Abstract
Background:
While the complex roles of macrophages in myocardial injury is widely appreciated, the function of neutrophils in non-ischemic cardiac pathology has received relatively little attention.
Methods:
To examine the regulation and function of neutrophils in pressure overload-induced cardiac hypertrophy, mice underwent treatment with Ly6G antibody to deplete neutrophils and then subjected to transverse aortic constriction (TAC).
Results:
Neutrophil depletion diminished TAC-induced hypertrophy and inflammation, and preserved cardiac function. Myeloid deficiency of Wnt5a, a non-canonical Wnt, suppressed neutrophil infiltration to the hearts of TAC-treated mice and produced a phenotype that was similar to the neutropenic conditions. Conversely, mice overexpressing Wnt5a in myeloid cells displayed greater hypertrophic growth, inflammation and cardiac dysfunction. Neutrophil depletion reversed the Wnt5a overexpression-induced cardiac pathology and eliminated differences in cardiac parameters between wild-type and myeloid-specific Wnt5a transgenic mice.
Conclusions:
These findings reveal that Wnt5a-regulated neutrophil infiltration has a critical role in pressure overload-induced heart failure.
Keywords: inflammation, heart, hematopoiesis
Introduction
Heart failure is a growing public health burden, with over 37.7 million individuals estimated to be living with this condition worldwide1. Heart failure is a complex clinical syndrome that results from any functional impairment of ventricular filling or ejection. Thus, a variety of cardiac conditions can result in heart failure and patients display mixed etiologies. Among these, pressure overload-mediated cardiac hypertrophy is recognized as a risk factor for the development of heart failure2,3. Transverse aortic constriction (TAC) is a model of pressure overload hypertrophy that initially results in compensatory left ventricular growth, but at later time points the excessive hypertrophic stimuli will cause heart failure due to capillary rarefaction, cardiac myocyte death and fibrosis in rodents4–6.
The profound inflammatory response to acute myocardial ischemia has been extensively documented with much attention focused on monocytes and macrophages7,8. A population of cardiac macrophages are present in the uninjured myocardium, and macrophage numbers increase following injury due to the infiltration of blood-derived monocytes and their local proliferation9–14. Neutrophils are among the earliest cells recruited to the ischemic myocardium15. It is well established that neutrophil depletion will diminish infarct size and protect the heart from dysfunction in experimental models of myocardial infarction16–19. In contrast, relatively little is known about the roles that myeloid cells play in cardiac hypertrophy that is not associated with acute ischemic injury. Recent studies have shown that mechanical stress or hypertension will lead to the recruitment of Ly6Chi monocytes to the heart where they differentiate into macrophage populations and contribute to cardiac dysfunction20,21. While pressure overload hypertrophy also leads to the recruitment of neutrophils prior to monocyte infiltration22,23, the role that neutrophils play in cardiac hypertrophy and the mechanism that control their recruitment to the heart are not understood.
Methods
The data, analytical methods, and study materials are available from the corresponding author upon request.
Mice
C57Bl/6J wild type mice (Stock# 000664) and C57Bl/6J LysM-Cre mice (Stock# 004781) were obtained from Jackson Laboratories. Wnt5-floxed mice and Wnt5a-transgenic mice were provided by Terry P. Yamaguchi24,25. Male mice were used for both in vivo and in vitro experiments. Myeloid-specific Myelo-KO and TG mice are defined as Wnt5afl/fl, LysMCre/+ (Myelo-KO) and Rosa-Myelo-TG, LysMCre/+ (Myelo-TG) respectively. In all the experiments, Wnt5afl/fl, LysM+/+ mice were used as a control to Myelo-KO mice, and LysMCre/+ mice were used as a control to Myelo-TG mice. Mice were maintained on a 12-h light/dark schedule in a specific pathogen-free animal facility and given food and water ad libitum. The number of mice included in each study is indicated in the figures or the associated legends. The Institutional Animal Care and Use Committee (IACUC) of Boston University and the University of Virginia approved all study protocols.
Neutrophil depletion
Neutrophil depletion was performed using monoclonal anti-mouse Ly6G antibody (Bio X Cell, clone 1A8, Cat# BP0075–1) as described previously26. 500 µg/injection/mouse of anti-Ly6G antibody was injected intraperitoneally to mice at multiple time points that are indicated in the figures. Control mice were injected with isotype control anti-trinitrophenol antibody (Bio X Cell, clone 2A3, Cat# BP0089).
Murine surgical models
Transverse aortic constriction (TAC) was performed as previously27. Briefly, isoflurane-anesthetized mice were subjected to ligation of transverse thoracic aorta between the innominate artery and left common carotid artery using a 27-gauge blunt needle -operated mice without constriction served as controls. Surgery to each group of mice was performed by individuals who were blinded to the identity of the mouse genotype. In some experiments, cells were isolated from ischemic hearts for analysis of Wnt5a transcript expression because ischemia led to greater inflammatory cell recruitment than TAC and thereby increased signal-to-noise ratio. Myocardial infarction was induced by permanent ligation of the left anterior descending coronary artery as previously described. In brief, mice were anesthetized with isoflurane (2%/2 liters O2), intubated and ventilated with an Inspira Advanced Safety Single Animal Pressure/Volume Controlled Ventilator (Harvard Apparatus). Left thoracotomy was performed in the fourth intercostal space after shaving the chest wall. The left ventricle was visualized and the LAD was ligated with monofilament 8–0 suture (Ethicon, Somerville, NJ). The chest and skin were closed with a 7–0 nylon suture followed by removal of air from the thorax via a pleural catheter.
Statistics
Data were expressed as mean ± SEM, except for the box plot where the whisker extends from minimum to maximum. Shapiro-Wilk normality test was used to evaluate data distribution, and F-test was used to evaluate homogeneity of variance. For normally distributed data with one experimental variable, statistical analyses were performed by parametric analysis: unpaired (two-tailed) Student’s t test (with Welch’s correction when variance was unequal) for two groups and one-way ANOVA with Tukey’s multiple comparison test for three groups. For non-normally distributed data with one experimental variable, statistical analyses were performed by non-parametric analysis: Mann-Whitney U test (two-tailed) for two groups and Kruskal-Wallis tests with post-hoc Dunn’s multiple comparison tests for three groups. Data with more than one experimental variable were evaluated by two-way ANOVA, with post-hoc Tukey’s or Sidak’s multiple comparison tests. Results of echocardiography were evaluated by two-way repeated measures ANOVA with post-hoc Sidak’s or Tukey’s multiple comparison tests. Results were considered significantly different at 0.05. All the statistical analyses were performed by GraphPad Prism 7 software (GraphPad Software).
Additional methods are provided in the Online Supplement.
Antibodies used for flow cytometry analyses are provided in Supplemental Table 1. Real-time PCR primers used in this study are provided in Supplemental Table 2.
Study approval
Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University and the University of Virginia.
Results
Immune cell activation by transverse aortic constriction
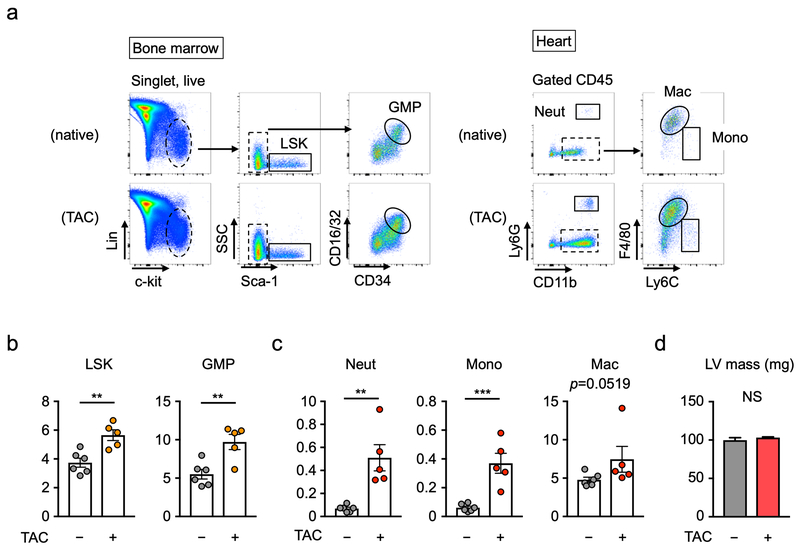
To test whether pressure overload injury to the heart activates the immune system in mice, hematopoietic cells in bone marrow and the heart were analyzed by flow cytometry analysis (Figure 1a). TAC lead to a significant expansion of the granulocyte-macrophage progenitor (GMP) cells, consistent with myeloid cell activation in bone marrow, and an increase in the number of linage-negative, Sca1+, c-Kit+ (LSK) cells at 3 days after TAC (Figure 1b). At this time point, significant Ly6G+ neutrophil and Ly6Chi monocytes infiltration into heart was observed, and cardiac F4/80+ macrophages showed a strong trend toward expansion (p=0.052) (Figure 1c). Notably, the infiltration of neutrophils and monocytes occurred prior to detectable changes in heart weight in response to TAC (Figure 1d).
Figure 1. Immune cell activation by myocardial pressure overload.
a. Flow cytometry gating strategy of bone marrow and heart at 3 days after TAC or sham surgery. Cells were defined as: (i) LSK cells (Lin−, c-Kit+, Sca-1+), (ii) GMP cells (Lin−, c-Kit+, Sca-1−, CD34hi, CD16/32hi), (iii) Neutrophils (CD45+, CD11b+, Ly6G+,), (iv) macrophages (CD45+, CD115+, Ly6G−, F4/80+), (v) Ly6Chi monocytes (CD45+, CD115+, Ly6G−, Ly6Chi). See also Supplemental Figure 1. b. Flow cytometry analysis of bone marrow from sham-treated mice or mice at 3 days after TAC. The absolute number of LSK cells and GMP cells in the bones of a single leg (femur and tibia) are shown. Statistical analysis was performed using unpaired (two-tailed) Student’s t test. c. Flow cytometry analysis of left ventricles from mice at sham state and 3 days after TAC. The absolute numbers of cardiac neutrophils, monocytes and macrophages are shown. Statistical analysis was performed using unpaired (two-tailed) Student’s t test with Welch’s correction (neutrophils and monocytes) and Mann-Whitney U test (macrophages). d. Left ventricle mass at sham state and at 3 days after TAC. Statistical analysis was performed using two-tailed unpaired Student’s t test (with Welch’s correction). For b-d, 5 TAC-treated and 6 mice sham mice were analyzed. **p<0.01, ***p<0.001. TAC=transverse aortic constriction, LSK= Lin−Sca-1+c-Kit+, GMP=granulocyte-macrophage progenitor, Neut= neutrophil, Mono= monocyte, Mac= macrophage, LV=left ventricle, NS = not significant.
Neutrophil depletion inhibits pathological cardiac hypertrophy
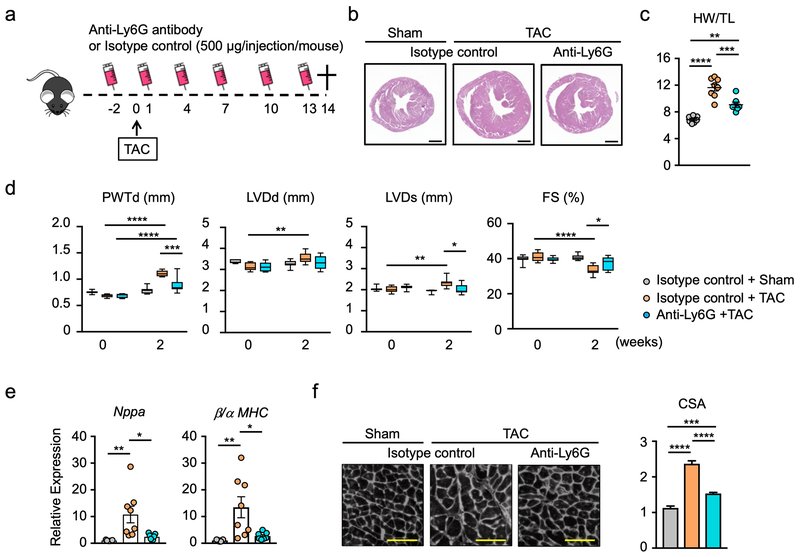
To test whether neutrophils functionally contribute to the pathogenesis of pressure overload hypertrophy, mice were treated with anti-Ly6G antibody to deplete neutrophils (Supplemental Figure 1), and then subjected to transverse aortic constriction (TAC) for 2 weeks (Figure 2a). Neutrophil depletion by this method markedly diminished the hypertrophic response to pressure overload (Figure 2b,c). Echocardiographic measurements showed that anti-Ly6G antibody treatment diminished the TAC-induced increase in posterior wall thickness and alleviated the reduction in fractional shortening (Figure 2d). Consistent with these data, neutrophil depletion markedly reduced the TAC-induced increase in markers of heart failure, including the transcript that encodes for atrial natriuretic peptide A and the ratio of transcripts that encode for myosin heavy chain β/α (Figure 2e). Correspondingly, neutrophil depletion led to a reduction in the TAC-induced increase in cardiac myocyte cross-sectional area (Figure 2f), further documenting that neutrophils exert pro-hypertrophic cardiac myocyte growth and cardiac dysfunction.
Figure 2. Neutropenic mice display attenuated cardiac dysfunction in response to pressure overload.
a. Schematic of the study. Mice were intraperitoneally administered 500 μg/injection/mouse of anti-ly6G (clone 1A8) (n=7) or isotype control (n=8) before and after TAC surgery at indicated time points. Heart from each group were analyzed at 14 days after TAC. Sham group (n=7) was injected isotype control antibody. b. Representative images of hematoxylin-eosin staining of the heart tissue from each experimental group at the end of study. Scale bar indicates 1 mm. c. Heart weight normalized by tibia length from each group at the end of study (n=7-8). Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison tests. d. Echocardiographic parameters of sham group (n=7) and groups injected with anti-Ly6G (n=7) or isotype control antibody (n=8) before and after TAC surgery. Statistical analysis was performed using two-way repeated measures ANOVA with Tukey’s multiples comparison tests. e. Analysis of transcript expression in the myocardium obtained from TAC- or sham-treated mice subjected to anti-Ly6G or isotype control antibody. 36b4 was used as a reference for normalization (n=7-8 in each group). Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison tests. f. Representative images and analysis of wheat germ agglutinin staining of the heart sections from each experimental group at the end of study (n=7–8 in each group). Scale bar indicates 100 μm. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison tests. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. TAC= transverse aortic constriction; HW= heart weight; TL= tibia length; PWTd= posterior wall thickness at diastole; LVDd= left ventricular diameter at end-diastole; LVDs=left ventricular diameter at end-systole; FS= fractional shortening; MHC=myosin heavy chain; CSA= cross sectional area of myocyte.
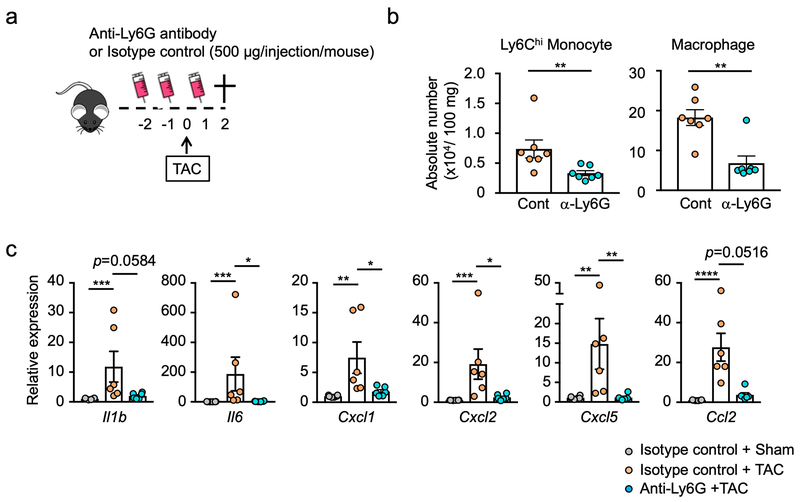
Treatment of mice with anti-Ly6G antibody led to a marked reduction in myocardial markers of inflammation at 2 days post-TAC (Figure 3a). Flow cytometric analysis revealed reductions in levels of infiltrating Ly6Chi monocytes and reduction in the tissue content of CD11b+, Ly6Clo, F4/80+ macrophages (Figure 3b, see gating strategy in Supplemental Figure 2). According, there was reduced expression of transcripts that encode for pro-inflammatory proteins including IL-1β, IL6, Cxcl1, Cxcl2, Cxcl5 and Ccl2 (Figure 3c).
Figure 3. Neutropenic mice display diminished Ly6Chi monocyte infiltration and decreased macrophage content after pressure overload.
a. Scheme of the study. Mice were administered mouse anti-ly6G (500 μg/injection/mouse) or isotype control antibody before and after TAC surgery as indicated. Analysis was performed 2 days after TAC surgery. b. Flow cytometry analysis of cardiac tissue from TAC-treated mice subjected to anti-Ly6G or isotype control antibody (n=7 in each group). Numbers of cells are normalized by tissue weight. Statistical analysis was performed using Mann-Whitney U tests. c. Analysis of transcript expression in the myocardium obtained from sham or TAC-treated mice subjected to anti-Ly6G or isotype control antibody. 36b4 was used as a reference for normalization (n=6 in each group). Statistical analysis was performed using Kruskal-Wallis test with Dunn’s multiple comparison tests (Il1b, Il6, Cxcl1, Cxcl5 and Ccl2) and one-way ANOVA with Tukey’s multiple comparison tests (Cxcl2). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. TAC= transverse aortic constriction.
The requirement of myeloid Wnt5a for pathological cardiac hypertrophy
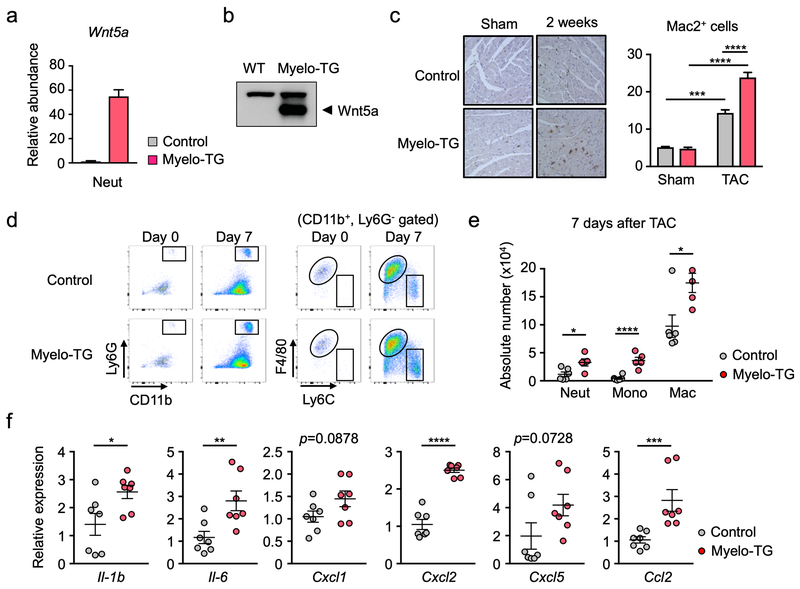
Our previous studies have documented that Wnt5a in myeloid cells regulates inflammatory processes in a diet-induced obesity model25, and it has been reported that Wnt5a stimulates chemotactic migration of neutrophils28. Thus, the role of Wnt5a as a potential regulator of cardiac neutrophils was investigated. Myeloid cells and fibroblasts were isolated from ischemic hearts and assayed for Wnt5a transcript expression. Wnt5a transcript was readily detected in cardiac fibroblasts and Ly6G+ neutrophils that had infiltrated the heart post-myocardial infarction (Supplemental Fig 3a) and post-TAC (Supplemental Fig 3b). Much lower levels of Wnt5a were detected in the CD11b+, Ly6Clo, F4/80+ macrophage fraction, and Wnt5a was undetectable in Ly6Chi monocytes. In contrast, Wnt5a expression was not detected in neutrophils isolated from blood, indicating that the potential source of Wnt5a is activated neutrophils. In separate assays, myeloid cell fractions were assessed for the expression of the Frizzled proteins that serve as Wnt receptors. Only high levels of Fzd5, a Wnt5a receptor29,30, were observed in the neutrophil fraction (Supplemental Figure 3c). Adjusting for 36b4 expression as an internal control, the expression of Fzd5 was much higher than what was observed in Ly6Chi or Ly6CLo monocyte fractions. To examine the action of Wnt5a on neutrophil function in vitro, a chemotaxis assay was performed on bone marrow-derived murine neutrophils using a modified Boyden chamber assay. Consistent with a previous report28, the addition of recombinant Wnt5a protein stimulated neutrophil migration (Supplemental Figure 3d). We also stimulated bone marrow neutrophils with recombinant Wnt5a at different time points to test whether signaling pathway that control neutrophil migration are activated by Wnt5a. The time-dependent activating phosphorylation of PI3K, JNK½ and Erk½ were detected by western blot (Supplemental Figure 3e), consistent with the previously described pro-migratory and pro-inflammatory actions of Wnt5a in neutrophils28.
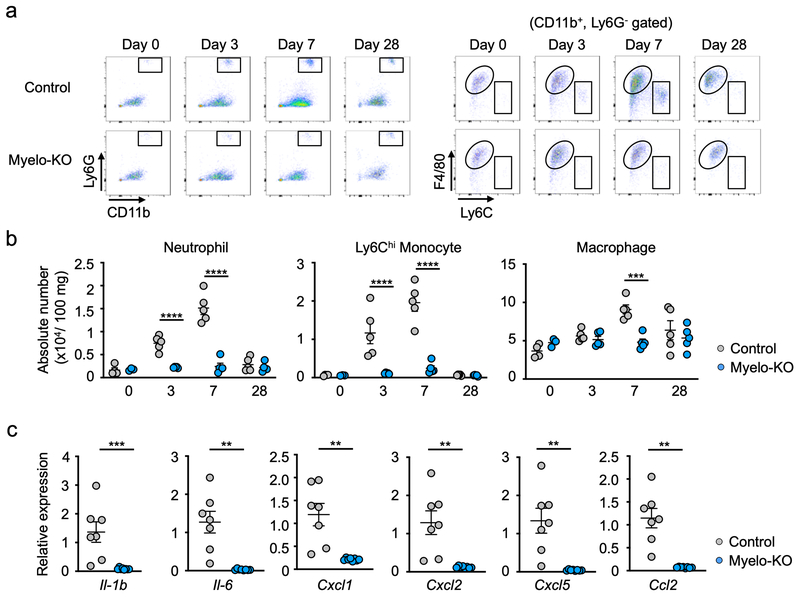
To examine the role of myeloid Wnt5a in pressure overload cardiac hypertrophy in vivo, targeted Wnt5a-deficient (Wnt5a knockout [KO]) mice were generated by crossing Wnt5a floxed mice with LysM-Cre mice25. Removal of exon 2 of Wnt5a was confirmed by PCR analysis of bone marrow derived neutrophils (Supplemental Figure 4). At baseline, there were no detectable differences between total white blood cells or percentages of myeloid and lymphoid cells between genotypes (Supplemental Figure 5). In response to TAC surgery, hearts from these mice displayed markedly attenuated Lys6G+ neutrophil infiltration and diminished CD11b+, Ly6G−, F4/80+ macrophage accumulation (Figure 4a). The absolute numbers of infiltrating neutrophils and Ly6Chi monocytes normalized to 100 μg of cardiac tissue weight were reduced in the Wnt5a-KO mice in response to TAC at 3 and 7 days post-TAC (Figure 4b). The isolation of residual cardiac neutrophils from the TAC-treated Wnt5a-KO mice revealed that they represented cells that had evaded Cre-mediated recombination (Supplemental Figure 6). Corresponding to the decrease in monocyte infiltration, there was a reduction in tissue macrophage number at 7 days post-TAC. Myeloid Wnt5a-deficiency also led to reductions in tissue macrophage proliferation (Supplemental Figure 7). Similar to what was observed by neutrophil deletion (Figure 3c), Wnt5a ablation in LysM-Cre-positive cells resulted in large decreases in transcripts that encode inflammatory mediators, including IL-1β, IL6, Cxcl1, Cxcl2, Cxcl5 and Ccl2, in hearts at 3 days post-TAC (Figure 4c).
Figure 4. Myeloid-specific Wnt5a deficiency reduces cardiac inflammation after pressure overload.
a. Flow cytometry analysis of cardiac tissue from Myelo-KO (Wnt5afl/fl, LysMCre/+) and littermate control (Wnt5afl/fl, LysM+/+) mice before and after TAC at indicated time points. Cells were defined as in Supplemental Figure 1. b. The absolute number of cardiac myeloid cells for each experimental group before and after TAC at the indicated time points (n=4–5 in control and n=3–5 in Myelo-KO group). Numbers are normalized by tissue weight analyzed. Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison tests. c. Analysis of transcript expression in the myocardium obtained from Myelo-KO and littermate control mice at 3 days after TAC surgery. 36b4 was used as a reference for normalization (n=7 in control and n=8 in Myelo-KO group). Statistical analysis was performed using Mann-Whitney U test (Il-1b) and unpaired (two-tailed) Student’s t test with Welch’s correction (Il6, Cxcl1, Cxcl2, Cxcl5 and Ccl2). **p<0.01, ***p<0.001, ****p<0.0001. Myelo-KO= myeloid-specific wnt5a knockout mice.
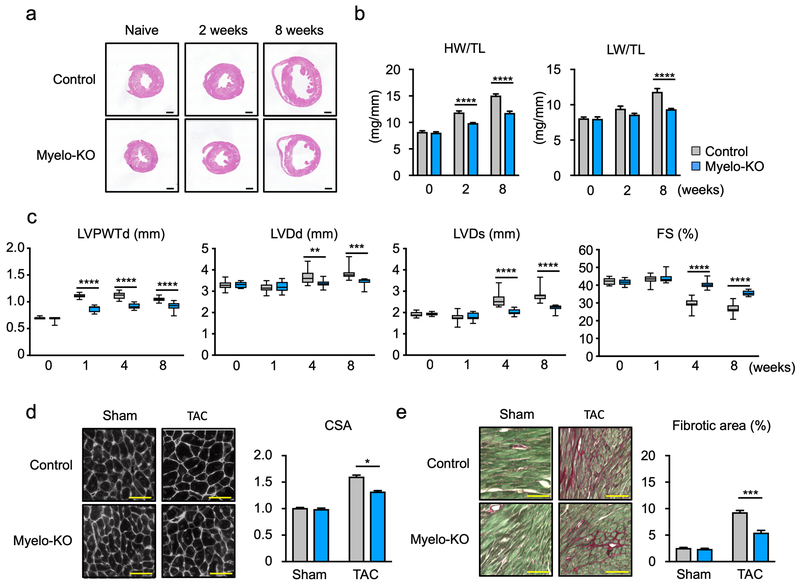
Measurements were performed to analyze cardiac function in the Myelo-KO mice at baseline and at different time points post-TAC. Hearts from Myelo-KO mice displayed less pressure-overload induced hypertrophy that could be observed by the examination of histological sections (Figure 5a) and by diminished heart weight/tibia length ratios at 2 and 8 weeks (Figure 5b). Myelo-KO mice also displayed reduced lung weight at 8 weeks post-TAC, indicative of diminished cardiac dysfunction. Echocardiographic measurements revealed diminished TAC-induced increases in left ventricle posterior wall thickness, and diastolic and systolic dimensions in the TAC-treated Myelo-KO mice compared to wild-type mice (Figure 5c). Correspondingly the Myelo-KO mice displayed improved fractional shortening. TAC-treated Myelo-KO mice also displayed a reduction in myocyte cross-sectional area (Figure 5d) and interstitial fibrosis relative to wild-type mice in response to TAC (Figure 5e).
Figure 5. Myeloid-specific Wnt5a deficiency attenuates cardiac hypertrophy and dysfunction after pressure overload.
a. Representative images of heart sections from Myelo-KO (Wnt5afl/fl, LysMCre/+) and littermate control (Wnt5afl/fl, LysM+/+) mice before and after TAC. Samples were collected as indicated time periods. b. Heart weight or lung weight relative tibia length for the experimental groups (n=5–12 in each group). Scale bar indicates 1 mm. Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison tests. c. Sequential echocardiographic analysis of Myelo-KO and littermate control mice after TAC at the indicated time points (n=12 in each group). Statistical analysis was performed using two-way repeated measures ANOVA with Sidak’s multiple comparison tests. d. Representative image and tabulation of myocyte cross-sectional analysis based upon wheat germ agglutinin staining of the heart sections from each group at the end of study (n=4 in sham and n=6 in TAC groups). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. e. Representative data and analysis of Picrosirius red staining of fibrosis in the heart sections from each experimental group of mice (n=4 in sham and n=6 in TAC groups). Scale bar indicates 100 μm. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Myelo-KO= myeloid-specific wnt5a knockout mice, HW= heart weight, LW= lung weight, TL= tibia length, LVPWTd= left ventricle posterior wall thickness at diastole, LVDd= left ventricle diameter at end-diastole, LVDs= left ventricle diameter at end-systole, FS= fractional shortening, TAC= transverse aortic constriction, CSA= cross sectional area of myocyte.
Myeloid Wnt5a overexpression exacerbates cardiac remodeling
The above work shows that neutrophil depletion protects against TAC-induced remodeling and heart failure and that Wnt5a-deficiency in LysM-Cre+ myeloid cells inhibits neutrophil infiltration to the TAC-injured heart and phenocopies aspects of the neutrophil depletion conditions. Thus, to test the effect of Wnt5a gain of function, a myeloid-restricted Wnt5a overexpression system was generated by crossing LysM-Cre mice with knock-in mice carrying a Cre-inducible Wnt5a transgene. This mouse line expresses high levels of Wnt5a transcript (Figure 6a) and protein (Figure 6b) in bone marrow-derived neutrophils and macrophages (not shown). At baseline, the transgenic overexpression of Wnt5a in myeloid cells had no detectable impact on leukocyte number or on the percentages of myeloid and lymphoid cells in blood (Supplemental Figure 8). Immunohistochemical staining of cardiac sections revealed an increase of Mac2+ macrophages in the Myelo-TG hearts following TAC surgery (Figure 6c). Flow cytometric analysis revealed greater infiltration of Ly6G neutrophils and increased CD11b+, Ly6G−, F4/80+ macrophage content in the heart post-TAC (Figure 6d). The absolute numbers of infiltrating neutrophils, Ly6Chi monocytes and macrophages (normalized to cardiac tissue weight) were greater in the Myelo-TG mice compared to control at 7 days post-surgery (Figure 6e). Greater macrophage proliferation was also observed in the post-TAC hearts of the Myelo-TG mice (Supplemental Figure 9). The overexpression of Wnt5a in LysM-Cre-positive cells led to statistically significant increases in the expression of transcripts that encode for IL-1β, IL6, Cxcl2, and Ccl2, in TAC-treated hearts at 3 days post-TAC and a trend towards increased expression of Cxcl1 and Cxcl5 transcripts (Figure 6f).
Figure 6. Myeloid-specific Wnt5a overexpression enhances cardiac inflammation after pressure overload.
a. Overexpression of Wnt5a in the neutrophils isolated from bone marrow was documented by qRT-PCR (n= 2). Error bar indicates the range of values. b. Representative immunoblot of Wnt5a protein increase within the myeloid lineage of Myelo-TG mice. c. Representative images and analysis of Mac2 staining of the sections of hearts from Myelo-TG (Rosa-Wnt5a, LysMCre/+) and littermate control (LysMCre/+) mice at 2 weeks after TAC or sham surgery (n=3 per sham groups and n=5 per TAC groups). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. d. Flow cytometry analysis of cardiac tissue from Myelo-TG and littermate control mice 7 days after TAC. Cells were defined as in Supplemental Figure 1. e. Absolute number of cardiac myeloid cells in each group 7days after TAC (n=6 in control and n=4 in Myelo-TG group). Numbers are normalized to tissue weight. Statistical analysis was performed using two-tailed unpaired Student’s t test or by Mann-Whitney U test for data which failed to pass the Shapiro-Wilk normality test. f. Analysis of transcript expression in the myocardium obtained from Myelo-TG and littermate control mice at 3 days post-TAC surgery. 36b4 was used as a reference for normalization (n=7 in each group). Statistical analysis was performed using unpaired (two-tailed) Student’s t test (Il-1b, Il6, Cxcl1, Cxcl2) and Mann-Whitney U test (Cxcl5 and Ccl2). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Myelo-TG= myeloid-specific wnt5a overexpression mice, TAC= transverse aortic constriction, Neut= neutrophil, Mono= monocyte, Mac= macrophage.
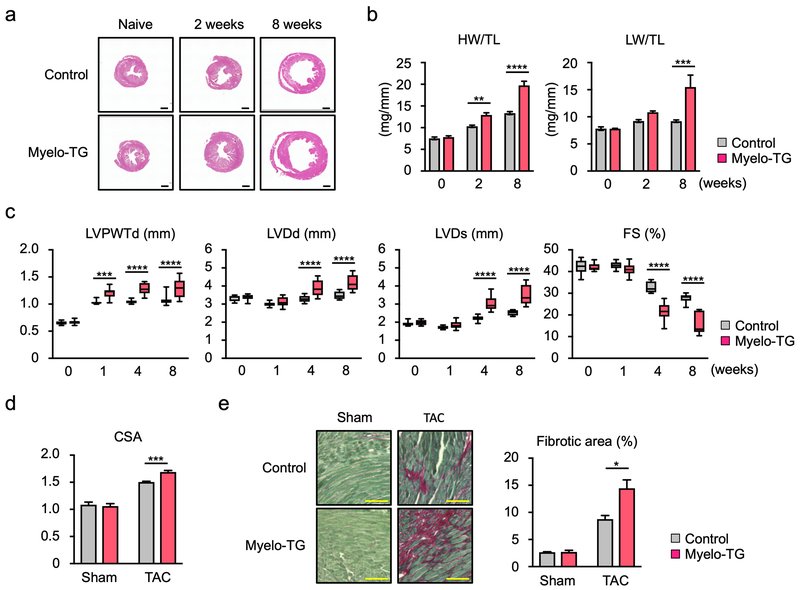
The increase in cardiac inflammation in the Myelo-TG mice was associated with greater cardiac hypertrophy and dysfunction in response to TAC. While no difference in phenotype was observed between the Myelo-TG and control at baseline, Wnt5a overexpression in myeloid cells led to a marked increase in the time-dependent hypertrophic response that could be observed in histological cross-sections of the heart and by increases in heart weight/tibia length ratios (Figure 7a,b). Consistent with greater cardiac failure, Myelo-TG mice also displayed an increase in lung weight to tibia length ratio at 8 weeks post-TAC. Echocardiographic measurements revealed that Myelo-TG mice displayed exacerbated TAC-induced increases in left ventricle posterior wall thickness and diastolic and systolic dimensions, and further reductions fractional shortening compared to wild-type mice that had undergone TAC (Figure 7c). Correspondingly, TAC-treated Myelo-TG mice displayed an increase in myocyte cross-sectional area (Figure 7d). Staining sections with Picrosirius red revealed that Myelo-TG mice also displayed an increase in TAC-induced interstitial fibrosis relative to wild-type mice (Figure 7e).
Figure 7. Myeloid-specific Wnt5a overexpression promotes cardiac hypertrophy and dysfunction in response to pressure overload.
a. Representative images of hematoxylin-eosin staining of heart sections from Myelo-TG (Rosa-Wnt5a, LysMCre/+) and littermate control (LysMCre/+) mice after TAC. Samples were collected as indicated time points. Scale bar indicates 1 mm. b. Heart or lung weights were adjusted by tibia length. (n=5–10 in each group). Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison tests. c. Sequential echocardiographic analysis of Myelo-TG and littermate control mice after TAC at indicated time points (n=10 in each group). Statistical analysis was performed using two-way repeated measures ANOVA with Sidak’s multiple comparison tests. d. Myocyte cross-sectional area was evaluated from the analysis of wheat germ agglutinin staining of the heart sections from each group at the end of study (n=4 in sham and n=6 in TAC groups). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. e. Representative data images and analysis of Picrosirius red staining of heart sections from each group of mice to assess fibrosis (n=4 in each group). Scale bar indicates 100 μm. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. *p<0.05, **p<0.05, ***p<0.001, ****p<0.0001. Myelo-TG= myeloid-specific wnt5a overexpression mice, HW= heart weight, LW= lung weight, TL= tibia length, LVPWTd= left ventricle posterior wall thickness at diastole, LVDd= left ventricle diameter at diastole, LVDs= left ventricle diameter at systole, FS= fractional shortening, CSA= cross sectional area of myocyte.
Neutrophil depletion reverses myeloid Wnt5a-mediated cardiac dysfunction
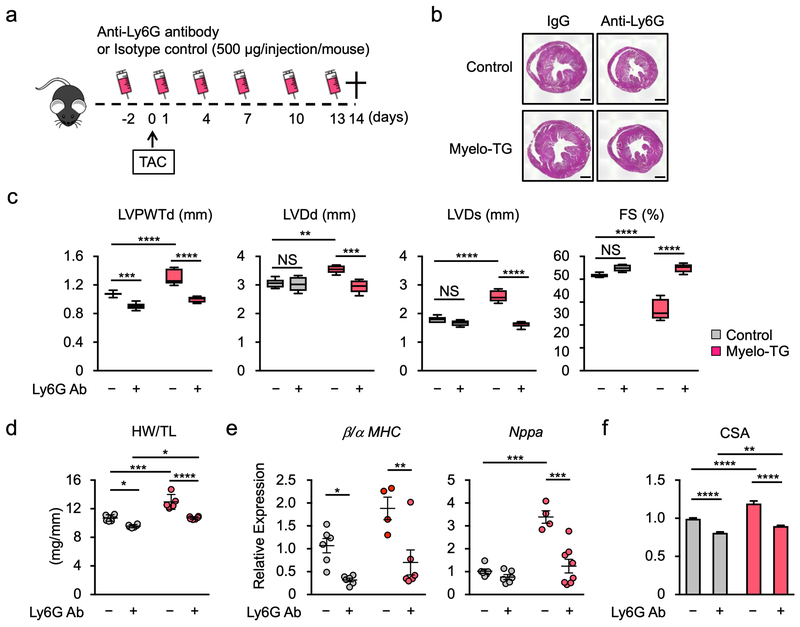
The LysM-Cre promoter, used to overexpress Wnt5a in neutrophils, will also ectopically express the transgene in monocytes and macrophages that express little or no endogenous Wnt5a, thereby creating a situation that could confound the interpretation of the underlying biology. Therefore, additional experiments were conducted to assess whether neutrophil depletion could reverse the exacerbated Myelo-TG phenotype in response to TAC (Figure 8a). In mice that underwent TAC surgery, treatment with Ly6G antibody in control mice and reversed the increase in the hypertrophic response caused by the transgenic overexpression of Wnt5a (Figure 8b, d). Correspondingly, echocardiographic analyses showed that neutrophil depletion normalized the exacerbated changes in the Myelo-TG mice and eliminated any detectable differences between control and Myelo-TG lines in these measurements (Figure 8c). Transcript markers of heart failure were analyzed to extend these analyses (Figure 8e). Neutrophil depletion reduced atrial natriuretic peptide A transcript expression and diminished the ratio of transcripts that encode for myosin heavy chain β/α in the Myelo-TG mice, and eliminated differences in these parameters between the control and transgenic strains. Neutrophil depletion also led to a reduction in the increased myocyte cross-sectional area caused by Wnt5a overexpression in myeloid cells and eliminated differences in this parameter between the wild-type and Myelo-TG strain (Figure 8f). Overall, the percentage impact of the neutrophil depletion was quantitatively greater in the Myelo-TG condition than in the wild-type mice regardless of the cardiac parameter that was analyzed.
Figure 8. Neutrophil depletion reverses the effects of Wnt5a overexpression on the pressure overloaded heart.
a. Schematics of the study. Myelo-TG (Rosa-Wnt5a, LysMCre/+) and littermate control (LysMCre/+) mice were administered anti-ly6G or isotype control antibody (500 μg/injection/mouse) before and after TAC surgery as indicated. Hearts from each group were analyzed at 14 days after TAC. b. Masson’s trichrome staining of the heart sections prepared from each experimental group at the end of study. Scale bar indicates 1 mm. c. Echocardiographic data of Myelo-TG and littermate control mice injected, with anti-Ly6G or isotype control antibody, at 14 days after TAC (n=6 in the control groups and n=5–6 in the Myelo-TG groups). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. d. Heart weight normalized by tibia length from each experimental group at the end of study. n=6 in per control groups and n=5–6 in per Myelo-TG groups. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. e. Analysis of transcript expression in the myocardium obtained from Myelo-TG and control mice at 2 weeks after TAC surgery. 36b4 was used as a reference for normalization (n=6 in control and n=4–6 in myelo-TG group). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. f. Myocyte cross-sectional analysis was determined from wheat germ agglutinin staining of the heart sections from each experimental group at the end of study (n=5–6 in control and n=6 in Myelo-TG groups). Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison tests. *p<0.01, **p<0.01, ***p<0.001, ****p<0.0001. NS= not significant, TAC= transverse aortic constriction, Myelo-TG= myeloid-specific wnt5a overexpression mice, LVPWTd= left ventricle posterior wall thickness at diastole, LVDd= left ventricle diameter at diastole, LVDs= left ventricle diameter at systole, FS= fractional shortening, HW= heart weight, TL= tibia length, MHC=myosin heavy chain, CSA= cross sectional area of myocyte.
Discussion
Neutrophils are abundant, highly migratory and short-lived cells of the innate immune system31,32. They are recruited to sites of injury within minutes of damage, where they become activated and propagate the inflammatory response. This response involves the recruitment of monocytes/macrophages through the abilities of neutrophils to release chemoattractant molecules and upregulate adhesion proteins on vascular endothelial cells. It is well established that neutrophils have critical roles in the acute ischemic injury to the heart and that neutrophil-depletion results in reduced myocardial infarct size in response to ischemia/reperfusion injury17–19. However, the potential role of neutrophils in models of cardiac injury that do not involve acute ischemia or myocardial necrosis has received little attention.
Here, it is shown that TAC leads to activation of hematopoietic cells in bone marrow as indicated by increases in hematopoietic LSK and GMP cells. TAC also leads to the early myocardial infiltration of neutrophils and monocytes, and these changes in the heart and bone marrow precede detectable cardiac hypertrophy. To understand the functional role of neutrophils in cardiac hypertrophy and dysfunction, experiments were performed where mice were depleted of neutrophils immediately prior to TAC surgery. This intervention led marked reductions in TAC-mediated cardiac hypertrophy and dysfunction. Accordingly, neutrophil depletion also led to a reduction in cardiac infiltration by monocytes, diminished macrophage content, and reduced expression of pro-inflammatory mediators in the myocardium. These findings are consistent with the hypothesis that neutrophil infiltration is an early and necessary step in the progression to cardiac hypertrophy and dysfunction that results from pressure overload hypertrophy. It has been reported that bone marrow-derived monocytes are required for TAC-induced cardiac remodeling20, and it is reasonable to hypothesize that the protective effects of neutrophil depletion on the heart are mediated in part by a lack of subsequent monocyte recruitment under these conditions. In support of this mechanism, we found that neutropenic mice display diminished Ly6Chi monocyte infiltration and diminished macrophage content following transverse aortic constriction. This effect is accompanied by large reductions in Cxcl1 chemokine expression that has been shown to mediate monocyte infiltration and cardiac remodeling during hypertrophy33.
Similar to neutropenic mice, mice lacking Wnt5a in myeloid cells display a diminished remodeling response to TAC. The Wnt5a-deficient mice display large reductions in TAC-induced neutrophil and monocyte infiltration to the heart, and a suppression of pro-inflammatory cytokines and chemokines. These mice also display reductions in TAC-induced cardiac hypertrophy and pathological cardiac remodeling. Conversely, mice that overexpress Wnt5a in myeloid cells display increased neutrophil and monocyte infiltration and greater cardiac dysfunction after TAC surgery. Importantly, neutrophil ablation is shown to protect against the progression of the cardiac hypertrophy and failure in both wild-type and myeloid-specific Wnt5a transgenic mice, and this treatment eliminated differences in cardiac parameters between these genotypes. Collectively, these data suggest that Wnt5a acts through its ability to modulate neutrophil recruitment to the heart. Consistent with this notion is the finding that Wnt5a promotes neutrophil migration in vitro and that it is selectively expressed by neutrophils isolated from the heart, suggesting that Wnt5a can function as an autocrine regulator of neutrophil function. However, we do not rule out the possibility that other cellular sources of Wnt5a in the heart contribute to neutrophil cell recruitment in a paracrine manner, or that neutrophils can be regulated in an endocrine manner by circulating Wnt5a or modulators of Wnt5a, such as Sfrp534. In this regard, cardiac myocyte non-canonical Wnt signaling has been shown to activate monocytes in experimental myocardial infarction35.
Wnt5a is involved in a variety of pathological processes in a context-dependent manner. Wnt5a is recognized as a relatively unique member of the Wnt family of secreted signaling molecules because it has direct pro-inflammatory activities through the activation of several non-canonical Wnt signaling pathways. WNT5A has been associated with clinical heart failure36,37, and inflammation caused by metabolic disease25,38 in patient cohorts. Previously, we have shown in ex vivo human tissues or mouse models that Wnt5a functions to impair vascular function and negatively regulate regenerative angiogenesis39–43, and promotes rheumatoid arthritis severity44 and the inflammation associated with metabolic disease25,38,45. In some studies, the effects mediated by Wnt5a have been associated with myeloid cells in general, but little information exists regarding the myeloid cell identity that mediates these effects in vivo. The current work represents the first in vivo study to show that Wnt5a is a regulator of pathological cardiac hypertrophy and that its effects can largely be attributed to neutrophil infiltration.
In addition to monocyte recruitment46, neutrophils also function to promote cardiovascular tissue injury by generating reactive oxygen species47 and the release of DNA, that comprises neutrophil extracellular traps (NETs), through a process that is known as NETosis48. A previous study found that deficiency in peptidylarginine deiminase 4 (PAD4) or treatment with DNase 1, that prevents the formation of NETs or promotes their degradation, respectively, diminishes myocardial collagen accumulation in a model of aortic ascending constriction49. However, it was reported that PAD4-deficiency or DNase1 treatment did not affect other aspects of pressure overload hypertrophy including posterior wall thickness and left ventricular dimensions. Collectively, these data suggest that NETosis promotes cardiac fibrosis but not the other components of the failing heart phenotype that are impacted by Wnt5a-mediated neutrophil infiltration.
In summary, we have shown that neutrophils play a critical role in the hypertrophic response to pressure overload hypertrophy and subsequent cardiac dysfunction. Under these conditions, early neutrophil infiltration is shown to be required for monocyte recruitment to the myocardium and the increase in cardiac macrophage content. These data are consistent with the observation that the subsequent recruitment of bone marrow-derived macrophages that participate for cardiac hypertrophy and dysfunction, at least in part through their ability to activate T cell expansion. It is also shown that non-canonical Wnt5 is essential for neutrophil infiltration of the myocardium and subsequent hypertrophic growth and cardiac dysfunction. These data further document the importance of inflammation in pathological cardiac hypertrophy and heart failure, and reinforce the concept of treating this condition with anti-inflammatory strategies.
Supplementary Material
Clinical Perspective.
What is new?
While numerous studies have elucidated the roles of macrophages in cardiac diseases, this study shows that neutrophils also play a critical role in the hypertrophy of the left ventricle that results from pressure overload in a murine model of heart failure.
Mechanistic studies identify that a non-canonical Wnt protein is essential for the recruitment of neutrophils to the injured myocardium.
What are the clinical implications?
This study expands our knowledge of how inflammatory cells, in particular neutrophils, contribute to the hypertrophy of the left ventricle under conditions that do not involve ischemia or myocardial necrosis.
Since cardiac hypertrophy is a risk factor for the development of heart failure, this study implicates Wnt5a-mediated neutrophil infiltration as an early step in the progression of this disease.
Acknowledgements
Y. Wang, S. Sano, K. Oshima, M. Sano, Y. Watanabe, Y. Katanasaka, Y. Yura, C. Jung, A. Anzai conducted experiments, and acquired and analyzed data. K. Walsh, Y. Wang and S. Sano wrote and edited the manuscript. K. Walsh, F. Swirski and N. Gokce provided supervision. N. Gokce and K. Walsh provided funding. The authors thank Marieke Jones, PhD and Data Services at the Health Sciences Library at the University of Virginia for advice on statistical analyses.
Funding Sources
Y. Wang is supported by the China Scholarship Council. S. Sano is supported by American Heart Association Grant 17POST33670076. N. Gokce is supported by NIH grants HL140836 and HL126141. K. Walsh is supported by NIH grants HL126141, HL131006, HL129120, and HL132564.
Footnotes
Conflict of Interest Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K, Rosendorff C, Yancy C. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e535–e578. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol. 1987;10 Suppl 6:S135–S140. [PubMed] [Google Scholar]
- 4.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009;2:138–144. [DOI] [PubMed] [Google Scholar]
- 5.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertens (Dallas, TX 1979). 2006;47:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frodermann V, Nahrendorf M. Macrophages and Cardiovascular Health. Physiol Rev. 2018;98:2523–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. [DOI] [PubMed] [Google Scholar]
- 10.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ Res. 2016;119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo J-L, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. [DOI] [PubMed] [Google Scholar]
- 16.Hoyer FF, Nahrendorf M. Neutrophil contributions to ischaemic heart disease. Eur Heart J. 2017;38:465–472. [DOI] [PubMed] [Google Scholar]
- 17.Litt MR, Jeremy RW, Weisman HF, Winkelstein JA, Becker LC. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation. 1989;80:1816–1827. [DOI] [PubMed] [Google Scholar]
- 18.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Jones SP, Suhara T, Greer JJM, Ware PD, Nguyen NP, Perlman H, Nelson DP, Lefer DJ, Walsh K. Endothelial cell overexpression of fas ligand attenuates ischemia-reperfusion injury in the heart. J Biol Chem. 2003;278:15185–15191. [DOI] [PubMed] [Google Scholar]
- 20.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2(+) Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC Basic to Transl Sci. 2018;3:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisheit C, Zhang Y, Faron A, Kopke O, Weisheit G, Steinstrasser A, Frede S, Meyer R, Boehm O, Hoeft A, Kurts C, Baumgarten G. Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PLoS One. 2014;9:e112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuster JJ, Zuriaga MA, Ngo DT-M, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim K, Hyun Y-M, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, Kim M. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1beta/NLRP3 Inflammasome. J Am Coll Cardiol. 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung YS, Lee HY, Kim SD, Park JS, Kim JK, Suh P-G, Bae Y-S. Wnt5a stimulates chemotactic migration and chemokine production in human neutrophils. Exp Mol Med. 2013;45:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. [DOI] [PubMed] [Google Scholar]
- 30.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. [DOI] [PubMed] [Google Scholar]
- 31.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. [DOI] [PubMed] [Google Scholar]
- 32.Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015;36:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Zhang Y-L, Lin Q-Y, Liu Y, Guan X-M, Ma X-L, Cao H-J, Liu Y, Bai J, Xia Y-L, Du J, Li H-H. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39:1818–1831. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Sano S, Fuster JJ, Kikuchi R, Shimizu I, Ohshima K, Katanasaka Y, Ouchi N, Walsh K. Secreted Frizzled-related Protein 5 Diminishes Cardiac Inflammation and Protects the Heart from Ischemia/Reperfusion Injury. J Biol Chem. 2016;291:2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer IS, Jungmann A, Dieterich C, Zhang M, Lasitschka F, Werkmeister S, Haas J, Muller OJ, Boutros M, Nahrendorf M, Katus HA, Hardt SE, Leuschner F. The cardiac microenvironment uses non-canonical WNT signaling to activate monocytes after myocardial infarction. EMBO Mol Med. 2017;9:1279–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraityte A, Lunde IG, Askevold ET, Michelsen AE, Christensen G, Aukrust P, Yndestad A, Fiane A, Andreassen A, Aakhus S, Dahl CP, Gullestad L, Broch K, Ueland T. Wnt5a is associated with right ventricular dysfunction and adverse outcome in dilated cardiomyopathy. Sci Rep. 2017;7:3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraityte A, Vinge LE, Askevold ET, Lekva T, Michelsen AE, Ranheim T, Alfsnes K, Fiane A, Aakhus S, Lunde IG, Dahl CP, Aukrust P, Christensen G, Gullestad L, Yndestad A, Ueland T. Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J Mol Med (Berl). 2017;95:767–777. [DOI] [PubMed] [Google Scholar]
- 38.Zuriaga MA, Fuster JJ, Farb MG, MacLauchlan S, Breton-Romero R, Karki S, Hess DT, Apovian CM, Hamburg NM, Gokce N, Walsh K. Activation of non-canonical WNT signaling in human visceral adipose tissue contributes to local and systemic inflammation. Sci Rep. 2017;7:17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breton-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, Gokce N, Fuster JJ, Walsh K, Hamburg NM. Endothelial Dysfunction in Human Diabetes Is Mediated by Wnt5a-JNK Signaling. Arterioscler Thromb Vasc Biol. 2016;36:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farb MG, Karki S, Park S-Y, Saggese SM, Carmine B, Hess DT, Apovian C, Fetterman JL, Breton-Romero R, Hamburg NM, Fuster JJ, Zuriaga MA, Walsh K, Gokce N. WNT5A-JNK regulation of vascular insulin resistance in human obesity. Vasc Med. 2016;21:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karki S, Ngo DTM, Farb MG, Park SY, Saggese SM, Hamburg NM, Carmine B, Hess DT, Walsh K, Gokce N. WNT5A regulates adipose tissue angiogenesis via antiangiogenic VEGF-A165b in obese humans. Am J Physiol Heart Circ Physiol. 2017;313:H200–H206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT-M, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murdoch CE, Shuler M, Haeussler DJF, Kikuchi R, Bearelly P, Han J, Watanabe Y, Fuster JJ, Walsh K, Ho Y-S, Bachschmid MM, Cohen RA, Matsui R. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J Biol Chem. 2014;289:8633–8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLauchlan S, Zuriaga MA, Fuster JJ, Cuda CM, Jonason J, Behzadi F, Duffen JP, Haines GK 3rd, Aprahamian T, Perlman H, Walsh K. Genetic deficiency of Wnt5a diminishes disease severity in a murine model of rheumatoid arthritis. Arthritis Res Ther. 2017;19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. [DOI] [PubMed] [Google Scholar]
- 47.Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, Wagner DD. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med. 2017;214:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.