Abstract
Exposure to inorganic arsenic (iAs) remains a global public health problem. Urinary arsenicals are the current gold-standard for estimating both iAs exposure and iAs metabolism. However, the distribution of these arsenicals may differ between the urine and target organs. Instead, plasma arsenicals may better represent internal dose and capture target organ exposure to arsenicals. Drinking water iAs, plasma and urinary arsenicals were quantified individuals living in the Zimapan and Lagunera regions of Mexico. The relationship between drinking water iAs and plasma arsenicals was examined using both Spearman correlations and multivariable linear regression models. In addition, the distribution of arsenicals in plasma and urine was examined and the association between plasma and urinary arsenicals was assessed using both Spearman correlations and multivariable linear regression models. Levels of iAs in drinking water were significantly associated with plasma arsenicals in unadjusted and adjusted analyses and the strength of these associations was similar to that of drinking water iAs and urinary arsenicals. These results suggest that plasma arsenicals are reliable biomarkers of iAs exposure via drinking water. However, there were notable differences between the profiles of arsenicals in the plasma and the urine. Key differences between the proportions of arsenicals in plasma and urine may indicate that urine and plasma arsenicals reflect different aspects of iAs toxicokinetics, including metabolism and excretion..
Keywords: Inorganic Arsenic, Plasma Arsenic, Arsenic Biomarkers
Background
Arsenic is a ubiquitous metalloid found in the environment and is the highest priority contaminant on the Agency for Toxic Substances and Disease Registry’s (ATSDR) 2017 Substance Priority List (1). Exposure to inorganic arsenic (iAs) is a global public health problem, impacting communities in the United States (U.S.), Mexico, Bangladesh, and China, among others (2). Importantly, iAs exposure has been linked to a wide range of chronic health outcomes, including cancers of the skin, lung, liver, and bladder; diabetes, immunosuppression; and pregnancy complications (2, 3).
Given the global impact of iAs exposure on human health, identifying reliable biomarkers of iAs exposure is an important task. The concentrations of total or speciated arsenic in the blood, urine, saliva, hair, or toenails have been used as biomarkers of iAs exposure in both population-based or clinical studies (4). Among these biomarkers, the urinary concentrations of iAs and its methylated metabolites, monomethylated arsenic (MMAs) and dimethylated arsenic (DMAs), are currently considered the gold-standard for iAs exposure assessment (4). Importantly, these measures have also been used to characterize the individual capacity to metabolize (detoxify) iAs and to estimate the risk of disease associated with iAs exposure. Differences in the concentrations or proportions of iAs, MMAs, and DMAs have been linked to susceptibility to a variety of adverse health effects of iAs exposure (5, 6). For example, high proportions of urinary MMAs (%U-MMAs) have been associated with higher risk of cancers and skin lesions (5, 6), while high %U-DMAs has been associated with diabetes risk (7). However, the concentrations of urinary arsenicals reflect only recent iAs exposure. In addition, some studies suggest that the distribution of arsenicals in the urine does not represent the distribution found in target organs (8). Therefore, there is a clear need to examine other biological matrices which could serve as sources of reliable biomarkers of iAs exposure, iAs metabolism, and/or disease risk in target tissues.
The concentrations of arsenic species in blood plasma may serve as alternatives to urinary arsenicals, as they represent an internal exposure level and reflect the amounts and composition of iAs and its metabolites that directly interact with target organs (8, 9). This has biological significance because unbound arsenicals in the plasma are available for transport into target tissues and, therefore, may more closely represent target organ-specific exposure to individual arsenic species than urinary arsenicals. However, quantitative speciation analysis of arsenic in plasma is difficult because the concentrations of arsenicals are low and these arsenicals are, in part, bound to plasma proteins (10). To date, only two human studies have measured levels of arsenicals in plasma. One of these studies examined speciation of arsenic in both red blood cells and the plasma of a small cohort of adults living in West Bengal, India that were exposed to iAs via drinking water (10), and the other linked the concentrations and proportions of plasma arsenicals to the odds of type-1 and type-2 diabetes among adolescents in a U.S. cohort (9). However, neither study has confirmed that measures of arsenic species in plasma reflect iAs exposure by examining the relationship between the concentrations of arsenicals in plasma with those in urine, or with measures of iAs in food, soil, or drinking water.
The goal of this present study was to determine if iAs and/or its methylated metabolites in plasma can serve as biomarkers of iAs exposure or metabolism. To achieve this goal, we quantified arsenic species in plasma collected from individuals living in the Zimapan and Lagunera regions of Mexico, for whom we have previously reported drinking water iAs and urinary arsenicals (7). To investigate whether plasma arsenicals could serve as biomarkers of iAs exposure, the association between the concentrations of plasma arsenicals and drinking water iAs was examined. Proportions of plasma arsenicals were also compared to drinking water iAs levels to investigate whether plasma arsenicals could provide information about the efficiency of iAs metabolism. In addition, plasma arsenicals were compared to urinary arsenicals, the current gold-standard biomarker of both iAs exposure and metabolism. These results suggest that the concentrations of iAs and its metabolites in plasma are reliable biomarkers of iAs exposure and that key differences between the distribution of plasma and urinary arsenicals may have larger implications for the use of urinary biomarkers of iAs exposure and metabolism.
Methods
Study Recruitment
Study recruitment has been described elsewhere (7, 11). Briefly, study participants were recruited among the residents, both adults and children, of the Zimapan and Lagunera regions in Mexico, where chronic exposures to iAs in drinking water have frequently been reported (12–15). Potential participants gave written informed consent and completed a detailed questionnaire to assess eligibility and collect information regarding demographics, the use and sources of drinking water, history of disease, and medication use. Participants were eligible for recruitment if they had lived in the study areas for a minimum of 2 years and reported no occupational exposure to iAs. Individuals were further excluded from enrollment if they self-reported being pregnant, an alcoholic, having chronic or acute urinary tract diseases, or type 1 diabetes. In addition, only genetically unrelated individuals were recruited into this cohort to facilitate genotypic analyses described in the original reports (7, 11). All procedures involving human subjects in the original and this current study have been approved by Institutional Review Boards of the Centro de Investagacion y de Estudios Avanzados del Instituto Politecnico Nacional (Cinvestav-IPN) and/or the University of North Carolina at Chapel Hill (UNC-CH). The final study population was composed of 258 individuals.
Sample Collection
Participants enrolled in this study underwent medical examination where body weight, height, and blood pressure were recorded. Spot urine and fasting venous blood samples were collected from each individual during the examination. Urine was aliquoted and snap-frozen in dry ice immediately following collection. Fasting venous blood was collected into EDTA-vacutainers (Becton, Dickinson and Co., Franklin Lakes, NJ). Plasma was prepared from the whole blood by centrifugation at 4°C and immediately snap-frozen in dry ice. At the medical examination, participants also provided a sample of their drinking water. The water, urine, and plasma samples were packed on dry ice and transported to Cinvestav-IPN or the Universidad Juarez del Estado de Durango and stored at −80°C until analysis. Drinking water and urine samples were analyzed at Cinvestav-IPN or the Universidad Juarez del Estado de Durango. Plasma samples were later shipped on dry ice to UNC-CH for analysis.
Arsenic Quantification in Urine, Plasma, and Drinking Water
The analysis of arsenic species in drinking water and in urine was carried out in the original study (7) and has been previously described in detail (16, 17). Briefly, the concentration of iAs in drinking water was measured by hydride generation (HG)-atomic fluorescence spectrometry. The concentrations of iAs and its methylated metabolites in urine (U-iAs, U MMAs, and U-DMAs) were determined using HG-atomic absorption spectrometry with cryotrapping (CT) (16, 18). In this method, pentavalent and trivalent species of arsenic (i.e. iAsIII, iAsV, MMAsIII, MMAsV, DMAsIII, and DMAsV) were measured separately and summed to calculate U-iAs, U-MMAs and U-DMAs. The sum of U-iAs + U-MMAs + U-DMAs was further calculated to assess total urinary arsenic (U-tAs). The limit of detection (LOD) for pentavalent and trivalent arsenic species was 0.1 ng/mL. Samples below the LOD were imputed as LOD/√2. No samples were below the LOD for U-iAsIII, 15% < LOD for U-iAsV, 26% < LOD for U MMAsIII, 0.8% < LOD for U-MMAsV, 14% < LOD for U-DMAsIII, and 0.3% < LOD for U DMAsV. The proportions of U-iAs, U-MMAs, and U-DMAs (expressed as %U-iAs, %U-MMAs, and %U-DMAs) were calculated by dividing by U-tAs and multiplying by 100. The concentrations of arsenic species in urine are expressed as μg/L and are specific gravity (SG) adjusted according to the following formula: ArsenicSG = Arsenic[(1.013–1)/(SG-1), where ArsenicSG is the specific gravity-corrected arsenical concentration and 1.013 is the average SG in the Zimapan and Lagunera cohort. SG was measured for each urine sample using a digital Atago PAL refractometer (Atago USA). Urine samples were collected from 257 of the participants and samples with a SG exactly equal to 1.0 (N=25) were excluded from further analysis, leaving 232 participants with urinary arsenicals in this study.
The concentration of plasma arsenicals (P-iAs, P-MMAs, and P-DMAs) were measured in the present study using HG-CT-inductively coupled plasma-mass spectrometry (ICP-MS) (9, 10, 19). The LODs were 0.63 ng As/L for iAs and 0.05 ng As/L for MMAs and DMAs. The LOD for iAs is higher than the LOD for MMAs and DMAs as a result of the higher background levels of iAs in blank samples. The inter-assay coefficients of variation were 3.6% for iAs, 3.8% for MAs, and 3.3% for DMAs. To ensure accuracy of the analysis, a standard reference material, Arsenic Species in Frozen Human Urine (SRM 2669; National Institute of Standards and Technology) diluted in deionized water was analyzed with approximately every other group of samples for a total of 34 times. The average concentrations of iAs, MMAs, and DMAs in SRM 2669 determined by HG-CT-ICP-MS represented 90.4%, 94.5%, and 79.6% of the certified values, respectively. For additional quality control, SRM 2669 was diluted in a sample of human plasma. The averaged concentrations determined by 12 independent analyses represented 97.1%, 98.6%, and 89% of the certified values for iAs, MMAs, and DMAs, respectively. The sum of P-iAs + P-MMAs + P-DMAs was calculated to produce total plasma arsenic (P-tAs). The proportion of plasma arsenic species (expressed as %P-iAs, %P-MMAs, and %P-DMAs) were calculated as described above for urine.
Statistical Analysis
Differences in the proportions of arsenicals in plasma and urine and the distribution of plasma arsenicals by demographic factors were examined and statistical significance was assessed using Kruskal-Wallis tests. In order to evaluate the potential for speciated plasma arsenicals to serve as biomarkers of iAs exposure, we investigated the relationship between iAs concentrations in drinking water, concentrations and proportions of plasma arsenicals, and concentrations and proportions of urine arsenicals using Spearman correlation coefficients. Additionally, the potential for non-linear associations between indicators of iAs exposure were investigated using locally weighted scatterplot smoothing (LoWeSS).
The relationships between drinking water iAs, speciated plasma arsenicals, and speciated urine arsenicals were examined using multivariable linear regression models to adjust for relevant variables that may be potential confounders. Relevant variables were selected based on the identification of demographic factors associated with plasma arsenicals. The following variables were considered for inclusion in these models: sex (male/female), age (≤ 18 vs. 19–35 vs. 36–55 vs. ≥55), body mass index (BMI; underweight vs. normal vs. overweight vs. obese), smoking status (yes/no), alcohol consumption (yes/no), and seafood consumption in the past week (yes/no). BMI was categorized according to the World Health Organization (WHO) recommendations: Underweight < 18.5 kg/m2; 18.5 kg/m2 ≤ Normal BMI < 25 kg/m2; 25 kg/m2 ≤ Overweight BMI < 30 kg/m2; Obese ≥ 30 kg/m2 (20). Levels of drinking water iAs, plasma and urine arsenicals, but not their proportions, were log-transformed to approximate a normal distribution. For models where both the exposure and outcome were log-transformed, the percent change in the outcome associated with a doubling of the exposure was reported. For models where only the exposure was log-transformed, the mean difference in the outcome associated with a doubling of the exposure was reported. Given that LoWeSS lines indicated the relationship between drinking water iAs and the proportions of plasma arsenicals may be quadratic, polynomial linear regression models where drinking water iAs was coded as a quadratic function were also examined. All statistical analyses were carried out using SAS version 9.4 (Cary, NC) and statistical significance was set at p < 0.05.
Code Availability
Code underlying these analyses have not been made publically available.
Results
Cohort Demographics and Arsenic Exposure Indicators
The study comprises 258 individuals living in the Zimapan and Lagunera areas of Mexico. Demographic characteristics are summarized in Table 1. The majority of participants were women (67%), non-smokers (91%), did not self-report alcohol consumption (79%), and did not self-report seafood consumption within the past week (89%). The median age was 35 years, ranging from 5 – 88 years. Levels of drinking water iAs and urinary arsenicals in this population have been reported elsewhere (7). Briefly, water iAs levels ranged from 3.1 – 215.2 ppb, with a median of 22.1 ppb. The median urine iAs levels were 3.5 ppb, 3.9 ppb, 19.4 ppb and 27.7 ppb for U-iAs, U-MMAs, U-DMAs, and U-tAs, respectively.
Table 1.
Demographic characteristics, measures of water, urine, and plasma arsenic species in the Zimapan and Lagunera cohort.
| N (%) | Median (IQR) | Range | |
|---|---|---|---|
| Age (years) | 258 (100) | 35 (32) | 5 – 88 |
| Sex | |||
| Male | 84 (32.6) | ||
| Female | 174 (67.4) | ||
| BMI1 | |||
| Underweight | 30 (11.9) | ||
| Normal | 68 (27.0) | ||
| Overweight | 72 (28.6) | ||
| Obese | 82 (32.5) | ||
| Smoking | |||
| Non-Smoker | 234 (90.7) | ||
| Smoker | 24 (9.3) | ||
| Alcohol Consumption | |||
| No | 203 (78.7) | ||
| Yes | 55 (21.3) | ||
| Recent Seafood Consumption2 | |||
| No | 230 (89.5) | ||
| Yes | 27 (10.5) | ||
| Water iAs (μg/L) | 258 (100) | 22.1 (41.6) | 3.1 – 215.2 |
| Urinary Arsenic Measures3 (μg/L) | 232 (90) | ||
| U-iAs | 3.5 (4.3) | 0.3 – 26.9 | |
| U-MMAs | 3.9 (4.3) | 0.4 – 24.2 | |
| U-DMAs | 19.4 (26.5) | 1.4 – 144.8 | |
| U-tAs | 27.7 (34.4) | 3.8 – 164.1 | |
| Plasma Arsenic Measures (ng/L)4 | 242 (94) | ||
| P-iAs | 185.4 (128.0) | 10.8 – 5,071.7 | |
| P-MMAs | 188.3 (217.2) | 47.5 – 2844.5 | |
| P-DMAs | 336.4 (326.2) | 83.4 – 3,155.3 | |
| P-tAs | 718.4 (598.3) | 221.3 – 9,669.9 | |
| Urinary Arsenic Measures3 (%) | 232 (90) | ||
| %U-iAs | 12.4 (8.0) | 3.5 – 64.4 | |
| %U-MMAs | 14.8 (7.7) | 3.3 – 48.0 | |
| %U-DMAs | 71.0 (13.5) | 18.9 – 90.8 | |
| Plasma Arsenic Measures (%)4 | 242 (94) | ||
| %P-iAs | 23.7 (21.6) | 3.2 – 62.2 | |
| %P-MMAs | 27.5 (9.1) | 11.4 – 47.0 | |
| %P-DMAs | 46.0 (16.7) | 17.5 – 72.2 |
n = 6 missing;
n = 1 missing;
Urine arsenic measures shown are specific gravity-adjusted; samples with a specific gravity equal to 1.0 (N = 25) were omitted;
n = 16 missing. Abbreviations: IQR = inter-quartile range; BMI = body mass index.
Levels and Distribution of Arsenic Species in Plasma
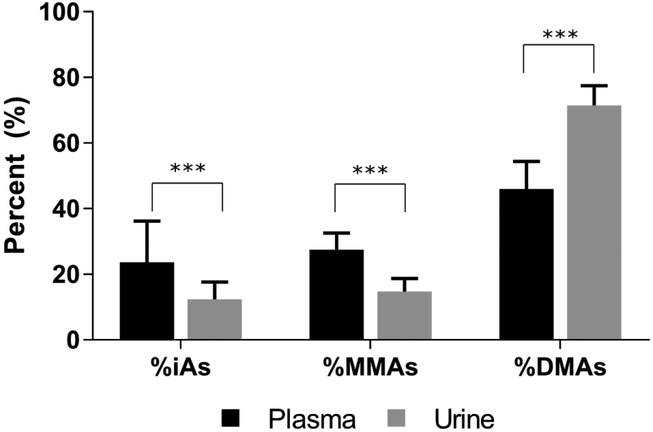
Plasma samples from 242 (94%) of the participants were available for arsenic analysis in this study (Table 1). No samples were below the LOD. The levels of arsenicals in plasma were one to two orders of magnitude lower than those in urine, with a median of 185.4 ppt for P-iAs, 188.3 ppt for P-MMAs, 336.4 ppt for P-DMAs, and 718.4 ppt for P-tAs (Table 1). Interestingly, the distribution of plasma arsenicals differed from the distribution of arsenicals in urine (Figure 1). Specifically, there were higher %iAs and %MMAs and lower %DMAs in plasma compared to urine. Notably, the levels and distribution of plasma arsenicals differed by sex and age (Table 2). The median levels of P-MMAs and %P-MMAs were lower in females compared to males. The levels of P-tAs increased significantly with age, suggesting that older individuals had greater exposure to iAs or that the excretion of iAs into urine is impaired. In addition, levels of P MMAs, P-DMAs and %P-DMAs increased, while %P-iAs decreased with increasing age. This may suggest that older individuals in the cohort also methylated iAs more efficiently than younger individuals. Additionally, %P-iAs decreased with increasing BMI, while %P-MMAs and %P-DMAs increased, which is consistent with higher methylation efficiency in overweight and obese individuals as compared to those with normal weight. Seafood consumption was also associated with lower P-iAs and %P-iAs and higher P-DMAs and %P-DMAs. Lastly, %P MMAs were marginally lower among non-smokers. Alcohol consumption was not associated with changes in either the levels or proportions of arsenicals in plasma.
Figure 1.
The proportions of arsenic species in plasma and urine (Median (+ 75th Percentile)). Wilcoxon rank sum test: ***: p-value < 0.0001.
Table 2.
Differences in the concentrations and proportions of plasma arsenicals (ng/L) by demographic characteristics (N=242).
| Characteristic | N (%) | P-iAs Median (IQR) |
P-MMAs Median (IQR) |
P-DMAs Median (IQR) |
P-tAs Median (IQR) |
%P-iAs Median (IQR) |
%P-MMAs Median (IQR) |
%P-DMAs Median (IQR) |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 76 (31.4) | 206.2 (150.7) | 243.0 (281.1) | 356.0 (403.0) | 838.9 (764.4) | 20.2 (19.1) | 29.8 (9.4) | 46.3 (17.5) |
| Female | 166 (68.6) | 182.8 (112.6) | 183.0 (185.9) * | 328.3 (298.9) | 690.1 (517.1) | 26.8 (22.7) | 26.2 (9.0) ** | 45.9 (15.5) |
| Age | ||||||||
| < 18 | 68 (28.1) | 219.7 (111.3) | 166.6 (235.0) | 271.6 (273.5) | 664.1 (671.2) | 30.1 (24.0) | 21.4 (9.8) | 40.9 (16.7) |
| 19 – 35 | 59 (24.4) | 171.8 (136.7) | 162.6 (143.3) | 247.3 (259.9) | 573.4 (465.2) | 25.2 (22.6) | 27.5 (9.8) | 43.1 (1.5) |
| 36 – 55 | 83 (34.3) | 183.0 (126.0) | 184.6 (169.8) | 351.9 (263.4) | 728.3 (476.4) | 23.9 (20.7) | 26.3 (9.5) | 48.5 (14.8) |
| > 55 | 32 (13.2) | 193.3 (112.9) | 348.7 (277.5) ** | 655.8 (519.4) *** | 1168.2 (865.4) *** | 14.7 (10.1) ** | 30.3 (9.5) | 54.4 (8.9) *** |
| BMI1 | ||||||||
| Underweight | 28 (11.9) | 241.4 (180.7) | 153.8 (189.0) | 234.5 (254.0) | 629.9 (595.5) | 37.2 (29.5) | 25.8 (7.5) | 41.7 (15.0) |
| Normal | 64 (27.1) | 185.6 (118.9) | 186.6 (254.7) | 296.3 (343.9) | 705.1 (684.6) | 25.2 (22.5) | 28.3 (9.1) | 41.6 (19.0) |
| Overweight | 67 (28.4) | 117.1 (143.6) | 205.0 (226.8) | 321.3 (376.5) | 731.1 (597.3) | 20.6 (19.7) | 30.0 (10.5) | 48.5 (12.6) |
| Obese | 77 (32.6) | 184.8 (89.1) | 191.1 (162.5) | 381.0 (306.0) | 750.5 (539.3) | 22.4 (19.5) * | 26.2 (9.2) ** | 50.2 (16.2) ** |
| Smoking | ||||||||
| No | 222 (91.7) | 185.4 (121.9) | 188.3 (215.3) | 337.3 (325.8) | 717.7 (610.4) | 23.6 (20.7) | 27.3 (9.3) | 46.5 (15.9) |
| Yes | 20 (8.3) | 184.4 (204.0) | 203.2 (247.0) | 332.6 (293.6) | 834.2 (517.5) | 24.5 (23.3) | 30.9 (8.2) * | 41.2 (19.4) |
| Alcohol | ||||||||
| Consumption | ||||||||
| No | 192 (79.3) | 185.0 (129.3) | 188.0 (216.0) | 337.3 (329.1) | 717.7 (607.8) | 23.6 (22.6) | 27.3 (9.2) | 46.6 (17.0) |
| Yes | 50 (20.7) | 188.7 (112.4) | 197.6 (259.5) | 332.6 (288.4) | 808.9 (576.4) | 24.1 (18.0) | 27.6 (8.8) | 43.1 (13.8) |
| Seafood | ||||||||
| Consumption2 | ||||||||
| No | 217 (90.0) | 188.9 (113.4) | 185.1 (211.6) | 324.5 (323.4) | 707.9 (596,0) | 25.9 (22.2) | 27.3 (9.3) | 45.3 (16.5) |
| Yes | 24 (10.0) | 125.0 (145.3) ** | 219.3 (229.7) | 412.8 (302.3) * | 802.8 (563.2) | 15.7 (11.2) ** | 30.2 (8.0) | 53.9 (17.3) ** |
: p<0.1 by Kruskal-Wallis Test;
: p < 0.05 by Kruskal-Wallis Test;
: p < 0.0001 by Kruskal-Wallis Test.
n = 6 missing;
n = 1 missing
Associations between Drinking Water iAs and Levels of Plasma and Urinary Arsenicals
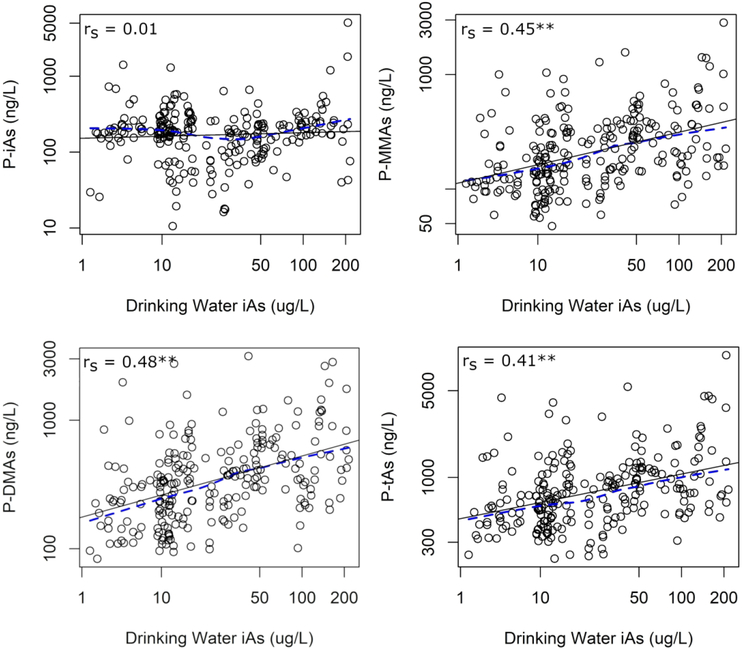
In order to determine whether plasma arsenicals may serve as biomarkers of iAs exposure or metabolism, we examined the association between drinking water iAs levels and the concentrations of plasma arsenicals. In univariate comparisons, we found moderate correlations between measures of drinking water iAs and the concentrations of P-MMAs (rs = 0.45), P-DMAs (rs = 0.48), and P-tAs (rs = 0.41) (Figure 2; Supplemental Table 1). The strength of these correlations were similar to those found between drinking water iAs levels and concentrations of urinary arsenicals in this cohort (Supplemental Table 1). Interestingly, no correlation was found between drinking water iAs and P-iAs (rs = 0.01). These relationships remained consistent after adjusting for age, sex, BMI, smoking status, and seafood consumption. A doubling of the concentration of drinking water iAs levels was associated with higher P-iAs (6.3%, 95% Confidence Interval [CI] = −0.7, 13.9), P-MMAs (21.0%, 95% CI: 14.4, 27.9), P-DMAs (21.5%, 95% CI: 15.0, 28.3), and P-tAs (17.0%, 95% CI: 11.3, 23.0) (Table 3).
Figure 2:
Univariate relationship between concentration of iAs in drinking water and concentrations of arsenicals in plasma. Abbreviations: rs = Spearman correlation coefficient, LoWeSS = locally-weighted scatter smoothing. Key: Black solid line, regression line; blue dashed line, LoWeSS line; *, p<0.1; **, p<0.05.
Table 3.
Percent change in concentration of plasma arsenicals associated with drinking water iAs.
| Percent Change (95% CI) in Plasma Arsenicals | ||||
|---|---|---|---|---|
| P-iAs | P-MMAs | P-DMAs | P-tAs | |
| Drinking Water iAs1 | 6.3% (−0.7, 13.9) | 21.0% (14.4, 27.9) | 21.5% (15.0, 28.3) | 17.0% (11.3, 23.0) |
| Sex | ||||
| Males | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Females | 8.6% (−15.5, 39.5) | −13.0% (−29.1, 6.6) | −13.7% (−29.3, 5.2) | −7.6% (−23.0, 10.9) |
| Age | ||||
| < 18 | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| 18 – 35 | −13.8% (−39.4, 22.7) | −2.4% (−26.8, 30.1) | −5.3% (−28.5, 25.3) | −5.6% (−27.0, 22.0) |
| 35 – 55 | 4.7% (−27.2, 50.6) | 13.6% (−15.5, 52.6) | 32.7% (−0.5, 77.1) | 21.2% (−6.9, 57.9) |
| ≥ 55 | 10.7% (−27.6, 69.2) | 54.0% (9.0, 117.5) | 77.7% (26.8, 148.9) | 53.6% (12.8, 109.3) |
| BMI | ||||
| Underweight | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Normal | −5.8% (−36.0, 38.6) | 0.9% (−26.3, 38.1) | −7.7% (−32.1, 25.4) | −8.6% (−31.0,21.0) |
| Overweight | −17.6% (−47.7, 29.7) | −1.2% (−31.7, 43.0) | −7.8% (−35.7, 32.3) | −13.8% (−38.1, 20.0) |
| Obese | −10.9% (−43.7, 40.8) | −10.3% (−38.2, 30.3) | −6.5% (−35.1, 34.5) | −13.8% (−38.3, 20.4) |
| Smoking | ||||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | −13.4% (−41.0, 27.2) | −11.6% (−35.4, 20.9) | 5.3% (−22.4, 42.9) | −4.7% (−28.0,26.1) |
| Seafood Consumption | ||||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | −33.2% (−53.0, −5.2) | −13.8% (−35.2, 14.8) | −8.1% (−30.5, 21.4) | −15.6% (−34.6, 9.0) |
percent change in plasma arsenicals associated with a doubling of the concentration of drinking water iAs.
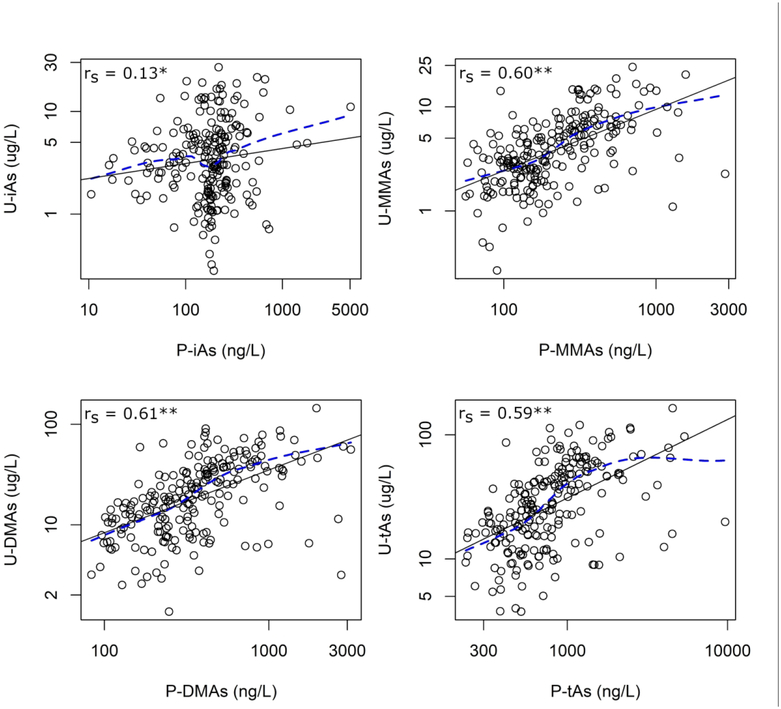
Levels of plasma and urinary arsenicals were compared to determine whether there are important differences between plasma arsenicals and an established biomarker of iAs exposure. We observed strong positive correlations between P-MMAs and U-MMAs (rs = 0.60), P-DMAs and U-DMAs (rs = 0.61), and P-tAs and U-tAs (rs = 0.59) (Figure 3). Again, the correlation between P-iAs and U-iAs was weak (rs = 0.13), although it did reach marginal statistical significance. These relationships remained largely consistent after adjusting for age, sex, BMI, smoking status, and seafood consumption (Table 4). Notably, the association between P-iAs and U-iAs was stronger following adjustment for confounders, with a doubling of P-iAs associated with a mean U-iAs that was 12.9% (95% CI: 2.5, 24.2) higher.
Figure 3.
Univariate relationship between concentrations of arsenicals in plasma and urine. Abbreviations: rs = Spearman correlation coefficient, LoWeSS = locally-weighted scatter smoothing. Key: Black solid line, regression line; blue dashed line, LoWeSS line; *, p<0.1; **, p<0.05.
Table 4.
Percent change in concentration of urinary arsenicals associated with plasma arsenicals.
| Percent Increase (95% CI) in Urinary Arsenicals | ||||
|---|---|---|---|---|
| U-iAs | U-MMAs | U-DMAs | U-tAs | |
| P-iAs1 | 12.9% (2.5, 24.2) | |||
| P-MMAs1 | 45.9% (34.8, 57.9) | |||
| P-DMAs1 | 47.7% (34.2, 62.5) | |||
| P-tAs1 | 49.8% (36.0, 64.9) | |||
| Sex | ||||
| Males | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Females | −35.0% (−50.1,−15.3) | −18.6% (−33.1,−0.9) | −26.0% (−41.3,−6.6) | −27.5% (−41.2,−10.6) |
| Age | ||||
| < 18 | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| 18 – 35 | −23.0% (−46.9, 11.5) | −14.0% (−34.5, 12.9) | −17.6% (−40.4, 13.9) | −15.2% (−36.7, 13.6) |
| 35 – 55 | −16.5% (−43.3, 22.9) | −5.2% (−28.7, 26.0) | −7.5% (−34.1, 29.7) | −7.3% (−31.7, 25.9) |
| ≥ 55 | 21.1% (−22.8, 90.1) | 28.1% (−8.6, 79.5) | 8.0% (−27.9, 61.7) | 12.9% (−21.4, 62.3) |
| BMI | ||||
| Underweight | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Normal | 12.4% (−25.7, 70.0) | −18.0% (−39.5, 11.3) | −2.7% (−32.2, 39.8) | −1.3% (−28.8, 36.9) |
| Overweight | 33.2% (−17.1, 114.0) | −25.9% (−47.7, 4.9) | 2.4% (−32.3, 54.8) | 8.6% (−25.3, 57.8) |
| Obese | 11.0% (−31.5, 79.8) | −25.4% (−47.7, 6.4) | 8.7% (−28.7, 65.7) | 8.4% (−25.9, 58.7) |
| Smoking | ||||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | 23.8% (−17.6, 86.0) | 2.7% (−24.0, 38.6) | 32.1% (−7.7, 88.9) | 29.4% (−6.3, 78.7) |
| Seafood | ||||
| Consumption | ||||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | 28.3% (−9.5, 81.8) | −13.7% (−33.1, 11.4) | 9.1% (−19.4, 47.7) | 13.4% (−13.7, 49.0) |
percent change associated with a doubling of the concentration of plasma arsenicals
Associations between Drinking Water iAs and Proportions of Plasma and Urinary Arsenicals
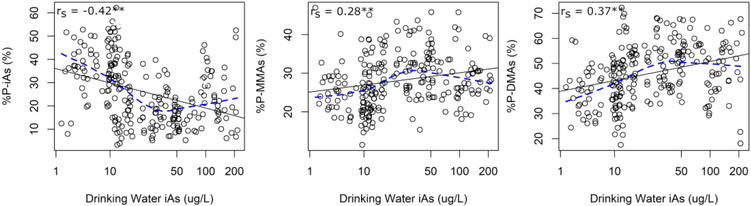
In order to investigate whether plasma arsenicals may serve as biomarkers of iAs metabolism, we examined the associations between drinking water iAs levels and the proportions of plasma arsenicals. Again, we also compared the proportions of plasma arsenicals to the proportions of urinary arsenicals, which are the currently accepted biomarkers of iAs metabolism. Drinking water iAs levels were negatively correlated with %P-iAs (rs = −0.42) and positively correlated with %P-MMAs (rs = 0.28) and %P-DMAs (rs = 0.37) (Figure 4; Supplemental Table 2). The strength of the association between the proportions of drinking water iAs and the proportions of urinary arsenicals tended to be stronger than for plasma arsenicals, except for %U-iAs, which was uncorrelated with drinking water iAs levels (Supplemental Table 2). Associations remained consistent following adjustment for age, sex, BMI, smoking status, and seafood consumption (Table 5). After adjusting for these relevant variables, a doubling in the concentration of drinking water iAs was associated with lower %P-iAs (−3.6%, 95% CI: −5.1, −2.1), and higher %P-MMAs (1.2%, 95% CI: 0.4, 2.0), and %P-DMAs (2.4%, 95% CI: 1.2, 3.7). Interestingly, LoWeSS lines indicated a potentially curvilinear relationship between drinking water iAs and %P-iAs, %P-MMAs, and %P-DMAs, which may suggest a possible saturation of iAs methylation at higher levels of iAs exposure (Figure 4). Polynomial regression models indicated that there was a significantly curvilinear relationship between drinking water iAs and the proportions of each of the plasma arsenicals (Supplemental Table 3).
Figure 4.
Univariate relationship between concentration of iAs in drinking water and proportions of arsenicals in plasma. Abbreviations: rs = Spearman correlation coefficient, LoWeSS = locally-weighted scatter smoothing. Key: Black solid line, regression line; blue dashed line, LoWeSS line; *, p<0.1; **, p<0.05.
Table 5.
Mean difference in proportions of plasma arsenicals associated with drinking water iAs
| Mean Difference (95% CI) in Plasma Arsenicals | |||
|---|---|---|---|
| %P-iAs | %P-MMAs | %P-DMAs | |
| Drinking Water iAs1 | −3.6% (−5.1, −2.1) | 1.2% (0.4, 2.0) | 2.4% (1.2, 3.7) |
| Sex | |||
| Males | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Females | 4.7% (0.9, 8.5) | −1.9% (−3.9, 0.1) | −2.8% (−6.1, 0.5) |
| Age | |||
| < 18 | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| 18 – 35 | −1.0% (−6.3, 4.4) | 0.5% (−2.4, 3.3) | 0.6% (−4.0, 5.2) |
| 35 – 55 | −2.2% (−7.8, 3.3) | −2.3% (−5.2, 0.6) | 4.5% (−0.2, 9.2) |
| ≥ 55 | −6.4% (−12.9, 0.1) | −0.5% (−3.9, 2.9) | 6.9% (1.8, 12.4) |
| BMI | |||
| Underweight | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Normal | −2.1% (−8.0, 3.7) | 3.0% (−0.2, 6.1) | −0.8% (−5.9, 4.2) |
| Overweight | −5.1% (−12.0, 1.8) | 4.0% (0.4, 7.7) | 1.0% (−4.9, 7.0) |
| Obese | −3.5% (−10.5, 3.5) | 1.5% (−2.2, 5.2) | 2.0% (−4.0, 8.0) |
| Smoking | |||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | −2.5% (−8.3, 3.4) | −1.9% (−5.0, 1.2) | 4.4% (−0.6, 9.4) |
| Seafood Consumption | |||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | −4.5% (−9.8, 0.9) | 0.7% (−2.2, 3.5) | 3.8% (−0.8, 8.4) |
percent change in plasma arsenicals associated with a doubling of the concentration of drinking water iAs.
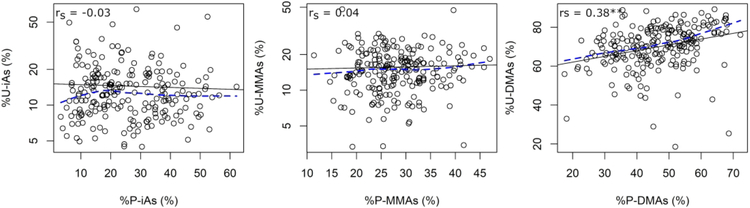
With respect to urinary arsenicals, there was a moderate positive correlation only between %P-DMAs and %U-DMAs (rs = 0.38) (Figure 5). This association remained significant after adjusting for age, sex, BMI, smoking, and seafood consumption, where a 10 percentage point increase in %P-DMAs was associated with an average %U-DMAs that was 1.6 (95% CI: 0.1, 3.1) percentage points higher (Table 6). The associations between %P-iAs and %U-iAs and between %P-MMAs and %U-MMAs remained null.
Figure 5.
Univariate relationship between the proportions of arsenicals in plasma and urine. Abbreviations: rs = Spearman correlation coefficient, LoWeSS = locally-weighted scatter smoothing. Key: Black solid line, regression line; blue dashed line, LoWeSS line; *, p<0.1; **, p<0.05.
Table 6.
Mean difference in the proportion of urinary arsenicals associated with the proportion of plasma arsenicals.
| Mean Difference (95% CI) in %Urine Arsenicals | |||
|---|---|---|---|
| %U-iAs | %U-MMAs | %U-DMAs | |
| %P-iAs1 | −0.8% (−1.9, 0.3) | ||
| %P-MMAs1 | 0.4% (−0.8, 1.7) | ||
| %P-DMAs1 | 1.6% (0.1, 3.1) | ||
| Sex | |||
| Males | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Females | 0.6% (−2.9, 4.0) | 1.3% (−0.7, 3.3) | −0.9% (−4.8, 3.0) |
| Age | |||
| < 18 | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| 18 – 35 | 0.7% (−4.1, 5.5) | 1.4% (−1.4,4.3) | −2.2% (−7.6, 3.3) |
| 35 – 55 | −1.2% (−6.2, 3.8) | 0.2% (−2.7, 3.2) | 0.2% (−5.4, 5.9) |
| ≥ 55 | −1.2% (−7.0, 4.6) | 1.1% (−2.3, 4.5) | −1.8% (−8.4, 4.9) |
| BMI | |||
| Underweight | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Normal | 0.5% (−4.8, 5.8) | −2.0% (−5.1, 1.2) | 1.2% (−4.8, 7.1) |
| Overweight | 2.9% (−3.4, 9.1) | −4.8% (−8.5, −1.2) | 1.0% (−6.0, 8.0) |
| Obese | −0.8% (−7.1, 5.5) | −4.8% (−8.5, −1.1) | 4.7% (−2.3, 11.8) |
| Smoking | |||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | −1.5% (−6.8, 3.8) | −3.1% (−6.2, 0.0) | 3.5% (−2.6, 9.6) |
| Seafood | |||
| Consumption | |||
| No | 0.0% (ref) | 0.0% (ref) | 0.0% (ref) |
| Yes | −0.4% (−5.1, 4.3) | −3.3% (−6.1,−0.6) | 2.1% (−3.2, 7.5) |
mean difference in plasma arsenicals associated with a 10% increase in the proportion of plasma arsenical
Discussion
In the present study, plasma arsenicals were evaluated as potential biomarkers of exposure to iAs in a cross-sectional cohort of individuals living in the Zimapan and Lagunera regions of Mexico. There were three primary findings in this study. First, the levels and proportions of plasma arsenicals were quantified and their distribution was found to vary across important sociodemographic factors, such as sex and age. Second, drinking water iAs levels were correlated with P-tAs and many individual plasma arsenicals, supporting its potential as a biomarker of iAs exposure and metabolism. Third, we compared the concentrations and proportions of arsenicals in urine, the current gold-standard biomarker of iAs exposure and metabolism, with those in plasma. We showed that, in spite of significant correlations between concentrations of MMAs, DMAs and tAs measures, there were key differences in the arsenical distribution between plasma and urine.
Plasma levels of arsenic species were much lower than the levels in urine, likely due to rapid metabolism and excretion of iAs (4). Still, P-tAs concentrations (0.22 – 9.67 μg As/L) were higher than those previously reported in the plasma of children and adolescents in the SEARCH for Diabetes in Youth Case-Control (SEARCH-CC) cohort (0.026 – 1.25 μg As/L) (9). This difference reflects the fact that levels of iAs exposure in the Zimapan and Lagunera regions of Mexico are higher than those observed in the general U.S. population (12–14). Interestingly, the distribution of arsenicals differed between the plasma and the urine, with higher %iAs and %MMAs and lower %DMAs in plasma. Similar proportions of plasma arsenicals were previously reported in an iAs-exposed population in India (10). However, these values differ from proportions reported in the SEARCH-CC cohort (9). For example, a median of 63.4% P-iAs was reported among participants in the SEARCH-CC cohort, while a median of 23.7% P-iAs was observed in this study. These differences may stem from differences in age and magnitude of iAs exposure in this cohort, which we have shown to be negatively associated with %P-iAs. Additionally, differences in ethnicity and arsenic (+3 oxidation state) methyltransferase (AS3MT) genotype may also be related to differences in the distribution of plasma arsenicals between these populations (21).
It has been previously suggested that the distribution of arsenicals in urine may differ from the distribution in tissues. We have previously shown that %iAs is higher and %DMAs lower in bladder epithelial cells compared to urine of individuals exposed to iAs in drinking water (8). These observations are consistent with what we observed in plasma arsenicals in the present study. Thus, the proportions of arsenicals in plasma may better reflect the distribution of arsenicals retained in tissues, including target tissues such as the bladder (8). The finding that %DMAs is higher in urine while %iAs is higher in tissues is consistent with the concept of iAs methylation as a detoxification pathway, where DMAs formation facilitates excretion of arsenic from the body. Liver is thought to be the primary site of iAs methylation (22). However, our observation that the distribution of arsenicals in the plasma differs from that in the urine suggests that iAs in plasma may undergo additional methylation prior to excretion. Possible sites of further iAs methylation are the kidneys, which have been shown to have iAs methylation activity in mice (23). Alternatively, kidney function may explain the discrepancy between the distribution of arsenicals in the plasma and the urine. For example, preferential excretion of DMAs and reabsorption of iAs by the kidneys could explain higher %iAs and lower %DMAs in the plasma compared to urine. This is supported by Rosen and colleagues, who have shown that mammalian glucose transporter GLUT1 facilitates transport of trivalent iAs (24). They suggested that trivalent iAs can form trimers that resemble a six-membered cyclic oxo-bridged rings of glucose (25), making it a potential substrate for GLUT-mediated transport. Thus, iAs might be reabsorbed from primary urine along with glucose via glucose transporters.
The concentrations of plasma arsenicals, specifically P-tAs, P-MMAs and P-DMAs, were positively associated with the concentration of iAs in drinking water, indicating that these arsenic measures can serve as reliable biomarkers of iAs exposure from drinking water. The strength of the correlations between most of the plasma arsenicals and water iAs was similar to that of the correlation between urinary arsenicals and drinking water iAs in this cohort, which are generally accepted as biomarkers of chronic iAs exposure. Interestingly, P-iAs was not correlated with drinking water iAs and was only marginally correlated with U-iAs, although it was significantly associated with U-iAs in our multivariable linear regression models. Despite the fact that P-iAs was not associated with iAs exposure via drinking water, it is an internal measure of iAs exposure. Therefore, it may still better approximate target organ-specific iAs exposures than U-iAs.
Interestingly, we observed that drinking water iAs was negatively associated with %P-iAs and positively associated with %P-MMAs and %P-DMAs. This is in contrast with existing data on proportions of urinary arsenicals, including a recent meta-analysis of factors affecting iAs metabolism, which report that iAs exposure was associated with higher %U-iAs and lower %U-DMAs (26). However, it is worth noting that drinking water iAs and %U-DMAs are also positively associated in this cohort. Additionally, we observed that the relationship between drinking water iAs and the proportion of plasma arsenicals was curvilinear, with a plateauing of this association as drinking water iAs levels increased. This may suggest a possible saturation of the pathway for iAs methylation at higher levels of iAs exposure. Similar observations have been published in other studies examining the relationship between iAs exposure and proportions of urinary arsenicals. For example, Lindberg and colleagues found that %U-DMAs production was inhibited by increasing exposure to iAs, starting around 50 μg/L As in the urine (27). However, while the proportions of arsenic species are often believed to represent changes in iAs metabolism, they actually represent several processes that include metabolism, along with other processes, such as transport across cell membranes and excretion of arsenicals into urine.
This study has both strengths and limitations. These data are from a moderately-sized cohort with previously reported measures of drinking water iAs and urine arsenicals, in which we conducted additional analysis of arsenicals in stored plasma samples. This study is among the first to report measurements of arsenicals in both plasma and urine, although plasma arsenicals have been previously measured in other cohorts (9, 10). However, while this study collected detailed demographics information, it is missing information on several factors that may be important contributors to iAs exposure or metabolism. First, indicators of socioeconomic status were not collected. Second, dietary factors that could affect iAs metabolism, such as dietary nutrients involved in one-carbon metabolism (e.g. folate or methylcobalamine), were not assessed. Dietary intake of these nutrients may be potential sources of uncontrolled confounding in the analyses (28, 29). On the other hand, diet has also been shown to be an important source of iAs exposure in the Lagunera region of Mexico (14). The addition of this information could further inform the role of plasma arsenicals as biomarkers of iAs exposure. Third, the analytical methods used in both the original and present study did not measure organic arsenicals, such as arsenobetaine, which could have been used to better isolate the amount of plasma and urine arsenicals represented by exposure to iAs (30). Lastly, information regarding kidney function was not collected, but is likely an important factor controlling the transport of arsenic species from the plasma to the urine. It has been previously shown that kidney function is related to changes in urinary biomarkers of iAs exposure and metabolism. Specifically, deficiencies in kidney function are associated with lower %U-iAs and higher %U-DMAs in the urine (31, 32). Adjusting for kidney function may explain the differences in the distribution of arsenicals between the plasma and the urine observed in this study, and may improve the strength of the association between iAs in drinking water and arsenicals in plasma and urine.
In conclusion, this study is among the first to comprehensively examine plasma arsenicals as potential biomarkers of iAs exposure and metabolism. Significant associations between drinking water iAs and plasma arsenicals were observed. However, while levels of plasma arsenicals were associated with levels of urine arsenicals, the proportions of plasma arsenicals were largely unassociated with proportions of urine arsenicals. These results have important implications for the use of the proportion of urinary arsenicals as biomarkers of the internal dose of iAs exposure or as a measure of the efficiency of iAs metabolism.
Supplementary Material
Acknowledgements:
Funding for this work was provided by the National Institute of Environmental Health Sciences (T32-ES007018 and 5R01-ES015326–02) and the Gillings Innovation Laboratory.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.ATSDR. Substance Priority List. 2017.
- 2.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013. March;121(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vahter M Effects of arsenic on maternal and fetal health. Annual review of nutrition. 2009;29:381–99. [DOI] [PubMed] [Google Scholar]
- 4.Hughes MF. Biomarkers of Exposure: A Case Study with Inorganic Arsenic. Environmental Health Perspectives. 2006;114(11):1790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A. The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ Health Perspect. 2017. August 1;125(8):087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamboa-Loira B, Cebrian ME, Franco-Marina F, Lopez-Carrillo L. Arsenic metabolism and cancer risk: A meta-analysis. Environmental research. 2017. July;156:551–8. [DOI] [PubMed] [Google Scholar]
- 7.Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environmental health : a global access science source. 2011;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currier JM, Ishida MC, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Gutierrez-Torres DS, et al. Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environ Health Perspect. 2014. October;122(10):1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grau-Perez M, Kuo CC, Spratlen M, Thayer KA, Mendez MA, Hamman RF, et al. The Association of Arsenic Exposure and Metabolism With Type 1 and Type 2 Diabetes in Youth: The SEARCH Case-Control Study. Diabetes care. 2017. January;40(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal BK, Suzuki KT, Anzai K. Impact of arsenic in foodstuffs on the people living in the arsenic-affected areas of West Bengal, India. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2007. October;42(12):1741–52. [DOI] [PubMed] [Google Scholar]
- 11.Drobna Z, Del Razo LM, Garcia-Vargas GG, Sanchez-Pena LC, Barrera-Hernandez A, Styblo M, et al. Environmental exposure to arsenic, AS3MT polymorphism and prevalence of diabetes in Mexico. Journal of exposure science & environmental epidemiology. 2013. March;23(2):151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Razo LM, Arellano MA, Cebrian ME. The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Environmental pollution (Barking, Essex : 1987). 1990;64(2):143–53. [DOI] [PubMed] [Google Scholar]
- 13.Armienta M, Rodríguez C. Evaluación de la presencia de Arsénico en el valle de Zimapan Hidalgo Universidad Nacional Autónoma de México, 1993. [Google Scholar]
- 14.Del Razo LM, Garcia-Vargas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, et al. Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2002. October;40(10):1423–31. [DOI] [PubMed] [Google Scholar]
- 15.SARH (Secretaría de Agricultura y Recursos Hidraúlicas) Estudio de Hidroarsenicismo en la Comarca Lagunera, México. Cinvestav-IPN, Comisión de Aguas del Valle de México de SARH, 1989. [Google Scholar]
- 16.Devesa V, Maria Del Razo L, Adair B, Drobna Z, Waters SB, Hughes MF, et al. Comprehensive analysis of arsenic metabolites by pH-specific hydride generation atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry. 2004;19(11):1460–7. [Google Scholar]
- 17.Le XC, Ma M. Short-column liquid chromatography with hydride generation atomic fluorescence detection for the speciation of arsenic. Analytical chemistry. 1998. May 1;70(9):1926–33. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Zavala A, Matousek T, Drobna Z, Paul DS, Walton F, Adair BM, et al. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer). J Anal At Spectrom. 2008;23:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matoušek T, Currier JM, Trojánková N, Saunders RJ, Ishida MC, González-Horta C, et al. Selective hydride generation-cryotrapping-ICP-MS for arsenic speciation analysis at picogram levels: analysis of river and sea water reference materials and human bladder epithelial cells. Journal of analytical atomic spectrometry. 2013. June/21;28(9):1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Physical Status: The Use and Interpretation of Anthropometry. Geneva: World Health Organization: 1995. [Google Scholar]
- 21.Agusa T, Fujihara J, Takeshita H, Iwata H. Individual variations in inorganic arsenic metabolism associated with AS3MT genetic polymorphisms. International journal of molecular sciences. 2011;12(4):2351–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drobna Z, Walton FS, Paul DS, Xing W, Thomas DJ, Styblo M. Metabolism of arsenic in human liver: the role of membrane transporters. Archives of toxicology. 2010. January;84(1):3–16. [DOI] [PubMed] [Google Scholar]
- 23.Healy SM, Casarez EA, Ayala-Fierro F, Aposhian H. Enzymatic methylation of arsenic compounds. V. Arsenite methyltransferase activity in tissues of mice. Toxicol Appl Pharmacol. 1998. January;148(1):65–70. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X, McDermott JR, Ajees AA, Rosen BP, Liu Z. Trivalent arsenicals and glucose use different translocation pathways in mammalian GLUT1. Metallomics : integrated biometal science. 2010. March;2(3):211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Boles E, Rosen BP. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. The Journal of biological chemistry. 2004. April 23;279(17):17312–8. [DOI] [PubMed] [Google Scholar]
- 26.Shen H, Niu Q, Xu M, Rui D, Xu S, Feng G, et al. Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans: A Systematic Review and Meta-Analysis. International journal of environmental research and public health. 2016;13(2):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindberg AL, Ekstrom EC, Nermell B, Rahman M, Lonnerdal B, Persson LA, et al. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environmental research. 2008. January;106(1):110–20. [DOI] [PubMed] [Google Scholar]
- 28.Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005. December;113(12):1683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MN, Gamble MV. Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. Journal of toxicology. 2012;2012:595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, et al. Estimation of Inorganic Arsenic Exposure in Populations With Frequent Seafood Intake: Evidence From MESA and NHANES. Am J Epidemiol. 2016. October 15;184(8):590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters BA, Hall MN, Liu X, Neugut YD, Pilsner JR, Levy D, et al. Creatinine, arsenic metabolism, and renal function in an arsenic-exposed population in Bangladesh. PLoS One. 2014;9(12):e113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters BA, Hall MN, Liu X, Slavkovich V, Ilievski V, Alam S, et al. Renal function is associated with indicators of arsenic methylation capacity in Bangladeshi adults. Environmental research. 2015. November;143(Pt A):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.