Abstract
Background: Early diagnosis of invasive fungal disease (IFD) is challenging. High-resolution computed tomography (CT) may improve IFD diagnosis; however, there are no definitive imaging signs for differentiating between bacterial pneumonia and IFD. Methods: We retrospectively evaluated CT images of 208 patients with IFD (n = 102) or bacterial pneumonia (n = 106). We classified pulmonary opacities as consolidations, ground-glass opacities (GGOs), or nodules and recorded the presence of perinodular ground-glass halos, reversed halo sign (RSH), and cavitation (crescent-shaped or not). Results: Consolidation appeared in 83.3% and 92.5% of patients with IFD and bacterial pneumonia, respectively. Multifocal non-segmental consolidation was more common in IFD (48%) than bacterial pneumonia (22.6%; P < 0.05). Segmental or subsegmental consolidation was more common in bacterial pneumonia (43.4%) than IFD (7.8%; P < 0.01). GGOs and nodules were more common in IFD than bacterial pneumonia (60.8% vs. 24.5% and 54.9% vs. 15.1%, respectively; each P < 0.05). Consolidation combined with GGO, nodules, or both GGO and nodules was more frequent in IFD than in bacterial pneumonia (each P < 0.05). Nodules with halo sign (n = 23) appeared in 22.5% and 3.8% of patients with IFD and bacterial pneumonia, respectively. Nodules with RSH appeared only in IFD, and those with cavitation appeared in 11.8% and 1.9% of patients with IFD and bacterial pneumonia, respectively. Conclusions: Consolidation plus GGO and nodules or consolidation plus nodules is suggestive for IFD. Segmental or subsegmental consolidations are more frequent in bacterial pneumonia than in IFD. Large nodules, as well as nodules with halo sign or both small and large nodules, are related to IFD.
Keywords: Invasive fungal disease, bacterial pneumonia, high-resolution computed tomography
Introduction
Invasive fungal disease (IFD) of the lung is a common complication in immunocompromised patients and is associated with high morbidity and mortality [1]. Early diagnosis of IFD is often difficult, but it is imperative to improve patient survival. Serum galactomannan testing may have a role in diagnosis, but it has low sensitivity and frequent false-positive results [2-4]. Computed tomography (CT) plays an important role in the diagnosis and management of patients with fungal infections due to its ability to reveal early predictive signs of fungal infection [5-8]. In recognition of advances in diagnostic technology, a consensus committee of the European Organization for Research and Treatment of Cancer (EORTC) Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Disease Mycoses Study Group (MSG) updated the guidelines for IFD classification [9]. The updated guidelines include an increased role for CT scans. CT is probably the most important tool for the management of early IFD and may be superior to the mycological criteria established by the EORTC/MSG [6,7,10]. However, pulmonary CT findings are nonspecific. The diagnostic sensitivity of the radiologic criteria specified by the EORTC/MSG definition of invasive aspergillosis is low compared with the gold standard of autopsy examination [11]. Several studies have focused on describing specific CT signs of pulmonary aspergillosis [6,12,13], but there is little information about CT features that may allow IFD to be distinguished from bacterial pneumonia. The aim of this study was to distinguish the CT features of IFD from those of bacterial pneumonia to improve the early diagnosis of IFD.
Materials and methods
Patient population
This study is a retrospective evaluation of 208 consecutive patients diagnosed with IFD or bacterial pneumonia between October 2014 and September 2015. The 102 patients with IFD included 55 females and 47 males, with a mean age of 43.6 (range 22-65) years. The 106 patients with bacterial pneumonia included 43 females and 63 males, with a mean age of 47.9 (range 19-67) years. All of the patients with IFD had a proven or probable diagnosis of IFD according to the consensus EORTC/MSG criteria [9]. Proven IFD was defined by histological evidence of tissue invasion. Probable IFD was defined by the presence of host factors; clinical findings such as a halo sign, air-crescent sign, or cavity within an area of consolidation on CT; and mycological evidence of fungal infection from culture, cytological analysis of bronchoalveolar lavage (BAL) fluid, or galactomannan measurement in the serum or BAL. Confirmation of IFD patients consisted of a positive culture of BAL fluid and associated radiologic findings. Diagnosis of bacterial pneumonia was based on a positive culture of sputum or bronchoscopic aspirate in addition to positive blood or pleural fluid cultures. CT scans were performed when IFD or bacterial pneumonia was clinically suspected. According to the regulations of the Ethics Committee of Southwest Hospital, informed consent was not necessary for this retrospective study.
Computed tomography
CT examinations were performed using a dual-source CT scanner (Somatom Definition, Siemens, Erlangen, Germany). The scanning parameters for the chest CT were 120 kVp, 120 mAs, 1.25 mm collimation, pitch of 1, and routine 512 × 512 matrix. Clinical images were reconstructed with 5-mm-thick axial images (width 1200 HU, centered on a density of -600 HU) and 2-mm-thick axial images using high spatial frequency algorithm intervals with a sharp kernel (B46). All patients were scanned in the supine position from the lung apex to the base of the chest during full inspiration. In most cases, no intravenous contrast was applied.
Image analysis
Lung lesions were interpreted on a (picture archiving and communication system (PACS). Three radiologists, each with 10-15 years of experience in the interpretation of thoracic CT examinations (C.W., C.Y.L., and C.P.), analyzed the CT scans and made decisions about the pattern, distribution, and extent of pulmonary abnormalities by means of consensus. We classified the pattern as consolidation, ground-glass opacity (GGO), nodules, or a combination of two or more of those findings [14]. Consolidation and nodules were defined as areas of dense increase in attenuation, with obscuration of the underlying vessels. We further subcategorized consolidation as segmental or non-segmental and as patchy or focal. We defined patchy consolidation as a wedge-shaped area with shape and size corresponding to a secondary pulmonary lobule or subsegment. We defined GGO as a hazy increase in lung attenuation without obscuration of the underlying pulmonary vasculature. A nodule was defined as an area with well or poorly defined, rounded opacity that was no greater than 3 cm. We defined nodules < 1 cm in diameter as small and those > 1 cm in diameter as large. We defined halo sign as an area of GGO surrounding a nodule. We used the term “tree-in-bud pattern” to describe centrilobular branching structures that resembled a budding tree. A cavity was defined as a gas-filled space, visible as a lucency within a pulmonary lesion, and air crescents surrounding soft-tissue lesions to constitute a crescent sign.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Fisher exact probability and Chi-square tests were used to determine the statistical significance of differences in the presence of CT features between patient groups. P-values < 0.05 were considered statistically significant.
Results
The frequencies of the different features of parenchymal involvement in IFD and bacterial pneumonia are summarized in Table 1. There was no significant difference in the frequency of consolidation between bacterial pneumonia (93 of 106 patients, 62.3%) and IFD (85 of 102 patients, 83.3%). In IFD, the consolidation was focal and non-segmental in 14 patients (13.7%), multifocal and non-segmental in 49 patients (48%), segmental or subsegmental in 8 patients (7.8%), and both segmental and non-segmental in 14 patients (13.7%). In bacterial pneumonia, the consolidation was focal and non-segmental in 10 patients (9%), multifocal and non-segmental in 24 patients (22.6%), segmental or subsegmental in 46 patients (43.4%), and both segmental and non-segmental in 13 patients (12.3%; Figure 1). Non-segmental consolidation was more common in IFD than in bacterial pneumonia (48% vs. 22.6%; P < 0.05). Segmental or subsegmental consolidation was more common in bacterial pneumonia than in IFD (43.4% vs. 7.8%; P < 0.01).
Table 1.
CT findings of pulmonary infections
| CT findings | Classification | IFD (n = 102) | Bacterial pneumonia (n = 106) | P valuea |
|---|---|---|---|---|
| Consolidation | 85 (83.3) | 98 (92.5) | ||
| Shape | Non-segmental focal | 14 (13.7) | 15 (14.2) | < 0.05 |
| Non-segmental multifocal | 49 (48) | 24 (22.6) | < 0.01 | |
| Segmental or subsegmental | 8 (7.8) | 46 (43.4) | ||
| Segmental + non-segmental | 14 (13.7) | 13 (12.3) | ||
| With cavitation (including air crescent sign) | 4 | 2 | ||
| Reversed halo sign | 8 | |||
| GGO | 62 (60.8) | 26 (24.5) | < 0.05 | |
| Diffuse | 26 (25.4) | 8 (7.5) | < 0.05 | |
| Patchy | 36 (35.2) | 18 (16.9) | < 0.05 | |
| Nodules | 63 (61.8) | 23 (21.7) | < 0.01 | |
| Size | Small | 15 (14.7) | 14 (13.2) | |
| Large | 20 (19.6) | 3 (2.8) | < 0.05 | |
| Both small and large | 28 (27.5) | 6 (5.7) | < 0.05 | |
| Tree-in-bud | 12 (11.8) | 9 (8.5) | ||
| Halo | 23 (22.5) | 4 (3.8) | < 0.05 | |
| With cavitation (incl. air crescent sign) | 6 | |||
| Pleural effusion | 21 (20.6) | 52 (49.1) | < 0.05 | |
Data are given as the number (percentage) of patients.
Calculated using the Fisher exact test.
IFD: invasive fungal disease, GGO: ground-glass opacitie.
Figure 1.
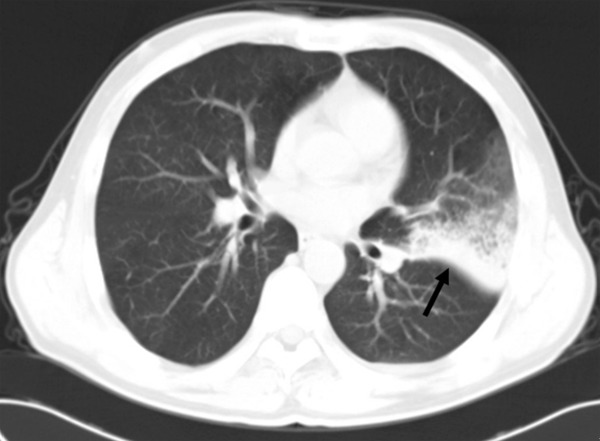
High-resolution CT scans of a 62-year-old man with chronic myeloid leukemia, with a consolidation area due to bacterial pneumonia just anterior to the left major fissure.
GGO was more common in IFD than in bacterial pneumonia (60.8% vs. 24.5%; P < 0.05). Both patchy and diffuse GGO distributions were more common in patients with IFD than in those with bacterial pneumonia (each P < 0.05; Table 1).
Nodules were more common in IFD than in bacterial pneumonia (60.8% vs. 16.9%; P < 0.05). Furthermore, both large nodules and mixtures of small and large nodules were more common in IFD than in bacterial pneumonia (19.6% vs. 0.9% and 27.5% vs. 5.7%, respectively; each P < 0.001). There was no significant difference in the frequency of tree-in-bud opacities between the two diseases. Six patients with IFD had nodules with cavitation, whereas no patients with bacterial pneumonia had them.
In terms of combinations of consolidation, GGO, and nodules, consolidation alone was less common in IFD than in bacterial pneumonia (9.8% vs. 62.3%; P < 0.01; Figure 1). Among the patients with IFD, four (3.9%) had only GGO, and three (2.1%) had only nodules. Similarly, among the patients with bacterial pneumonia, three (2.8%) had only GGO, and two (1.8%) had only nodules. The remaining 85 patients with IFD and 40 patients with bacterial pneumonia had various combinations of consolidation, GGO, and nodules (Table 2). There were several combinations that appeared more frequently in IFD than in bacterial pneumonia: consolidation plus GGO (24.5% vs. 13.2%, P < 0.05; Figure 2), at least one segmental area of consolidation plus at least one nodule (26.4% vs. 11.3%, P < 0.05; Figures 3 and 4), GGO plus at least one nodule (9.8% vs. 2.8%, P < 0.05; Figure 5), and at least one segmental area of consolidation plus GGO and at least one nodule 22.5% vs. 5.7%, P < 0.01; Figures 6, 7 and 8).
Table 2.
Combinations of CT features of pulmonary infections
| CT pattern | IFD (n = 85) | Bacterial pneumonia (n = 35) | P value |
|---|---|---|---|
| Consolidation + GGO | 25 (24.5) | 14 (13.2) | < 0.05 |
| Consolidation + nodules | 27 (26.5) | 12 (11.3) | < 0.05 |
| Consolidation + nodules + GGO | 23 (22.5) | 6 (5.7) | < 0.05 |
| GGO + nodules | 10 (9.8) | 3 (2.8) | < 0.05 |
Data are given as the number (percentage) of patients unless otherwise indicated. Percentages may not equal 100% because of rounding. IFD: invasive fungal disease, GGO: ground-glass opacitie.
Figure 2.
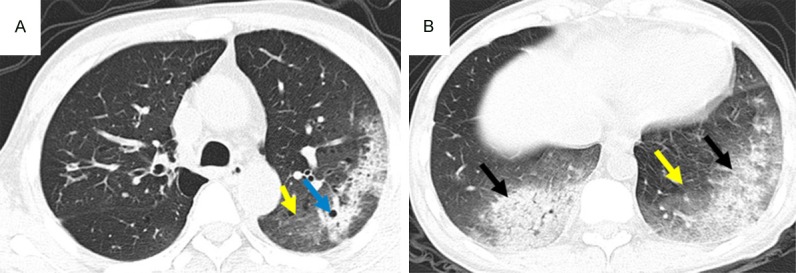
CT pattern of consolidation + GGO, High-resolution CT scans of a 54-year-old man with myelodysplastic syndrome. Areas of consolidation (black arrows) with associated cavitation (blue arrow) and ground-glass attenuation (yellow arrows) in the left lower lobe due to IFD.
Figure 3.
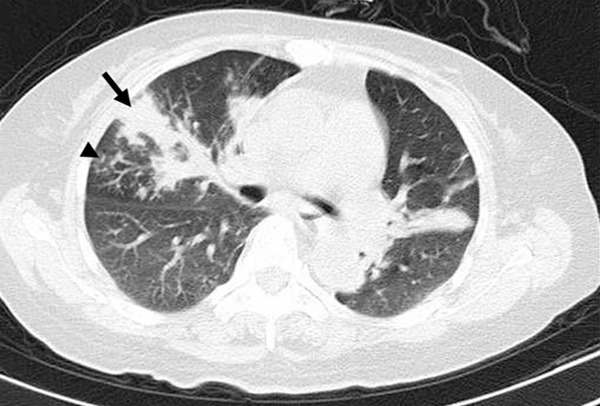
CT pattern of consolidation + nodules, High-resolution CT scans of a 53-year-old woman with multiple myeloma. An area of consolidation (black arrow) associated with small nodular opacities with a tree-in-bud appearance (arrowhead) in the right middle lobe due to IFD.
Figure 4.
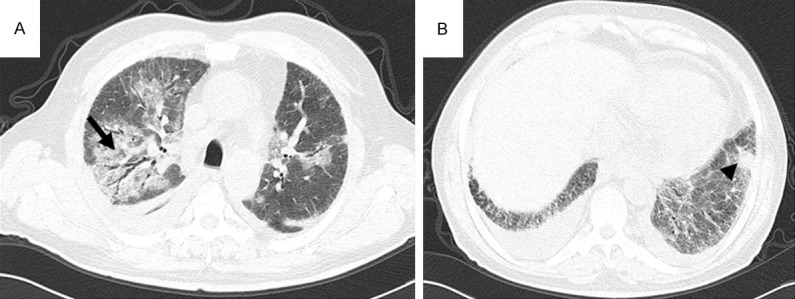
CT pattern of consolidation + nodules, High-resolution CT scans of a 46-year-old man with myelodysplastic syndrome. A: A consolidation area with air bronchogram in the right upper pulmonary lobe (arrow) due to IFD. B: An irregular nodular appearance in the left lower lobe (arrowhead) due to IFD.
Figure 5.
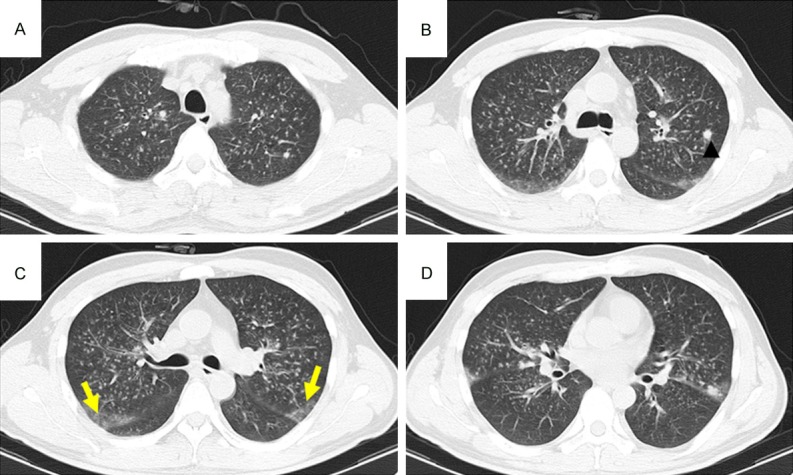
CT pattern of GGO + nodules High-resolution CT scans of a 51-year-old man with non-Hodgkin’s lymphoma. A-D: Diffuse, bilateral, ill-defined nodular opacities with a tree-in-bud appearance, multiple large nodules (arrowhead), and patchy ground-glass attenuation in the right upper lobe (yellow arrows) due to IFD.
Figure 6.
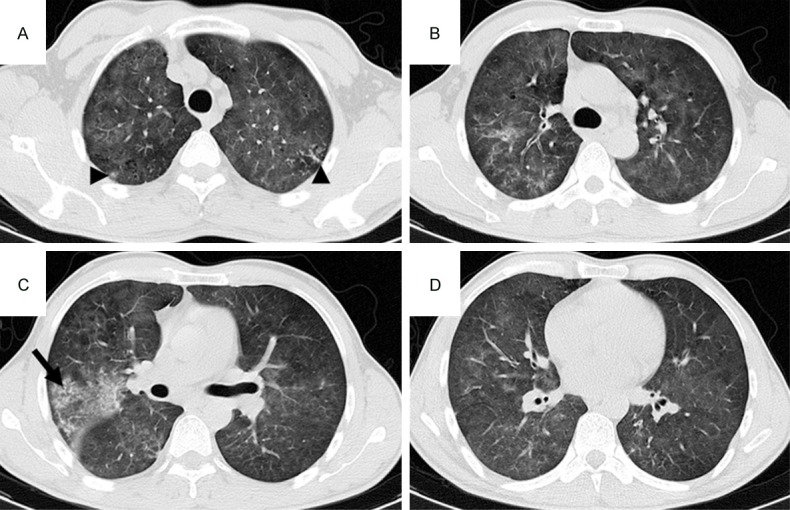
CT pattern of consolidation + GGO + nodules, High-resolution CT scans of a 56-year-old man with acute myeloid leukemia-M3. A-D: Diffuse ground-glass attenuation, patchy consolidation in the right upper lobe (arrow), and ill-defined small nodular opacities with a tree-in-bud appearance in the bilateral upper lobes (arrowheads) due to IFD.
Figure 7.
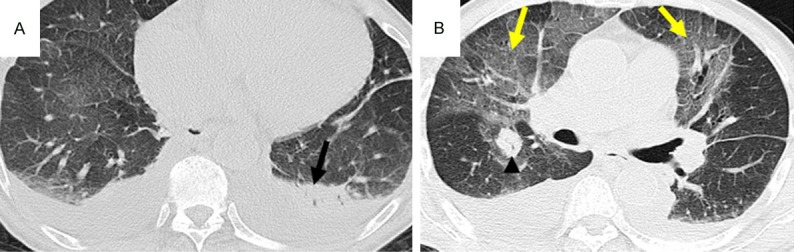
CT pattern of consolidation + GGO, High-resolution CT scans of a 77-year-old man with multiple myeloma. A: A segmental area of consolidation in the left lower lobe (black arrow). B: Diffuse ground-glass attenuation in the lung (yellow arrow) and a nodular appearance in the right upper lobe due to IFD.
Figure 8.
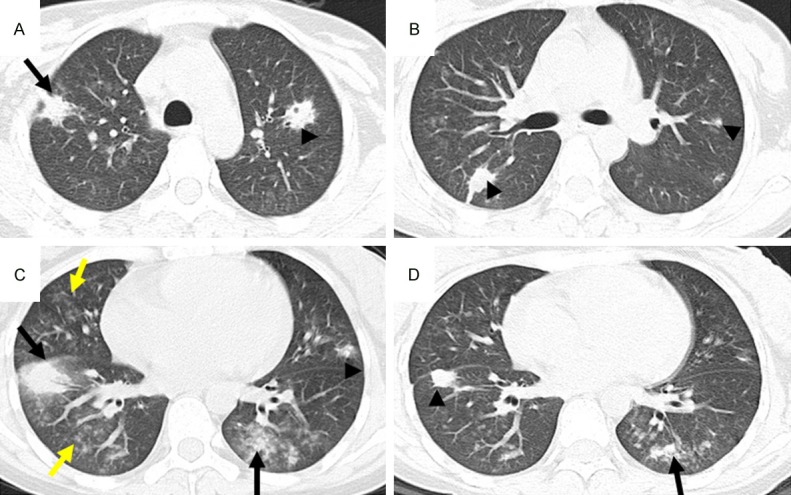
CT pattern of consolidation + GGO + nodules High-resolution CT scans of a 27-year-old woman with lymphocytic leukemia. A-D: Multiple areas of consolidation (black arrows), ground-glass attenuation (yellow arrows), and multiple ill-defined nodules (arrowheads) due to IFD. Note the nodule with a thick-walled cavity in the left upper lobe.
The halo sign appeared in 22.5% of the patients with IFD and 3.8% of the patients with bacterial pneumonia (P < 0.05). The reversed halo sign (RHS) appeared in 7.8% of the patients with IFD and none of the patients with bacterial pneumonia. The tree-in-bud pattern appeared in 11.8% of the patients with IFD and in 8.5% of the patients with bacterial pneumonia. Cavitation appeared in 9.8% of the patients with IFD (Figure 2) and only 1.9% of the patients with bacterial pneumonia (Table 1).
Discussion
Mortality rates from IFD are exceedingly high among immunocompromised patients. Early diagnosis of IFD followed by anti-fungal therapy is expected to improve those outcomes. Empiric therapy is usually started as soon as there is clinical suspicion of fungal infection; however, antifungal drugs are expensive and can have severe side effects. CT signs of IFD have been shown to precede serum galactomannan positivity [10]. In a previous study, a CT-based treatment strategy for IFD in immunocompromised patients seemed feasible, and a CT-based preemptive strategy reduced the use of parenteral antifungal agents by 68% [18]. Those results highlight the importance of early diagnosis and treatment in IFD. In practice, the most challenging task is to differentiate between bacterial pneumonia and IFD.
The clinical and radiological manifestations of pulmonary IFD have been studied extensively and are well known [15-20]. The revised EORTC/MSG criteria extended the guidelines for the radiological diagnosis of IFD [9], but there are still no definitive, highly specific imaging signs for early recognition. In our study, 22.5% of the patients with IFD presented with dense, well-circumscribed lesions (nodules) accompanied by a halo sign, thus meeting the radiological criteria for IFD as defined by the EORTC/MSG. The halo sign is neither sensitive nor specific for IFD, however, and is occasionally present in patients with other diseases [22-25]. Our radiological findings in patients with IFD consisted mostly of ill-defined consolidations, nodules, and ground-glass infiltrates. IFD often presents as a nonspecific air-space consolidation. Christe et al. reported that wedge-shaped, pleural-based consolidations were indicative of pulmonary IFD with a specificity of 100% and a sensitivity of 43-46% [26]. However, other studies showed a low sensitivity (25%) of wedge-shaped consolidation for IFD diagnosis [21]. Extensive vascular permeation and apparent occlusion of small-to-medium-sized arteries by fungal hyphae, with or without thrombus formation, are suggestive of pulmonary aspergillosis [12]. In contrast, the presence of lobar or segmental consolidation areas suggests bacterial pneumonia [27,28]. In our series, three out of 102 patients with IFD presented wedge-shaped consolidations, which may not be helpful in early diagnosis. The late appearance of wedge-shaped consolidations during the course of pulmonary IFD limits its usefulness for early diagnosis.
Consolidation was the most common CT finding in both IFD and bacterial pneumonia in our study. There was no significant difference in the overall frequency of that finding between the two diseases; however, 62.3% of the patients with bacterial pneumonia exhibited segmental or subsegmental consolidation without GGO or nodules. In contrast, multifocal, non-segmental consolidation occurred significantly more often in patients with IFD than in those with bacterial pneumonia. Therefore, segmental or subsegmental consolidation is suggestive of bacterial pneumonia [26,29], whereas multifocal non-segmental consolidation suggests IFD [4,12,17].
The EORTC/MSG consensus recently showed confidence in the etiologic significance of morphologic features of pulmonary opacities, defining any new occurrence of halo sign, cavitation, or air-crescent sign as a major criterion for fungal infection [9]. Nevertheless, some authors are skeptical about the possibility of CT-based etiologic diagnosis of pulmonary infections in immunocompromised patients [27,30]. The results of our study suggest that some CT patterns are clinically significant in distinguishing IFD from bacterial pneumonia.
Specific CT patterns that differentiate IFD from other pulmonary infections would be very useful in the early diagnosis of IFD. The CT patterns of segmental consolidation plus GGO and nodules, or segmental consolidation plus nodules, occurred significantly more often in IFD than in bacterial pneumonia, and thus appear to have a high predictive value for IFD. In our series, the halo sign was present in 22.5% of the patients with IFD but only 3.8% of those with bacterial pneumonia. The RHS has been reported in association with other infectious conditions and some non-infectious processes [31,32]. It was initially described as a relatively specific finding in the diagnosis of cryptogenic organizing pneumonia [7,33], but it was subsequently reported in a wide spectrum of diseases including infectious and non-infectious conditions [34-36]. The RHS lesion differs from the pattern of necrosis and cavitation. Legouge reported that the presence of the RHS on CT was a strong indicator of pulmonary mucormycosis in leukemic patients with neutropenia [37,38]. In our study, eight out of 102 patients with IFD exhibited a typical RHS, but none of the patients with bacterial pneumonia exhibited that sign. Hence, we suggest that the RHS could be added to the list of diagnostic CT findings of IFD. Cavitation or air-crescent formation occurs later in the course of the disease and is considered highly suggestive of IFD [39]; however, the sensitivity of those signs in IFD diagnosis did not exceed 27% in previous studies [38]. We observed nodules with cavitation in six patients with IFD but not in any patients with bacterial pneumonia.
Our study has some limitations. The number of patients was small, and we did not perform lung biopsies. Our inclusion criteria could also have caused a selection bias. Another limitation was the lack of comparison with other pulmonary infections.
Conclusion
Our results show that a CT pattern of either segmental consolidation plus GGO and nodules or segmental consolidation plus nodules can be considered suggestive of IFD. Segmental or subsegmental consolidation appeared more frequently in bacterial pneumonia than in IFD. Large nodules, nodules with a halo sign, or both small and large nodules were associated with IFD. Those results suggest that CT scan can be useful for the early differential diagnosis of IFD.
Disclosure of conflict of interest
None.
References
- 1.Oh YW, Effmann EL, Godwin JD. Pulmonary infections in immunocompromised hosts: the importance of correlating the conventional radiologic appearance with the clinical setting. Radiology. 2000;217:647–656. doi: 10.1148/radiology.217.3.r00dc35647. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762–1769. doi: 10.1086/429921. [DOI] [PubMed] [Google Scholar]
- 3.Duarte RF, Sanchez-Ortega I, Cuesta I, Arnan M, Patino B, Fernandez de Sevilla A, Gudiol C, Ayats J, Cuenca-Estrella M. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis. 2014;59:1696–1702. doi: 10.1093/cid/ciu673. [DOI] [PubMed] [Google Scholar]
- 4.Brown MJ, Miller RR, Muller NL. Acute lung disease in the immunocompromised host: CT and pathologic examination findings. Radiology. 1994;190:247–254. doi: 10.1148/radiology.190.1.8259414. [DOI] [PubMed] [Google Scholar]
- 5.Kim MJ, Lee KS, Kim J, Jung KJ, Lee HG, Kim TS. Crescent sign in invasive pulmonary aspergillosis: frequency and related CT and clinical factors. J Comput Assist Tomogr. 2001;25:305–310. doi: 10.1097/00004728-200103000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 7.Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52:1144–1155. doi: 10.1093/cid/cir122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal BH. Aspergillosis. N Engl J Med. 2009;360:1870–1884. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 9.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisser M, Rausch C, Droll A, Simcock M, Sendi P, Steffen I, Buitrago C, Sonnet S, Gratwohl A, Passweg J, Fluckiger U. Galactomannan does not precede major signs on a pulmonary computerized tomographic scan suggestive of invasive aspergillosis in patients with hematological malignancies. Clin Infect Dis. 2005;41:1143–1149. doi: 10.1086/444462. [DOI] [PubMed] [Google Scholar]
- 11.Sinko J, Csomor J, Nikolova R, Lueff S, Krivan G, Remenyi P, Batai A, Masszi T. Invasive fungal disease in allogeneic hematopoietic stem cell transplant recipients: an autopsy-driven survey. Transpl Infect Dis. 2008;10:106–109. doi: 10.1111/j.1399-3062.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 12.Franquet T, Muller NL, Gimenez A, Guembe P, de La Torre J, Bague S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics. 2001;21:825–837. doi: 10.1148/radiographics.21.4.g01jl03825. [DOI] [PubMed] [Google Scholar]
- 13.Brook O, Guralnik L, Hardak E, Oren I, Sprecher H, Zuckerman T, Engel A, Yigla M. Radiological findings of early invasive pulmonary aspergillosis in immune-compromised patients. Hematol Oncol. 2009;27:102–106. doi: 10.1002/hon.879. [DOI] [PubMed] [Google Scholar]
- 14.Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, Webb WR, Zerhouni EA. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200:327–331. doi: 10.1148/radiology.200.2.8685321. [DOI] [PubMed] [Google Scholar]
- 15.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 16.Won HJ, Lee KS, Cheon JE, Hwang JH, Kim TS, Lee HG, Han J. Invasive pulmonary aspergillosis: prediction at thin-section CT in patients with neutropenia--a prospective study. Radiology. 1998;208:777–782. doi: 10.1148/radiology.208.3.9722859. [DOI] [PubMed] [Google Scholar]
- 17.Horger M, Hebart H, Einsele H, Lengerke C, Claussen CD, Vonthein R, Pfannenberg C. Initial CT manifestations of invasive pulmonary aspergillosis in 45 non-HIV immunocompromised patients: association with patient outcome? Eur J Radiol. 2005;55:437–444. doi: 10.1016/j.ejrad.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Dignan FL, Evans SO, Ethell ME, Shaw BE, Davies FE, Dearden CE, Treleaven JG, Riley UB, Morgan GJ, Potter MN. An early CT-diagnosis-based treatment strategy for invasive fungal infection in allogeneic transplant recipients using caspofungin first line: an effective strategy with low mortality. Bone Marrow Transplant. 2009;44:51–56. doi: 10.1038/bmt.2008.427. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, Kim SH, Choi SH, Sung H, Kim MN, Woo JH, Kim YS, Park SK, Lee JH, Lee KH, Lee SG, Han DJ, Lee SO. Clinical and radiological features of invasive pulmonary aspergillosis in transplant recipients and neutropenic patients. Transpl Infect Dis. 2010;12:309–315. doi: 10.1111/j.1399-3062.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 20.Stanzani M, Battista G, Sassi C, Lewis RE, Tolomelli G, Clissa C, Femia R, Bazzocchi A, Tumietto F, Viale P, Ambretti S, Baccarani M, Vianelli N. Computed tomographic pulmonary angiography for diagnosis of invasive mold diseases in patients with hematological malignancies. Clin Infect Dis. 2012;54:610–616. doi: 10.1093/cid/cir861. [DOI] [PubMed] [Google Scholar]
- 21.Bruno C, Minniti S, Vassanelli A, Pozzi-Mucelli R. Comparison of CT features of Aspergillus and bacterial pneumonia in severely neutropenic patients. J Thorac Imaging. 2007;22:160–165. doi: 10.1097/RTI.0b013e31805f6a42. [DOI] [PubMed] [Google Scholar]
- 22.Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol. 2005;78:862–865. doi: 10.1259/bjr/77712845. [DOI] [PubMed] [Google Scholar]
- 23.Yogi A, Miyara T, Ogawa K, Iraha S, Matori S, Haranaga S, Murayama S. Pulmonary metastases from angiosarcoma: a spectrum of CT findings. Acta Radiol. 2016;57:41–46. doi: 10.1177/0284185115571789. [DOI] [PubMed] [Google Scholar]
- 24.Shrot S, Schachter J, Shapira-Frommer R, Besser MJ, Apter S. CT halo sign as an imaging marker for response to adoptive cell therapy in metastatic melanoma with pulmonary metastases. Eur Radiol. 2014;24:1251–1256. doi: 10.1007/s00330-014-3129-6. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi D, Bargagli E, Calabro AG, Torricelli E, Giannelli F, Cavigli E, Miele V. Atypical HRCT manifestations of pulmonary sarcoidosis. Radiol Med. 2018;123:174–184. doi: 10.1007/s11547-017-0830-y. [DOI] [PubMed] [Google Scholar]
- 26.Kawel N, Schorer GM, Desbiolles L, Seifert B, Marincek B, Boehm T. Discrimination between invasive pulmonary aspergillosis and pulmonary lymphoma using CT. Eur J Radiol. 2011;77:417–425. doi: 10.1016/j.ejrad.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Reittner P, Ward S, Heyneman L, Johkoh T, Muller NL. Pneumonia: high-resolution CT findings in 114 patients. Eur Radiol. 2003;13:515–521. doi: 10.1007/s00330-002-1490-3. [DOI] [PubMed] [Google Scholar]
- 28.Nie Y, Li C, Zhang J, Wang H, Han P, Lv X, Xu X, Guo M. Clinical application of high-resolution computed tomographic imaging features of community-acquired pneumonia. Med Sci Monit. 2016;22:1053–1061. doi: 10.12659/MSM.895638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conces DJ Jr. Bacterial pneumonia in immunocompromised patients. J Thorac Imaging. 1998;13:261–270. doi: 10.1097/00005382-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Franquet T, Muller NL, Gimenez A, Martinez S, Madrid M, Domingo P. Infectious pulmonary nodules in immunocompromised patients: usefulness of computed tomography in predicting their etiology. J Comput Assist Tomogr. 2003;27:461–468. doi: 10.1097/00004728-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Gaeta M, Blandino A, Scribano E, Minutoli F, Volta S, Pandolfo I. Computed tomography halo sign in pulmonary nodules: frequency and diagnostic value. J Thorac Imaging. 1999;14:109–113. doi: 10.1097/00005382-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Algin O, Gokalp G, Topal U. Signs in chest imaging. Diagn Interv Radiol. 2011;17:18–29. doi: 10.4261/1305-3825.DIR.2901-09.1. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, Sung KJ. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol. 2003;180:1251–1254. doi: 10.2214/ajr.180.5.1801251. [DOI] [PubMed] [Google Scholar]
- 34.Queiroz RM, Gomes MP, Valentin MV. Pulmonary paracoccidioidomycosis showing reversed halo sign with nodular/coarse contour. Radiol Bras. 2016;49:59–60. doi: 10.1590/0100-3984.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barreto MM, Marchiori E, de Brito A, Escuissato DL, Hochhegger B, Souza AS, Rodrigues RS. CT morphological features of the reversed halo sign in pulmonary paracoccidioidomycosis. Br J Radiol. 2015;88:20150246. doi: 10.1259/bjr.20150246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchiori E, Zanetti G, Hochhegger B, Irion KL, Carvalho AC, Godoy MC. Reversed halo sign on computed tomography: state-of-the-art review. Lung. 2012;190:389–394. doi: 10.1007/s00408-012-9392-x. [DOI] [PubMed] [Google Scholar]
- 37.Legouge C, Caillot D, Chretien ML, Lafon I, Ferrant E, Audia S, Pages PB, Roques M, Estivalet L, Martin L, Maitre T, Bastie JN, Dalle F. The reversed halo sign: pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin Infect Dis. 2014;58:672–678. doi: 10.1093/cid/cit929. [DOI] [PubMed] [Google Scholar]
- 38.Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–614. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 39.Caillot D, Couaillier JF, Bernard A, Casasnovas O, Denning DW, Mannone L, Lopez J, Couillault G, Piard F, Vagner O, Guy H. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J. Clin. Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]


