Abstract
Introduction
Warfarin continues to be the most widely used anticoagulant in clinical practice around the world for the prevention of thromboembolic events in patients with atrial fibrillation (AF). The evaluation of the quality of anticoagulation control, estimated by time in therapeutic range (TTR), is accepted as a good method to evaluate the quality of anticoagulation. The variability of TTR can be explained by the presence of variants of the CYP2C9 and VKORC1 genes.
Methods
This study examined the association between polymorphisms of the CYP2C9 and VKORC1 genes and control of oral anticoagulation, through TTR, in patients with AF. A cross-sectional study was conducted within a cohort follow-up. The study comprised of 317 patients with AF, using warfarin, who were followed up for one year. The genotyping of genes CYP2C9 (rs1057910), (rs1799853) and VKORC1 (rs923231) was performed by PCR in real time, using TaqMan probes.
Results
Patients who had variant genotypes for the CYP2C9*3 gene (rs1057910) presented higher TTR (TTR 81–100%) when compared to when compared to the <45% and 46–60% TTR groups (p=0.005 and p=0.002, respectively). Regarding VKORC1 (rs923231), patients who had the variant genotype for the VKORC1 (rs923231) gene also presented a higher TTR (TTR 81–100%), when when compared to the <45% and 46–60% TTR groups (p=0.005 and p=0.004, respectively). In a multivariate model, VKORC1 (rs923231) remained associated for comparisons with the TTR groups (<45% vs 81–100% groups, p=0.01; and 46–60% vs 81–100% groups, p=0.01).
Conclusion
The genotypes of the CYP2C9*3 (AA) and VKORC1 -1639 (GG) genes were associated with the worst quality of anticoagulation control (TTR) in patients with AF using warfarin in the northeast of Brazil.
Keywords: atrial fibrillation, time in therapeutic range, oral anticoagulant, genetic polymorphism
Introduction
Atrial fibrillation (AF) is the most common supraventricular arrhythmia in clinical practice.1,2 In view of the increase in the elderly population, it is estimated that AF may affect 6–12 million people in the United States by 2050 and 9 million people in Europe by 2060.3 In the Brazilian population, it is estimated that 1.5 million individuals have AF and 33% of hospital admissions occur due to this arrhythmia.4,5
AF is responsible for approximately one-third of hospitalizations for heart rhythm disorders.5 AF, although absent from heart valve disease and preexisting cardiovascular disease, caused mortality in both sexes to double. It is associated with an unfavorable prognosis and a three-fold increase in the incidence of stroke, as well as a three-fold increase in heart failure.6
Oral anticoagulants (OAC) have demonstrated adequate efficacy and safety, being considered a good option in clinical practice with warfarin being the most prescribed OAC for patients with AF.7–10
The clinical benefit and the risk of OAC therapy are associated with the time in which theinternational normalized ratio (INR) values remain between 2 and 3, the time within the time in therapeutic range (TTR).10 Measurement of the quality of anticoagulation evaluates whether therapy is being maintained within a therapeutic range. A high TTR is associated with a reduced risk of thromboembolic and/or hemorrhagic events, indicating a good quality of treatment.11 For TTR values below 60%, the use of strategies that promote the stimulation of adherence and other related actions for patients with TTR below the range is recommended.12,13,14
A maintenance dose of warfarin depends on several factors, such as diet, age, body weight, polypharmacy and genetic polymorphisms.15,16 The most important genetic factors in the dosage of warfarin are the CYP2C9*2 (rs1799853-allele T) and CYP2C9*3 (rs1057910-allele C) polymorphisms and the gene VKORC1 -1639G>A (rs9923231-allele A).16 The presence of these polymorphisms confers increased warfarin sensitivity, leading to an increased risk of bleeding or improved TTR levels during warfarin therapy and suggests the use of lower doses of warfarin to achieve adequate INR and decrease the risk of bleeding events.17–19 Thus, the present study examined the association between polymorphisms of the CYP2C9 and VKORC1 genes and the control of oral anticoagulation through TTR in patients with AF.
Materials and methods
Patients and study design
This is a cross-sectional study within a cohort follow-up, based on the comparison of groups with monitoring of the pharmacological response to warfarin. Three hundred and seventeen subjects, randomly selected, were enrolled in this study. As inclusion criteria, individuals had to be older than 18 years, with AF diagnosed by clinical examination, confirmed by conventional electrocardiographic recording or by 24-hr electrocardiographic record (Holter), in an outpatient clinic, using warfarin with therapeutic target INR between 2 and 3, accompanied by the oral anticoagulation clinic. Individuals who used enzymatic inducers, who were with mechanical valve and patients with diagnosed liver disease were excluded from the study.
Genotyping
The biological material (blood) was collected by vacuum venipuncture for DNA extraction with a commercial kit. The DNA samples were extracted from whole blood (EDTA) with QIAamp Mini Spin Columns kit (Qiagen) following the instructions from the manufacturer. In the next step, the DNA samples were stored at −20ºC until the time during which the genotyping assays were performed. Genotyping methodology was used in real-time PCR, through the TAQMAN® system by using fluorescent probe CYP2C9*2 (rs1799853) assay (C__25625805_10), CYP2C9*3 (rs1057910) assay (C_27104892_10) and VKORC1 -1639G>A (rs9923231) assay (C_30403261_20). Reactions were carried out using TaqMan Genotyping mastermix (Thermo Fisher Scientific, MA, USA) according to the instructions from the manufacturer. For genotyping, the 5 QuantiStudio equipment (Thermo Fisher Scientific, MA, USA) was used. All reactions were performed at the Instituto Aggeu Magalhães-FIOCRUZ-PE.
Calculation of TTR
The calculation of TTR was performed using the Rosendaal method for percentage of INR in the therapeutic range; this method was calculated by incorporating the frequency of measurements of INR and its actual values, assuming that the changes between consecutive INR measurements are linear over time.20 The time of follow-up to evaluate the TTR consisted of 10 appointments in 1 year (2016–2017). The subjects were divided into four groups according to the calculated therapeutic index ranging from 0% to 100%, in which 100% represents the best response to warfarin, group 1 TTR (0–45%), group 2 (TTR 46–60%), group 3 (TTR 61–80%) and group 4 (TTR 81–100%).
Statistical analysis
Data were analyzed through descriptive and inferential statistics, using Epi-info 7.0, GraphPad Prism (version 6) and SPSS 20.0 software. The Kolmogorov–Smirnov test was used to test normality. The chi-square test was used to evaluate the Hardy–Weinberg equilibrium. The allele and genotypic frequencies of the patients grouped according to the TTR were evaluated using the Fisher’s exact test or the chi-square, when appropriate. The differences were considered significant for p-values<0.05. The magnitude of these associations was estimated by OR, using confidence intervals of 95%. Binary logistic regression was used to adjust OR, and the variables with p<0.20 of the univariate model were included as possible confounding factors for the TTR.
Ethical considerations
The study was conducted in accordance with the declaration of Helsinki; all patients provided written informed consent, and the study was evaluated by the Research in Ethics Committee of the Universidade de Pernambuco and approved in document no. 1.337.133 and CAAE: 51283215.0.0000.5192.
Results
Table 1 shows the clinical and demographic characteristics of patients with AF using warfarin. The individuals were divided according to the TTR values and presented a mean age of 60.63±16.11 years; 166 (52.4%) were female and 188 (59%) stated their dominant color to be brown. The main comorbidities were arterial hypertension – 281 (88.64%), heart failure – 163 (51.24%), dyslipidemia – 135 (42.6%) and valvulopathy – 129 (40.75%). Overweight patients – 135 (42.6%) – had the most prevalent body mass index (BMI). Polypharmacy (more than 5 drugs) was found in 110 patients (34.7%). There was no statistical association with the results of demographic and clinical characteristics among the groups, except that male gender was more prevalent in the group with the best therapeutic range (group 4) and the worst therapeutic range (group 1) (p=0.02). The main complications were stroke, 77 (24.3%), and bleeding, 7 (2.2%), in the total population. There was a statistical association when comparing patients with complications with established TTR groups. The frequency of patients with stroke increased with the TTR improvement in TTR (p<0.0001), and in Group 2, in which the TTR is outside the therapeutic range, a predominance of patients with bleeding (p=0.033) was found. The HAS-BLED scores lower than 2, which may be associated with a low risk of bleeding, were more frequent in individuals with a higher TTR (94%) and less frequent in individuals with a lower TTR (65.5%) (p=0.0004).
Table 1.
Sociodemographic and clinical characterization of patients with atrial fibrillation using warfarin (n=317)
| Variable | Total | Group 1, n=98 |
Group 2, n=74 |
Group 3, n=94 |
Group 4, n=51 |
Test χ2 (p-value) |
|---|---|---|---|---|---|---|
| Sociodemographic | ||||||
| Age (mean ± sd) | 60.63±16.11 | 60.26±11.32 | 60.32±12.7 | 64.19±12.59 | 62.59±12.37 | 0.1 |
| Sex | ||||||
| Male | 151 (47.6) | 57 (58) | 25(34) | 42 (45) | 27 (53) | 0.02 |
| Female | 166 (52.4) | 41 (42) | 49 (66) | 52 (55) | 24 (47) | |
| Color/race* | ||||||
| White | 78 (25) | 20 (20.4) | 17 (23) | 27 (28.7) | 14 (27.5) | 0.93 |
| Brown | 188 (59) | 62 (63.3) | 44 (59.5) | 51 (54.3) | 31 (60.8) | |
| Black | 51 (16) | 16 (16.3) | 13 (15.5) | 16 (17) | 6 (11.7) | |
| Clinic | ||||||
| Arterial hypertension | 281 (88.64) | 84 (85.7) | 66 (89.2) | 85 (90.43) | 46 (90.2) | 0.86 |
| Heart failure | 163 (51.24) | 61 (62.24) | 35 (47.3) | 43 (45.74) | 24 (47.1) | 0.15 |
| Coronary artery disease | 38 (12) | 12 (12.24) | 8 (10.8) | 11 (11.7) | 7 (13.73) | 0.99 |
| Valvopathy | 129 (40.7) | 41 (41.84) | 35 (47.3) | 34 (36.2) | 19 (37.25) | 0.65 |
| Diabetes | 59 (18.6) | 16 (16.33) | 14 (18.9) | 22 (23.4) | 7 (13.73) | 0.63 |
| Dyslipidemia | 135 (42.6) | 39 (39.8) | 29 (36.2) | 48 (51.06) | 19 (37.25) | 0.4 |
| Smoker | 119 (37.54) | 42 (44.86) | 25 (33.8) | 35 (37.23) | 17 (33.33) | 0.73 |
| Alcohol user | 106 (33.44) | 56 (57.14) | 49 (66.2) | 59 (62.77) | 34 (66.67) | 0.23 |
| Body mass index | ||||||
| Normal | 90 (28.4) | 24 (24.4) | 24 (32.4) | 29 (30.85) | 13 (25.5) | 0.92 |
| Overweight | 135 (42.6) | 44 (45) | 27 (36.5) | 42 (44.68) | 22 (43) | |
| Obese | 92 (29) | 30 (30.6) | 23 (32) | 23 (24.47) | 16 (31.5) | |
| Complications | ||||||
| Stroke | 77 (24.3) | 21 (21.4) | 15 (20.27) | 25 (26.6) | 16 (31.37) | <0.0001 |
| Bleeding | 7 (2.2) | 0 | 5 (6.8) | 2 (2.1) | 0 | 0.033 |
| Scores | ||||||
| CHADS2VASc <2 | 104 (32.8) | 37 (37.7) | 29 (39.1) | 25 (26.6) | 13 (25.5) | 0.14 |
| CHADS2VASc >2 | 213 (67.2) | 61 (62.3) | 45 (60.9) | 69 (73.4) | 38 (74.5) | |
| HAS-BLED <2 | 234 (73.8) | 64 (65.5) | 50 (67.5) | 75 (79.8) | 48 (94) | 0.0004 |
| HAS-BLED >2 | 83 (26.2) | 34 (34.7) | 24 (32.5) | 19 (20.2) | 3 (6) |
Notes: *Self-referenced; Group 1: TTR 0–45%; Group 2: TTR=46–60%; Group 3: TTR=61–80%; Group 4: TTR=81–100%. All statistically significant associations were highlighted in bold.
Abbreviation: TTR, time in therapeutic range.
The distribution of the genotypes for the CYP2C9 and VKORC1 -1639 G>A gene polymorphisms was in agreement with the Hardy–Weinberg Euilibrium (HWE) (Table 2). The frequency of the variant allele for CYP2C9*2 and CYP2C9*3 was 8.36% and 6% for alleles T and C, respectively, and 16.4% and 12% for CT/TT and AC/CC genotypes, respectively. The variant A allele of the VKORC1 gene 1639 G>A had a frequency of 29.6%, and for the GA/AA variant genotypes it was 48.88%.The mean of TTR was compared between the CC vs CT/TT carries for CYP2C9*2, AA vs AC/CC carries for CYP29*3 and GG vs GA/AA for VKORC1; however, this analysis did not show any statistical association, with p=0.76, p=0.30 and p=0.06, respectively.
Table 2.
Frequency of polymorphisms in CYP2C9 and VKORC1 genes in patients with atrial fibrillation using warfarin (n=317)
| Polymorphism | Allele (n) | AF N (%) | Group 1 n=98 (%) | Group 2 n=74 (%) |
Group 3 n=94 (%) |
Group 4 n=51 (%) |
Test χ2 (p-value) OR (CI 95%)* | ||
|---|---|---|---|---|---|---|---|---|---|
| G1 vs G4 | G2 vs G4 | G3 vs G4 | |||||||
| CYP2C9*2 (rs1799853) (n=317) | C | 581(91.64) | 178 (90.8) | 138 (93.2) | 171 (91) | 94 (92.1) | 0.68 0.82 (0.34–2.02) |
0.73 1.19 0.42–3.03 |
0.83 0.9 (0.36–2.31) |
| T | 53 (8.36) | 18 (19.2) | 10 (6.78) | 17 (9) | 8 (7.9) | ||||
| CC | 265 (83.6) | 80 (81.63) | 64 (86.5) | 78 (83) | 43 (84.3) | 0.82 0.8 (0.34–2.02) |
0.78 0.66 (0.26–1.64) |
0.99 0.9 (0.36–2.3) |
|
| CT | 51 (16.1) | 18 (18.37) | 10 (13.5) | 15 (16) | 8 (15.7) | ||||
| TT | 1 (0.3) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | ||||
| Polymorphism | Allele (n) | AF N (%) |
Group 1 n=79 (%) |
Group 2 n=57 (%) |
Group 3 n=79 (%) |
Group 4 n=51 (%) |
Test χ2 (p-value) OR (CI 95%) | ||
| G1 vs G4 | G2 vs G4 | G3 vs G4 | |||||||
| CYP2C9*3 (rs1057910) (n=266) | A | 490 (94) | 152 (96.2) | 111 (97;3) | 149 (94.3) | 88 (86.3) |
0.003 4.03 (1.59–10.58) |
0.002 5.88 (1.67–19.64) |
0.02 2.63 (1.09–6.33) |
| C | 32 (6) | 6 (3.8) | 3 (2.7) | 9 (5.7) | 14 (13.7) | ||||
| AA | 234 (88) | 73 (92.4) | 54 (94.3) | 70 (88.6) | 37 (72.5) |
0.005 4.6 (1.67–12.91) |
0.002 6.81 (2.0–23.17) |
0.003 2.94 (1.2–6.9) |
|
| AC | 32 (12) | 6 (7.6) | 3 (5.7) | 9 (11.4) | 14 (27.5) | ||||
| CC | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Polymorphism | Allele (n) | AF N (%) |
Group 1 n=96 (%) |
Group 2 n=70 (%) |
Group 3 n=94 (%) |
Group 4 n=51 (%) |
Test χ2 (p-value) OR (CI 95%) | ||
| G1 vs G4 | G2 vs G4 | G3 vs G4 | |||||||
| VKORC1 −1639G>A (rs9923231) (n=311) | G | 438 (70.4) | 138 (73.4) | 102 (72.8) | 140 (74.5) | 58 (56.8) |
0.009 1.93 (1.18–3.18) |
0.009 2.03 (1.2–3.5) |
0.002 2.21 (1.32–3.7) |
| A | 184 (29.6) | 54 (26.6) | 38 (27.2) | 48 (25.5) | 44 (43.2) | ||||
| GG | 159 (51.12) | 51 (53.1) | 39 (55.7) | 54 (57.4) | 15 (29.4) |
0.005 2.72 (1.33–5.73) |
0.004 3.01 (1.36–6.25) |
0.001 3.24 (1.58–6.89) |
|
| GA | 120 (38.58) | 36 (37.5) | 24 (34.3) | 32 (34) | 28 (54.9) | ||||
| AA | 32 (10.3) | 9 (9.4) | 7 (10) | 8 (8.6) | 8 (15.7) | ||||
Notes: Group 1: TTR 0–45%; Group 2: TTR=46–60%; Group 3: TTR=61–80%; Group 4: TTR=81–100%. G1= Group 1; G2= Group 2; G3= Group 3; G4= Group 4. * For statistical analysis, the dominant genetic comparison model was utilized. All statistically significant associations were highlighted in bold.
Abbreviations: AF, atrial fibrillation; TTR, time in therapeutic range.
In the group comparison analysis, Group 4 was used as the comparison group. All comparisons of alleles and genotypes for the CYP2C9*3 gene (rs1057910) were statistically significant, with Group 1 vs Group 4 (p=0.003, OR=4.03, CI 1.59–10.58), Group 2 vs Group 4 (p=0.002, OR=5.88, CI 1.67–19.64) and Group 3 vs Group 4 (p=0.02,OR=2.63, CI 1.09–6.33). In related to the genotypes the findings were as follows: Group 1 vs Group 4 (p=0.005, OR=4.6, CI 1.67–12.91), Group 2 vs Group 4 (p=0.002, OR=6.81, CI 2,0–23,17) and Group 3 vs Group 4 (p=0.003, OR=2.94, CI 1.2–6.9). When comparing the selected groups with VKORC1, statistical significance was also found for the alleles G vs A: Group 1 vs Group 4 (p=0.009, OR=1.93, CI 1.18–3.18), Group 2 vs Group 4 (p=0.009, OR=2.03, CI 1.2–3.5) and Group 3 vs Group 4 (p=0.002, OR=2.21, CI 1.32–3.7), and genotypes (GG vs GA/AA): Group 1 vs Group 4 (p=0.005, OR=2.72, CI 1.3–5.73), Group 2 vs Group 4 (p=0.004, OR=3.01, CI 1.36–6.25) and Group 3 vs Group 4 (p=0.001, OR=3.24, CI 1.58–6.89). There was no statistical significance regarding CYP2C9*2 alleles and genotypes.
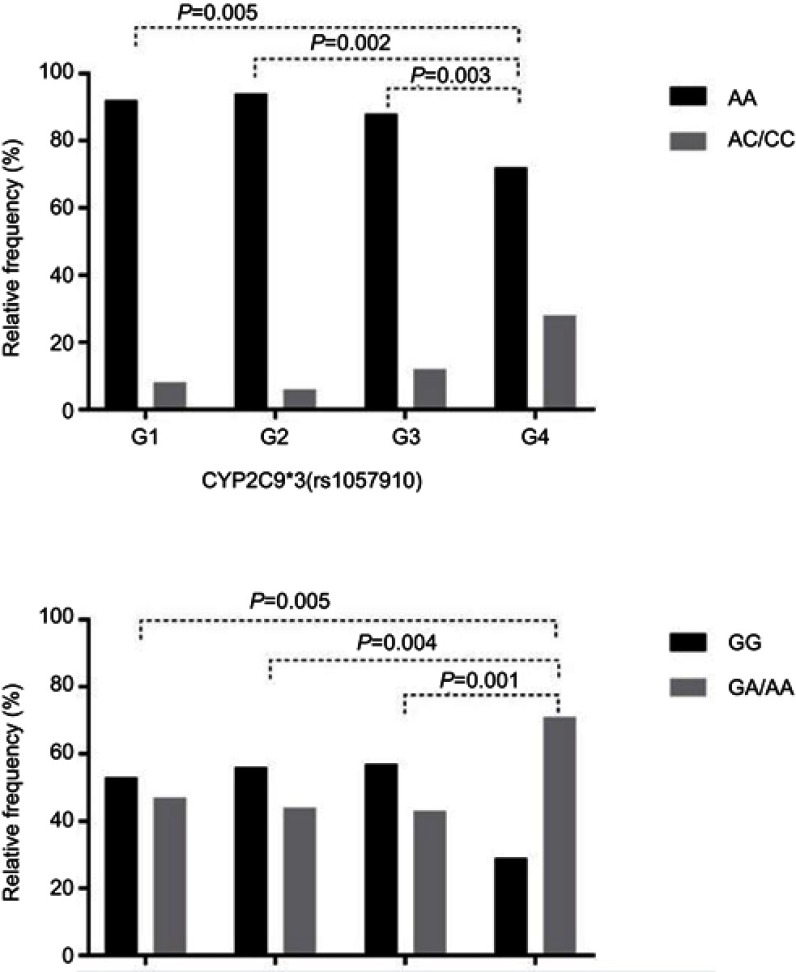
Figure 1 summarizes the findings of Table 2 regarding the frequency and distribution of genotypes in the groups by TTR of the polymorphisms in the CYP2C9*3 and VKORC1 genes and the comparison with the reference group (group 4).
Figure 1.
Relative frequency of genotypes of the CYP2C9*3 (rs1057910) and VKORC1 −1639 G> A (rs923231) genes in patients with atrial fibrillation. G1 (time in therapeutic range (TTR) 0–45%), G2 (TTR 46–60%), G3 (TTR 61–80%), G4 (TTR 81–100%).
Notes: G1= Group 1; G2= Group 2; G3= Group 3; G4= Group 4.
Binary logistic regression analysis was performed for patients with better quality of anticoagulation (group 4), when compared with the group with the worst therapeutic response (group 1). It did not differ in relation to age, the female gender was predominant in Group 2; however, heart failure and the GK genotype of VKORC1 (rs923231) were independent predictors factors for a worse therapeutic response (p=0.03, OR=2.45, CI 1.08–5.55) and (p=0.01, OR=3.05, CI 1.27–7.27), respectively (Table 3).
Table 3.
Binary logistic regression to predict worse therapeutic response in patients with atrial fibrillation using warfarin
| Variable | Group 1 vs group 4 | Group 2 vs group 4 | ||||
|---|---|---|---|---|---|---|
| p | OR | CI 95% | p | OR | CI 95% | |
| Age | 0.72 | 1.00 | (0.98–1.02) | 0.89 | 1.00 | (0.97–1.02) |
| Female sex | 0.66 | 0.82 | (0.35–1.92) | 0.01 | 3.57 | (1.35–9.09) |
| BMI | 0.94 | 1.03 | (0.41–2.58) | 0.95 | 1.02 | (0.37–2.8) |
| Heart failure | 0.03 | 2.45 | (1.08–5.55) | 0.16 | 1.96 | (0.75–5.08) |
| CYP2C9*3 (AA) | 0.47 | 0.58 | (0.13–2.53) | 0.08 | 0.18 | (0.02–1.28) |
| VKORC1 (GG) | 0.01 | 3.05 | (1.27–7.27) | 0.01 | 3.46 | (1.34–8.9) |
Notes: Group 1: TTR=0–45%; Group 2: TTR=46–60%; Group 4: TTR=81–100%. All statistically significant associations were highlighted in bold.
Abbreviation: BMI, body mass index.
In a comparison between group 4 and group 2, being female was observed as a risk factor for a TTR 46–60% (p=0.01, OR=3.57, CI 1.35–9.09) and, again, the GG genotype of VKORC1 (p=0.01, OR=3.46, CI 1.34–8.90) had a direct association with out-of-range TTR, regardless of other factors that may contribute to the poor quality of anticoagulation. In order to summarize the main studies using similar strategy of analysis, we done a table with studies using same genes with TTR response (Table 4).
Table 4.
Comparison of studies in polymorphisms of genes CYP2C9*2, CYP2C9*3 and VKORC with TTR response
| Study | Gene | SNP ID | Genotype | TTR response |
|---|---|---|---|---|
| Mili et al,31 2017 | VKORC1 - 1639 | rs9923231 | GA/AA | Better |
| Wypasek et al,18 2015 | CYP2C9*2 | rs1799853 | CT/TT | Worse |
| CYP2C9*3 | rs1057910 | AC/AA | Worse | |
| Almeida et al,30 2014 | CYP2C9*2 | rs1799853 | CT/TT | Better |
| CYP2C9*3 | rs1057910 | AC/AA | Better | |
| Skov et al,26 2013 | VKORC1 – 1639 | rs9923231 | GA/AA | Better |
| CYP2C9*2 | rs1799853 | CT/TT | Worse | |
| CYP2C9*3 | rs1057910 | CC | Better |
Abbreviations: TTR, time in therapeutic range; SNP, single nucleotide polymorphisms.
Discussion
The present study revealed that the presence of the A and G alleles in the polymorphisms of CYP2C9*3 and VKORC1 genes, respectively, decreases the sensitivity to warfarin by TTR analysis. Furthermore, it is associated with a poorer quality of anticoagulation, the GG genotype of VKORC1 gene being independently associated with a three-fold greater risk for a poorer therapeutic response in a multivariate model.
Singer et al21 demonstrated that female patients (53.3±21.3) presented a lower TTR than males (56.4±21.4 p<0.001). Skov et al26 revealed that the group with TTR>75% had a lower proportion of females (38%) than the TTR group <60% (46.5%, p<0.001)26 Pokorney et al10 found a lower frequency of females in the group with the worst TTR (38%), which is in agreement with those found in the present study. In the multivariate analysis, the female gender is associated as an independent predictor of TTR below the desired level (46–60%) p=0.01.
The clinical variables (hypertension, heart failure, diabetes mellitus, among others) did not influence the TTR. As complications, the study presented a higher frequency of stroke in the group with a higher TTR, a finding that differs from the literature, as in the Pokorney study,10 which showed that the value of TTR was associated with clinical outcomes and that patients with a higher TTR had fewer thromboembolic events. However, in the research in question, the moment at which the patient presented the thromboembolic event was unknown, occurring either prior to OAC therapy or during treatment. Hemorrhage was found more frequently in patients with a lower TTR (p=0.033), corroborating the findings of Pokorney et al10 and White et al22 However, other studies show that the impact of genetic factors on the excessive risk of anticoagulation or bleeding in patients treated with warfarin occurs primarily during the beginning of treatment.10,23
The frequencies of genetic polymorphisms were very similar in the population studied by Santos et al,25 with the following findings: for CYP2C9*2 variant allele T 12.9% and genotype (CT/TT) 22.8%, for CYP2C9*3 variant C allele 3.2%, and genotype (CA/CC) 5.8%.24 In a study by Santos et al25 in a population from the southeast of Brazil, the frequencies of the variant A allele and the variant homozygous genotype for VKORC1 −1639G> A were 32.5% and 12.3%, respectively, while the frequencies of the variant allele of CYP2C9*2 and CYP2C9*3 were 10.8% and 4.6%, respectively.
In a study with patients of Danish origin,26 the variant allele for CYP2C9*3 and VKORC1 −1639 G>A presented a lower TTR and an increased risk of high INR, but the researchers report that this finding cannot be explained by the clinical characteristics of the patients. It therefore differs from that found in the present study, which revealed that patients with a variant allele for the cited polymorphisms presented a higher TTR, a finding that can be explained by the increased sensitivity to warfarin in patients with the variant allele for these polymorphisms.
Patients with a variant allele for the CYP2C9*3 and VKORC1 −1639 G>A polymorphisms require a lower dose of warfarin to maintain a stable INR. Due to the low catalytic activity, the drug remains circulating longer, in a higher concentration and in its most active form (S-enantiomer), and consequently, the sensitivity of warfarin increases, so that patients who have variants in these polymorphisms are considered hypersensitive.19
The follow-up of patients with AF using OAC through TTR is a strategy used in many countries. The use of genotype-guided therapies may provide the patient with an adequate response based on their pharmacogenetic profile.27,28 In this study, the TTR with the best therapeutic range was associated with the variant alleles for CYP2C9*3 and VKORC1 -1639 G>A polymorphisms. The hypersensitivity to warfarin associated with the risk of bleeding can be explained by the presence of the variant alleles; however, in the present study, this association could not be evaluated, due to the presence of an out-of-range TTR, which includes patients with high catalytic activity through the INR above 3, which is associated with the polymorphisms of the genes studied. In fact, the increased risk for bleeding can be observed in patients with polymorphisms in the CYP2C9 and VKORC1 genes.9,19 In the present study, the presence of hemorrhagic events was very low; in addition, it was observed that patients with TTR<45% had a higher HAS-BLED>2 when compared to the groups with a better TTR (p=0.0004).
The presence of variant alleles and genotypes in the abovementioned polymorphisms is intended to assist in the prescription of warfarin dosage in an individualized way, allowing a correct treatment with a decrease in thromboembolic and hemorrhagic events. Since 2007, the Food Drug and Administration (FDA) has changed the label of the warfarin drug, stating that information on the CYP2C9 and VKORC1 genotypes, when available, may be helpful in the prescription of the starting dose.29 However, the FDA does not have a specific algorithm, so the authors have validated the algorithms found in the literature for each population studied.
The present study evaluated the INR of patients with AF from a single clinical center for 1 year; however, it is necessary to confirm the association observed in an independent cohort in other centers. In addition, a knowledge of the dosage used by patients during treatment is important for a dose–response analysis. However, it is believed that the results of this study may alert other clinical centers to the importance using genotypes of CYP2C9 and VKORC1 genes as predictors of response to warfarin and to the way in which it behaves in this population.
Conclusion
The CYP2C9*3 (AA) and VKORC1 -1639 (GG) genotypes were associated with a lower effectiveness of anticoagulation control (TTR) in patients with AF using warfarin. These findings emphasize the need to use a dose prediction algorithm that takes into account the presence of genetic polymorphism in the population studied.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Soeiro AM, Leal TC, Oiveira Jr MT, et al. Manual de Condutas Práticas na Unidade de Emergência do INCORr São Paulo: Ed Manole; 2015. [Google Scholar]
- 2.Heinisch RH, Figueiredo LB, Araújo LR, Joaquim MVG. Clinical and epidemiological profile of patients with atrial fibrillation. Arq Catarin Med. 2013;42(1):409. [Google Scholar]
- 3.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magalhães LP, Figueiredo MJO, Cintra FD, et al. II Brazilian guidelines for atrial fibrillation. Arq Braz of Cardiol. 2016;106:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeida ED, Guimarães RB, Stephan LS, et al. Clinical differences between subtypes of atrial fibrillation and flutter: cross-sectional registry of 407 patients. Arq Braz of Cardiol 2015;105(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira Júnior AC Classification of atrial fibrillation using kurtosis [dissertation]. São Luís, MA; 2017. [Google Scholar]
- 7.Grillo TA, Mirande RC. The new oral anticoagulants in clinical pratice. J Med Minas Gerais. 2014;24(Supl 8):S87–S95. [Google Scholar]
- 8.Pivatto Junior F, Teixeira Filho GF, Sant´Anna JRM, et al. Advanced age and incidence of atrial fibrillation in the postoperative period of aortic valve replacement. J Braz Cir Cardiovasc. 2014;29(1):45–50. doi: 10.5935/1678-9741.20140010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina FT, Zanusso Júnior G. Coumarin anticoagulants: actions, risks and monitoring of therapy. Journal Health E Biol. 2014;9(2):75–82. [Google Scholar]
- 10.Pokorney SD, Simon DN, Thomas L, et al. Patients’ time in therapeutic range on warfarin among US patients with atrial fibrillation: results from ORBIT-AF registry. Am Heart J. 2015;170:141–8.e1. doi: 10.1016/j.ahj.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 11.Marcatto LR, Sacilotto L, Darrieux FCDC, et al. Age is associated with time in therapeutic range for warfarin therapy in patients with atrial fibrillation. Oncotarget. 2016;7(34):54194–54199. doi: 10.18632/oncotarget.10944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa JM, Pimento MC, Groia RCS, Costa MA, Antunes MISS, Martins MAP. Measurements of the time in therapeutic range in patients taking oral anticoagulant. J Braz Farm Health. 2016;7(1):13–16. [Google Scholar]
- 13.Ferreira J, Mirco A. Systematic review of cost-effectiveness analyzes of new oral anticoagulants in the prevention of stroke in atrial fibrillation. J Port Cardiol. 2015;34(3):179–191. doi: 10.1016/j.repc.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 14.Carvalho ARS, Ciol MA, Tiu F, Rossi LA, Dantas RAS. Oral anticoagulation: impact of therapy on health-related quality of life over six months. Journal Latin-Am Nurs. 2013;21(Spec):08 telas. [DOI] [PubMed] [Google Scholar]
- 15.Wypasek E, Mazuri P, Bochenek P, et al. Factors influencing quality of anticoagulation control and warfarin dosage in patients after aortic valve replacement within the 3 months of follow up. J Physiol Pharmacol. 2016;67(3):385–393. [PubMed] [Google Scholar]
- 16.Baranova EV, Asselbergs FW, Boer A, Maitland-van der Zee AH. The COAG and EU-PACT trials: what is the clinical benefit of pharmacogenetic-guided coumarin dosing during therapy initiation? Curr Mol Med. 2014;14(7):841–848. doi: 10.2174/1566524014666140811114906 [DOI] [PubMed] [Google Scholar]
- 17.Jia L, Wang Z, Men J, Cai H, Wei M. Polymorphisms of VKORC1 and CYP2C9 are associated with warfarin sensitivity in Chinese population. Ther Clin Risk Manag. 2017;13:421–425. doi: 10.2147/TCRM.S130198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wypasek E, Cieśla M, Suder B, Janik L, Sadowski J, Undas A. CYP2C9 polymorphism and unstable anticoagulation with warfarin in patients within the first 3 months following heart valve replacement. Adv Clin Exp Med. 2015;24(4):607–614. doi: 10.17219/acem/32577 [DOI] [PubMed] [Google Scholar]
- 19.Gracia BH, Ambrosio EP, Della-Rosa VA. The polymorphism of the CYP2C9 and VKORC1 genes and their influence on the anticoagulant action of warfarin. Journal Health E Biol. 2014;9(2):93–103. [Google Scholar]
- 20.Rosendaal FR, Cannegieter SC, Van Der Meer FMJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. doi: 10.1055/s-0038-1651587 [DOI] [PubMed] [Google Scholar]
- 21.Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2:e000067. doi: 10.1161/JAHA.112.000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167:239–245. doi: 10.1001/archinte.167.3.239 [DOI] [PubMed] [Google Scholar]
- 23.Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW, et al. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427(6974):541–544. doi: 10.1038/nature02254 [DOI] [PubMed] [Google Scholar]
- 24.Santos PC, Dinardo CL, Schettert IT, et al. CYP2C9 and VKORC1 polymorphisms influence warfarin dose variability in patients on long-term anticoagulation. Eur J Clin Pharmacol. 2013;69:789. doi: 10.1007/s00228-012-1404-5 [DOI] [PubMed] [Google Scholar]
- 25.Santos PC, Marcatto LR, Duarte NE, et al. Development of a pharmacogenetic-based warfarin dosing algorithm and its performance in Brazilian patients: highlighting the importance of population-specific calibration. Pharmacogenomics. 2015;16(8)865-876. [DOI] [PubMed] [Google Scholar]
- 26.Skov J, Bladbjerg EM, Leppin A, Jespersen J. The influence of VKORC1 and CYP2C9 gene sequence variants on the stability of maintenance phase warfarin treatment. Thromb Res. 2013. doi: 10.1016/j.thromres.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Pirmohamed M, Burnside FRCP, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386 [DOI] [PubMed] [Google Scholar]
- 28.Meghan J, Arwood PD, Deng J, et al. Anticoagulation endpoints with clinical implementation of warfarin pharmacogenetic dosing in a real-world setting – a proposal for a new pharmacogenetic dosing approach. Clin Pharmacol Ther. 2017;101(5):675–683. doi: 10.1002/cpt.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA approves updates warfarin (Coumadin) prescribing information: new genetic information may help providers initial dosing estimates of the anticoagulation for individual patients. Silver Spring: U.S. Food and Drug Administration; 2009. [Google Scholar]
- 30.Almeida VCO, Ribeiro DD, Gomes KB, Godard ALB. Polymorphisms of CYP2C9, VKORC1, MDR1, APOE and UGT1A1 genes and the therapeutic warfarin dose in Brazilian patients with thrombosis: a prospective cohort study. Mol Diagn Ther. 2014;18(6):675–683. doi: 10.1007/s40291-014-0121-4 [DOI] [PubMed] [Google Scholar]
- 31.Mili FD, Allen T, Wadell PW, et al. VKORC1-1639A allele influences warfarin maintenance dosage among Blacks receiving warfarin anticoagulation: a retrospective cohort study. Future Cardiol. 2018;14(1):15–26. doi: 10.2217/fca-2017-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]