Abstract
Resident memory T (TRM) cells in the lung are vital for heterologous protection against influenza A virus (IAV). Environmental factors are necessary to establish lung TRM, however the role of T cell intrinsic factors like T cell receptor (TCR) signal strength have not been elucidated. Here we investigated the impact of TCR signal strength on the generation and maintenance of lung TRM cells after IAV infection. We inserted high and low affinity OT-I epitopes into IAV and infected mice after transfer of OT-I T cells. We uncovered a bias in TRM formation in the lung elicited by lower affinity TCR stimulation. TCR affinity did not impact the overall phenotype or long-term maintenance of lung TRM cells. Overall, these findings demonstrate that TRM formation is negatively correlated with increased TCR signal strength. Lower affinity cells may have an advantage in forming TRM to ensure diversity in the antigen-specific repertoire in tissues.
INTRODUCTION
Following infection, naïve antigen specific-CD8+ T cells are primed in secondary lymphoid organs (SLOs) by antigen-bearing dendritic cells (DCs). These antigen-specific CD8+ T cells rapidly expand, differentiate, and traffic to the site of infection. After contraction a proportion survive to become memory cells which can be subdivided into three broad subsets. Two subsets are present primarily in the circulation, and can be distinguished by the expression of CD62L. Effector memory T (TEM) cells lack CD62L expression and preferentially circulate through the blood, splenic red pulp and non-lymphoid tissues (NLTs) (1). Central memory T (TCM) cells express CD62L and circulate through SLOs and the blood (1). The third memory population, tissue resident memory T (TRM) cells, reside in NLTs and are poised to rapidly respond to a secondary infection (2). TRMs can mediate protection against many virus infections and are critical for control of reinfection with influenza A viruses (IAVs) (3–6). While TRM cells in most NLTs appear durable (7–9), TRM cells in the lung wane rapidly with negative consequences for protection against IAV (3–6). Understanding the intrinsic and extrinsic signals that drive formation and maintenance of lung TRM cells will be critical for the design of broadly protective durable IAV vaccines.
Environmental cues are critical drivers of establishing TRM cells (5, 10–12). While some environmental cues needed for TRM formation are known, there may also be a role for cell intrinsic signals. TRM cells are also impacted by antigen presentation and TRM formation in the lung may require local antigen (13–16). In addition to antigen presentation, TCR affinity has been demonstrated to shape memory CD8+ T cell differentiation. Higher affinity TCR interactions push CD8+ T cells towards a TEM cell fate, while reduced TCR affinity stimulation preferentially generates TCM cells (17). Given that TRM share some traits with TEM cells (10), it is possible that high affinity simulation will favor TRM. Conversely, TCR signal strength was shown to be inversely correlated with TRM in the brain (18). However, a separate study found that brain TRM cells had overall higher affinity TCR than peripheral memory counterparts (19). Together these data highlight the potential impacts of TCR affinity in the differentiation of TRM. However, the consensus rules of how TCR affinity effects TRM ontogeny are not yet clear, and this issue has not been investigated in the lung or other mucosal tissues.
To investigate the role of TCR signal strength in TRM cell formation after IAV infection we generated viruses expressing the cognate antigen for OT-I T cells, OVA257–264 SIINFEKL (N4) peptide, or altered peptide ligands (APLs) with reduced affinity. B6 mice bearing naïve OT-I T cells were infected with IAVs containing OT-I epitopes and lung TRM cell formation was assessed. Consistent with published reports, affinity correlated with clonal burst size (20). As expected, cells stimulated by higher affinity ligands also were more likely to form TEM than TCM. Interestingly, decreased affinity stimulated CD8+ T cells had an advantage in forming TRM in the lung. Transcriptional profiling demonstrates that low affinity stimulated cells have increased survival factors suggesting a potential mechanism for the disparity. Though low affinity cells were more likely to form TRM, high and low affinity TRM cells exhibited similar phenotypes and functions. These data suggest that in addition to environmental cues, TCR affinity also has a significant impact on programing TRM differentiation. This is consistent with a model where terminal differentiation negatively impacts TRM, shedding new light on the ontogeny of TRM.
MATERIALS AND METHODS
Mice
Female C57Bl/6 mice were purchased from The Jackson Laboratory. CD45.1 OT-I TCR-Tg mice were provided by Dr. Vezys (University of Minnesota). All experimental protocols involving the use of mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Flow cytometry and reagents
Single cell suspensions were washed with 1 X PBS and stained with a fixable viability dye for 30 min on ice, Ghost Dye™ Red 780 or UV 450 (Tonbo). Cells were washed once with FACS buffer (cold HBSS supplemented with 2% bovine serum), stained with surface Abs, then washed before flow cytometric detection on a BD LSRFortessa (Becton Dickinson). For IFN-γ staining, spleen and lung single cell suspensions were incubated in complete T cell media with/without 1 or 0.1 μg/mL SIINFEKL or SIITFEKL peptides for 4 h at 37° in the presence of GolgiPlug (BD Biosciences). For positive intracellular staining controls, cells were stimulated with eBioscience™ Cell Stimulation Cocktail. Cells were washed 2x with FACS buffer and stained as above. For intracellular staining cells were fixed with BD Cytofix/Cytoperm (BD Biosciences), incubated on ice for 30 min, washed 2x with 1 X BD Perm/Wash buffer, then incubated with Abs for 30 min. Cells were washed 2x with 1 X BD Perm/Wash buffer and resuspended in FACS buffer. Abs used include: B220 (cRA3–6B2), Bcl-2 (BCL.10C4), CD4 (GK1.5), CD8-β (YTS156.7.7), CD44 (IM7), CD45.2 (104), CD62L (H1.2F3), CD103 (2E7), CD127 (SB/199), CX3CR1 (SA011F11), F4/80 (BM8), IFN-γ (XMG1.2), Ki67 (16A8), and KLRG1 (2F1/KLRG1) (Biolegend); CXCR3 (CXCR3–173) (BD Horizon); CD45.1 (A20) (eBioscience); CD8-α (53–6.7) (Tonbo); Cleaved Caspase-3 (D3E9) (Cell Signaling Technology); and Granzyme B (GB11) (ThermoFisher Scientific). H-2Db-PA224 (SSLENFRAYV) and H-2Db-NP366 (ASNENMETM) (NIH Tetramer Core Facility). Complete T cell media consisted of RPMI 1640 with 10% FBS, 4 mM L-glutamate, 0.1mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin and streptomycin, 10 mM HEPES, and 5 mM 2-ME. OT-I cells were gated as Live/Dead−, Dump− (F4/80, CD4, B220), CD8β+, CD45.2−, CD45.1+.
Plasmid design & virus rescue
In-Fusion primers (Takara Bio) were designed for insertion of epitope sequences into nucleotide position 207 in the stalk of NA of influenza A/Puerto Rico/8/1934 (PR8). This site has been previously used to insert exogenous sequences without attenuation (21, 22). The SIINFEKL (N4)- AGTATAATCAACTTTGAAAAACTG, epitope or APL epitopes: SIYNFEKL (Y3)- AGTATATACAATTTTGAAAAACTG or SIITFEKL (T4) AGTATAATCACTTTTGAAAAACTG, were amplified and recombined into pDZ NA via In-Fusion HD cloning. Viruses were rescued via HEK293T transfection and amplified in embryonated chicken eggs as previously described (23). Rescued viruses were sequence confirmed and titered on Madin-Darby canine kidney (MDCK) cells (ATCC).
Cell culture and plaque assay
MDCK cells were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% FBS and 1% pen-strep. Infections were carried out in infection medium PBS with 10% CaMg, 1% pen-strep, 5% bovine serum albumin) at 37°C for 1 hr. Infection medium was replaced with an agar overlay (MEM, 1 mg/mL tosyl_sulfonyl phenylalanyl chloromethlyl ketone trypsin, 1% DEAE-dextran, 5% NaCO3, 2% agar), and cells were cultured at 37°C for 40 h and then fixed with 4% formaldehyde. Blocking and immunostaining were done for 1 hr at 25°C in 5% milk using the following antibodies: polyclonal anti-IAV PR8/34, 1:5,000 (V301-511-552), and peroxidase rabbit anti-chicken IgG, 1:5,000 (303-035-003; Jackson Immuno Research). TrueBlue peroxidase substrate (Kirkegard & Perry Laboratories) was used as directed for detection of virus plaques.
OT-I T cell isolation, adoptive transfer, and IAV infection
Lymph nodes were harvested from OT-I TCR-Tg CD45.1 mice and a single cell suspension was generated. The purity of CD8+ OT-I+ T cells was verified by flow cytometry and 50,000 or 1,000 OT-I T cells were transferred into C57Bl/6 CD45.2 mice via retro-orbital injection. For IAV infection, mice were anesthetized using a weight-based dose of ketamine/xylazine delivered intraperitoneally. Mice were infected intranasally with 40 plaque forming units (PFUs) of IAV APL viruses. During infection, all mice having weight loss exceeding 25% of their starting weight were sacrificed.
In vivo iv injection of anti-CD8 antibody and lymphocyte isolation from tissues
To discriminate parenchymal cells from blood-borne cells, mice were given an intravenous (iv) injection of anti-CD8α (3 μg) for 3 min, as described (24, 25). Mice were euthanized and spleen, lung and female reproductive tract (FRT) were harvested. Tissues were minced and washed 2x with harvest buffer (cold RPMI 1640 supplemented with 5% bovine serum, 4 mM L-glutamate and 10 mM HEPES). Lungs were incubated in a solution of RPMI 1640/ 10% bovine serum/ 2 mM MgCl2/ 2mM CaCl2/ 10 mM HEPES/ 4 mM L-glutamate medium containing 100 U/mL of collagenase type I (Worthington) for 45 min at 37°C. Lung pieces were then incubated in a solution of RPMI 1640/ 10% bovine serum/ 10 mM HEPES/ 4 mM L-glutamate medium containing 1.3 mM EDTA (Calbiochem) for 45 min at 37°C. FRTs were incubated in a solution of RPMI 1640/ 10% bovine serum/ 2 mM MgCl2/ 2mM CaCl2/ 10 mM HEPES/ 4 mM L-glutamate medium containing 50 mg/100 mL of collagenase type IV (Sigma-Aldrich) for 1 h at 37°C. Single cell suspensions of all tissues were generated and stained for flow cytometry as described above.
Tissue preparation and microscopy
C57Bl/6 mice previously infected with IAV_N4 or IAV_T4 were injected iv with anti-CD8α for three minutes prior to takedown. Lungs were fixed in 2% PFA, inflated and flash frozen in optimum cutting temperature compound. 7 μm sections were cut from each block with a Leica CM1950 cryostat and stained for imaging on a Leica DM6000B EPI fluorescent microscope (violet LED). A minimum of 500 and a maximum of 30,000 CD45.1+/CD8α− cells per section were counted using ImageJ software for a percentage of the total number of nucleated cells. Abs used were: CD8α (53–6.7) (Tonbo), CD45.1 (clone A20) (Biolegend) and donkey anti-rat (polyclonal) (Jackson ImmunoResearch).
Next generation mRNA-sequencing sample preparation.
OT-I cells from IAV_N4 and IAV_T4 infected mice were sorted and RNA was extracted using RNeasy Micro Plus kit (Qiagen). The cDNA library was prepared using strand-specific RNA-seq protocols. Samples were run on an Illumina HiSeq 2500 (50 base pair single-end). We obtained an average of 14.5 million reads per sample. Sequencing reads were mapped to the mouse (mm10) genome using Bowtie aligner (bowtie2 version 2.3.4) with local mode, -L 22 and -N 1 parameters (26). Reads were sorted and filtered by removing the PCR duplicates with Samtools (version 1.9) (27) and assigned to Ensembl gene models (Mus_musculus.GRCm38.87.gtf) with featureCounts of the Subread software package (version 1.5) (28). Differentially expressed genes were compared between IAV_N4 and IAV_T4 samples. Statistical analysis was performed using the edgeR bioconductor package (29, 30). Multidimensional scaling was performed by with edgeR using the top 500 differentially expressed genes across samples. Sequencing data deposited under GEO series accession number GSE130609 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE130609)
Statistical analysis
GraphPad Prism was used to determine statistical significance. Student unpaired two-tailed t test or one-way ANOVA. A p value of < 0.05 was considered statistically significant.
RESULTS
Experimental Model
To determine how TCR signal strength impacts lung TRM formation, we inserted the high affinity OT-I SIINFEKL (N4) epitope or low affinity APLs, SIYNFEKL (Y3) and SIITFEKL (T4) (20), into the stalk of neuraminidase (NA). First, we assessed whether insertion of the epitopes into the NA stalk altered the dynamics of the endogenous IAV-specific CD8+ T cell response. We tracked PA224 and NP366-specific CD8+ T cells after transfer of OT-I T cells and infection with IAV epitope viruses. Prior to euthanasia, mice were injected iv with anti-CD8α antibody to distinguish between cells in vascular contiguous compartments, such as lung capillaries (antibody accessible, iv+) and the lung parenchyma (antibody inaccessible, iv−) (24, 25). At 10 and 34 days post infection (dpi) the number of PA224 and NP366-specific CD8+ T cells was similar between the IAV_N4 and IAV_T4 infections (Fig. S1A–D). In the lung, there was a reduction in the number of endogenous IAV-specific CD8+ T cells 10 days after IAV_N4 infection, likely due to the presence of OT-I T cells and the high affinity ligand (Fig. S1A, S1B, bottom) (31). Importantly, both viruses undergo similar replication kinetics and clearance in vivo (Fig. S1G). These data indicate that the IAV_N4 and IAV_T4 viruses are replicating comparably and therefore any differences in OT-I T cell expansion, contraction and TRM formation will not be due to alterations in engineered virus replication.
Low affinity TCR stimulation favors TRM establishment
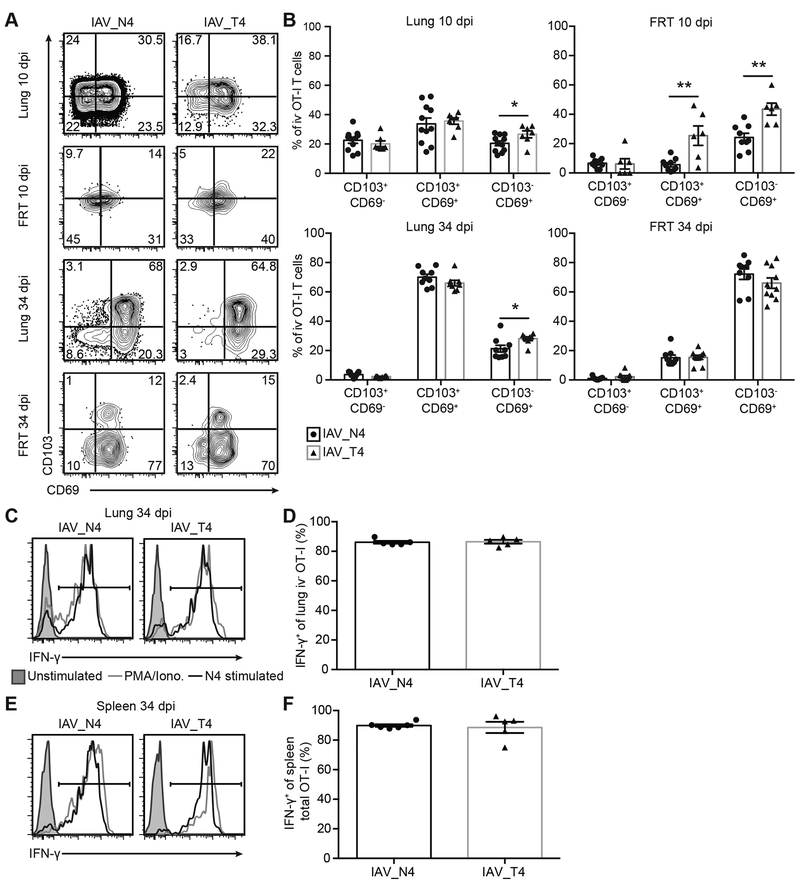
To assess how TCR signal strength impacts lung TRM formation, naïve OT-I T cells were transferred into congenically distinct hosts, infected with IAV_N4, IAV_Y3 or IAV_T4, and tracked over time. As expected, at 10 dpi in the spleen and lung high affinity (N4) stimulated OT-I T cells had undergone a larger clonal burst than low affinity (Y3) or (T4) stimulated cells (Fig. 1A, 1B, S. Table 1). Reduced numbers of low affinity stimulated OT-I T cells were also observed after contraction at 34 dpi (Fig. 1C, 1D, S. Table 1). Due to differences in the clonal burst size between high and low affinity stimulated cells, we decreased the precursor number by 50x and challenged with IAV_N4 in an attempt to approximate the effector pool observed during low affinity stimulation. At 10 dpi in the lung, the 50x reduced precursor cells had reduced expansion, albeit still at higher levels than low affinity stimulation. After contraction, we still observed greater numbers of the 50x reduced high affinity stimulated cells than low affinity stimulated cells (Fig. 1A–D, S. Table 1).
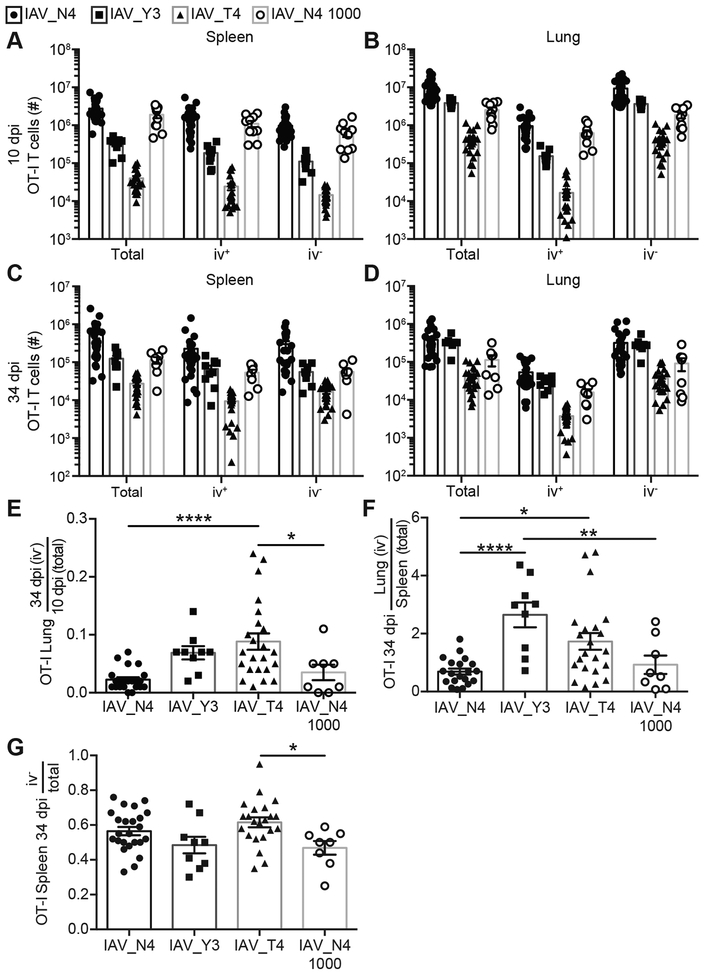
Figure 1. CD8+ T cells stimulated with high or low affinity ligands can form lung TRM.
CD45.1+ OT-I T cells were transferred into CD45.2+ hosts and were infected with 40 PFU of IAV_N4, IAV_Y3 or IAV_T4. Spleen and lungs were harvested after anti-CD8α iv injections. (A-D) The number of OT-I T cells, either total (iv+ and iv− combined), iv+, or iv− cells in the spleen (A, C) and lung (B, D) from mice infected IAV_N4, IAV_Y3, IAV_T4, or IAV_N4 with 1000 OT-I precursors at 10 (A, B) or 34 (C, D) dpi. (E) The number of iv− OT-I T cells in the lung at 34 dpi was divided by the average number of total OT-I T cells in the lung at 10 dpi. (F) The number of iv− OT-I T cells in the lung at 34 dpi was divided by the number of total OT-I T cells in the spleen at 34 dpi, for each mouse. (G) The number of iv− OT-I T cells in the spleen at 34 dpi was divided by the number of total OT-I T cells in the spleen at 34 dpi, for each mouse. The results are compiled from at 2–5 independent experiments with at least 4 mice per group, per experiment (± SEM). A-D one-way ANOVA (see table S1). E-G *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 (unpaired t test).
To determine the capability of differentially stimulated OT-I T cells to form lung TRM, we assessed the ratio of TRM cells at 34 dpi to the total lung OT-I T cells at 10 dpi in the lung (Fig. 1E). Because the clonal burst size is dramatically different between high and low affinity peptide stimulations, normalizing the number of TRM cells at 34 dpi to the number of OT-I cells present in the whole lung at 10 dpi for each peptide directly compares TRM formation. The OT-Is in the lung at 10 dpi represent the cells that could form TRM. If TCR signal strength impacted TRM formation, this ratio would be different between the high and low affinity ligands. An increased proportion of normalized OT-I T cells are present in the lung parenchyma at 34 dpi of IAV_T4 infected mice, compared to IAV_N4 infected mice and IAV_N4 infected mice with 50x reduced OT-I precursors, indicating an advantage in TRM formation by lower affinity T cells (Fig. 1E). When we performed this same calculation on endogenous IAV-specific CD8+ T cells, we did not observe a difference between IAV_N4 and IAV_T4 infections (Fig. S1E). As a way to resolve the ability of cells to differentiate into TRM, we compared the number of lung OT-I TRM cells to the total number of spleen OT-I cells at 34 dpi. A greater proportion of low affinity cells (Y3 or T4 stimulated) than high affinity stimulated cells are lung TRM (Fig. 1F). There was no difference in the ratios of endogenous IAV-specific CD8+ T cells (Fig. S1F). As an additional control we compared the number of splenic OT-I iv− cells to total OT-I cells at 34 dpi as it would be expected that there would not be a difference between low and high affinity stimulation. As expected these data do not show an alteration between the different epitope viruses demonstrating the TRM bias is present in non-lymphoid tissues (Fig. 1G). Importantly, reducing the precursor frequency of high affinity stimulated cells by 50x resulted in a decreased effector pool size, but still resulted in a lower ratio of TRM, suggesting that the size of the effector pool alone is not altering the capacity to form TRM. These data indicate that TCR signal strength can impact lung TRM formation after IAV infection, where lower affinity stimulated cells may be more likely to form TRM.
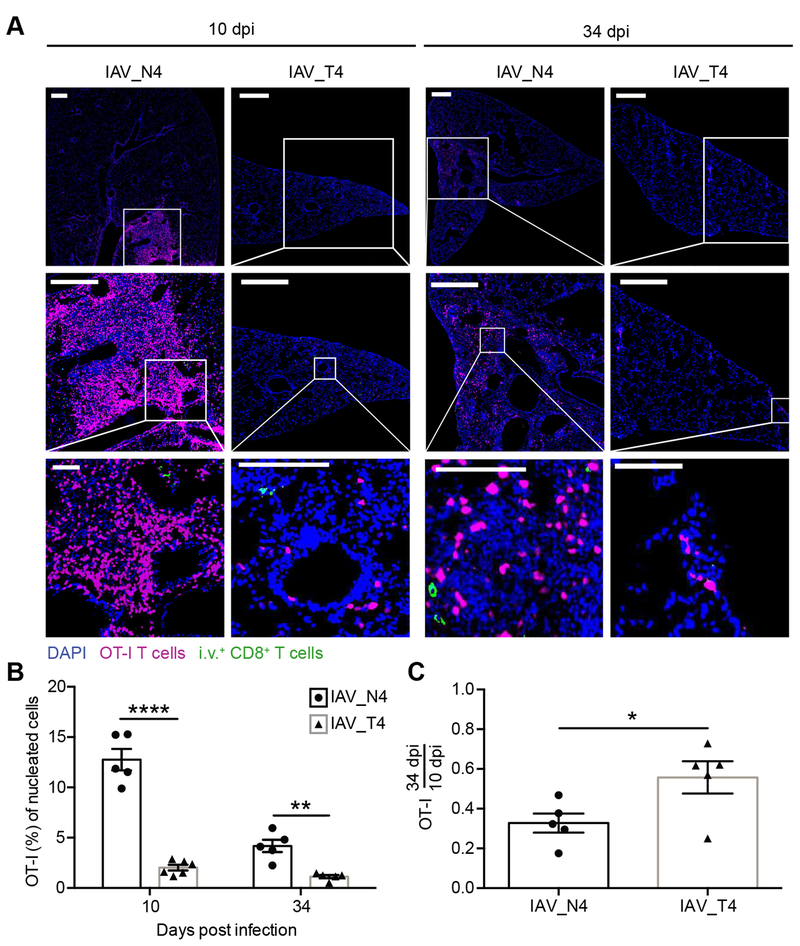
To alleviate technique-specific caveats (2, 32) and to determine the tissue localization we next assessed TRM cells from viruses eliciting varying TCR signal strength by microscopy. Less than 5% of lung OT-I T cells from IAV_N4 or IAV_T4 infected mice were iv antibody positive. We observed increased frequencies of OT-I T cells in the lungs of IAV_N4 infected mice compared to IAV_T4 infected mice at both effector and memory time points (Fig. 2A, 2B). To determine if signal strength impacted memory formation we calculated the ratio of OT-I T cells for each ligand from 10 to 34 dpi. Similar to what we observed by flow cytometry analysis, there was an increased proportion of T4 stimulated OT-I T cells to form TRM by microscopy (Fig. 2C). Together these data further support the concept that the ability to form TRM is impacted by TCR signal strength.
Figure 2. Confirmation of TRM formation by CD8+ T cells stimulated with high or low affinity peptides by microscopy.
Challenged mice were generated as in Figure 1. Tissue sections were stained for DAPI (blue), CD45.1 (magenta) and donkey anti-rat (green) to identify nucleated cells, OT-I T cells and iv+ cells, respectively. (A) Representative images of lungs from IAV_N4 or IAV_T4 epitopes at 10 or 34 dpi. White bars = 500 μm for top and middle rows, 100 μm for bottom row. (B) The percentage of OT-I T cells (CD45.1+) of nucleated cells (DAPI) in the lung from IAV_N4 or IAV_T4 infected mice. Each point represents a mouse, from two distinct lung sections, which were taken at least 100 μm apart. (C) The percentage of OT-I T cells in the lung at 34 dpi was divided by the average number of OT-I T cells in the lung at 10 dpi for each infection. The results (B, C) are compiled from 2 independent experiments with 2–3 mice per group, per experiment (± SEM). *p < 0.05, ** p < 0.01, **** p < 0.0001 (unpaired t test).
TCR signal strength alters TRM formation in a distal uninfected mucosal tissue
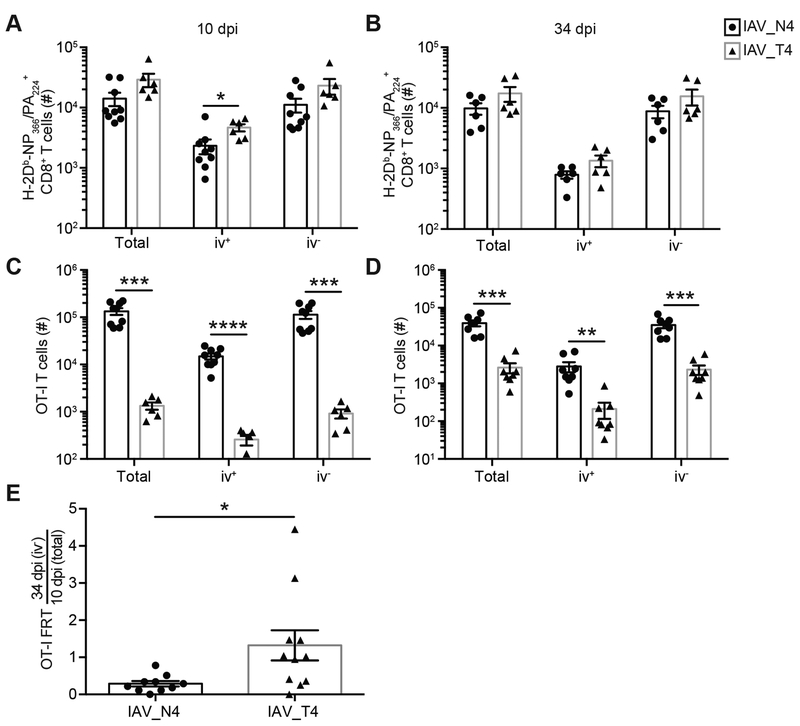
To determine if this was a lung-specific phenotype, we assessed TRM formation in a distal uninfected tissue, the female reproductive tract (FRT), which would lack antigen and inflammation from the IAV infection. Equivalent numbers of endogenous IAV-specific CD8+ T cells were detected in the FRT after IAV_N4 or IAV_T4 infections (Fig. 3A, 3B). Consistent with the spleen and lung, increased numbers of OT-I T cells were present in the FRT after IAV_N4 infection, when compared to IAV_T4 infection (Fig. 3C, 3D). Again, we compared the ratio of TRM cells in the FRT at 34 dpi to the total TRM precursors at 10 dpi which demonstrated an increased proportion of TRM cells generated after IAV_T4 infection (Fig. 3E). These data are consistent with the concept that TRM formation is impacted by the strength of antigen-specific stimulation.
Figure 3. TCR signal strength alters TRM formation in a distal uninfected mucosal tissue.
Challenged mice were generated as in Figure 1. On indicated dpi, FRTs were harvested after anti-CD8α iv injections. (A, B) The number of endogenous (CD45.1− CD45.2+) CD8+ H-2Db-PA224/NP366 tetramer+ CD44hi cells, either total, iv+, or iv− cells in the FRT from mice infected with IAV_N4 or IAV_T4 at 10 (A) or 34 (B) dpi. (C, D) The number of OT-I T cells in the FRT at 10 (C) or 34 (D) dpi. (E) The number of iv− OT-I T cells in the FRT at 34 dpi was divided by the average number of total OT-I T cells in the FRT at 10 dpi. The results (A-E) are representative of 2 independent experiments with at least 3 mice per group (± SEM). *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 (unpaired t test).
TCR signal strength does not impact survival, apoptosis or proliferation during contraction
To understand the disparity in TRM formation between high and low affinity stimulated cells, we analyzed these cells early during contraction at 15dpi. Similar to 10 and 34 dpi, greater numbers of OT-I T cells were present after IAV_N4 challenge at 15 dpi (Fig. S2A). We assessed the expression of Ki67 (proliferation), cleaved caspase-3 (apoptosis) and Bcl-2 (prosurvival molecule) in IAV_N4 or IAV_T4 primed OT-I T cells. Equal expression of Ki67, cleaved caspase-3 and Bcl-2 was seen (Fig. S2B,C). These data suggest, at least at this time point, that alteration in TRM between cells with low and high TCR stimulation is not due to alterations in contraction.
Strength of TCR simulation correlates with T cell differentiation status
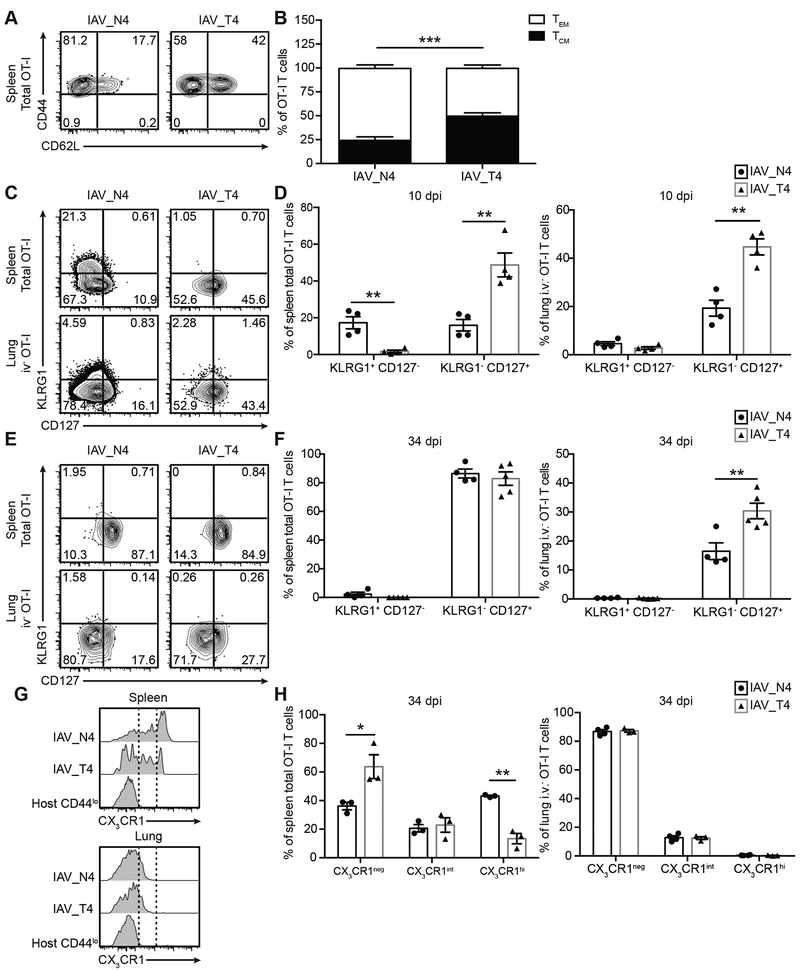
Memory CD8+ T cell differentiation is shaped by the strength of TCR stimulation, where higher affinity interaction correlates with greater TEM generation over TCM generation (17), elevated KLRG1 expression, reduced CD127 (IL-7Rα) expression (33, 34), and increased CX3CR1 expression (35, 36). We assessed these correlations in our IAV model and determined the TCM/TEM ratio of OT-I T cells in the spleen after IAV_N4 or IAV_T4 infections. At 34 dpi, N4 stimulated OT-I T cells had an increased percentage of TEM cells when compared to low affinity stimulated OT-I cells (Fig. 4A, 4B). At 10 dpi in the spleen, an increased frequency of high affinity stimulated OT-I T cells expressed KLRG1 (Fig. 4C, 4D). In both the spleen and lung, a greater percentage of low affinity stimulated OT-I T cells expressed CD127 (Fig. 4C, 4D). This trend was maintained in the lung at 34 dpi (Fig. 4E, 4F). Additionally, we observed a greater frequency of low affinity stimulated cells expressing no or intermediate levels of CX3CR1 in the spleen (Fig. 4G, 4H). Consistent with published results, the majority of lung TRM cells are CX3CR1neg (Fig. 4G, 4H) (35), and the low and high affinity stimulated cells expressed equivalent amounts of CX3CR1 (Fig. 4G, 4H). Overall, our data are consistent with previous reports where high affinity stimulated cells form more TEM than TCM, express more KLRG1 and less CD127, and express higher amounts of CX3CR1 (17, 33–36). These data are consistent with the idea that high affinity stimulated cells are more differentiated towards an effector fate than low affinity stimulated cells, with negative consequence for TRM cell differentiation.
Figure 4. TCR affinity simulation correlates with the differentiation status of OT-I T cells.
Challenged mice were generated as in Figure 1. (A) Representative flow plots of CD62L and CD44 staining of total splenic OT-I T cells from mice infected with IAV_N4 (left) or IAV_T4 (right) at 34 dpi. (B) The percentage of CD44+ CD62L+ (TCM) or CD44+ CD62L− (TEM) OT-I T cells. (C, E) Representative flow plots of CD127 and KLRG1 staining of total splenic (top) or iv− lung OT-I T cells (bottom) at 10 (C) and 34 (E) dpi with IAV_N4 or IAV_T4. (D, F) The percentage of total splenic (left) or iv− lung (right) KLRG1+ CD127− or KLRG1− CD127+ OT-I T cells at 10 (D) and 34 (F) dpi. (G) Representative flow plots of CX3CR1 staining of total splenic (top) or iv− lung OT-I T cells at 34 dpi. Naïve host CD44lo cells are included for reference. Dashed line indicates gates. (H, I). The percentage of total splenic (H) or iv− lung (I) OT-I T cells that CX3CR1neg, int or high at 34 dpi. The results (B) are compiled from 2 independent experiments with at least 4 mice per group, per experiment (± SEM). The results (D, F) are representative of 2 independent experiments, with at least 4 mice per group. The results (H, I) are from 1 experiment with 3–4 mice per group. *p < 0.05, ** p < 0.01, *** p < 0.001 (unpaired t test).
Lung TRM cell phenotype is independent of TCR signal strength
CD103, which binds to E-cadherin promoting residency and CD69, which antagonizes S1PR1 preventing tissue egress, have been classically used as markers for TRM (37). To determine the heterogeneity of cells in the lungs after high and low TCR stimulation, we assessed CD103 and CD69 expression on iv− OT-I T cells on 10 and 34 dpi. At 10 dpi, approximately 50% of iv− OT-I T cells had upregulated CD103 in the lung, with or without CD69 expression, regardless of TCR affinity stimulation (Fig. 5A, 5B). At 34 dpi, the frequency of CD103+ OT-I T cells in the tissue increased to approximately 75%, regardless of the TCR ligand affinity (Fig. 5A, 5B). The majority of CD103+ cells were also CD69+ (Fig. 5A, 5B). At both 10 and 34 dpi we observed slightly more low affinity CD103− CD69− cells in the lung. We also assessed CD103 and CD69 expression of iv− OT-I T cells in the FRT. At 10 dpi more low affinity cells were CD103+ CD69+ and CD103− CD69+, but by 34 dpi, low and high affinity cells were expressing similar levels with the majority of cells expressing CD103− CD69+ (Fig. 5A, 5B) These data indicate that TRM expression of CD103 and CD69 in the lung is independent of TCR affinity.
Figure 5. Phenotype of lung TRM cells is independent of TCR signal strength.
Challenged mice were generated as in Figure 1. (A) Representative flow plots of CD69 and CD103 staining on iv− OT-I T cells in the lung and FRT on 10 and 34 dpi from mice. (B) The percentage of iv− OT-I T cells expressing CD103+CD69−, CD103+CD69+ or CD103−CD69+ (C-F) On 34 dpi, lungs (C, D) and spleens (E, F) were harvested and cells were stimulated ex vivo with 1 μg/mL N4 peptide. As a positive control, cells were stimulated with PMA/Ionomycin. (C, E) Representative IFN-γ staining of iv− lung (C) or total spleen (E) OT-I T cells unstimulated, stimulated with PMA/Ionomycin or N4 peptide. (D, F) Percentage of iv− lung (D) or total spleen (F) IFN-γ+ OT-I T cells after ex vivo N4 stimulation. The results (B) are combined from 2 independent experiments with at least 3 mice per group, per experiment (± SEM). The results (D,F) are 1 representative experiment of 2 independent experiments with at least 5 mice per group, per experiment (± SEM). *p < 0.05, ** p < 0.01 (unpaired t test).
Production of IFN-γ has been demonstrated to be a critical mediator of the sensing and alarm function of TRM to rapidly protect tissues from reinfection (38, 39). The ability of lung TRM cells generated by high or low TCR affinity stimulation to produce IFN-γ was not impacted as measured by ex vivo stimulation with the high affinity N4 peptide (Fig. 5C, 5D). OT-I memory cells in the spleen also produced equal amounts of IFN-γ after stimulation (Fig. 5E, 5F). We also assessed granzyme B production by lung TRMs. Similar to IFN-γ, granzyme B production was equivalent between low and high affinity primed cells (Fig. S3A–B). We also assessed IFN-γ and granzyme B expression in the spleen at 10 dpi and observed equal amounts between low and high affinity primed cells after stimulation (Fig. S3C–F).
To determine functional dynamics of activation ex vivo we stimulated OT-Is from IAV_N4 or IAV_T4 primed mice with two concentrations of either N4 or T4 peptide. Low affinity primed cells stimulated ex vivo with a 10-fold reduced concentration of N4 peptide produced less IFN-γ (Fig. S3G). Additionally, even with low affinity T4 peptide restimulation, high and low affinity primed cells produced equivalent IFN-γ at high peptide concentration (Fig. S3G). These data demonstrate that low affinity TCR interactions during a primary immune response does not alter the functionality of TRM cells upon restimulation, as measured by IFN-γ and granzyme B. Together these data indicate that TCR signal strength does not alter markers of residency in the lung or the response of TRM cells to stimulation.
TRM longevity is independent of TCR affinity
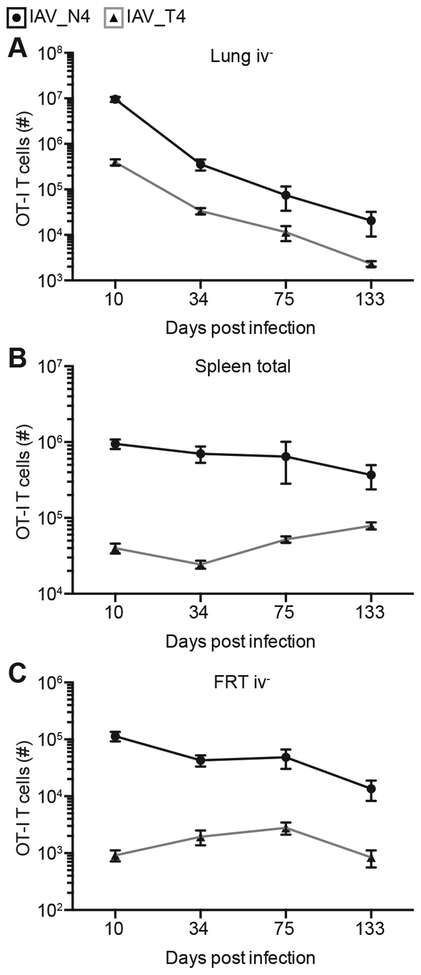
After IAV infection, the number of lung TRM cells and protection against secondary infection wanes with time (4, 5). To determine if TRM maintenance in the lung is impacted by varying TCR signal strength, we assessed the number of OT-I TRM cells at late memory time points after IAV_N4 or IAV_T4 infection. From 34 to 133 dpi, the number of both high and low affinity stimulated OT-I TRM cells decreased at a similar rate in the lung and FRT (Fig. 6A and C). Memory was maintained in the spleen regardless of TCR affinity (Fig. 6B). These data suggest that while TCR signal strength impacts TRM formation it does not alter the longevity of these cells in the tissues, therefore cell extrinsic factors may be critical for long-term maintenance within the lung.
Figure 6. TRM longevity is independent of TCR affinity.
Challenged mice were generated as in Figure 1. On indicated dpi, lungs (A), spleen (B) and FRTs (C) were harvested after anti-CD8α iv injections from mice infected with IAV_N4 or IAV_T4. The results (75 and 133 dpi) are of 1 experiment with at least 4 mice per group (± SEM). The data graphed for 10 and 34 dpi are shown in Figures 1 and 3.
Distinct transcriptomes of high and low affinity lung TRM cells
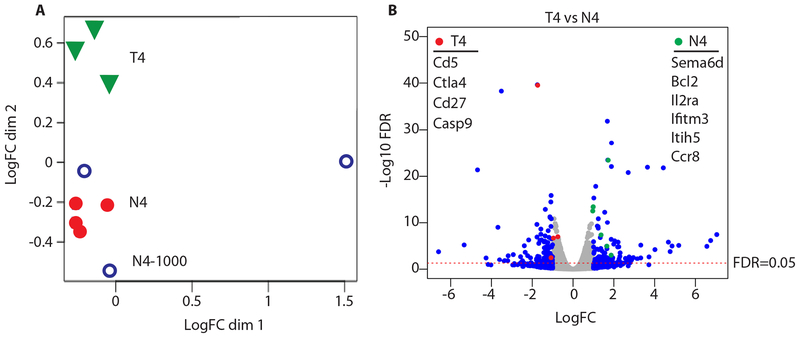
To determine the potential transcriptional signatures leading to increased TRM during low affinity stimulation we sorted OT-I TRM from the lungs of IAV_N4 or IAV_T4 infected mice and performed RNA-seq. Multidimensional scaling demonstrates alterations in the transcriptomes of low and high affinity stimulated cells. Importantly there were no major differences if 50x lower numbers of OT-I were transferred for IAV_N4 stimulation. These data suggest that TCR stimulation and not clonal burst size are responsible for the majority of the transcriptional changes. There were 816 differentially expressed genes between IAV_N4 and IAV_T4 stimulation (FDR < 0.05) (Fig. 7A). Interestingly, several genes involved cell survival were increased in low affinity stimulated cells (Il2ra, Bcl2, Sema6d, Ifitm3) (Fig. 7B). Additionally, there was increased expression of genes that may promote residency (e.g. Ccr8 a marker of residency for TRM in the skin (40) and Itih5 which blocks tumor metastases (41, 42)). Conversely, high affinity stimulated cells exhibited increased cell death and activation markers (Casp9, Cd5, Ctla4, and Cd27) (Fig. 7B). Together these data demonstrate that low affinity stimulation drives a transcriptional program that enhances the potential to survive in the lung tissues.
Figure 7. Transcriptional changes in low and high affinity TRM.
Challenged mice were generated as in Figure 1. CD45.1+ OT-I CD8+ T cells were purified by FACS and RNA profiled by mRNA-seq. (A) Multidimensional scaling plot demonstrating transcript alterations between groups. (B) Volcano plot comparing expression changes between IAV_N4 and IAV_T4 primed OT-I T cells, dotted line FDR=0.05. Blue symbols ± 1 Log2FC. Genes highlighted by red and green circles are listed by Log10 FDR.
DISCUSSION
In this study, we assessed the ability of TCR signal strength to regulate lung TRM formation. We inserted N4 peptide or lower affinity APLs, Y3 and T4, into IAV to assess TRM formation, heterogeneity, response to stimulation and maintenance over time. We analyzed TRM formation by using two independent readouts, flow cytometry and microscopy, to alleviate technique-specific caveats (2, 32). We discovered that lower affinity TCR stimulation elicits a bias towards TRM formation in the lung after IAV infection. Interestingly this low affinity bias was also observed in a distal uninfected mucosal tissue. Lower TCR stimulation drove a distinct transcriptional profile in TRM within the lungs resulting increased expression of pro-survival genes. Additionally, TCR signal strength impacted the differentiation of memory cells, where high affinity stimulated cells formed more TEM cells and expressed greater levels of KLRG1 and CX3CR1 than low affinity stimulated cells, consistent with previous studies (17, 33–36). However, there was no impact of TCR affinity on the expression of TRM markers, or the response of TRM to stimulation, suggesting that TRM quality was not altered. TRM in the lung are short lived, which negatively impacts protection over time (3–6). Importantly, the maintenance of TRM was not impacted by TCR signal strength. Interestingly, in humans IAV-specific memory CD8+ T cells of different clones reacting to the same antigen have different ratios of TRM formation in the lung suggesting the possibility that a range of TCR signal strength can differentially drive TRM in people (43). In conclusion, we demonstrated that T cell intrinsic factors can impact TRM as increased TCR signal strength is negatively correlated with TRM formation. These findings have implications for vaccine design and T cell-based cancer therapeutics by suggesting that excessive T cell stimulation may bias the population away from TRM differentiation.
We chose to use a fixed TCR and alter the affinity of the peptide in the context of IAV infection to study TRM formation in the lung. This system allowed us to transfer in a known number of antigen-specific cells that are easily trackable during all stages of the immune response by both flow cytometry and microscopy. IAV_N4 and IAV_T4 viruses replicated equivalently, ensuring that the OT-I T cells were present in a lung environment with equivalent viral load, antigen load, length of antigen presentation and access to the same populations of antigen presenting cells. Importantly, the presence of endogenous CD8+ T cells does not alter the response of OT-I T cells to low affinity antigens (20). Additionally, all peptides used in this study bind equivalently to the same MHC-I, H-2Kb, thereby eliminating a potential caveat (20). Together this reductionist system allowed us to specifically assess the role of TCR signal strength, eliminating many of the potentially confounding variables found using endogenous epitopes and polyclonal T cell responses. Therefore, we were able to conclude that TRM formation is at least partially influenced by TCR affinity. However, the presence of a large precursor number can alter the ability of stimulated CD8+ T cells to form memory (44). To ensure that a large effector population was not solely responsible we decreased the precursor frequency by 50x to reduce the clonal burst size. Reduced precursor cells stimulated with high affinity antigen were still less effective at forming TRM than low affinity stimulated cells and had similar transcriptional profiles to high affinity cells with a larger number of precursors. These data further suggest that strength of TCR negatively correlates with the capacity to form TRM.
The environment is a critical driver of TRM formation in NLTs. Signals that drive TRM can be expressed in the steady state, and can be enhanced or induced by virus infection. While extracellular queues are required for TRM formation, presence of antigen within the tissue is unclear. Previous studies have demonstrated that depending on the tissue, infection, and site of replication antigen can either be required or dispensable for establishment of TRM (45–50). Non-specific inflammation can pull CD8+ T cells into a NLT which then become resident (48, 49). In contrast, non-pulmonary antigen delivery in the context of lung inflammation does not induce lung TRM (14). Additionally, the presence of cognate antigen in the lung is necessary for optimal sustained expression of CD103 and CD69 by TRM precursors (14). This is analogous with the antigen two hit model for effector T cell function and survival within the lung (51, 52). Conversely, TRM formation after IAV infection occurred in the FRT in the absence of local antigen or inflammation, suggesting that TCR signaling during priming in secondary lymphoid organs imprints memory differentiation including TRM formation. This further highlights the importance of TCR signal strength during priming. It is interesting to speculate on the different requirements of residency in various mucosal tissues. Some may simply be due to tissue-specific factors, such as what cell types are present and the basal production of cytokines and chemokines. This is further supported by the distinct transcriptional profiles of TRM from the lung, skin and gut (10). The plasticity of TRM formation in diverse NLTs ensures adequate responses at the site of a local infection.
During IAV infections CD8+ memory responses are impacted by the dynamics of antigen presentation. Stimulation of IAV-specific CD8+ T cells by different immunodominant epitopes result in differential expression of CD103 and CD69, and differentially regulated transcriptomes in TRM cells (53). This could be driven by multiple factors including antigen load, TCR avidity and the APC subset presenting antigen. CD8+ T cells bind to H-2Db-PA224 with higher avidity than H-2Db-NP366 leading to greater IFN-γ ex vivo (54). In contrast, OT-I TRM cells generated from both high and low affinity antigen can produce equivalent amounts of IFN-γ ex vivo. These epitope-specific differences could be driven by several factors. Not all cells can present NP336 and PA224 epitopes after IAV infection, and the levels of antigen presentation for each epitope varies (15, 55). Furthermore, antigen abundance can dictate levels of memory and TRM during IAV infection (6, 55). Together these data suggest that in addition to environment, the antigen load, TCR avidity, and APC subset all can also affect lung TRM formation. Our system bypasses antigen load and APC subset by expressing the low and high affinity epitopes off of the same IAV gene segment, allowing for direct assessment of the role of TCR signaling in TRM ontogeny. Using this reductionist approach, we identified distinct transcriptional signatures between low and high affinity stimulated TRMs. Interestingly several of the genes increased in the low affinity cells are involved in cellular survival and may underpin the enhanced ability to form TRM. Sema6d is involved in stabilizing CD127 surface expression on memory CD4+ T cells (56). Differences were also seen Ifitm3 which may help low affinity cells survive reinfection and help to prevent the possibility of pathogen escape by having a diverse array of TRMs in the lung (57, 58). We also identified increased expression of Itih5, an inter-alpha trypsin inhibitor, which is involved in extracellular matrix stabilization and prevention of tumor metastases (41, 42, 59). Together these data suggest that transcriptional changes that promote survival and retention in the tissue may be responsible for increased capacity of lower affinity stimulated cells to form TRM.
Using a reductionist approach, we were able to demonstrate T cell intrinsic properties can impact the differentiation of TRM cells during an acute viral infection. Lower affinity stimulation leads to increased proportion of cells capable of forming TRM. While higher affinity stimulations induce larger clonal burst sizes leading to a greater total number of TRM cells, having a bias in TRM formation for lower affinity cells helps to ensure a broad diversity in the TRM pool. This may be critical in preventing escape from CD8+ T cell-mediated pathogen control upon reinfection.
Supplementary Material
KEY POINTS.
Low affinity stimulated CD8+ T cells have an advantage in forming TRM.
High and low affinity stimulated TRMs have distinct transcriptomes.
TRM longevity is not impacted by strength of TCR stimulation.
ACKNOWLEDGEMENTS
We thank Dr. Steve Jameson for helpful discussions regarding this project and Dr. Vaiva Vezys for the gift of CD45.1+ OT-I mice. We acknowledge the NIH Tetramer Core Facility for providing H-2Db-PA224 and H-2Db-NP366 tetramers. We also thank the UMN Flow Cytometry Resource Facility, Genomics Center, and University Imaging Center for expert technical assistance, especially Dr. Jason Mitchell.
GRANT SUPPORT
This work was supported by NIH K22 AI110581 and NIH R01 AI132962 to RAL. JKF was supported by T32 HL007741 and EJF by T32 AI007313. This project was also funded in part with Federal funds from NIAID, NIH and the department of Health and Human Services, under CEIRS Contract No. HHSN272201400005C.
Footnotes
Disclosure
The authors declare no conflicts of interests
REFERENCES
- 1.Sallusto F, Lenig D, Forster R, Lipp M, and Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. [DOI] [PubMed] [Google Scholar]
- 2.Schenkel JM, and Masopust D. 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, and Woodland DL. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol 166: 1813–1822. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, and Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, and Harty JT. 2017. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, Reading PC, and Wakim LM. 2017. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2. [DOI] [PubMed] [Google Scholar]
- 7.Masopust D, Vezys V, Marzo AL, and Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. [DOI] [PubMed] [Google Scholar]
- 8.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, and Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, and Gebhardt T. 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 11.Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, and Samson M. 2011. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol 45: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 12.DeBerge MP, Ely KH, and Enelow RI. 2014. Soluble, but not transmembrane, TNF-alpha is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology. J Immunol 192: 5839–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, and Miyazawa M. 2016. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. The Journal of experimental medicine 213: 3057–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMaster SR, Wein AN, Dunbar PR, Hayward SL, Cartwright EK, Denning TL, and Kohlmeier JE. 2018. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballesteros-Tato A, Leon B, Lee BO, Lund FE, and Randall TD. 2014. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity 41: 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iborra S, Martinez-Lopez M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, Del Fresno C, and Sancho D. 2016. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1(+) Dendritic Cells. Immunity 45: 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith-Garvin JE, Burns JC, Gohil M, Zou T, Kim JS, Maltzman JS, Wherry EJ, Koretzky GA, and Jordan MS. 2010. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood 116: 5548–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maru S, Jin G, Schell TD, and Lukacher AE. 2017. TCR stimulation strength is inversely associated with establishment of functional brain-resident memory CD8 T cells during persistent viral infection. PLoS Pathog 13: e1006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost EL, Kersh AE, Evavold BD, and Lukacher AE. 2015. Cutting Edge: Resident Memory CD8 T Cells Express High-Affinity TCRs. J Immunol 195: 3520–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zehn D, Lee SY, and Bevan MJ. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaton NS, Sachs D, Chen CJ, Hai R, and Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A 110: 20248–20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, and Varga SM. 2018. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS pathogens 14: e1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langlois RA, Varble A, Chua MA, Garcia-Sastre A, and tenOever BR. 2012. Hematopoietic-specific targeting of influenza A virus reveals replication requirements for induction of antiviral immune responses. Proceedings of the National Academy of Sciences of the United States of America 109: 12117–12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, and Braciale TJ. 2005. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest 115: 3473–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, and Masopust D. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langmead B, and Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and Genome S Project Data Processing. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Smyth GK, and Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. [DOI] [PubMed] [Google Scholar]
- 29.Robinson MD, McCarthy DJ, and Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy DJ, Chen Y, and Smyth GK. 2012. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins MR, Webby R, Doherty PC, and Turner SJ. 2006. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol 177: 2917–2925. [DOI] [PubMed] [Google Scholar]
- 32.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, and Masopust D. 2015. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161: 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, and Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, and Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 35.Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, and von Andrian UH. 2016. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 45: 1270–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Hochst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, Flecken T, Giesen D, Engel D, Jung S, Busch DH, Protzer U, Thimme R, Mann M, Kurts C, Schultze JL, Kastenmuller W, and Knolle PA. 2015. Functional classification of memory CD8(+) T cells by CX3CR1 expression. Nat Commun 6: 8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masopust D, and Soerens AG. 2019. Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37: 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, and Masopust D. 2014. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenkel JM, Fraser KA, Vezys V, and Masopust D. 2013. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCully ML, Ladell K, Andrews R, Jones RE, Miners KL, Roger L, Baird DM, Cameron MJ, Jessop ZM, Whitaker IS, Davies EL, Price DA, and Moser B. 2018. CCR8 Expression Defines Tissue-Resident Memory T Cells in Human Skin. J Immunol 200: 1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veeck J, Chorovicer M, Naami A, Breuer E, Zafrakas M, Bektas N, Durst M, Kristiansen G, Wild PJ, Hartmann A, Knuechel R, and Dahl E. 2008. The extracellular matrix protein ITIH5 is a novel prognostic marker in invasive node-negative breast cancer and its aberrant expression is caused by promoter hypermethylation. Oncogene 27: 865–876. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki K, Kurahara H, Young ED, Natsugoe S, Ijichi A, Iwakuma T, and Welch DR. 2017. Genome-wide in vivo RNAi screen identifies ITIH5 as a metastasis suppressor in pancreatic cancer. Clin Exp Metastasis 34: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizzolla A, Nguyen TH, Sant S, Jaffar J, Loudovaris T, Mannering SI, Thomas PG, Westall GP, Kedzierska K, and Wakim LM. 2018. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J Clin Invest 128: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badovinac VP, Haring JS, and Harty JT. 2007. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity 26: 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMaster SR, Wilson JJ, Wang H, and Kohlmeier JE. 2015. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol 195: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, Konig PA, Tao S, Tao R, Heikenwalder M, Busch DH, Korn T, Kastenmuller W, Drexler I, and Gasteiger G. 2016. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. The Journal of experimental medicine 213: 3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan TN, Mooster JL, Kilgore AM, Osborn JF, and Nolz JC. 2016. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. The Journal of experimental medicine 213: 951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, and Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proceedings of the National Academy of Sciences of the United States of America 109: 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin H, and Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, and Farber DL. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGill J, Van Rooijen N, and Legge KL. 2008. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. The Journal of experimental medicine 205: 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGill J, Van Rooijen N, and Legge KL. 2010. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med 207: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizawa A, Bi K, Keskin DB, Zhang G, Reinhold B, and Reinherz EL. 2018. TCR-pMHC encounter differentially regulates transcriptomes of tissue-resident CD8 T cells. Eur J Immunol 48: 128–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Gruta NL, Turner SJ, and Doherty PC. 2004. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol 172: 5553–5560. [DOI] [PubMed] [Google Scholar]
- 55.La Gruta NL, Kedzierska K, Pang K, Webby R, Davenport M, Chen W, Turner SJ, and Doherty PC. 2006. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proceedings of the National Academy of Sciences of the United States of America 103: 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Connor BP, Eun SY, Ye Z, Zozulya AL, Lich JD, Moore CB, Iocca HA, Roney KE, Holl EK, Wu QP, van Deventer HW, Fabry Z, and Ting JP. 2008. Semaphorin 6D regulates the late phase of CD4+ T cell primary immune responses. Proceedings of the National Academy of Sciences of the United States of America 105: 13015–13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen EK, Randolph AG, Bhangale T, Dogra P, Ohlson M, Oshansky CM, Zamora AE, Shannon JP, Finkelstein D, Dressen A, DeVincenzo J, Caniza M, Youngblood B, Rosenberger CM, and Thomas PG. 2017. SNP-mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat Med 23: 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wakim LM, Gupta N, Mintern JD, and Villadangos JA. 2013. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol 14: 238–245. [DOI] [PubMed] [Google Scholar]
- 59.Chen L, Mao SJ, McLean LR, Powers RW, and Larsen WJ. 1994. Proteins of the inter-alpha-trypsin inhibitor family stabilize the cumulus extracellular matrix through their direct binding with hyaluronic acid. J Biol Chem 269: 28282–28287. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.