Abstract
This study aimed to evaluate the operational performance, embolization effect and safety of CalliSpheres microspheres (CSM) in a porcine renal model. 24 healthy normal pigs were randomized into CSM group and Embosphere microspheres (ESM) group equally, and embolized with CSM or ESM in the lower pole of right kidneys, respectively. On D2, D7 and D28 after the operation, angiography and biochemistry examination were performed, and then the pigs were subjected to renal macroscopic and histopathological examination after euthanasia (4 pigs/group per each time point). The embolization was performed successfully in all pigs without obvious adverse events or deaths, and there was no difference in operation assessments (including number of embolized arteries, volume of injected microspheres and vessel recanalization rate) between CSM group and ESM group. After the operation, all pigs in CSM group and ESM group presented with renal necrosis, stasis and capsule exudation on D2 and D7, and capsule thickening, fibrosis and renal atrophy on D28, and there was no difference in these macroscopic or microscopic manifestations between two groups. For biochemistry indexes (including blood routine, coagulation function, liver function and renal function), most of them were similar between two groups at each time point. CSM embolizes porcine renal artery effectively and safely without obvious adverse events or deaths, which might be a good option for embolization therapy in clinical practices.
Keywords: CalliSpheres microspheres, renal artery embolization, effectivity, safety, necrosis
Introduction
Transcatheter embolization is a well-established and commonly performed technique in vascular interventional therapy which has been accepted for treating tumors, vascular lesions and hemorrhages [1-3]. The increasing demand for transcatheter embolization in clinical practices arouses great interests on the development of novel embolization agents [4-6]. Microspheres, also known as spherical particles, are novel embolization agents that overcome a number of drawbacks of non-spherical particles (such as imprecisely calibrated diameters, potential catheter obstruction, incomplete occlusion and unpredictable in vivo behaviors) [7,8]. Currently, there are several microsphere products commercially available. Among these, the first microsphere product is Embosphere microspheres (ESM) (Biosphere Medical, Roissy, France) that is made from tris-acrylic gelatin and marketed in 1996, and other common microsphere products include Contour SE (Boston Scientific, Natick, USA), Bead Block (Biocompatibles, Farnham, UK), LC Beads (Biocompatibles, Farnham, UK), EmboGold (Merit Medical Systems, South Jordan, USA), Embozene (Boston Scientific, Natick, USA), Quadrasphere (Merit Medical Systems, South Jordan, USA) and so on [8-10]. Each microsphere product presents with distinct elasticity, compressibility, rigidity and other biochemical properties, which exert great impacts on their functions and performance [8,11,12]. For instance, Contour SE microspheres display better deformability compared with Bead Block and ESM, suggesting that Contour SE microspheres are able to achieve more distant embolization than that of Bead Block and ESM [8]. Therefore, it is of great importance to evaluate the embolization performance and in vivo behaviors for a new microsphere product.
As a novel microsphere product used for embolization, CalliSpheres Microsphere (CSM) is independently developed by local Chinese pharmaceutical company in 2015, which possesses several outstanding features as follows: Firstly, CSM is made of nonabsorbable and nontoxic polyvinyl alcohol, and it blocks target arteries for a longer time compared to those absorbable embolization materials, which means that CSM provides better treatment efficacy for some diseases (such as renal tumors and vascular malformations) [13-18]. Secondly, CSM presents with smooth surface and favorable stability, elasticity as well as biocompatibility, implying that CSM is less likely to induce microcatheter occlusion and adverse events [13-18]. Thirdly, CSM is negatively charged, and it is able to load a number of positively charged chemotherapeutic drugs (such as doxorubicin, pirarubicin and arsenic trioxide) and then releases them stably in target sites [13-18]. Although there are a number of studies focusing on the application of CSM in drug-eluting bead-transcatheter artery chemotherapy in liver cancer patients, the operational performance and in vivo behaviors of CSM remain to be further investigated [13-18].
In the present experiment, we aimed to evaluate the operational performance, embolization effect and safety of CSM, and compare it with ESM in a porcine renal model.
Methods
Experimental animals
A total of 24 healthy white pigs (23~25 weeks old) were purchased from Shanghai Jiagan Biotechnology Co., Ltd. (Shanghai, China), which consisted of 10 males weighing from 49.5 to 56.5 Kg and 14 nonpregnant females weighing from 48 to 57.5 kg. After delivery, physical examination and quarantine were performed, and the health status of pigs was confirmed by veterinarian. All pigs were housed in approved stainless-steel cages and acclimatized for 7 days. During the whole experiment, pigs were free to drink water, fed with high fiber pannage and raised in the animal room where the average temperature was kept between 18.3°C and 24.0°C, the relative humidity was kept between 33% and 68%, and the light and dark cycles were alternated about 9/15 hours a day. This study was conducted in Gateway Medical Innovation Center (Shanghai, China) according to “Guidance for Industry and FDA Staff-Class II Special Controls Guidance Document: Vascular and Neurovascular Embolization Devices”, and the protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the center before initiation of the experiment.
Study design
Twenty-four experimental pigs were randomly assigned to CSM group (N = 12) or ESM group (N = 12) using simple randomization method, and then partial renal artery embolization was performed using CSM or ESM respectively according to its group. There were three postoperative observation time points including day 2 (D2), day 7 (D7) and day 28 (D28) after the operation. On D2, D7 and D28, angiography and biochemistry examination were performed respectively on 4 pigs in each group, then euthanasia was carried out for those pigs, and the euthanized pigs were subjected to macroscopic and histopathological examination of embolized kidneys.
Embolization procedure
Pigs were fasted within 12 hours before anesthesia. All operations were performed with aseptic technique under general anesthesia, and the electrocardiograph, heart rate and invasive blood pressure were monitored and recorded in real time. Before operation, sedation (xylazine, 4 mg/kg) and anesthesia (propofol, 1-8 mg/kg) were performed on pigs. After successful induction of anesthesia, the pigs were connected with the ventilator after oral intubation, and the respiratory pathway was established, so that the pigs continued to inhale the mixture of anesthetics and oxygen to maintain anesthesia.
The embolization procedures were conducted as follows. Firstly, under digital subtraction aortography (DSA) (Innova 2100; GE Healthcare, Milwaukee, USA), the 5F catheter (Terumo Corporation, Tokyo, Japan) was pushed to the entrance of right renal artery with the guidance of 0.035-inch super-slip guide wire (Terumo Corporation, Tokyo, Japan), then the guide wire was withdrawn. Next, angiography of the entire right kidney was performed using a high-pressure syringe combined with DSA to observe the main branches of renal artery and to determine the target artery for embolization, and the target artery information selected for embolization was recorded in detail on the surgical record sheet, meanwhile, clear angiograms of the right renal artery were preserved. The target artery should be relatively straight and easy to be superselected in order to avoid vascular injury. Besides, if branches of one artery in the lower pole entered the upper pole, then that artery should not be the target artery. Subsequently, 2.7F microcatheter (Terumo Corporation, Tokyo, Japan) was inserted into tertiary branch arteries of right renal lower pole under the guidance of 0.014-inch microwire (Terumo Corporation, Tokyo, Japan). The frontier position of the microcatheter as well as the anatomy and blood flow status of the target vessel were identified by DSA fluoroscopy. After determining the appropriate location of the microcatheter for embolization, the mixture of CSM (300-500 μm, Suzhou Hengrui Callisyn BioMedical Technology Co., Ltd, Suzhou, Jiangsu, China) and contrast agent (iodixanol injection, Jiangsu hengrui pharmaceutical Co., Ltd, Suzhou, Jiangsu, China, mixed as 1:2 before injection) or the mixture of ESM (300-500 μm, Biosphere Medical, Inc, Rockland, Mass., USA) and contrast agent (iodixanol injection, Jiangsu hengrui pharmaceutical Co., Ltd, Suzhou, Jiangsu, China, mixed as 1:1 before injection) was injected into the target artery through the catheter, with an average injection speed of less than 0.5 mL/min. During embolization, catheter position and embolization status were monitored closely by DSA to avoid catheter displacement and reflux of embolic agent due to overdose injection. After embolization of all target arteries, the 2.7F microcatheter was withdrawn, and the actual volume of injected microspheres as well as the completion time of embolization were recorded. The 5F catheter was placed at the entrance of renal artery, and the angiography of the right kidney was performed through DSA combined with a high-pressure syringe to confirm that the target arteries were successfully embolized and the end point of embolization was realized. And the end point of embolization was defined as the renal artery appeared as a “tree-trunk appearance in winter” under DSA after stopping embolization, and no target vessel was recanalized after waiting for 5 to 7 cardiac cycles. Moreover, the blood flow status of upper and lower poles was recorded, and the clear angiograms the end of embolization were preserved.
After resuscitation, the pigs were transferred to the animal room and fed for further observation. During the postoperative observation period, following the requirements of animal care standards, the temperature of the animal room was managed in a normal range, medicine for reducing pain and infection was administered, and adequate water and electrolytes were provided to pigs. All postoperative care and medication use were recorded in the corresponding tables.
Operation assessments
After the operation, the operation time, injection time, the obstruction of microcatheter by microspheres and the injection volume and speed of microspheres were recorded. And the number of embolized arteries, the number of broken arteries, the number of embolization of non-target arteries and embolization outcome were identified by DSA. Embolization outcome was classified as follows: (1) incomplete embolization: the contrast staining of target area was weakened, and there was no obvious change of blood flow in the lower pole artery, or target arteries below grade 2 had obvious changes in blood flow, but not all of them were embolized; (2) complete embolization: the angiographic staining of target area was disappeared, and all the target arteries below grade 2 were embolized; (3) excessive embolization: both target arteries and non-targeted arteries were embolized.
Physical examination
Physical examinations of all pigs were conducted regularly, which consisted of weight, rectal temperature, food-intake, activity and adverse events.
Sample collection and determination
Blood samples for evaluation of hematology, coagulation, renal and liver function were collected from pigs before the operation, on D2, D7 and D28 after the operation. Pigs were fasted overnight prior to blood collection. Blood sample processing and determination was performed by WuXi AppTec (Suzhou) Co., Ltd (Suzhou, China). The blood samples for hematology testing were stored at 2-8°C during storage and transportation; the blood samples for renal and liver function and coagulation function testing were centrifugally separated (centrifugal time: 15 minutes, rotational speed: 3000RPM) to obtain serum and plasma and then were frozen preserved (less than -60°C). (Transport under dry ice storage conditions). Hematological indexes, such as white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), platelet count (PLT) and so on, were determined by Advia 2120 Hematology Analyzer; coagulation function indexes including prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB) and thrombin time (TT) were measured by BE XRM Coagulation Analyzer; and the renal and liver function indexes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), alkaline phosphatase (ALP), urea (UREA), creatinine (CRE), glucose (GLU) and so on, were detected by Hitachi 7180 Biochemistry Analyzer.
Recanalization evaluation of embolized arteries
Recanalization evaluation of embolized arteries was determined by DSA on D2, D7 and D28 after the operation. The vascular recanalization status was classified as: (1) no recanalization: no vascular recanalization occurred; (2) partial recanalization: some target arteries were recanalized, but the number of recanalized arteries was less than half of total embolized arteries; (3) complete recanalization: recanalization was found in all target-embolized vessel locations. Recanalization rate was defined as the percentage of pigs with partial or complete recanalization during the whole study in each group.
Gross observation and histopathologic examination
After euthanasia, pig samples were collected for gross observation and histopathological examination. Necropsies were performed by Gateway Medical Innovation Center, and the formalin-fixed right kidneys from all pigs were transferred to Wuxi AppTec for tissues trimming, slides preparation and microscopic examination. On D2, D7 and D28 after the operation, 4 pigs in each group were euthanized by injection of high concentration potassium chloride solution respectively, and general anatomy and gross inspection were performed on main organs such as heart, liver, spleen and lung. Subsequently, 10% neutral formalin was perfused into the renal tissue in situ, and the kidney was cut into slices (the selection of section was performed as shown in Figure 1 [19]). There were 10 consecutive sections at the lower poles (A1-A10) and upper (B1-B10) respectively, with an interval of about 0.3 cm. In order to avoid cross contamination, the control side was taken first, and then the embolization side was taken. After sampling, dehydration, paraffin embedding, sectioning, hematoxylin and eosin staining and microscopic examination were performed. Histopathological examinations of the sections at lower poles (A1-A10) and upper poles (B1-B10) were performed by pathologists under an optical microscope. Histopathological assessment items included (1) inflammatory response, (2) necrosis, (3) vessel damage, (4) vascular congestion, and (5) vascular regeneration. The severity of each item was scored based upon a scale of within normal limits (0 points), minimal (1 point), mild (2 points), moderate (3 points), marked (4 points) and severe (5 points). And two average scores of each item in the lower pole (10 consecutive sections: A1-A10) and the upper (10 consecutive sections: B1-B10) pole were respectively calculated. (Since most of the upper pole (B1-B10) scores were 0 points, there was no report in this paper.) Besides, whether microspheres drifted to the non-target sites (the upper pole) was also identified by histopathological examinations, and if microspheres were found in the upper pole, it was defined as instrument drift.
Figure 1.
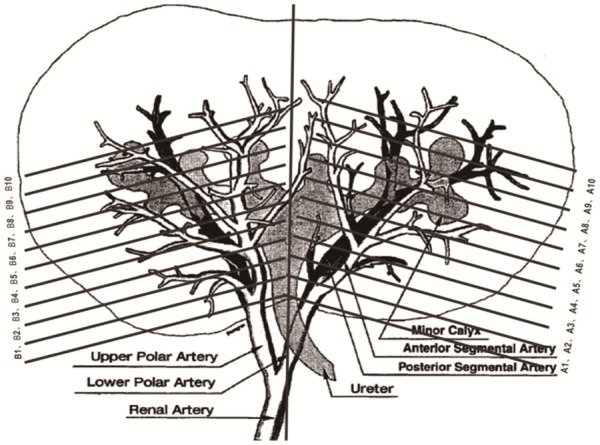
The partition of the kidney [19]. The figure originated from Anat Rec 1996; 246: 217-23.
Statistical analysis
Data were expressed as mean ± standard deviation or count (percentage). Normal distribution of continuous variables used t-test and the categorical variables used Chi-square test. SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and GraphPad Prism 6.02 (GraphPad Software, La Jolla, CA, USA) was used for plotting. All tests were 2-sided and P value < 0.05 indicated a significant difference.
Results
Comparison of overall operation assessments between CSM group and ESM group
The operation was successfully performed in all pigs without death or obvious adverse events, and all pigs presented with normal weight, rectal temperature, food-intake and activity after the operation (data did not show). In CSM group, the mean operation time was 83.25 ± 22.37 minutes, injection volume of microspheres was 0.53 ± 0.15 ml, number of excessively embolized arteries was 3 (25.0%), and number of embolized arteries was 46 (100.0%) (Table 1). In ESM group, the mean operation time was 80.58 ± 24.02 minutes, injection volume of microspheres was 0.64 ± 0.11 ml, number of excessively embolized arteries was 3 (25.0%), and number of embolized arteries was 47 (100.0%). There was no difference in operation indexes between the two groups (all P > 0.05, Table 1).
Table 1.
Operation indexes
| Items | ESM group (N = 12) | CSM group (N = 12) | P value |
|---|---|---|---|
| Operation time (min) | 80.58 ± 24.02 | 83.25 ± 22.37 | 0.781 |
| Injection time (min) | 49.58 ± 17.88 | 41.83 ± 17.42 | 0.294 |
| Injection speed (ml/min) | 0.23 ± 0.08 | 0.19 ± 0.06 | 0.167 |
| Injection volume of microspheres (ml) | 0.64 ± 0.11 | 0.53 ± 0.15 | 0.050 |
| Number of animals occurred excessive embolization (n/%) | 3 (25.0) | 3 (25.0) | 1.000 |
| Number of animals occurred microcatheter obstruction (n/%) | 3 (25.0) | 0 (0.0) | 0.217 |
| Number of embolized arteries (n/%) | 47 (100.0) | 46 (100.0) | 1.000 |
| Number of broken arteries (n/%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Number of nontarget-embolized arteries (n/%) | 0 (0.0) | 0 (0.0) | 1.000 |
Data were presented as mean value ± standard deviation or count (percentage). All comparisons between CSM group and ESM group were determined by t test or Chi-Square test. P value < 0.05 was considered significant.
Comparison of vessel recanalization and instrument drift between CSM group and ESM group
5 (41.7%) pigs in CSM group and 7 (58.3%) pigs in ESM group occurred vessel recanalization after the operation (Figure 2A); meanwhile, 7 (58.3%) pigs in CSM group and 9 (75.0%) pigs in ESM group occurred instrument drift (Figure 2B). There was no difference in vessel recanalization rate (P > 0.05) or instrument drift rate (P > 0.05) between the two groups. Notably, the vessel recanalization did not influence the embolization effect because the lower pole of the right kidney was atrophic in all pigs after the operation. Besides, the instrument drift (which might derive from the relatively high pressure of injector during the angiography procedure) did not influence the blood flow in the upper pole of right kidney either.
Figure 2.
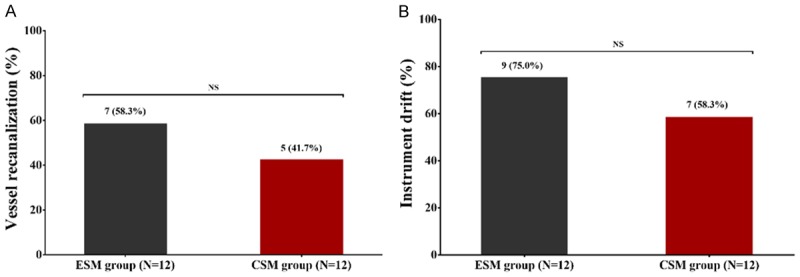
Vessel recanalization and instrument drift in CSM group and ESM group. 5 (41.7%) pigs in CSM group and 7 (58.3%) pigs in ESM group occurred vessel recanalization (A). 7 (58.3%) pigs in CSM group and 9 (75.0%) pigs in ESM group occurred instrument drift (B). No difference of vessel recanalization or instrument drift was discovered between two groups. Comparison between two groups was determined via Chi-square test. P value < 0.05 was considered significant. *P < 0.05. CSM, CalliSpheres microspheres; ESM, Embosphere microspheres; NS, no significance.
The angiogram in CSM group and ESM group before the operation, right after the operation, on D2, D7 and D28 after the operation
Before operation, the staining of the renal parenchyma and the blood vessel was explicit in CSM group (Figure 3A) and ESM group (Figure 3F). Right after the operation, the staining of the renal parenchyma and the blood vessel in lower pole was disappeared in CSM group (Figure 3B) and ESM group (Figure 3G), indicating that the embolization was successful. On D2 after the operation, the target arteries were embolized persistently in two groups (Figure 3C, 3H). On D7 after the operation, the blood flow was partly reinstated in proximal arteries (Figure 3D, 3I), and on D28 after the operation, the blood flow was partly reinstated in a proportion of distal arteries (Figure 3E, 3J). In addition, the blood flow of upper pole was unobstructed at each time point in all pigs, suggesting that the microspheres did not embolize the upper pole arteries even though instrument drift was observed in some pigs. To sum up, both CSM and ESM embolized target arteries effectively in a sustained manner.
Figure 3.
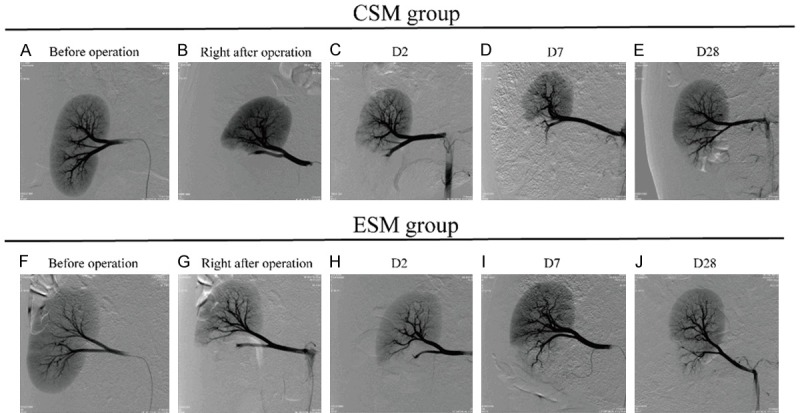
The angiogram before the operation, right after the operation, on D2, D7 and D28 after the operation in CSM group and ESM group. Before operation, the staining of blood vessel and renal parenchyma could be clearly seen in CSM group (A) and ESM group (F). Right after the operation, the staining of the renal parenchyma and the blood vessel in lower pole were disappeared in CSM group (B) and ESM group (G). On D2 after treatment, the target arteries were embolized persistently in two groups (C, H). On D7 after treatment, the blood flow was partly reinstated in proximal arteries (D, I), and on D28 after treatment, the blood flow was partly reinstated in a proportion of distal arteries (E, J). CSM, CalliSpheres microspheres; ESM, Embosphere microspheres.
Macroscopic findings of the lower pole in CSM group and ESM group on D2, D7 and D28 after the operation
On D2 after the operation, both CSM group (Figure 4A) and ESM group (Figure 4D) presented with renal necrosis and stasis in the lower segment, exudation in the right renal capsule, and swollen in the right kidney. On D7 after the operation, both CSM group (Figure 4B) and ESM group (Figure 4E) presented with swollen, adhesion, necrosis, atrophy, and exudation in the renal capsule. On D28 after the operation, both CSM group (Figure 4C) and ESM group (Figure 4F) presented with decrescent, yellowing, firm, and uneven surface of the lower pole. These finds were in accordance with microscopic findings (cortical necrosis, thrombus in the vascular lumen, inflammation, fibrosis and so on) which were described in the next paragraph. Taken together, macroscopic findings were similar between CSM group and ESM group.
Figure 4.
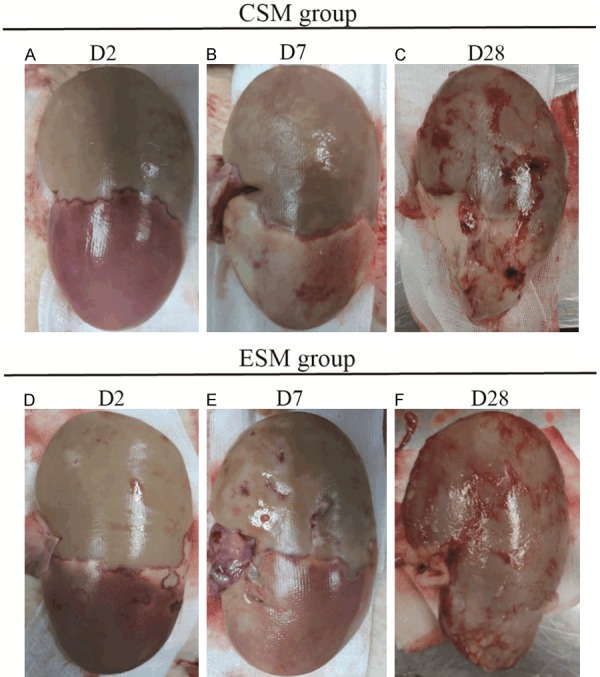
Gross findings of the lower pole in CSM group and ESM group on D2, D7 and D28 after the operation. On D2 after the operation, both CSM group (A) and ESM group (D) presented with renal necrosis and stasis in the lower segment, and exudation in the right renal capsule, and swollen in the right kidney. On D7 after the operation, both CSM group (B) and ESM group (E) presented with swollen, renal capsule adhesion, necrosis, atrophy, and exudation in renal capsule. On D28 after the operation, both CSM group (C) and ESM group (F) presented with decrescent, yellowing, firm, and uneven surface of the lower pole. CSM, CalliSpheres microspheres; ESM, Embosphere microspheres.
Microscopic findings of the lower pole in CSM group and ESM group on D2, D7 and D28 after the operation
On D2 after the operation, both CSM group (Figure 5A) and ESM group (Figure 5E) presented with wedge-shaped or widespread area of cortical necrosis, cell debris and hemorrhage (congestion) in the periphery rim, and congestion was also observed in the medulla in both groups. In CSM group, the target arteries changed greatly which included thrombosis in the vascular lumen, hemorrhage in the vascular wall (with occasional presence of multinucleated giant cells and occasional necrosis) and inflammation in the perivascular areas (Figure 5B); in ESM group, the target arteries also changed obviously which presented with perivascular inflammation and hemorrhage with multinucleated giant cells on the surface of microspheres and thrombosis in the vascular lumen (Figure 5F). On D7 after the operation, cortical necrosis persisted, and the inflammatory cell infiltrations were more prominent compared with D2 in CSM group (Figure 5C) and ESM group (Figure 5G). Besides, both groups existed basophilic tubule lining with flattened epithelial cells, and a large amount of spindle cells as well as neovascularization in the periphery of necrotic area, indicating that the reparative process had initiated. On D28 after the operation, necrotic area was reduced and was gradually replaced by inflammatory cells, regenerative tubules (glomeruli), new blood vessels and fibrosis in both groups. Also, the cortex became thin and fibrous became thickened in the capsule. In addition, the structure of some arteries was disrupted and difficult to identify, and some microspheres seemed to be phagocytized by multinucleated giant cells in CSM group (Figure 5D) and ESM group (Figure 5H). Notably, ESM was fragmentized microscopically and incomplete in shape, which might be an artifact generated during the slide preparations. In brief, there was no difference between CSM group and ESM group in terms of inflammation, necrosis, congestion, vessel damage and regeneration in the lower pole of the kidney.
Figure 5.
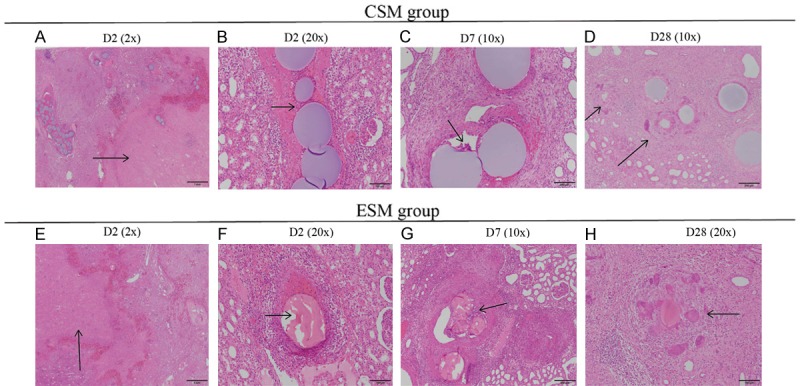
Histopathology findings of the lower pole in CSM group and ESM group on D2, D7 and D28 after the operation. On D2 after the operation, there was cortical necrosis with hemorrhage/congestion in the periphery rim and congestion in medulla in CSM group (arrows) (A) and ESM group (E). Besides, inflammatory cells infiltrated in the perivascular area and there existed thrombosis in both groups (arrows) (B, F). The ESM was fragmented and incomplete in shape, which was most likely to be an artifact generated during slides preparation. On D7 after the operation, the microspheres were surrounded by multinucleated giant cells (arrows) in both groups (C, G). On D28 after the operation, microspheres were phagocytized by multinucleated giant cells in CSM group (D) and ESM group (arrows) (H). Specimen samples were examined using hematoxylin-eosin (H-E) staining. CSM, CalliSpheres microspheres; ESM, Embosphere microspheres; H-E, hematoxylin-eosin.
Comparison of histopathology scores at the lower pole of the kidney between CSM group and ESM group
Necrosis score was 4.68 ± 0.47, 4.55 ± 0.60 and 1.20 ± 0.85 on D2, D7 and D28 after the operation in CSM group, respectively, and was 4.30 ± 0.56, 4.58 ± 0.55 and 0.68 ± 1.21 on D2, D7 and D28 after the operation in ESM group, respectively. No difference of necrosis scores was observed between CSM group and ESM group at each time point (all P > 0.05, Table 2). Besides, inflammation score, congestion score, vessel damage score or regeneration score was also of no difference between the two groups at each time point (all P > 0.05). These data implied that embolization of renal artery with CSM and ESM produced a similar effect on the kidney.
Table 2.
Histopathology scores at lower pole artery
| Items | ESM group | CSM group | P value |
|---|---|---|---|
| Necrosis score* | |||
| D2 | 4.30 ± 0.56 | 4.68 ± 0.47 | 0.340 |
| D7 | 4.58 ± 0.55 | 4.55 ± 0.60 | 0.944 |
| D28 | 0.68 ± 1.21 | 1.20 ± 0.85 | 0.511 |
| Inflammation score* | |||
| D2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| D7 | 2.43 ± 0.59 | 2.40 ± 0.55 | 0.943 |
| D28 | 2.35 ± 0.62 | 2.33 ± 0.66 | 0.966 |
| Congestion score* | |||
| D2 | 0.68 ± 1.16 | 0.68 ± 0.97 | 1.000 |
| D7 | 0.00 ± 0.00 | 0.08 ± 0.35 | 0.679 |
| D28 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| Vessel damage score* | |||
| D2 | 1.55 ± 0.71 | 1.85 ± 0.92 | 0.625 |
| D7 | 1.98 ± 0.86 | 2.48 ± 0.60 | 0.381 |
| D28 | 2.83 ± 1.32 | 1.85 ± 1.23 | 0.319 |
| Regeneration score* | |||
| D2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| D7 | 2.85 ± 0.43 | 2.70 ± 0.46 | 0.651 |
| D28 | 2.93 ± 0.69 | 3.03 ± 0.66 | 0.841 |
Data were presented as mean value ± standard deviation. All comparisons between CSM group and ESM group were determined by t test. P < 0.05 was considered significant.
Symptom severity was scored based upon a scale of within normal limits (0 points), minimal (1 point), mild (2 points), moderate (3 points), marked (4 points) and severe (5 points).
Comparison of renal function indexes between CSM group and ESM group
There was no difference in GLU (Figure 6A), UREA (Figure 6B) or CRE (Figure 6C) between CSM group and ESM group on D2, D7 or D28 (all P > 0.05), indicating that the renal function was similar after kidney being embolized with CSM or ESM.
Figure 6.
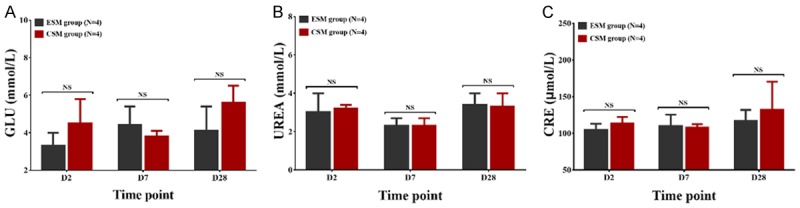
Renal function indexes in CSM group and ESM group. GLU (A), UREA (B) or CRE (C) were similar between CSM group and ESM group on D2, D7 or D28 after treatment. Comparison between two groups was determined by t test. P value < 0.05 was considered significant. *P < 0.05. CSM, CalliSpheres microspheres; ESM, Embosphere microspheres; GLU, glucose; UREA, urea; CRE, creatinine; NS, no significance.
Comparison of blood routine, coagulation function indexes and liver function indexes between CSM group and ESM group
For blood routine examination, the RBC (Figure 7A), WBC (Figure 7B), HGB (Figure 7C) and PLT (Figure 7D) were similar between CSM group and ESM group on D2, D7 and D28 after the operation (all P > 0.05). For coagulation function indexes, the APTT was larger in CSM group compared with ESM group on D28 (P < 0.01) while was similar between two groups on D2 (P > 0.05) and D7 (P > 0.05) after the operation (Figure 7F). No difference in PT (Figure 7E), TT (Figure 7G) or FIB (Figure 7H) was observed between the two groups on D2, D7 or D28 after the operation (all P > 0.05). As for liver function indexes, the ALT (Figure 7I), AST (Figure 7J), ALP (Figure 7K) or TBIL (Figure 7L) was of no difference between CSM group and ESM group at each time point, either (all P > 0.05). These data suggested that CSM exerted a similar impact on the physical state of pigs compared with ESM.
Figure 7.
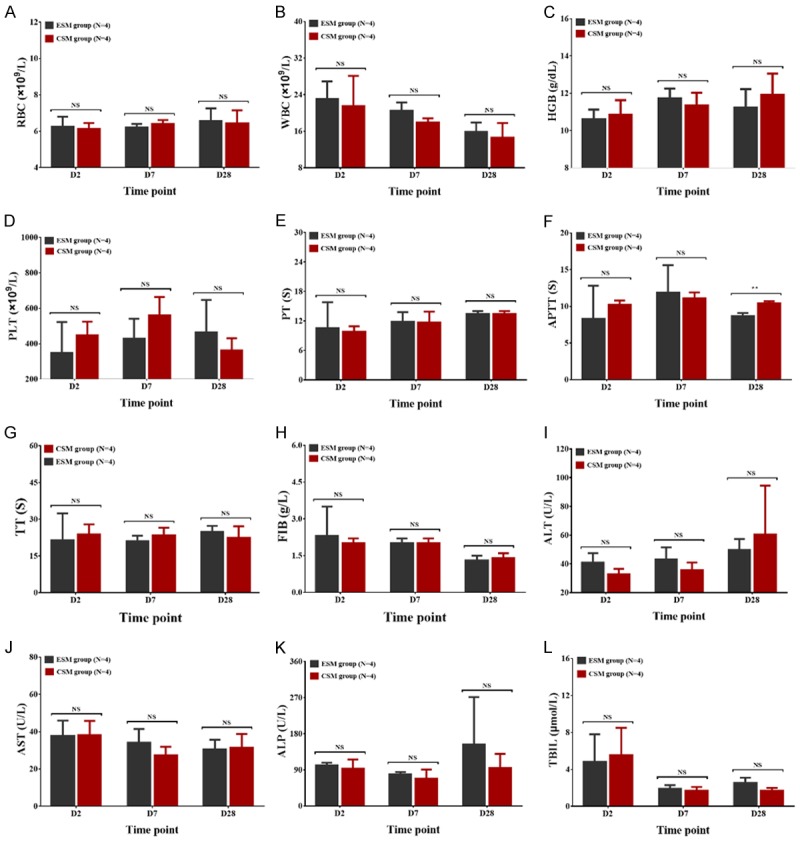
Blood routine, coagulation function indexes and liver function indexes in CSM group and ESM group. For blood routine examination, the RBC (A), WBC (B), HGB (C) or PLT (D) was of no difference between CSM group and ESM group on D2, D7 or D28 after the operation. For coagulation function indexes, the APTT (F) was increased on D28 while was similar on D2 and D7 after treatment in CSM group compared to ESM group; besides, there was no difference in PT (E), TT (G) or FIB (H) between the two groups on D2, D7 or D28 after treatment. As for liver function indexes, the ALT (I), AST (J), ALP (K) and TBIL (L) were similar between CSM group and ESM group. Comparison between two groups was determined by t test. P value < 0.05 was considered significant. **P < 0.01. CSM, CalliSpheres microspheres; ESM, Embosphere microspheres; RBC, red blood cell; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; FIB, fibrinogen. ALT, glutamic-pyruvic transaminase; AST, glutamic oxalacetic transaminase; ALP, alkaline phosphatase; TBIL, total bilirubin; NS, no significance.
Discussion
In the current study, we observed several interesting results: (1) All pigs in CSM group and ESM group received renal artery embolization successfully in the lower pole, and the two groups presented with similar overall operation assessments, including the vessel recanalization rate and instrumental drift rate. (2) Both CSM and ESM embolized target arteries accurately, effectively and long-lastingly, which also caused tissue necrosis, inflammation, congestion, vascular damage and regeneration in the lower pole at similar levels. (3) CSM and ESM group presented with similar blood routine, coagulation function, liver function and renal function indexes at each visit after the operation.
A lot of animal models have been established in order to investigate the in vivo performance of microspheres, such as rats, rabbits, sheep and pigs [9,20-22]. Among these animal models, we chose pigs as embolization model in the current study for several reasons. Firstly, pigs are the most widely used animal models in preclinical studies of microspheres [23,24]. Secondly, porcine models are not difficult to obtain, and pigs exhibit similar renal sizes, renal vessel diameters and lengths compared to human beings, which make them suitable for embolization [19]. Thirdly, if the microspheres occur non-target embolization in porcine kidneys, they could be distinguished easily (according to the anatomy) [4]. Fourthly, the overall embolization procedure for pigs is much the same with the embolization procedure conducted in clinical practices. Thus, embolization using porcine models is able to simulate the transcatheter arterial embolization in clinical practices. Last but not least, pigs have two kidneys, whereas we only embolized the secondary branch in the lower pole of the right kidney, which minimized the porcine sufferings and did not affect their survivals. In the present study, we utilized ESM as a control rather than LC bead or Bead Block (CSM are more similar to LC bead or Bead Block) because these two products were unavailable in China, and ESM exhibited similar features compared with LC Bead and Bead Block, including indications, particle sizes, compressibility and sterilization method.
CSM is made of polyvinyl alcohol and presents with smooth surface, superb arterial compliance and great biocompatibility, which embolizes target arteries accurately and long-lastingly [13-17]. In addition, CSM has 5 ranges of caliber (100-300 μm, 300-500 μm, 500-700 μm, 700-900 μm and 900-1200 μm), which fully meet different clinical needs [13-17]. Up to now, CSM has been reported to be an effective chemoembolization agent in treating hepatocellular carcinoma and soft tissue sarcoma patients [14-17]. In order to obtain more in-depth knowledge of its performance, the lower pole of the porcine kidney was embolized using CSM and ESM in this study, which revealed that the embolization was performed in all pigs without death or obvious adverse events. Besides, the overall operation evaluations were similar in pigs embolized with CSM and in pigs embolized with ESM, including vessel recanalization and instrument drift. The possible explanations for our results might be that: (1) CSM and ESM presented with similar characteristics, which suggested that they exhibited similar performances. (2) The relatively small sample size and short evaluation time might also influence the results. Interestingly, the recanalization rate in our study was lower than a previous study. In that study, the recanalization rate of target arteries was more than 60% after porcine renal being embolized with polyethylene glycol hydrogel-based resorbable microspheres [23]. The possible reason for the lower recanalization rate in our study might be due to that: both CSM and ESM were made of nonabsorbable materials, which embolized target arteries in a longer time compared with those microspheres (which were made of absorbable materials). In addition, we also observed instrument drift in both groups, which meant that a small proportion of microspheres were drifted to the upper pole. However, the drift derived from the relatively high pressure of injector during injection, which was not associated with the characteristics of CSM or ESM, and the drift did not affect upper pole of renal obviously, either (as depicted in Figure 4).
Generally, the embolization of arteries causes tissue injuries, which present with tissue ischemia, vascular damage, necrosis and inflammation, and the severity of tissue injuries positively correlates with embolization effectivity to some extent [25-27]. Besides, the microspheres also induce body reaction, which presents as infiltration of mononuclear inflammatory cells, and subsequently the occurrence of fibroplasia and neovascularization [28]. In an animal study, resorbable carboxymethyl cellulose/Chitosan microspheres are embolized to the target arteries in a rabbit renal model, which shows that the embolization leads to the renal malformation, discoloration and atrophy within 6 months after embolization. And microscopic observations discover that the embolization brings in fibering, tubular casts, multifocal mineralization, neovascularization and the loss of renal parenchyma [28]. In the other two studies, similar results are observed in porcine models using other material-based microspheres [27,29]. In the current study, the macroscopic and microscopic changes of the lower pole were also analyzed and compared between CSM group and ESM group. It showed that on D2 and D7, the lower pole presented with necrosis, stasis and capsule exudation, and on D28, the lower pole presented with decrescent, firm and uneven surface in CSM group and ESM group. These macroscopic observations were in line with microscopic observations, such as necrosis on D2, inflammation on D7 and D28, and fibrosis on D28. And all of these manifestations in lower pole were stable, chronic healing responses without any active or acute changes such as neutrophilic inflammation. In addition, no obvious difference was observed between CSM group and ESM group regarding the macroscopic or microscopic manifestations, suggesting that CSM was as safe as ESM for embolization, and it was suitable for long-term embolization. The possible reason for the similar results between CSM group and ESM group probably derived from the similar embolization effectivity. In the present study, we also discovered that the ESM was fragmented and incomplete in shape microscopically, which might be an artifact generated during slides preparation. Thus it could not influence the final results.
As discussed above, the artery embolization by microspheres brings in tissue ischemia, vascular damage, necrosis and inflammation. The evaluation of tissue injury could not only rely on macroscopic or microscopic manifestations, but also rely on biochemical tests such as blood routine, liver function, and renal function indexes [26,30]. For instance, a previous study utilizes drug-eluting microspheres for liver artery embolization in pigs, which leads to a transient increase in liver function indexes [30]. In another study, both the liver and renal function indexes of rabbit models are elevated transiently after their renal artery being embolized via Bletilla striata polysaccharide-based microspheres or polyvinyl alcohol-based microspheres, and there is no difference in the liver or renal function indexes between the two groups [26]. In the current study, the blood routine, coagulation function, liver function and renal function indexes of pigs were assessed after embolization with CSM or ESM, and we found most of these indexes were similar between CSM group and ESM group, confirming that the embolization using CSM was equally safe compared with embolization using ESM.
Some potential limitations of the present study should be mentioned. Firstly, we utilized the CSM and ESM for renal artery embolization with a size of 300-500 μm, and considering that different sized microspheres might present with different in vivo performance, other sized CSM and ESM should be compared in the further studies. Secondly, the comparison of macroscopic and microscopic observations between CSM group and ESM group was [30] comprehensive comparisons need to be conducted in further studies. Lastly, the sample size of animals was relatively small, which might decrease statistical power.
In summary, CSM embolizes porcine renal artery effectively and safely without obvious adverse events or deaths, which might be a good option for embolization therapy in clinical practices.
Acknowledgements
This study was supported by Jiangsu Province’s Key Provincial Talents Program (NO. ZDRCA2016038).
Disclosure of conflict of interest
None.
References
- 1.Dogan N, Nas OF, Canver B, Ozturk K, Gokalp G. Selective bilateral renal artery embolization with tris-acryl microspheres in focal segmental glomerulosclerosis. Diagn Interv Imaging. 2017;98:277–278. doi: 10.1016/j.diii.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Sommer CM, Do TD, Schlett CL, Flechsig P, Gockner TL, Kuthning A, Vollherbst DF, Pereira PL, Kauczor HU, Macher-Goppinger S. In vivo characterization of a new type of biodegradable starch microsphere for transarterial embolization. J Biomater Appl. 2018;32:932–944. doi: 10.1177/0885328217746674. [DOI] [PubMed] [Google Scholar]
- 3.Karalli A, Ghaffarpour R, Axelsson R, Lundell L, Bozoki B, Brismar T, Gustafsson O. Transarterial chemoembolization of renal cell carcinoma: a prospective controlled trial. J Vasc Interv Radiol. 2017;28:1664–1672. doi: 10.1016/j.jvir.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 4.de Silva S, Mackie S, Aslan P, Cade D, Delprado W. Histological comparison of kidney tissue following radioembolization with yttrium-90 resin microspheres and embolization with bland microspheres. Cardiovasc Intervent Radiol. 2016;39:1743–1749. doi: 10.1007/s00270-016-1482-3. [DOI] [PubMed] [Google Scholar]
- 5.Lubarsky M, Ray C, Funaki B. Embolization agents-which one should be used when? Part 2: small-vessel embolization. Semin Intervent Radiol. 2010;27:99–104. doi: 10.1055/s-0030-1247891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz MJ, Smith EB, Trost DW, Vaughan ED Jr. Renal artery embolization: clinical indications and experience from over 100 cases. BJU Int. 2007;99:881–886. doi: 10.1111/j.1464-410X.2006.06653.x. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa Lde A, Caldas JG, Conti ML, Malheiros DM, Ramos FF Jr. Effect of renal embolization with trisacryl and PAVc. Clinics (Sao Paulo) 2009;64:1105–1112. doi: 10.1590/S1807-59322009001100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilbao JI, de Luis E, Garcia de Jalon JA, de Martino A, Lozano MD, de la Cuesta AM, Sangro B. Comparative study of four different spherical embolic particles in an animal model: a morphologic and histologic evaluation. J Vasc Interv Radiol. 2008;19:1625–1638. doi: 10.1016/j.jvir.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Verret V, Ghegediban SH, Wassef M, Pelage JP, Golzarian J, Laurent A. The arterial distribution of embozene and embosphere microspheres in sheep kidney and uterus embolization models. J Vasc Interv Radiol. 2011;22:220–228. doi: 10.1016/j.jvir.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Sheth RA, Sabir S, Krishnamurthy S, Avery RK, Zhang YS, Khademhosseini A, Oklu R. Endovascular embolization by transcatheter delivery of particles: past, present, and future. J Funct Biomater. 2017;8 doi: 10.3390/jfb8020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stampfl S, Bellemann N, Stampfl U, Radeleff B, Lopez-Benitez R, Sommer CM, Thierjung H, Berger I, Richter GM. Inflammation and recanalization of four different spherical embolization agents in the porcine kidney model. J Vasc Interv Radiol. 2008;19:577–586. doi: 10.1016/j.jvir.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Stampfl U, Stampfl S, Bellemann N, Sommer CM, Lopez-Benitez R, Thierjung H, Radeleff B, Berger I, Richter GM. Experimental liver embolization with four different spherical embolic materials: impact on inflammatory tissue and foreign body reaction. Cardiovasc Intervent Radiol. 2009;32:303–312. doi: 10.1007/s00270-008-9495-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Huang C, Li Z, Yang Y, Bao T, Chen H, Zou Y, Song L. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and callispheres beads in rabbit livers. Drug Deliv. 2017;24:1011–1017. doi: 10.1080/10717544.2017.1344336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni JY, Sun HL, Chen YT, Luo JH, Wang WD, Jiang XY, Chen D, Xu LF. Drug-eluting bead transarterial chemoembolization in the treatment for unresectable soft tissue sarcoma refractory to systemic chemotherapy: a preliminary evaluation of efficacy and safety. J Cancer Res Clin Oncol. 2018;144:157–163. doi: 10.1007/s00432-017-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Z, Zhou G, Yu W, Shao G. Abstract No. 511 the comprehensive analysis of efficacy and safety of CalliSpheres® drug-eluting beads transarterial chemoembolization in 367 patients with liver cancer: a multiple-center, prospective cohort study (CTILC study) J Vasc Interv Radiol. 2018;29:S215. [Google Scholar]
- 16.Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16:69. doi: 10.1186/s12957-018-1368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou GH, Han J, Sun JH, Zhang YL, Zhou TY, Nie CH, Zhu TY, Chen SQ, Wang BQ, Yu ZN, Wang HL, Chen LM, Wang WL, Zheng SS. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres(R) beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18:644. doi: 10.1186/s12885-018-4566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Zhang D, Ying Y, Wang Z, Tao W, Zhu H, Zhang J, Peng Z. Clinical investigation on transarterial chemoembolization with indigenous drug-eluting beads in treatment of unresectable hepatocellular carcinoma. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2017;46:44–51. doi: 10.3785/j.issn.1008-9292.2017.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evan AP, Connors BA, Lingeman JE, Blomgren P, Willis LR. Branching patterns of the renal artery of the pig. Anat Rec. 1996;246:217–223. doi: 10.1002/(SICI)1097-0185(199610)246:2<217::AID-AR8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Kurzidem M, Seidensticker P, Rassweiler J. Renal chemoembolization with mitomycin c/ethibloc: pharmacokinetics and efficacy in an animal model. J Endourol. 2002;16:515–518. doi: 10.1089/089277902760367485. [DOI] [PubMed] [Google Scholar]
- 21.Sternlicht M, Sales SF, Daniels JR, Daniels A. Renal cisplatin chemoembolization with angiostat, gelfoam, and ethiodol in the rabbit: renal platinum distributions. Radiology. 1989;170:1073–1075. doi: 10.1148/radiology.170.3.2536948. [DOI] [PubMed] [Google Scholar]
- 22.Torii S, Jinnouchi H, Sakamoto A, Romero ME, Kolodgie FD, Virmani R, Finn AV. Comparison of biologic effect and particulate embolization after femoral artery treatment with three drug-coated balloons in healthy swine model. J Vasc Interv Radiol. 2019;30:103–109. doi: 10.1016/j.jvir.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Maeda N, Verret V, Moine L, Bedouet L, Louguet S, Servais E, Osuga K, Tomiyama N, Wassef M, Laurent A. Targeting and recanalization after embolization with calibrated resorbable microspheres versus hand-cut gelatin sponge particles in a porcine kidney model. J Vasc Interv Radiol. 2013;24:1391–1398. doi: 10.1016/j.jvir.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 24.Stampfl S, Bellemann N, Stampfl U, Sommer CM, Thierjung H, Lopez-Benitez R, Radeleff B, Berger I, Richter GM. Arterial distribution characteristics of embozene particles and comparison with other spherical embolic agents in the porcine acute embolization model. J Vasc Interv Radiol. 2009;20:1597–1607. doi: 10.1016/j.jvir.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Laurent A, Velzenberger E, Wassef M, Pelage JP, Lewis AL. Do microspheres with narrow or standard size distributions localize differently in vasculature? An experimental study in sheep kidney and uterus. J Vasc Interv Radiol. 2008;19:1733–1739. doi: 10.1016/j.jvir.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Luo SH, Song SL, Zheng CS, Li WY, Wang Y, Xia XW, Feng GS. Embolic effects of Bletilla striata microspheres in renal artery and transplanted VX2 liver tumor model in rabbits. Chin J Integr Med. 2019;25:431–438. doi: 10.1007/s11655-017-2953-3. [DOI] [PubMed] [Google Scholar]
- 27.Shomura Y, Tanigawa N, Shibutani M, Wakimoto S, Tsuji K, Tokuda T, Terada J, Kariya S, Kojima H, Komemushi A, Sawada S. Water-soluble polyvinyl alcohol microspheres for temporary embolization: development and in vivo characteristics in a pig kidney model. J Vasc Interv Radiol. 2011;22:212–219. doi: 10.1016/j.jvir.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Weng L, Seelig D, Souresrafil O. Longterm implantability of resorbable carboxymethyl cellulose/chitosan microspheres in a rabbit renal arterial embolization model. Cardiovasc Intervent Radiol. 2018;41:951–958. doi: 10.1007/s00270-018-1931-2. [DOI] [PubMed] [Google Scholar]
- 29.Yasutaka B, Sadao H, Shunichiro I, Michiyo H, Masayuki N. Experimental renal and hepatic artery embolization with a new embolic agent, atelocollagen, in a porcine model. Diagn Interv Radiol. 2013;19:141–144. doi: 10.4261/1305-3825.DIR.6086-12.1. [DOI] [PubMed] [Google Scholar]
- 30.Daniels JR, Sternlicht M, Daniels AM. Collagen chemoembolization: pharmacokinetics and tissue tolerance of cis-diamminedichloroplatinum(II) in porcine liver and rabbit kidney. Cancer Res. 1988;48:2446–2450. [PubMed] [Google Scholar]


