Abstract
Purpose:
To report the one-year progression of visual impairment on psychophysical tests of visual function in patients with early and intermediate AMD.
Design:
Prospective, observational study
Subjects and Controls:
Subjects with early and intermediate AMD were enrolled from the existing population at the Duke Eye Center, and healthy age-matched control subjects were recruited from family members or friends of the AMD subjects and from the Duke optometry and comprehensive eye clinics.
Methods:
Subjects and controls recruited during the baseline study were assessed at both 6 and 12 months after the initial study visit. Measurements of visual function included best-corrected visual acuity (BCVA), low luminance visual acuity (LLVA), low luminance deficit (LLD), microperimetry (MP) percent reduced threshold (PRT) and average threshold (AT), and cone contrast tests (CCT).
Main Outcome Measures:
Changes in BCVA, LLVA, LLD, MP PRT and AT, and CCT from baseline to 6-months and 12-months were assessed.
Results:
85 subjects completed the 12-month examination (19 controls, 27 early AMD, 39 intermediate AMD). Longitudinal analysis detected significant changes from baseline within each group in MP PRT and AT, and in the intermediate AMD group only for BCVA and CCT (p<0.05).
Conclusions:
Microperimetry and CCT are able to detect functional changes due to progression of dry AMD within a time period as short as 12 months. These functional markers may be useful endpoints in future clinical trials that assess the effect of potential treatments for AMD.
Precis
In this prospective, observational natural history study, we report the 12-month progression of impairment on visual function tests in patients with early and intermediate dry age-related macular degeneration compared to controls.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in Americans aged 60 years or older1, and is projected to affect 196 million people worldwide by 20202. Individuals with AMD are classified into early, intermediate, or advanced AMD stages based on the Age-Related Eye Disease Study (AREDS) classification3. AREDS vitamins have been shown to slow progression to advanced AMD3,4, and the advent of anti-vascular endothelial growth factor medications has led to substantial progress in the treatment of exudative, or “wet,” AMD characterized by choroidal neovascularization. However, no definitive or curative therapies currently exist for non-exudative or “dry” early to severe AMD, which affects the majority of patients with AMD.
In order to develop potential therapeutics to treat dry AMD, endpoints to monitor progression of disease are necessary, particularly to allow for earlier detection and treatment. While the rapidly progressive course of exudative AMD can be monitored with changes in best-corrected visual acuity (BCVA), this standard clinical endpoint used in the majority of ophthalmic clinical trials is not a sensitive measure for visual changes occurring during the slower and earlier course of dry AMD5. There is a need for the identification of sensitive functional endpoints with regulatory acceptance that can detect a clinically meaningful change in early to intermediate dry AMD in a reasonable timeframe. Endpoints that meet these needs are necessary for the development of potential treatments for dry AMD.
Previous studies offer insight into potential functional endpoints that may be used to monitor the natural history of dry AMD as well as potential changes due to interventions performed in clinical trials. Many patients with dry AMD report difficulty with vision in dim lighting6–8. This is thought to be caused by impaired function in the rod system that may result in impaired scotopic vision and delayed dark adaptation9. In all stages of AMD, histopathology has demonstrated that the rod photoreceptors degenerate earlier than cones10,11. Thus, measures that capture rod dysfunction may be useful functional markers of disease progression in dry AMD. In addition to functional visual changes in dim lighting and dark adaptation, loss of cones involved in blue color contrast sensitivity has also been reported12. Thus, psychophysical tests that monitor such rod-mediated functional deficits in a sensitive manner may prove useful as future clinical trial endpoints across the different stages of AMD over time13–15.
In this prospective, longitudinal, observational natural history study, we aimed to characterize the progression of early and intermediate dry AMD over one year using a variety of psychophysical tests that we believed would be able to capture progression of visual function deficits in dry AMD, such as low luminance visual acuity (LLVA), mesopic microperimetry, and cone contrast test (CCT). We have previously shown that these tests are able to distinguish between the early, intermediate AMD and normal controls in both a pilot study of 30 patients13 as well as the cross-sectional baseline study of 101 patients16. Thus, these measures may not only be able to distinguish early dry AMD stages, but also would have the potential to detect their progression. The hypothesis in the current study was that patients with intermediate dry AMD would show a significantly greater decline in their performance on specific psychophysical tests of visual function than age-matched controls after six months and one year of longitudinal follow-up.
Methods
The study subjects recruited during the baseline study16 were followed and assessed at both 6 and 12 months after the initial study visit. As described previously by Cocce et al.16, the subjects with early and intermediate AMD were enrolled from the existing population at the Duke Eye Center, and healthy age-matched control subjects were recruited from family members or friends of the AMD subjects and from the Duke optometry and comprehensive eye clinics. Written informed consent was obtained from all subjects. The study NCT01822873 was approved by the Duke University Health System Institutional Review Board (IRB), and was conducted in accordance with Good Clinical Practice (GCP) using the guidance documents and practices offered by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) or applicable international regulatory authority laws, regulations, and guidelines. Subjects were compensated $20 per visit and were reimbursed for parking costs during the study visit.
A baseline evaluation was performed when the subjects were enrolled, and subjects were classified as healthy controls (Age-Related Eye Disease Study, (AREDS) category 1), early AMD (AREDS category 2), or intermediate AMD (AREDS category 3)3. Control subjects had fewer than 5 small drusen <63 μm in size and otherwise no signs of AMD in either eye. Early AMD subjects were characterized by multiple small drusen measuring 63–124 m in diameter and/or retinal pigment epithelium (RPE) abnormalities. Intermediate AMD subjects had extensive intermediate drusen and least one large drusen >125 μm3. Subjects meeting the following criteria for advanced AMD were excluded: any geographic atrophy of the RPE and choriocapillaris, neovascular maculopathy, detachment of the sensory retina or RPE, retinal hard exudates, subretinal or sub-RPE fibrovascular proliferation or a disciform scar in either eye. After the baseline evaluation, follow-up assessments for each subject were performed at approximately 6 months and 12 months (+/− 2 months) based on the standard-of-care clinic visits for AMD subjects17.
At each visit, subjects were imaged with stereo color fundus photography (Zeiss FF 450 Plus IR; Carl Zeiss Meditec Inc., Dublin, CA), fundus autofluorescence (Spectralis 3 mode; Heidelberg Engineering GmbH, Heidelberg, Germany),15 and spectral domain optical coherence tomography (SD-OCT; Spectralis 6-mode; Heidelberg Engineering US). Ophthalmologists (EML, SC, LV, AH) performed clinical exams, and a medical retinal specialist (EML) evaluated color fundus photos for the extent of pigmentary changes and drusen size. Study subject demographics, ocular and medical history, and full ophthalmic examination were also recorded.
The following psychophysical tests of visual function that had been performed at baseline, as previously described by Cocce et al.16, were repeated at the 6-month and 12-month visit, in the following order: BCVA, LLVA, CCT for red, green, and blue, and mesopic microperimetry. BCVA, assessed using an Early Treatment in Diabetic Retinopathy Study (ETDRS) chart (85 cd/m2; Good-Lite, Elgin, IL, USA), was reported in the number of letters read18,19. LLVA was assessed by having subjects read the ETDRS chart through a 2.0-log neutral density filter that reduces luminance by 100-fold20. Low luminance deficit (LLD) was defined as the difference between BCVA and LLVA in ETDRS letters. CCT (Innova Systems, Burr Ridge, IL, USA) assessed for deficits in cone color discrimination13 by requiring subjects to distinguish colored letters that were detectable by a single cone type (long, medium, or short wavelength photoreceptors for red, green, and blue colors, respectively) on a gray background. Letters were shown in decreasing steps of color contrast to determine the threshold for distinguishing color. CCT results were scored on a normalized 100-point scale, with 90–100% representing normal cone function and scores below 75% representing color deficiency. This allowed the assessment of the function of each of the different cones types. Subjects were then dilated with tropicamide 1% and phenylephrine 2.5% for mesopic microperimetry testing (Macular Integrity Assessment [MAIA]; CenterVue, San Jose, CA, USA), during which retinal sensitivity was assessed using the standard 37 loci, 10° (37–10) MAIA grid. Microperimetry results were reported as percent reduced threshold (PRT), defined as the percentage of abnormal retinal sensitivity thresholds below 25 dB, and as average threshold (AT), defined as the average of retinal sensitivity values from all loci tested.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc, Cary, NC). Prior to the start of the study, we determined the necessary sample size using standard LLVA values in letters from all the three groups that were obtained in our pilot study13 for a two-sample t-test with an alpha-level of 0.05 and 80% power. The resulting sample size was inflated by 5% to account for the use of the non-parametric Wilcoxon rank sum test in the analysis and potential subject attrition at 12 months of up to 20%. The resulting necessary sample size was approximately 13–25 in the control group, 25–57 in the early group, and 13–57 in the intermediate group. The number of subjects subsequently enrolled in each group matched the planned sample size determined by the power calculation. Data collected from case report forms were entered into the Research Electronic Data Capture (RedCap) database by certified data entry analysts from the Duke Office of Clinical Research.
Cross-sectional analyses of the psychophysical test results from the 6-month and 12-month study visits were performed for the normal control, early AMD, and intermediate AMD groups. An overall p-value comparing the three groups was calculated, and correction for multiple comparisons for each variable was applied with a Bonferroni test (adjusted alpha level of p=0.025 for the p-value overall, and adjusted alpha level of p=0.0167 for the pair-wise comparisons). Furthermore, due to the exploratory, observational nature of this study, we also compared the groups in a pairwise fashion using the nonparametric Wilcoxon rank-sum test to detect any potential associations of interest, especially between the intermediate AMD and normal control groups and between intermediate and early AMD groups, with the expectation that these exploratory analyses will be reassessed and confirmed in a future larger study21,22,23.
Longitudinal analyses of the change from baseline to 12 months within each group were performed using Wilcoxon signed-rank tests. Comparisons between groups of the change from baseline to 12 months for each psychophysical test were assessed using the Wilcoxon rank sum test. To assess for possible progression of dry AMD greater than would be expected due to normal aging on each psychophysical test, the mean plus two times the standard deviation (SD) of the normal control group at baseline was computed as a cut-point. Fisher’s exact test was used to assess the significance of the difference among groups with respect to proportions exceeding the cut points.
Results
A total of 85 subjects (19 normal controls, 27 early AMD, 39 intermediate AMD) enrolled at baseline and completed the 12-month study visit (Table 1). Four of the 19 study eyes in the control group had less than 5 drusen <65 μm at baseline. All 85 subjects underwent a dilated fundus exam along with assessment of BCVA, LLVA, and LLD during the baseline and 12-month visit. Of the 85 subjects, 78 subjects completed the microperimetry testing, and 83 subjects completed cone contrast testing at the 12-month visit.
Table 1.
Subject demographics at the one-year follow-up visit.
| Variable | Statistic | Normal Control | Early AMD | Intermediate AMD |
|---|---|---|---|---|
| Age | N | 19 | 27 | 39 |
| Mean (SD) | 71.8 (7.2) | 72.7 (8.4) | 70.8 (6.7) | |
| Min, median, max | 61. 74, 81 | 58, 72, 90 | 51, 70, 82 | |
| Sex | ||||
| Male | N (%) | 13 (68) | 16 (59) | 26 (67) |
| Female | N (%) | 6 (32) | 11 (41) | 13 (33) |
| Race | ||||
| White (Caucasian) | N (%) | 19 (100) | 27 (100) | 37 (95) |
| Black or African American | N (%) | 0 | 0 | 1 (3) |
| Native Hawaiin or Pacific Islander | N (%) | 0 | 0 | 1 (3) |
| Ethnicity | ||||
| Not Hispanic or Latino | N (%) | 18 (95) | 22 (85) | 37 (95) |
| Hispanic or Latino | N (%) | 0 | 1 (4) | 0 |
| Unknown/Not reported | N (%) | 1 (5) | 3 (12) | 2 (5) |
Cross-sectional disease classification of subjects was confirmed using structural assessments (dilated fundus examination, color fundus photography, and SD-OCT), none of which showed obvious progression at the 12-month visit. Overall, the results of cross-sectional analysis of the psychophysical tests were similar from baseline to the 12-month visit, with a few exceptions: microperimetry PRT and AT showed significant differences at 6 and 12 months between the intermediate AMD group as compared to the control and early AMD groups; LLVA and LLD showed significant difference at 12 months between the intermediate AMD and control groups; and CCT Red and Blue were significantly different at 12 months for the early vs. intermediate AMD groups. BCVA did not significantly differ between the groups at the baseline, 6-month, and 12-month follow-up visits (p>0.0167, Table 2), and LLVA and LLD were unrevealing as well, with the exception of LLVA and LLD at 12 months for control vs. intermediate AMD. None of the tests displayed significant differences between the early AMD vs. control group at any of the visits. Significant differences between the intermediate and early AMD groups were detected in microperimetry PRT and AT at 6 and 12 months, and for the red and blue CCT at 12 months (p<<0.0167).
Table 2.
Cross-sectional analysis at the 6 and 12 month visits for normal control, early AMD, and intermediate AMD groups for best-corrected visual acuity (BCVA), low luminance visual acuity (LLVA), and low luminance deficit (LLD). Units of BCVA, LLVA and LLD are Early Treatment in Diabetic Retinopathy Study (ETDRS) letters. P-values for pair-wise comparisons between groups were performed using the Wilcoxon rank sum test.
| Test | Statistic | Normal Control | Early AMD | Intermediate AMD | Overall p-value | Normal Control vs. Early AMD | Normal Control vs. Intermediate AMD | Early vs. Intermediate AMD |
|---|---|---|---|---|---|---|---|---|
| BCVA (baseline) |
N | 19 | 27 | 39 | ||||
| Mean (SD) | 83.5 (4.5) | 81.4 (5.4) | 81.4 (5.8) | 0.400 | 0.223 | 0.230 | 0.927 | |
| Min, Median, Max | 73, 83, 90 | 69, 82, 90 | 64, 83, 92 | |||||
| BCVA (6 months) |
N | 17 | 27 | 37 | ||||
| Mean (SD) | 84.0 (5.7) | 80.4 (5.1) | 81.1 (6.4) | 0.072 | 0.020 | 0.078 | 0.668 | |
| Min, Median, Max | 73, 85, 95 | 72, 81, 91 | 67, 81, 95 | |||||
| BCVA (12 months) |
N | 19 | 27 | 39 | ||||
| Mean (SD) | 82.2 (5.5) | 82.4 (4.9) | 79.8 (7.7) | 0.299 | 0.671 | 0.242 | 0.180 | |
| Min, Median, Max | 68, 84, 89 | 71, 82, 90 | 54, 80, 91 | |||||
| LLVA (baseline) |
N | 19 | 27 | 39 | ||||
| Mean (SD) | 72.1 (4.8) | 70.8 (6.6) | 68.0 (7.5) | 0.134 | 0.531 | 0.045 | 0.249 | |
| Min, Median, Max | 63, 73, 80 | 60, 70, 83 | 41, 70, 80 | |||||
| LLVA (6 months) |
N | 17 | 27 | 37 | ||||
| Mean (SD) | 71.5 (8.9) | 70.8 (6.0) | 67.4 (11.2) | 0.173 | 0.426 | 0.095 | 0.208 | |
| Min, Median, Max | 47, 73, 82 | 56, 71, 82 | 16, 69, 81 | |||||
| LLVA (12 months) |
N | 19 | 27 | 39 | ||||
| Mean (SD) | 73.0 (5.6) | 70.4 (6.4) | 65.8 (10.2) | 0.018 | 0.282 | 0.008 | 0.069 | |
| Min, Median, Max | 63, 72, 84 | 55, 71, 82 | 28, 67, 80 | |||||
| LLD (baseline) |
N | 19 | 27 | 39 | ||||
| Mean (SD) | 11.4 (3.2) | 10.6 (4.2) | 13.4 (6.1) | 0.147 | 0.453 | 0.262 | 0.065 | |
| Min, Median, Max | 7, 11, 17 | 4, 11, 20 | 4, 12, 34 | |||||
| LLD (6 months) |
N | 17 | 27 | 37 | ||||
| Mean (SD) | 12.5 (8.5) | 9.6 (5.1) | 13.7 (9.5) | 0.110 | 0.460 | 0.277 | 0.042 | |
| Min, Median, Max | 5, 10, 42 | −3, 10, 19 | 3, 12, 64 | |||||
| LLD (12 months) |
N | 19 | 27 | 39 | ||||
| Mean (SD) | 9.2 (4.5) | 12.0 (2.8) | 14.0 (5.3) | 0.003 | 0.027 | 0.002 | 0.062 | |
| Min, Median, Max | −2, 9, 16 | 7, 11, 20 | 4, 14, 26 |
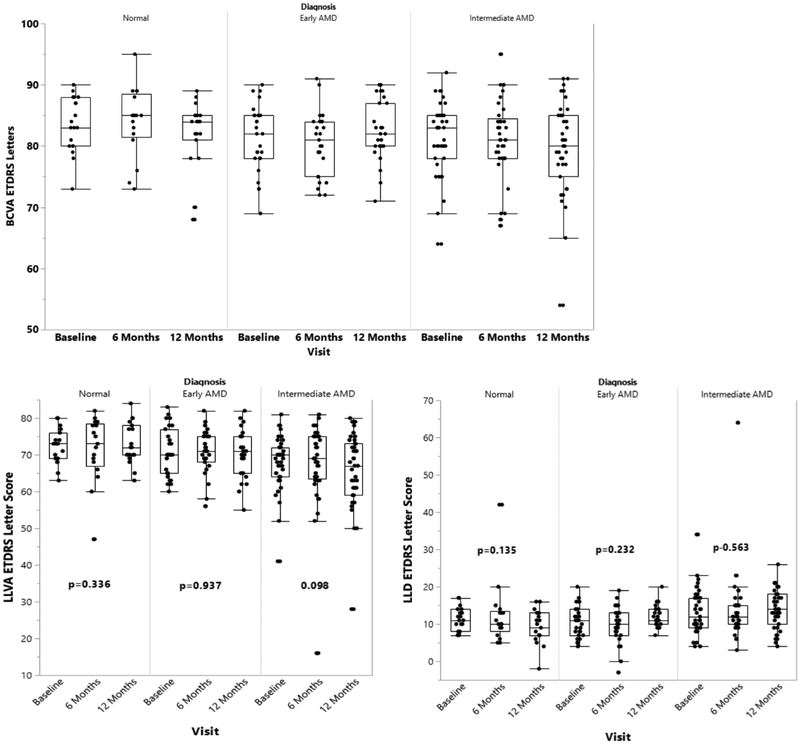
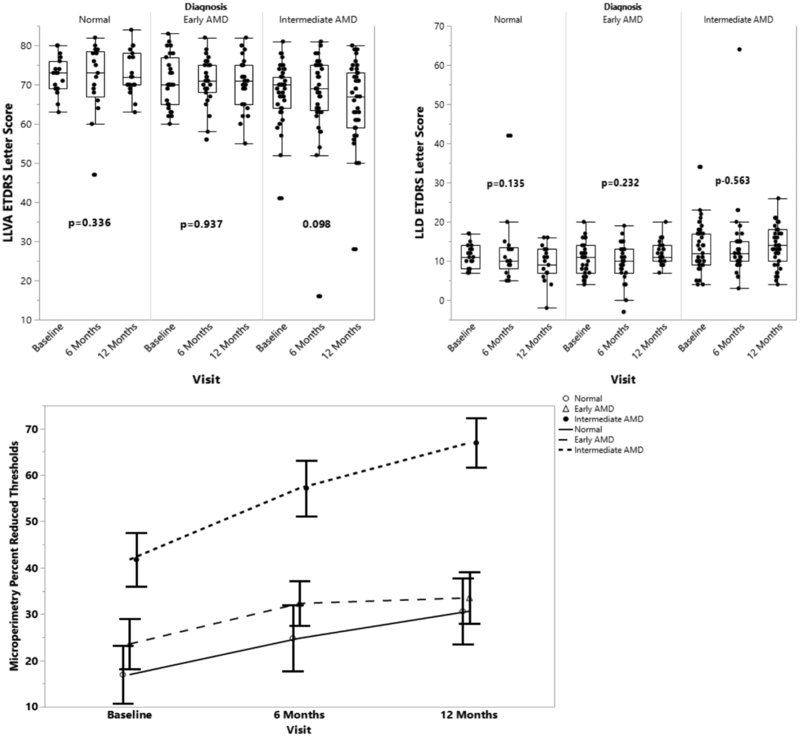
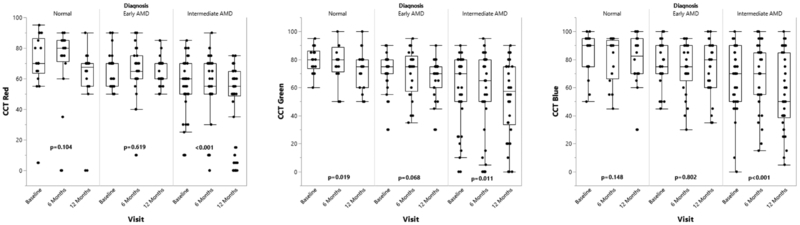
Longitudinal analyses of the visual function tests were performed to evaluate for significant changes from baseline to 12 months within each of the control, early AMD, and intermediate AMD groups. The intermediate AMD group demonstrated a statistically significant decline in some of the visual function measures over the first year as assessed by several psychophysical tests (Tables 2–4): BCVA (Figure 1), microperimetry PRT and AT (Figure 2), and CCT red, green, and blue (Figure 3) (p<0.05 for all metrics) showed a significant worsening compared to baseline, while LLVA and LLD did not significantly decline from baseline to 12 months. In the univariate analyses, changes from baseline to 12 months for BCVA (p=0.021), microperimetry AT (p=0.047), CCT red (p=0.012), and CCT blue (p=0.019) in the intermediate AMD group were significantly larger compared to those in the early AMD group. Compared to the change observed over 12 months in the normal control group, the intermediate AMD group showed a significantly greater change in microperimetry AT from baseline to 12 months (p=0.029), thus emphasizing the robustness of the change observed in microperimetry.
Table 4.
Cross-sectional analysis at the 6 and 12 month visits for normal control, early AMD, and intermediate AMD groups for cone contrast tests (CCT) of red, green, and blue cones. P-values for pair-wise comparisons between groups were performed using the Wilcoxon rank sum test.
| Test | Statistic | Normal Control | Early AMD | Intermediate AMD | Overall p-value | Normal Control vs. Early AMD† | Normal Control vs. Intermediate AMD† | Early vs. Intermediate AMD† |
|---|---|---|---|---|---|---|---|---|
| CCT Red (%) (baseline) |
N | 18 | 27 | 38 | ||||
| Mean (SD) | 70.28 (20.40) | 65.56 (11.29) | 56.71 (18.43) | 0.011 | 0.100 | 0.005 | 0.095 | |
| Min, Median, Max | 5, 70, 95 | 50, 70, 90 | 10, 60, 85 | |||||
| CCT Red (%) (6 months) |
N | 16 | 25 | 35 | ||||
| Mean (SD) | 71.9 (23.4) | 64.6 (17.3) | 55.7 (21.3) | 0.002 | 0.028 | <0.001 | 0.084 | |
| Min, Median, Max | 0, 80, 90 | 10, 65, 90 | 0, 55, 90 | |||||
| CCT Red (%) (12 months) |
N | 18 | 27 | 38 | ||||
| Mean (SD) | 62.8 (18.3) | 64.3 (8.7) | 48.4 (22.5) | <0.001 | 0.704 | 0.004 | 0.001 | |
| Min, Median, Max | 0, 67.5, 90 | 50, 60, 85 | 0, 55, 75 | |||||
| CCT Green (%) (baseline) |
N | 18 | 27 | 38 | ||||
| Mean (SD) | 80.00 (9.07) | 71.85 (12.57) | 59.34 (23.71) | <0.001 | 0.021 | <0.001 | 0.056 | |
| Min, Median, Max | 60, 80, 95 | 30, 75, 90 | 0, 70, 90 | |||||
| CCT Green (%) (6 months) |
N | 16 | 25 | 35 | ||||
| Mean (SD) | 77.5 (13.4) | 71.0 (16.8) | 58.9 (29.0) | 0.085 | 0.280 | 0.037 | 0.200 | |
| Min, Median, Max | 50, 80, 100 | 35, 75, 95 | 0, 65, 95 | |||||
| CCT Green (%) (12 months) |
N | 18 | 27 | 38 | ||||
| Mean (SD) | 73.3 (13.9) | 68.2 (13.0) | 52.6 (26.7) | 0.009 | 0.188 | 0.008 | 0.033 | |
| Min, Median, Max | 50, 75, 100 | 30, 70, 90 | 0, 57.5, 90 | |||||
| CCT Blue (%) (baseline) |
N | 18 | 27 | 38 | ||||
| Mean (SD) | 84.44 (15.14) | 76.11 (16.54) | 66.97 (23.32) | 0.008 | 0.072 | 0.003 | 0.137 | |
| Min, Median, Max | 50, 90, 100 | 45, 75, 100 | 0, 70, 100 | |||||
| CCT Blue (%) (6 months) |
N | 16 | 25 | 35 | ||||
| Mean (SD) | 80.0 (16.8) | 74.0 (18.5) | 68.0 (23.0) | 0.154 | 0.280 | 0.062 | 0.346 | |
| Min, Median, Max | 45, 90, 95 | 30, 75, 95 | 15, 70, 100 | |||||
| CCT Blue (%) (12 months) |
N | 18 | 27 | 38 | ||||
| Mean (SD) | 79.4 (18.1) | 75.4 (19.9) | 55.5 (26.8) | 0.001 | 0.514 | 0.002 | 0.004 | |
| Min, Median, Max | 30, 82.5, 100 | 35, 80, 100 | 5, 50, 100 |
Units of BCVA, LLVA and LLD are Early Treatment in Diabetic Retinopathy Study (ETDRS) letters.
P-values for pair-wise comparisons between groups were performed using the Wilcoxon rank sum test.
Figure 1.
Longitudinal progression of best corrected visual acuity (BCVA), low luminance visual acuity (LLVA), and low luminance deficit (LLD) at baseline, 6 months, and 12 months for the normal control, early AMD, and intermediate AMD subjects, as shown by boxplots. LLVA was reported as the number of ETDRS letters the subject could read through a 2.0-log neutral density filter. LLD was calculated as the difference in the number of ETDRS letters the subject was able to read under standard conditions (BCVA) and through the neutral density filter (LLVA). P-values are calculated to assess for significant changes between baseline and 12 months within each group using the Wilcoxon signed-rank test.
Figure 2.
Longitudinal progression of microperimetry percent reduced threshold (PRT) and average threshold (AT) at baseline, 6 months, and 12 months for the normal control, early AMD, and intermediate AMD subjects as shown by boxplots (top) and in a line graph (bottom). PRT is defined as the percentage of abnormal retinal sensitivity thresholds below 25 dB, and AT is the average of retinal sensitivity values from all loci tested. P-values are calculated to assess for significant changes between baseline and 12 months within each group using the Wilcoxon signed-rank test.
Figure 3.
Longitudinal progression of cone contrast tests (CCT) red, green, and blue at baseline, 6 months, and 12 months for the normal control, early AMD, and intermediate AMD subjects as shown by boxplots. CCT results were scored on a 100-point scale, with 90–100% representing normal cone function and scores below 75% representing color deficiency. P-values are calculated to assess for significant changes between baseline and 12 months within each group using the Wilcoxon signed-rank test.
For the normal control and early AMD groups, no significant changes were observed between baseline and 12 months for BCVA, LLVA, LLD, and CCT red and blue (p>0.0167, Tables 2 and 3). For all the groups, microperimetry PRT and AT were significantly different between baseline and 12 months (p<0.0167, Table 4), with the exception that microperimetry PRT did not change for the early AMD subjects (p=0.125). Additionally, the change in LLD from baseline to 12 months was significantly greater for the early AMD group than the normal control group (p=0.037).
Table 3.
Cross-sectional analysis at the 6 and 12 month visits for normal control, early AMD, and intermediate AMD groups for microperimetry (MP) percent reduced threshold (PRT) and average threshold (AT). P-values for pair-wise comparisons between groups were performed using the Wilcoxon rank sum test.
| Test | Statistic | Normal Control | Early AMD | Intermediate AMD | Overall p-value | Normal Control vs. Early AMD† | Normal Control vs. Intermediate AMD† | Early vs. Intermediate AMD† |
|---|---|---|---|---|---|---|---|---|
| MP PRT (%) (baseline) |
N | 18 | 25 | 35 | ||||
| Mean (SD) | 16.96 (26.59) | 23.56 (27.00) | 41.78 (34.44) | 0.005 | 0.242 | 0.003 | 0.028 | |
| Min, Median, Max | 0.0, 2.7, 94.6 | 0.0, 21.6, 97.3 | 0.0, 37.8, 100.0 | |||||
| MP PRT (%) (6 months) |
N | 17 | 23 | 32 | ||||
| Mean (SD) | 24.8 (29.3) | 32.3 (23.0) | 57.2 (34.0) | 0.001 | 0.138 | 0.001 | 0.009 | |
| Min, Median, Max | 0.0, 10.8, 100.0 | 2.7, 29.7, 91.9 | 2.7, 70.3, 100.0 | |||||
| MP PRT (%) (12 months) |
N | 18 | 25 | 35 | ||||
| Mean (SD) | 30.6 (30.1) | 33.5 (27.8) | 67.0 (31.4) | <0.001 | 0.639 | <0.001 | <0.001 | |
| Min, Median, Max | 0.0, 23.0, 100.0 | 0.0, 29.7, 94.6 | 5.4, 81.1, 100.0 | |||||
| MP AT (dB) (baseline) |
N | 18 | 25 | 35 | ||||
| Mean (SD) | 26.90 (3.80) | 26.85 (2.49) | 25.30 (3.07) | 0.028 | 0.382 | 0.019 | 0.049 | |
| Min, Median, Max | 14.4, 27.7, 31.3 | 21.3, 26.4, 31.0 | 17.9, 25.2, 30.1 | |||||
| MP AT (dB) (6 months) |
N | 17 | 23 | 32 | ||||
| Mean (SD) | 25.4 (3.8) | 25.8 (1.6) | 23.5 (2.9) | 0.001 | 0.245 | 0.003 | 0.002 | |
| Min, Median, Max | 14.4, 26.9, 28.1 | 22.5, 25.8, 28.9 | 14.1, 23.1, 28.1 | |||||
| MP AT (dB) (12 months) |
N | 18 | 25 | 35 | ||||
| Mean (SD) | 25.4 (3.5) | 25.5 (2.1) | 22.3 (4.1) | <0.001 | 0.232 | <0.001 | <0.001 | |
| Min, Median, Max | 13.5, 26.7, 28.5 | 19.8, 25.9, 28.7 | 9.4, 22.5, 27.9 |
Units of BCVA, LLVA and LLD are Early Treatment in Diabetic Retinopathy Study (ETDRS) letters.
P-values for pair-wise comparisons between groups were performed using the Wilcoxon rank sum test.
In order to further characterize the progression in each of the three groups (control, early AMD and intermediate AMD) from baseline to 12-months, we calculated the proportion of subjects from each group that had results outside the range of 95% of the normal values, defined as greater than 2 standard deviations plus the mean of baseline normal control values (Table 5). The proportion of intermediate AMD subjects that were out of this normal range was significantly greater than the proportion of normal controls for both LLD and microperimetry PRT (p<0.0167) (Table 6).
Table 5.
Longitudinal changes from baseline to one year within group for normal control, early AMD, and intermediate AMD patients. P-values are calculated to assess for significant change within each group over one year using the Wilcoxon signed-rank test.
| Test | Statistic | Normal Control | Early AMD | Intermediate AMD |
|---|---|---|---|---|
| BCVA | N | 19 | 27 | 39 |
| Mean (SD) | −1.3 (5.2) | 1.0 (4.7) | −1.6 (6.8) | |
| Min, Median, Max | −15.0, −2.0, 6.0 | −9.0, 1.0, 15.0 | −15.0, −3.0, 25.0 | |
| P-value | 0.497 | 0.420 | 0.033 | |
| LLVA | N | 19 | 27 | 39 |
| Mean (SD) | 0.9 (5.1) | −0.4 (5.6) | −2.2 (7.5) | |
| Min, Median, Max | −12.0, 2.0, 8.0 | −16.0, 0.0, 9.0 | −24.0, −1.0, 12.0 | |
| P-value | 0.336 | 0.937 | 0.098 | |
| LLD | N | 19 | 27 | 39 |
| Mean (SD) | −2.2 (5.8) | 1.4 (5.4) | 0.6 (7.1) | |
| Min, Median, Max | −16.0, −2.0, 9.0 | −8.0, 1.0, 13.0 | −14.0, 0.0, 15.0 | |
| P-value | 0.135 | 0.232 | 0.563 | |
| Microperimetry PRT (%) |
N | 18 | 25 | 35 |
| Mean (SD) | 13.7 (20.5) | 10.0 (35.0) | 25.2 (27.4) | |
| Min, Median, Max | −18.9, 8.1, 62.2 | −75.7, 8.1, 73.0 | −18.9, 18.9, 94.6 | |
| P-value | 0.003 | 0.125 | <0.001 | |
| Microperimetry AT (dB) |
N | 18 | 25 | 35 |
| Mean (SD) | −1.5 (1.7) | −1.4 (2.7) | −3.0 (3.3) | |
| Min, Median, Max | −4.5, −0.9, 1.2 | −6.1, −2.1, 4.6 | −18.5, −2.6, 1.2 | |
| P-value | 0.002 | 0.017 | <0.001 | |
| CCT Red (%) |
N | 18 | 27 | 38 |
| Mean (SD) | −7.5 (17.8) | −1.3 (11.0) | −8.3 (12.7) | |
| Min, Median, Max | −40.0, −5.0, 20.0 | −30.0, 0.0, 25.0 | −45.0, −10.0, 20.0 | |
| P-value | 0.104 | 0.619 | <0.001 | |
| CCT Green (%) |
N | 18 | 27 | 38 |
| Mean (SD) | −6.7 (11.4) | −3.7 (10.1) | −6.7 (15.1) | |
| Min, Median, Max | −35.0, −5.0, 15.0 | −30.0, −5.0, 20.0 | −50.0, −5.0, 20.0 | |
| P-value | 0.019 | 0.068 | 0.011 | |
| CCT Blue (%) |
N | 18 | 27 | 38 |
| Mean (SD) | −5.0 (13.6) | −0.7 (17.4) | −11.5 (15.4) | |
| Min, Median, Max | −30.0, −5.0, 25.0 | −35.0, 0.0, 40.0 | −45.0, −10.0, 30.0 | |
| P-value | 0.148 | 0.802 | <0.001 |
Table 6.
The percentage of subjects at one year that were greater than the mean plus two SD of the normal control values at baseline for each of the psychophysical tests: best-corrected visual acuity (BCVA), low luminance visual acuity (LLVA), low luminance deficit (LLD), microperimetry percent reduced threshold (PRT) and average threshold (AT), and cone contrast test (CCT) red, blue, and green.
| Test | Normal Control | Early AMD | Intermediate AMD | P-Value* |
|---|---|---|---|---|
| BCVA ≥ 92.48 | 0 | 0 | 0 | --- |
| LLVA ≥ 81.65 | 1 (5%) | 1 (4%) | 0 | 0.328 |
| LLD ≥ 17.74 | 0 | 1 (4%) | 11 (28%) | 0.011 |
| Microperimetry PRT ≥ 70.15 | 2 (11%) | 3 (12%) | 21 (60%) | 0.001 |
| Microperimetry AT ≥ 34.51 | 0 | 0 | 0 | --- |
| CCT Red ≥ 111.08 | 0 | 0 | 0 | --- |
| CCT Green ≥ 98.15 | 1 (6%) | 0 | 0 | 0.321 |
| CCT Blue ≥ 114.72 | 0 | 0 | 0 | --- |
P-values are calculated to assess for significant differences between the normal and intermediate AMD groups using Fisher’s exact test of difference in proportions.
Discussion
Individuals affected by dry AMD currently lack effective treatment options to definitively halt or reverse the course of this prevalent degenerative disease, which becomes disabling in the advanced stages. This partly stems from a paucity of sensitive functional endpoints that can be used in clinical trials to assess disease progression in a short period of time and intervene in the early stages. In this natural history study, we compare the results of several psychophysical measures of visual function at six months and one year between early and intermediate AMD subjects and normal age-matched controls, and we document the longitudinal changes in these measures over the study period. Overall, the data shows that, although AMD progression is slow and the AREDS staging for the individual patients did not change significantly, mesopic microperimetry and CCT are able to detect functional changes due to progression of dry AMD within a time period as short as 12 months. Thus, this is a strong argument for inclusion of functional measures in addition to structural assessments of disease progression in intermediate AMD.
In this work, we have demonstrated at 6 and 12 months that functional measures in cross-sectional analyses can distinguish between intermediate AMD vs. control as well as intermediate vs. early AMD disease stages. Microperimetry PRT, a metric that illustrates the percentage of loci with abnormal retinal sensitivity over the entire area tested, may be the most robust indication of progression of AMD, as it showed a significant decline in function across all three disease groups and time points (Figure 2). As expected, the progression of the intermediate AMD group was most pronounced and statistically robust in microperimetry PRT, as significant changes were seen in both methods of statistical analysis. In a cross-sectional analysis, Wu and colleagues have demonstrated significant differences for BCVA, LLVA, and microperimetry for all AMD groups except AREDS stage 2 or “early AMD” relative to controls.15 They subsequently reported that eyes with intermediate AMD demonstrate a statistically significant decline in mesopic microperimetry mean pointwise sensitivity over a 12-month follow-up period (mean (standard error): −0.42 dB (0.12 dB), P < 0.001), but that LLVA and BCVA do not significantly change.14 Similarly,_Vujosevic et al. found that mean retinal sensitivity decreased over 6 years in eyes with AREDS 2 and 3 (mean (standard deviation): −10.8 (9.2), p=0.0028).24 In our study, we observed a decline in microperimetry mean pointwise sensitivity, also termed average threshold, of −3.0 dB (standard deviation 3.3 dB, P<0.001). While the direction of the change in MP is similar across these three studies, the magnitude differs slightly between the studies. Disparities in the magnitude could be related to variation in the level of severity and visual impairment in intermediate AMD patients at baseline, or to differences in the testing technique, grid employed and the length of follow-up. For example, Wu et al. utilized a customized grid pattern for MAIA which tested points in different locations than the 10 degree standard grid pattern employed in our study, and Vujosevic et al. followed patients for a much longer period of time (i.e. 6 years).
Although none of the subjects in our study had evidence of progression on clinical dilated fundus exam, the longitudinal changes observed in microperimetry and CCT among subjects with intermediate and/or early AMD suggest that these psychophysical tests may detect subclinical progression in individuals with dry AMD. The fact that we noted significant declines in BCVA for the intermediate AMD group within a 12-month window underscores the importance of timely therapeutic intervention in intermediate AMD. The psychophysical metrics of BCVA and CCT can also be used to monitor the stability of the disease in patients with early AMD, as no significant changes and moreover no improvements were appreciated in this subgroup over the 12-month study period. Given the sensitive nature of microperimetry15, it is possible that the decline in visual function observed in the normal controls for microperimetry AT from baseline to 12 months may be evidence of subclinical progression to dry AMD. Longer follow-up in this study and other longitudinal natural history studies are needed to assess whether these changes in visual function on microperimetry do in fact precede structural changes on exam. CCT of all cone types (red, green, and blue) also consistently differentiated between the normal control and intermediate AMD groups at baseline and 12 months, and showed significant longitudinal changes at 12 months within the intermediate AMD group. To our knowledge, our study is the most comprehensive longitudinal study to date to evaluate a range of psychophysical tests in early and intermediate AMD subjects, employing CCT in addition to LLVA and microperimetry tests. This study is also the first to assess longitudinal changes in CCT in early-intermediate AMD patients.
This study has limitations that may impact the interpretation of our results. First, the necessary sample size was based on a pilot study13 which focused on detecting the difference in LLVA between groups and used a smaller number of controls and AMD patients, even though the study population was very similar to the current larger study. Second, some subjects were unable or unwilling to complete parts of the study, such as the microperimetry tests, due to the need for pupil dilation or because of time constraints. For the 6-month visit, several subjects elected not to return or complete all of the tests; this resulted in a smaller sample size that likely decreased the ability in detecting potential differences between groups at this visit. Furthermore, in order to understand the reliability of predicting the changes assessed over time, test-retest reliability of the functional measurements will require rigorous assessment before serving as clinical trial endpoints. Continued longitudinal analysis of the groups at future time points will be necessary to confirm persistent changes between and within groups over time, as well as to provide additional important insights into the natural course of visual impairment in dry AMD. Furthermore, it is important to recognize the distinction between statistical and clinical significance. While the observed changes in MP over the 12-month period were small and subclinical, they represent a statistically significant decline in retinal sensitivity that may become more clinically significant as patients are followed over a longer period of time. If MP is able to detect early alterations in visual function before the patient appreciates a subjective change, interventions may be better targeted toward the prevention of disease progression and resulting subjective visual dysfunction.
Future analyses over additional 12 month of longitudinal follow-up in this study will explore the relationship between structure and function in AMD over time by correlating psychophysical tests with findings on optical coherence tomography such as geographic atrophy, RPE drusen complex25, photoreceptor layer thickness, and hyperreflective foci overlying drusen26. This may either confirm that functional impairment proceeds the current structural grading or aid in the search for morphological changes of finer granularity that predict functional impairment and progression in AMD. Suggestions of possible changes in function preceding changes in structure arise in our results that showed significant decline in visual function in the control and intermediate AMD groups as measured on microperimetry PRT and AT but without any observed changes on dilated fundus exams nor examination of retinal images. Additionally, longitudinal changes on the macular pigment optical density test, a measure of the macular carotenoid pigments lutein and zeaxanthin that are thought to be protective factors in AMD27,28, should be correlated to the progression of visual impairment on tests of visual function explored in our study. In the future, these psychophysical tests should be evaluated in subjects with genetically subtyped AMD to allow possibly early identification of patients at risk for disease progression.
In summary, the natural progression of disease in patients with intermediate AMD differs from those of normal aging controls and early AMD. Our study investigated functional markers that could be sensitive to diagnosing disease stage as well as monitoring disease progression in the shortest length of time possible. Of the psychophysical tests of visual function, microperimetry and sensitive tests of cone function such as CCT may be the most likely endpoints to deliver on this goal. Further follow-up of our cohorts is needed to verify these findings at longer time points. The one-year results of this study provide foundational knowledge which should be built upon by ongoing long-term studies of AMD. Such sensitive psychophysical tests may represent useful endpoints for future clinical trials of potential therapies for dry AMD, particularly as we seek to intervene as soon as possible in the early stages of disease.
Acknowledgments
Grant support: This work was supported by the National Institute of Health/ National Eye Institute (K23EY026988), Research to Prevent Blindness and, through Duke University, industry support from Hoffmann-La Roche.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Study on Visual Function Impairments in Dry Age-related Macular Degeneration, clinicaltrials.gov/ identifier:
References
- 1.Garrett D Report Released On One of the Most-Feared Disabilities. Prevent Blindness America: National Eye Institute;2002. [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014;2(2):e106–e116. [DOI] [PubMed] [Google Scholar]
- 3.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew EY, Clemons TE, Agrón E, et al. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013;120(8):1604–1611.e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesmes LA, Jackson ML, Bex P. Visual function endpoints to enable dry AMD clinical trials. Drug Discov Today Ther Strateg. 2013;10(1):e43–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109(7):1235–1242. [DOI] [PubMed] [Google Scholar]
- 7.Owsley C, McGwin JG, Scilley K, Kallies K. Development of a Questionnaire to Assess Vision Problems under Low Luminance in Age-Related Maculopathy. Invest Ophthalmol Vis Sci. 2006;47(2):528–535. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch’s membrane change. Br J Ophthalmol. 1993;77(9):549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41(1):267–273. [PubMed] [Google Scholar]
- 10.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(7):1236–1249. [PubMed] [Google Scholar]
- 11.Medeiros NE, Curcio CA. Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42(3):795–803. [PubMed] [Google Scholar]
- 12.Holz FG, Gross-Jendroska M, Eckstein A, Hogg CR, Arden GB, Bird AC. Colour contrast sensitivity in patients with age-related Bruch’s membrane changes. Ger J Ophthalmol. 1995;4(6):336–341. [PubMed] [Google Scholar]
- 13.Chandramohan A, Stinnett SS, Petrowski JT, et al. VISUAL FUNCTION MEASURES IN EARLY AND INTERMEDIATE AGE-RELATED MACULAR DEGENERATION. Retina. 2016;36(5):1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA ophthalmology. 2015;133(4):442–448. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z, Ayton LN, Guymer RH, Luu CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121(8):1612–1619. [DOI] [PubMed] [Google Scholar]
- 16.Cocce KJ, Stinnett SS, Luhmann UFO, et al. Visual Function Metrics in Early and Intermediate Dry Age-related Macular Degeneration for Use as Clinical Trial Endpoints. Am J Ophthalmol.189:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panel American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern Guidelines Age-Related Macular Degeneration. San Francisco, CA: American Academy of Ophthalmology; 2015. [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(5 Suppl):741–756. [DOI] [PubMed] [Google Scholar]
- 19.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 20.Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(9):1480–1488, 1488.e1481–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman KJ. No Adjustments Are Needed for Multiple Comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 22.Rothman KJ. Six Persistent Research Misconceptions. J Gen Intern Med. 2014;29(7):1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Althouse AD. Adjust for Multiple Comparisons? It’s Not That Simple. Ann Thorac Surg. 2016;101:1644–1645. [DOI] [PubMed] [Google Scholar]
- 24.Vujosevic S, Pucci P, Casciano M, et al. Long-term longitudinal modifications in mesopic microperimetry in early and intermediate age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):301–309. [DOI] [PubMed] [Google Scholar]
- 25.Farsiu S, Chiu SJ, O’Connell RV, et al. Quantitative classification of eyes with and without intermediate age-related macular degeneration using optical coherence tomography. Ophthalmology. 2014;121(1):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuman SG, Koreishi AF, Farsiu S, Jung S-h, Izatt JA, Toth CA. Photoreceptor Layer Thinning over Drusen in Eyes with Age-Related Macular Degeneration Imaged In Vivo with Spectral-Domain Optical Coherence Tomography. Ophthalmology. 2009;116(3):488–496.e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 28.Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124(8):1151–1162. [DOI] [PubMed] [Google Scholar]