev.02204 [DOI] [PubMed] [Google Scholar]
- Ross AC, & Zolfaghari R (2011). Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr, 31, 65–87. doi: 10.1146/annurev-nutr-072610-145127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, & D’Amore PA (2009). An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences of the United States of America, 106(44), 18751–18756. doi: 10.1073/pnas.0905010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Sugawara A, Uruno A, Kudo M, Kagechika H, Sato Y, … Ito S (2007). All-trans retinoic acid induces in vitro angiogenesis via retinoic acid receptor: possible involvement of paracrine effects of endogenous vascular endothelial growth factor signaling. Endocrinology, 148(3), 1412–1423. [DOI] [PubMed] [Google Scholar]
- Selvam S, Kumar T, & Fruttiger M (2018). Retinal vasculature development in health and disease. Progress in Retinal and Eye Research, 63, 1–19. doi: 10.1016/j.preteyeres.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Sharma B, Chang A, & Red-Horse K (2017). Coronary Artery Development: Progenitor Cells and Differentiation Pathways. Annu Rev Physiol, 79, 1–19. doi: 10.1146/annurev-physiol-022516-033953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, … Pleasure SJ (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell, 139(3), 597–609. doi: 10.1016/j.cell.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, … Smith LE (2010). The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci, 51(6), 2813–2826. doi: 10.1167/iovs.10-5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins MJ, Lippmann ES, Faubion MG, Daneman R, Palecek SP, & Shusta EV (2018). Activation of RARα, RARγ, or RXRα Increases Barrier Tightness in Human Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells. Biotechnol J, 13(2). doi: 10.1002/biot.201700093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, & McMahon AP (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science, 322(5905), 1247–1250. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, & Keshet E (1995). Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci, 15(7 Pt 1), 4738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvmar MH, & Mäkinen T (2016). Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res, 111(4), 310–321. doi: 10.1093/cvr/cvw175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach J, & Rando RR (1994). Isomerization of all-trans-retinoic acid to 9-cis-retinoic acid. Biochem J, 299 ( Pt 2), 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, & Hynes RO (2010). Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development, 137(14), 2439–2449. doi: 10.1242/dev.049551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shi P, Xu Z, Li J, Xie Y, Mitton K, … Yan Q (2014). Up-regulation of VEGF by retinoic acid during hyperoxia prevents retinal neovascularization and retinopathy. Invest Ophthalmol Vis Sci, 55(7), 4276–4287. doi: 10.1167/iovs.14-14170 [DOI] [PubMed] [Google Scholar]
- Wang S, Huang W, Castillo HA, Kane MA, Xavier-Neto J, Trainor PA, & Moise AR (2018). Alterations in retinoic acid signaling affect the development of the mouse coronary vasculature. Dev Dyn, 247(8), 976–991. doi: 10.1002/dvdy.24639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woik N, & Kroll J (2015). Regulation of lung development and regeneration by the vascular system. Cellular and molecular life sciences: CMLS, 72(14), 2709–2718. doi: 10.1007/s00018-015-1907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, … Zhou B (2012). Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell, 151(5), 1083–1096. doi: 10.1016/j.cell.2012.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, … Simons M (2017). FGF-dependent metabolic control of vascular development. Nature, 545(7653), 224–228. doi: 10.1038/nature22322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun EJ, Lorizio W, Seedorf G, Abman SH, & Vu TH (2016). VEGF and endothelium-derived retinoic acid regulate lung vascular and alveolar development. American Journal of Physiology. Lung Cellular and Molecular Physiology, 310(4), L287–298. doi: 10.1152/ajplung.00229.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, & Nathans J (2014). Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell, 31(2), 248–256. doi: 10.1016/j.devcel.2014.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, … Nathans J (2014). Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest, 124(9), 3825–3846. doi: 10.1172/JCI76431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zheng B, Ye L, Zhang H, Zhu S, Zheng X, … Xu H (2016). Retinoic Acid Prevents Disruption of Blood-Spinal Cord Barrier by Inducing Autophagic Flux After Spinal Cord Injury. Neurochem Res, 41(4), 813–825. doi: 10.1007/s11064-015-1756-1 [DOI] [PubMed] [Google Scholar]


