Abstract
Aberrant expression of long non-coding RNA DSCAM-AS1 (Down Syndrome Cell Adhesion Molecule antisense) has been observed in several cancers. However, the expression status, biological function and underling mechanism of DSCAM-AS1 in hepatocellular carcinoma (HCC) remain unclear. The expression of DSCAM-AS1 was detected in HCC tissues and serum from both HCC patients and healthy controls. MTS, wound healing and transwell invasion assays were used to examine the effects of DSCAM-AS1 on cell proliferation, migration, and invasion in HCC cells, respectively. MicroRNAs (miRNAs) targeted DSCAM-AS1 was predicated by Starbase2.0 and identified using luciferase reporter and RNA immunoprecipitation assays. The xenograft mice were established to examine the effect DSCAM-AS1 on tumor growth in vivo. We found that DSCAM-AS1 was up-regulated in HCC tissues relative to adjacent non-tumor tissues. Serum levels of DSCAM-AS1 were higher in HCC patients than that in healthy controls. Increased DSCAM-AS1 was associated with poor prognosis. Knockdown of DSCAM-AS1 significantly inhibited HCC cell proliferation, migration and invasion. Moreover, miR-338-3p was confirmed as a direct target of DSCAM-AS1 in HCC cells. The miR-338-3p inhibitor could partially reverse the inhibitory effect of DSCAM-AS1 depletion in HCC cells. DSCAM-AS1 positively regulated CyclinD1 and smoothened (SMO) expression (two targets of miR-338-3p) in HCC cells. Moreover, tumor growth was tremendously retarded in nude mice received injection of SMCC-7721 cells transfected with sh-DSCAM-AS1. Taken together, the present work suggested that DSCAM-AS1 functioned as an oncogenic lncRNA that promoted HCC progression by sponging miR-338-3p.
Keywords: Hepatocellular carcinoma, DSCAM-AS1, miR-338-3p, proliferation, invasion
Introduction
One of the most common lethal malignancies is hepatocellular carcinoma (HCC), with a poor prognosis due to the advanced stage of disease at initial diagnosis, high rate of recurrence and metastasis after hepatic resection and multi-drug resistance [1,2]. It is urgently need to explore the pathogenesis and biological features of HCC for early detection and treatment.
Long non-coding RNAs (lncRNAs) are a class of non-coding transcripts greater than 200 nucleotides in length and without protein-coding capacity [3]. In recent years, researches have showed that lncRNAs could serve as transcriptional or post-transcriptional regulators of gene expression [4]. LncRNAs were reported to be involved in a broad spectrum of cellular progression, especially in cell proliferation, apoptosis, invasion and metastasis [5,6]. Mounting evidence has suggested that lncRNAs might play oncogenic or tumor suppressive role in the regulation of carcinogenesis, tumor initiation and development [7,8]. Number of cancer-associated lncRNAs was reported to implicate in tumorigenesis and progression of HCC, and serve as biological maker for diagnosis and treatment of this cancer [9,10].
LncRNA Down Syndrome Cell Adhesion Molecule (DSCAM) antisense (DSCAM-AS1), located on 21q22.2, and is an important member of the immunoglobulin superfamily of cell adhesion molecules [11]. Several studies revealed that DSCAM-AS1 played crucial roles in tumor proliferation, migration and invasion in non-small-cell lung cancer and breast cancer [12-14]. However, the status, biological function and the regulatory mechanisms of DSCAM-AS1 in HCC are still unknown.
Here, we detected the expression levels of DSCAM-AS1 in tissues and serum from patients with HCC, and evaluated its clinical significance in patients with HCC. Moreover, we also examined the effects of DSCAM-AS1 on HCC growth and metastasis, and tested regulatory mechanisms of DSCAM-AS1 in HCC progression.
Materials and methods
Patients and samples
48 paired HCC tissues and adjacent non-tumor tissues were collected from patients who underwent hepatectomy for HCC at China-Japan Union Hospital of Jilin University (Changchun, China) between January 2013 and December 2013. Besides that, blood (approximately 8 ml) was harvested from the elbow vein of all 48 patients with HCC and 30 healthy controls. Serum of blood was obtained by centrifugation at 750 g for 15 min. Matched adjacent non-tumor specimens were obtained from a part of the resected specimen that was farthest from the cancer (>5 cm laterally from the edge of the cancerous region). All samples were immediately frozen in liquid nitrogen after surgery, and stored at -80°C until use. Clinicopathological characteristics of patients with HCC were listed in Table 1. None of patients had received radio-chemotherapy or other therapy prior to surgery. This study was reviewed and approved by the Ethics Committee of the Jilin University and was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. All subjects were informed of the study and signed consent forms before surgery.
Table 1.
Association of DSCAM-AS1 expression with clinicopathologic factors of 48 patients with HCC
| Variables | No. of cases | DSCAM-AS1 expression | P value | |
|---|---|---|---|---|
|
| ||||
| High (n%) | Low (n%) | |||
| Age (years) | P=0.7761 | |||
| <50 | 21 | 12 | 9 | |
| ≥50 | 27 | 14 | 13 | |
| Gender | P=0.3806 | |||
| Male | 28 | 17 | 11 | |
| Female | 20 | 9 | 11 | |
| TNM stage | P=0.0063 | |||
| I-II | 37 | 16 | 21 | |
| III-IV | 11 | 10 | 1 | |
| Differentiated | P=0.5257 | |||
| Well/Moderate | 34 | 17 | 17 | |
| Poor | 14 | 9 | 5 | |
| Serum AFP (ng/ml) | P=0.7704 | |||
| ≤20 | 20 | 10 | 10 | |
| >20 | 28 | 16 | 12 | |
| HCV antigen | P=0.0702 | |||
| Positive | 31 | 20 | 11 | |
| Negative | 17 | 6 | 11 | |
| Vascular invasion | P<0.0001 | |||
| No | 39 | 17 | 22 | |
| Yes | 9 | 9 | 0 | |
AFP: Alpha-Fetoprotein; HCV: hepatitis c virus.
Cell lines and transfection
Shanghai Institutes for Biological Sciences (Shanghai, China) is a source of normal human liver cells (LO2) and four human HCC cell lines (HepG2, Hep3B, Huh7 and SMMC7721). All cells were grown in a humidified 5% CO2 incubator in RPMI-1640 medium supplemented with 10% FBS (Gibco, Rockville, MD, USA) at 37°C.
Following synthesis of the DSCAM-AS1short-hairpin RNA (sh-DSCAM-AS1) and non-targeting shRNA (sh-NC) sequences, these shRNAs were inserted into pGPU6/GFP/Neo vectors (Gene-Pharama, Shanghai, China), then were transfected with SMMC-7721 cells using Lipofectamine 2000 (Invitrogen, USA), as per provided indications. Selection of stably transfected cells was performed using 0.5 mg/mL G418 (Sigma-Aldrich).
Inhibitors and mimics of miR-338-3p, and negative control miRNAs (miR-NC) (RiboBio; Guangzhou, China) were transfected into SMMC-7721 cells using Lipofectamine 2000.
RNA and miRNA extraction and RT-PCR
A TRIzolTMPlus RNA Purification Kit (Invitrogen) was utilized for the isolation and purification of total RNA from cultured cells and tissues. M-MLV Reverse Transcriptase (Promega, Madison WI, USA) was used to synthesis the first-strand complementary DNA (cDNA). qPCR reactions utilized the Power SYBR Green PCR Master Mix (Life Technologies, New York, USA) in an Applied Biosystems 7500 RT-PCR system. GAPDH and U6 served as internal reference controls for lncRNA/mRNA and miRNA quantification, respectively. The 2-ΔΔCt method was employed to assess relative gene expression, as described by Livak and Schmittgen [15].
Cell proliferation assay
The CellTiter96® Aqueous One Solution Cell Proliferation kit (Promega, USA) was utilized to evaluate cell proliferation. 24 h post-transfection, the cells (2×103 cells/well) were plated in a 96-well plate, and cultured 24-72 h. After indicated periods, 20 μl MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] was added into per well, and cultured another 2 h at 37°C. A Microplate Reader (Biotek, American) was utilized to assess absorbance at 490 nm.
Wound healing assay
Cell migratory ability was evaluated by wound healing assay. Briefly, transfected cells (5×104 cells/well) were seeded into a six-well plate, and grown up to 100% confluence. Wounds were created using a 200 μl plastic pipette tip, and cultured in free-serum medium for 24 h. The wound widths were measured and photographed at 0 h, 24 h after wound under an optical microscope (Olym-pus, Tokyo, Japan).
Transwell invasion assay
The cell invasion assay was performed using a 24-well Transwell chamber (Costar, USA) coated with Matrigel. A total of 2×105 transfected cells suspended in free-serum medium were plated into the upper chamber of Transwell assay inserts. DMEM media supplemented with 10% FBS was added into the lower chamber of Transwell assay inserts. 48 h later, the invaded cells were fixed with 100% methanol and stained with 0.1% crystal violet. Images were taken under an inverted microscope (Olympus). Five fields chosen at random were used to count number of invaded cells.
Luciferase reporter assay
Starbase2.0 (http://starbase.sysu.edu.cn) was used to predict the miRNAs that binds to DSCAM-AS1. miR-338-3p was predict to bind with DSCAM-AS1. To test this prediction, luciferase reporter activity was performed. To construct luciferase reporter vectors, 3’UTR fragments from DSCAM-AS1 cDNA containing the predicted miR-338-3p-binding sites were synthesized and inserted into downstream of the luciferase gene in the pGL3 Basic vector (Promega), yielding the WT-DSCAM-AS1. The 3’UTRs of DSCAM-AS1 with mutant sequence were synthesized and subcloned in the pGL3 Basic vector (Promega), yielding the MT-DSCAM-AS1.
For luciferase assays, SMMC-7721 cells were co-transfected with luciferase reporter vector WT-DSCAM-AS1 or MT-DSCAM-AS1 and miR-338-3p mimic or miR-NC using Lipofectamine 2000 reagent. Relative luciferase activity was analyzed at 48 h post-transfection by normalizing firefly luminescence to Renilla luminescence using Dual-Luciferase Reporter Assays (Promega, Madison, WI, USA) according to provided instructions.
RNA immunoprecipitation (RIP)
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA) was employed to verify the potential interaction between DSCAM-AS1 and miR-338-3p in HCC cells based on provided protocols.
Western blots
Radio immunoprecipitation assay lysis buffer containing proteinase inhibitors (Sigma-Aldrich, St. Louis, MO, USA) was used to extract protein from tissues and cultured cells. The concentrations of protein were determined using BCA Protein Assay kits (Vigorous Biotechnology Beijing, Beijing, China). Protein extracts (30 μg) were resolved on 10% SDS-PAGE gels and transferred onto nitrocellulose membrane (Millipore, Madison, WI, USA). After blocking for 2 h with 5% non-fat dry milk, the membranes were incubated with primary antibodies against CyclinD1, SMO and GAPDH (all antibody from Santa Cruz Biotechnology, CA, USA) at 4°C overnight, following incubated with secondary HRP-conjugated antibody (1:5000, Santa Cruz Biotechnology) for 1 h at room temperature. GAPDH was used as internal control. Protein bands were observed by enhanced chemiluminescence (Cell Signaling Technology, Danvers, MA, USA). A Quantity One software (Bio-Rad, Hercules, CA, USA) was used to quantify integrated densities of bands.
Tumor xenograft formation assay
The Animal Research Committee of Jilin University approved all animal experimental protocols and surgical procedures. Twenty male BALB/c nude mice (4-5 weeks old and weighing 18-20 g) were bought from the Laboratory of the Animal Center of Jilin University and raised in this Center. SMMC-7721 cells that had undergone stable transfection with sh-DSCAM-AS1 or sh-NC were injected s.c. into animals (2×106 cells/mouse) on the right flank. Tumor width (W) and length (L) were measured using a vernier caliper every 7 days to calculate tumor volumes (V) using the following formula: V = (L×W2)/2. At 35 days after implantation, mice were sacrificed to remove tumor tissues. After weighted, a part of tumor tissues were sorted at -80°C for further analysis, other part of tissues were fixed in 10% formalin solution for immunohistochemistry (IHC) assay.
IHC
Xenograft tumor tissue samples were fixed in 10% formalin solution and embedded in paraffin. Sections (5 mm) were stained with Ki-67 antibody (1:400; Santa Cruz Biotechnology) to evaluate proliferation in vivo. Sections were examined and photographed using a light microscope (Olympus).
Statistical analysis
All results are means ± SD of from at least 3 replicates measurements, and were analyzed using SPSS v19.0 (IBM, Chicago, IL, USA). Data from 2 groups was compared with Student’s t-tests, while one-way ANOVAs were used for comparisons between multiple groups (>2 groups). Pearson’s correlation was used to analyze correlations. Kaplan-Meier and log-rank tests were used for survival curves. The threshold of significance was *P<0.05, **P<0.01.
Results
DSCAM-AS1 is overexpressed and associated with poor prognosis of HCC
To investigate the expression of DSCAM-AS1 in HCC tissues, we first examined its expression levels in 48 pairs of HCC tissues and adjacent non-tumor tissues using qRT-PCR. As presented in Figure 1A, DSCAM-AS1expression was remarkably higher in HCC tissues than that in adjacent non-tumor tissues. We also detected the serum levels of DSCAM-AS1 in HCC patients (n=48) and heath donor (n=30) by qRT-PCR. Our results illustrated that serum levels of DSCAM-AS1 were significantly higher in HCC patients than in healthy controls (Figure 1B). Moreover, we revealed that DSCAM-AS1 level was tremendously elevated in 4 HCC cell lines (HepG2, Hep3B, Huh7 and SMMC7721) in comparison with normal human liver cells (LO2) (Figure 1C). To evaluate the clinical significance of DSCAM-AS1in patients with HCC, median expression of DSCAM-AS1 was used to as a cutoff point to divide patients into two group: High expression of DSCAM-AS1 (n=26, fold change ≥6.3) or low expression of DSCAM-AS1 (n=22, fold change <6.3). Statistical analysis indicated that increased DSCAM-AS1 was positively associated with vascular invasion and tumor-node-metastasis (TNM) stage in patients with HCC (Table 1). In addition, Kaplan-Meier analysis revealed that high expression of DSCAM-AS1 was negatively related to overall survival rate (Figure 1D). These results suggested that DSCAM-AS1might be involved in HCC progression.
Figure 1.
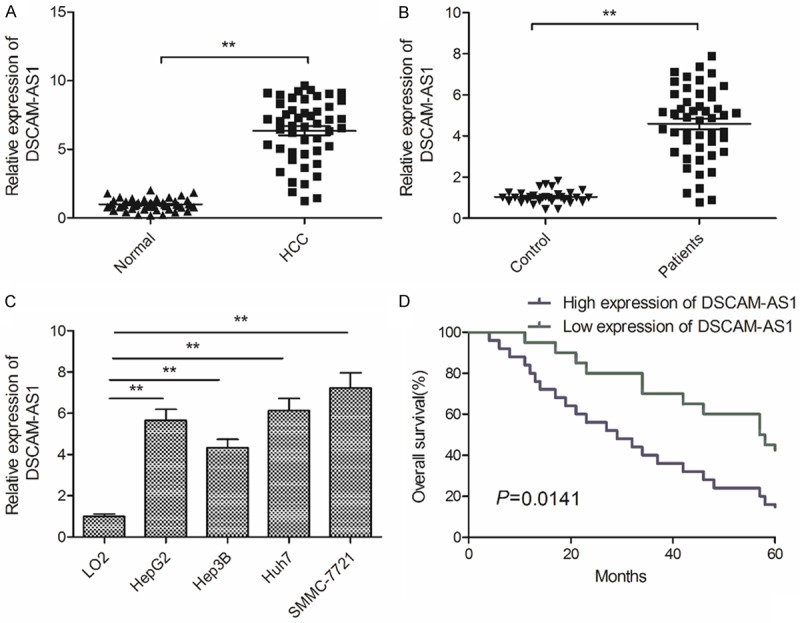
DSCAM-AS1 is overexpressed and associated with poor prognosis of HCC. A. qRT-PCR analysis was performed to examine the expression of DSCAM-AS1 in HCC tissues and adjacent non-tumor tissues. B. Serum levels of DSCAM-AS1 were detected in HCC patients (n=48) and heath donor (n=30) by qRT-PCR. C. The expression of DSCAM-AS1 was detected in 4 HCC cell lines (HepG2, Hep3B, Huh7 and SMMC7721) and normal human liver cells (LO2). D. Kaplan-Meier method analysis revealed that the high expression of DSCAM-AS1 group had shorter overall survival time compared to low expression of DSCAM-AS1 group. *P<0.01.
Knockdown of DSCAM-AS1 impairs HCC cell proliferation, migration and invasion
To further explore the role of DSCAM-AS1 in HCC, we successful knocked down DSCAM-AS1 in SMMC-7721 cells by transfection with sh-NC and sh-DSCAM-AS1 (Figure 2A). We subsequently explored the impact of DSCAM-AS1 knockdown on the proliferation of HCC using MTS assay. The result revealed that knockdown of DSCAM-AS1 significantly inhibits proliferation of SMMC-7721 cells at 48 h-72 h (Figure 2B). To determine the effect of DSCAM-AS1 on migration and invasion, wound healing and transwell invasion assays were performed. The results illustrated that DSCAM-AS1 knockdown significantly suppressed migratory and invasive capabilities (Figure 2C and 2D). These results suggested that DSCAM-AS1 knockdown impaired the ability of cell proliferation, migration and invasion of HCC cells.
Figure 2.
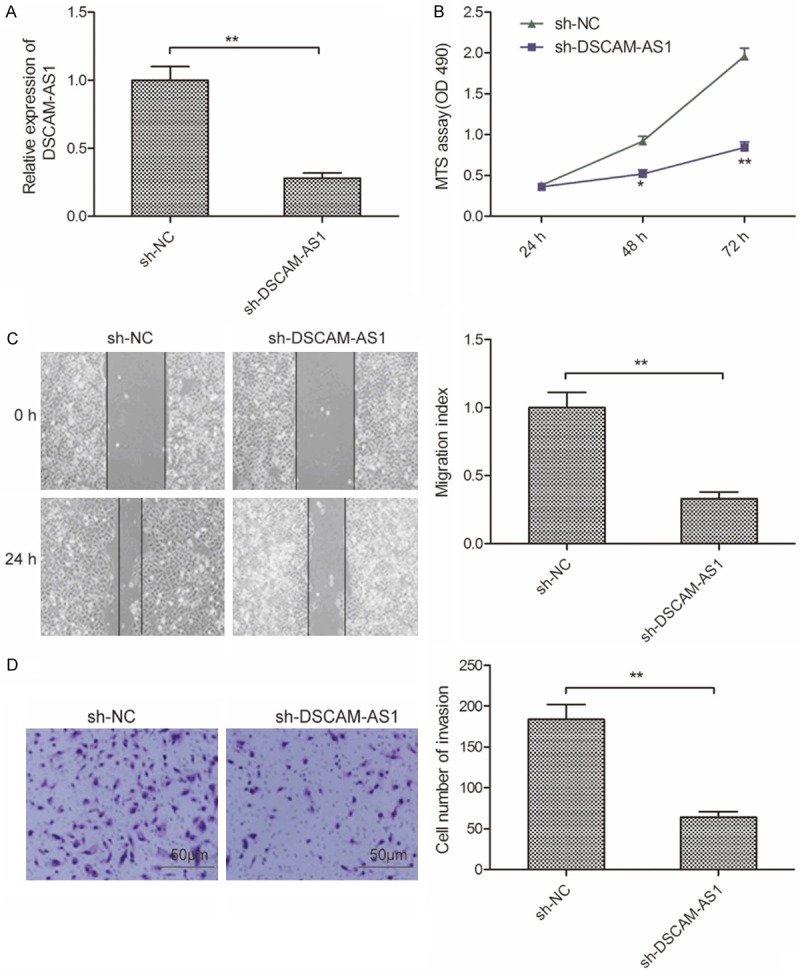
Knockdown of DSCAM-AS1 impairs HCC cell proliferation, migration and invasion. A. The expression of DSCAM-AS1 was examined in SMMC-7721 cells transfected with sh-NC and sh-DSCAM-AS1 by qRT-PCR. B-D. Cell proliferation, migration and invasion were determined in SMMC-7721 cells transfected with sh-NC and sh-DSCAM-AS1 by MTS, wound healing and transwell invasion assays, respectively. *P<0.05; **P<0.01.
miR-338-3p is a direct target of DSCAM-AS1 in HCC cells
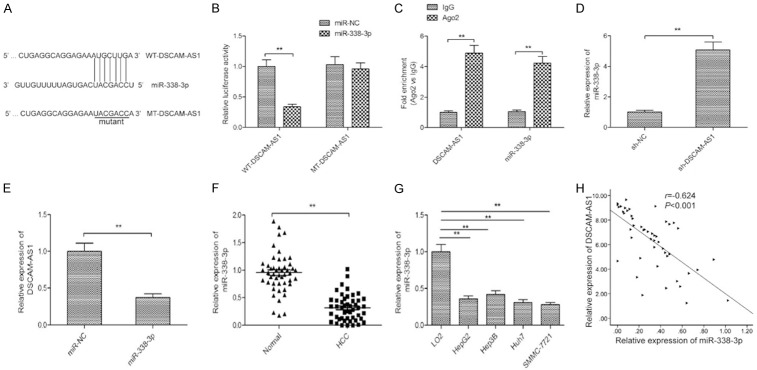
Growing evidence has suggested that lncRNA perform its regulatory functions via functioning as competing endogenous RNAs (ceRNA) [16]. To further investigate the potential mechanism by which DSCAM-AS1 exerted its role in HCC, a predication online tool (Starbse2.0) was used to predict the miRNAs that binds to DSCAM-AS1. We found that there was a potential binding site in DSCAM-AS1 with miR-338-3p (Figure 3A). To validate it, dual luciferase reporter assays were conducted. Results revealed that overexpression of miR-338-3p significantly inhibited the luciferase activity of WT-DSCAM-AS1 in SMMC-7721 cells, not but that of MT-DSCAM-AS1 (Figure 3B). RIP assay further confirm the ability of DSCAM-AS1 to act as a miR-338-3p sponge (Figure 3C). qRT-PCR further revealed miR-338-3p expression to be significantly higher in sh-DSCAM-AS1 transfected SMMC-7721 cells relative to controls (Figure 3D). Our results also showed that overexpression of miR-338-3p obviously decreased DSCAM-AS1 expression in SMMC-7721cells (Figure 3E). Besides, miR-338-3p expression was significantly downregulated in HCC tissues and cell lines (Figure 3F and 3G), and its expression was negative correlated with DSCAM-AS1 in HCC tissues (Figure 3H). There results suggested that miR-338-3p was a direct target of DSCAM-AS1 in HCC cells.
Figure 3.
miR-338-3p was a direct target of DSCAM-AS1 in HCC cells. A. Complementary sequences between miR-338-3p and DSCAM-AS1 were found using publicly available algorithms. The DSCAM-AS1 mutant sits (MT) is also shown. B. Relative luciferase activity was determined by luciferase reporter assays in SMMC-7721 cells co-transfected with WT-DSCAM-AS1 or MT-DSCAM-AS1 and miR-338-3p or miR-NC. C. The interaction between miR-338-3p and DSCAM-AS1 was determined with RIP assay. D. The expression of miR-338-3p was examined in SMMC-7721 cells transfected with sh-NC and sh-DSCAM-AS1 by qRT-PCR. E. The expression of DSCAM-AS1 was examined in SMMC-7721 cells transfected with miR-338-3p mimics or miR-NC by qRT-PCR. F. qRT-PCR analysis was performed to examine the expression of miR-338-3p in HCC tissues and adjacent normal tissues. G. The expression of miR-338-3p was detected in 4 HCC cell lines (HepG2, Hep3B, Huh7 and SMMC7721) and normal human liver cells (LO2). H. The correlation between DSCAM-AS1 and miR-338-3p was analyzed by Pearson’s correlation analysis. *P<0.05, **P<0.01.
miR-338-3p suppression reverses DSCAM-AS1 knockdown outcomes in HCC cells
To assess whether DSCAM-AS1 knockdown outcomes in HCC cells were mediated via miR-338-3p, a rescue experiment was performed by transfecting miR-338-3p inhibitor into SMMC-7721 cells in which DSCAM-AS1 had been stably knocked down. Inhibition of miR-338-3p partially reversed the upregulation of miR-338-3p which occurred following DSCAM-AS1 knockdown (Figure 4A). Moreover, inhibiting of miR-338-3p partially reversed the inhibitory effects of DSCAM-AS1 knockdown on the proliferation, migration and invasion of SMMC-7721 cells (Figure 4B-D). These results thus indicated that DSCAM-AS1 promoted HCC progression in part via miR-338-3p targeting.
Figure 4.
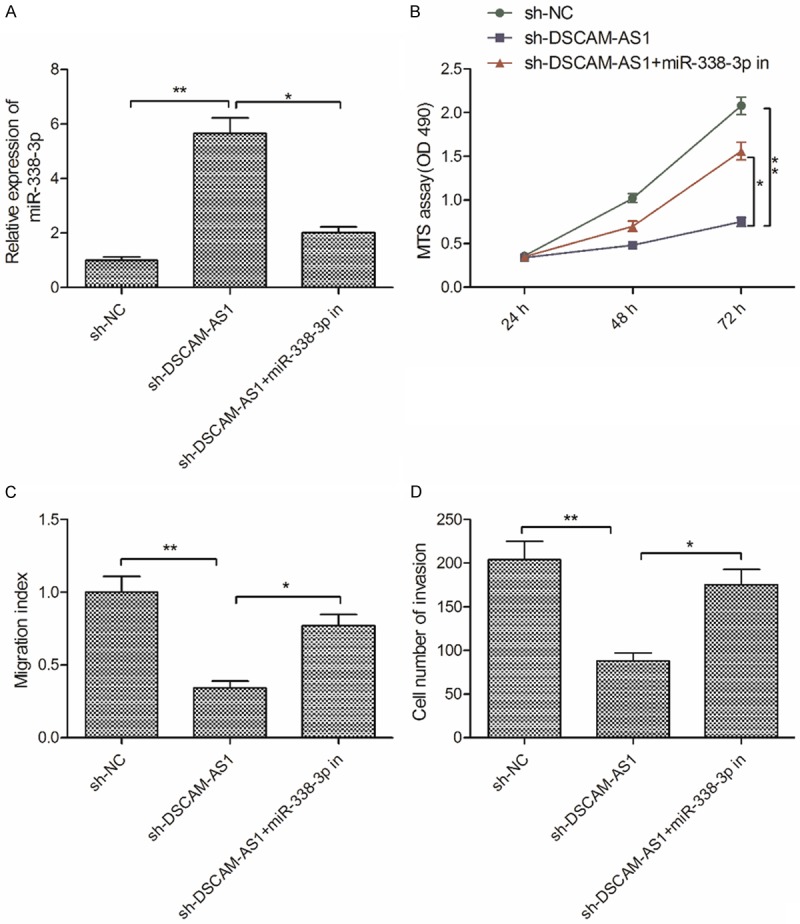
miR-338-3p suppression reverses DSCAM-AS1 knockdown outcomes in HCC cells. A. The expression of miR-338-3p was detected in SMMC-7721 cells transfected with sh-NC, sh-DSCAM-AS1 and sh-DSCAM-AS+miR-338-3p inhibitor (miR-338-3p in). B-D. Cell proliferation, migration and invasion were determined in SMMC-7721 cells transfected with sh-NC, sh-DSCAM-AS1 and sh-DSCAM-AS+miR-338-3p in. *P<0.05, **P<0.01.
DSCAM-AS1 modulated the expression of CyclinD1 and smoothened (SMO) by repressing miR-338-3p in HCC
It was well known that lncRNA could act as ceRNA of miRNA to regulate target genes of miRNA [17]. CyclinD1 and SMO have been confirmed to be targets of miR-338-3p in HCC [18,19]. To investigate whether DSCAM-AS1 controls CyclinD1 and SMO expression in HCC cells via miR-338-3p, SMMC-7721 cells were transfected with sh-NC, sh-DSCAM-AS1, or sh-DSCAM-AS1+miR-338-3p inhibitor, and then assessed CyclinD1 and SMO expression on above cells. We found that DSCAM-AS1 knockdown significantly decreased CyclinD1 and SMO levels, whereas miR-338-3p inhibition increased this expression in the context of DSCAM-AS1 knockdown (Figure 5A and 5B). For further proof, Pearson correlation analysis was applied to reveal that there was a positive relation between CyclinD1/SMO and DSCAM-AS1 in HCC tissues (Figure 5C and 5D), and that there was a negative relation between CyclinD1/SMO and miR-338-3p in HCC tissues (Figure 5E and 5F). In summary, consequences of these experiments suggested that DSCAM-AS1regulated CyclinD1 and SMO in HCC via sponging miR-338-3p.
Figure 5.
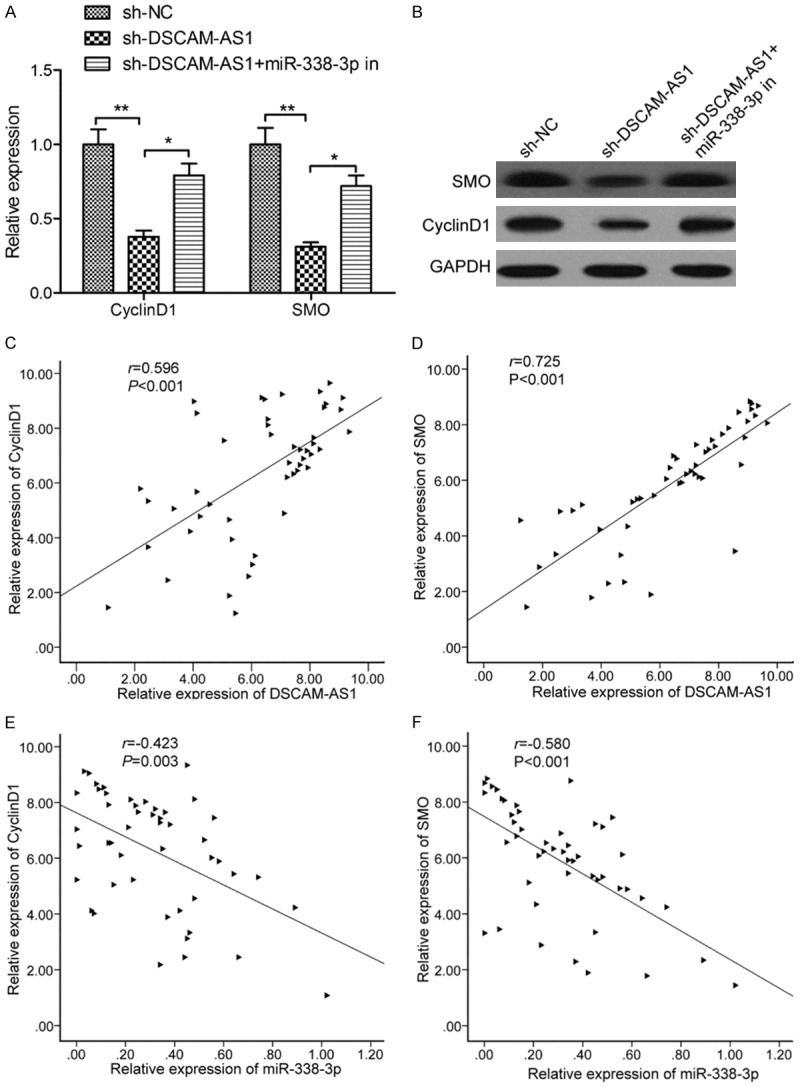
DSCAM-AS1 modulated the expression of cyclinD1 and smoothened (SMO) by repressing miR-338-3p in HCC. A. The CyclinD1 and SMO mRNA expression was measured in SMMC-7721 cells transfected with sh-NC, sh-DSCAM-AS1 and sh-DSCAM-AS+miR-338-3p in. B. The CyclinD1 and SMO protein expression was measured in SMMC-7721 cells transfected with sh-NC, sh-DSCAM-AS1 and sh-DSCAM-AS+miR-338-3p in. C. The correlation between DSCAM-AS1 and CyclinD1 was analyzed by Pearson’s correlation analysis. D. The correlation between DSCAM-AS1 and SMO was analyzed by Pearson’s correlation analysis. E. The correlation between miR-338-3p and CyclinD1 was analyzed by Pearson’s correlation analysis. F. The correlation between miR-338-3p and SMO was analyzed by Pearson’s correlation analysis. *P<0.05, **P<0.01.
DSCAM-AS1 knockdown retards tumor growth in vivo
To determine the effect of DSCAM-AS1 on tumor growth in vivo, we injected SMMC-7721 cells stable transfected with sh-DSCAM-AS1 or sh-NC into nude mice, then tumor growth was measured. The result showed that tumor growth was slower in sh-DSCAM-AS1 group than that in sh-NC group (Figure 6A). 35 days after injection, mice were killed, tumor were removed to weight. We found that tumor size and weight were reduced in sh-DSCAM-AS1 group compared with sh-NC group (Figure 6B and 6C). IHC analysis confirmed that knockdown of DSCAM-AS1 suppressed expression of Ki-67(a proliferation marker), which represents tumor proliferation ability (Figure 6D). In addition, we also detect the expression of DSCAM-AS1, miR-338-3p, CyclinD1 and SMO expression in xenograft tumor. The results showed that knockdown of DSCAM-AS1 expression lead a decrease of DSCAM-AS1 expression (Figure 6E), and increase of miR-338-3p expression (Figure 6F), as well as reduction of CyclinD1 and SMO expression (Figure 6G and 6H). These results suggested that DSCAM-AS1 knockdown suppressed HCC xenograft tumor growth in vivo.
Figure 6.
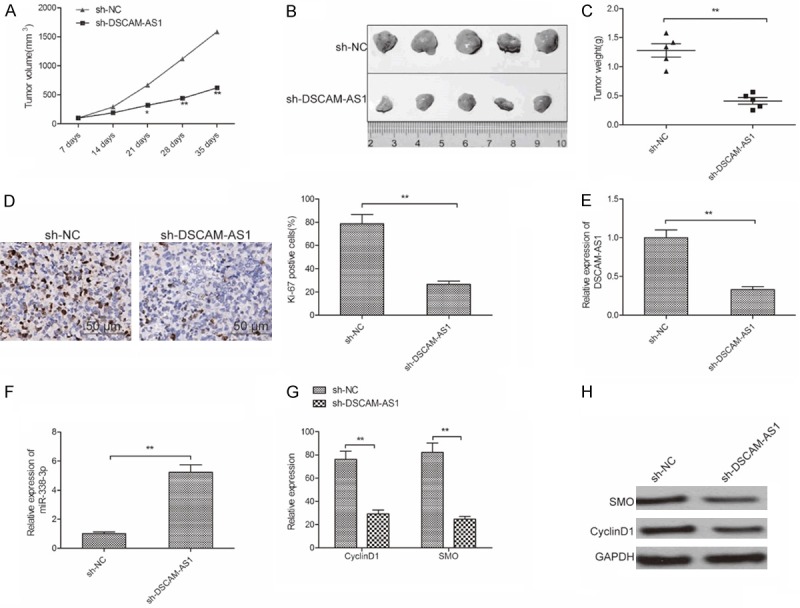
DSCAM-AS1 knockdown retards tumor growth in vivo. A. Tumor volumes were measured every 7 day until mice killed. B. Tumor image was taken at the end of experiments. C. Tumor weight was measured at the end of experiments. D. The Ki-67 expression was determined in xenograft tumor by IHC. E. The expression of DSCAM-AS1 was determined in xenograft tumor by qRT-PCR. F. The expression of miR-338-3p was determined in xenograft tumor by qRT-PCR. G, H. The CyclinD1 and SMO expression on mRNA and protein levels were measured in xenograft tumor by qRT-PCR and western blot assays, respectively. *P<0.05, **P<0.01.
Discussion
Accumulated studies in recent years have pointed out that lncRNAs served as cancer suppressors or oncogenes, contributing to tumor initiation and development through regulating various cancer cellular process, such as cell proliferation, apoptosis, differentiation, metastasis and invasion [7,8]. At present, many lncRNAs were identified to be involved in the tumorigenesis and metastasis of HCC [9,10,20]. For example, LINC01554 inhibited HCC cell growth in vitro and in vivo via regulating PKM2 expression and Akt/mTOR signaling pathway [21]. HCC cancer cells proliferation, migration and invasion were promoted by DLX6-AS1 through sponging miR-424-5p and increasing the expression of WEE1 [22]. MCM3AP-AS1 exerted an oncogenic role in HCC progression via regulating miR-194-5p/FOXA1axis [23]. However, the functional role in majority of deregulated lncRNAs in HCC has remained to be elucidated.
DSCAM-AS1, a discovery lncRNA, has been reported to be upregulated and play an oncogene role in non-small cell lung cancer and breast cancer [11-14]. However, the function of DSCAM-AS1 in HCC has not been investigated. Here, our results demonstrated that the expression of DSCAM-AS1 was upregulated in HCC tissues and cell lines, and its expression was closely associated with poor prognosis. Functional experiment revealed that knockdown of DSCAM-AS1 suppressed cell proliferation, invasion and migration. Further in vivo studies revealed that knockdown of DSCAM-AS1 retarded tumor growth in nude mice. Collectively, these results suggested that DSCAM-AS1 functioned as an oncogenic lncRNA in HCC.
Accumulating evidence suggested that lncRNAs implicated in various biological and pathological processes of cancer via interacting with miRNAs [16]. To further explore the underlying molecular mechanism that DSCAM-AS1 involved in HCC progression, we used software Starbase2.0 to select miRNAs interaction with DSCAM-AS1. Among miRNAs, miR-338-3p, an known tumor suppressor, was selected as a potential candidate. Several study confirmed that miR-338-3p inhibited HCC progression by regulating multiple genes [18,19,24-26]. We further confirmed that miR-338-3p could bind with DSCAM-AS1 in HCC cells through luciferase activity, RIP and qRT-PCR assays. We also found that there is a negative correlation between miR-338-3p and DSCAM-AS1 in HCC tissues. Importantly, the inhibitory effects on cell proliferation, migration and invasion mediated by DSCAM-AS1 depletion in HCC cells were partially reversed by inhibition of miR-338-3p. These results suggested that DSCAM-AS1 exerted oncogenic role in HCC by negatively regulating miR-338-3p.
LncRNAs have been reported to function as a ceRNA to regulate target gene expression or function by binding with miRNAs [27]. CyclinD1 and SMO were reportedly to be two target of miR-338-3p in HCC [18,19]. Here, we investigate whether DSCAM-AS1 could regulate CyclinD1 and SMO expression. Our result demonstrated that DSCAM-AS1 knockdown decreased CyclinD1 and SMO expression, whereas miR-338-3p inhibition reversed this trend. Moreover, Pearson correlation analysis revealed that CyclinD1/SMO expression was positive correlated with DSCAM-AS1 in HCC tissues, and was negative correlated with miR-338-3p in HCC tissues. These results suggested that DSCAM-AS1 functioned as a ceRNA for miR-338-3p to regulate CyclinD1 and SOD expression in HCC.
Taken together, the present study indicated that DSCAM-AS1 serves as oncogenic lncRNA that promoted HCC growth in vitro and in vivo. The results also suggested that DSCAM-AS1 positively regulated CyclinD1 and SOD expression by acting as a ceRNA for miR-338-3p binding. DSCAM-AS1 may thus be a viable clinical target in HCC. Further research will be needed to more accurately elucidate molecular mechanisms governing the function of DSCAM-AS1 in HCC progression.
Disclosure of conflict of interest
None.
Abbreviations
- AFP
Alpha-Fetoprotein
- cDNA
complementary DNA
- DSCAM-AS1
Down Syndrome Cell Adhesion Molecule antisense
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCC
hepatocellular carcinoma
- HCV
hepatitis c virus
- IHC
immunohistochemistry
- SMO
smoothened
- LncRNA
Long non-coding RNAs
- MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]
- NC
negative control
- qRT-PCR
Quantitative real-time PCR
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Do H, Kim W. Roles of oncogenic long non-coding RNAs in cancer development. Genomics Inform. 2018;16:e18. doi: 10.5808/GI.2018.16.4.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Egranov SD, Yang L, Lin C. Molecular mechanisms of long noncoding RNAs-mediated cancer metastasis. Genes Chromosomes Cancer. 2019;58:200–207. doi: 10.1002/gcc.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiStefano JK. Long noncoding RNAs in the initiation, progression, and metastasis of hepatocellular carcinoma. Noncoding RNA Res. 2017;2:129–136. doi: 10.1016/j.ncrna.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu L, Tang Q, Li G, Chen K. Long non-coding RNAs as biomarkers and therapeutic targets: recent insights into hepatocellular carcinoma. Life Sci. 2017;191:273–282. doi: 10.1016/j.lfs.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Liao J, Xie N. Long noncoding RNA DSCAM-AS1 functions as an oncogene in non-small cell lung cancer by targeting BCL11A. Eur Rev Med Pharmacol Sci. 2019;23:1087–1092. doi: 10.26355/eurrev_201902_16998. [DOI] [PubMed] [Google Scholar]
- 12.Khorshidi H, Azari I, Oskooei VK, Taheri M, Ghafouri-Fard S. DSCAM-AS1 up-regulation in invasive ductal carcinoma of breast and assessment of its potential as a diagnostic biomarker. Breast Dis. 2019;38:25–30. doi: 10.3233/BD-180351. [DOI] [PubMed] [Google Scholar]
- 13.Liang WH, Li N, Yuan ZQ, Qian XL, Wang ZH. DSCAM-AS1 promotes tumor growth of breast cancer by reducing miR-204-5p and up-regulating RRM2. Mol Carcinog. 2019;58:461–473. doi: 10.1002/mc.22941. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Bu D, Long J, Chai W, Dong J. LncRNA DSCAM-AS1 acts as a sponge of miR-137 to enhance tamoxifen resistance in breast cancer. J Cell Physiol. 2019;234:2880–2894. doi: 10.1002/jcp.27105. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, Wu D, Gao L, Liu X, Jin Y, Wang D, Wang T, Li X. Competing endogenous RNA networks in human cancer: hypothesis, validation, and perspectives. Oncotarget. 2016;7:13479–13490. doi: 10.18632/oncotarget.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu X, Tan D, Hou Z, Hu Z, Liu G. miR-338-3p is down-regulated by hepatitis B virus X and inhibits cell proliferation by targeting the 3’-UTR region of CyclinD1. Int J Mol Sci. 2012;13:8514–8539. doi: 10.3390/ijms13078514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XH, Chen JS, Wang Q, Chen XL, Wen L, Chen LZ, Bi J, Zhang LJ, Su Q, Zeng WT. miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J Pathol. 2011;225:463–472. doi: 10.1002/path.2877. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Jiang J, Xu Q, Ni C, Yang L, Huang D. A systematic review of long noncoding RNAs in hepatocellular carcinoma: molecular mechanism and clinical implications. Biomed Res Int. 2018;2018:8126208. doi: 10.1155/2018/8126208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng YL, Li L, Jia YX, Zhang BZ, Li JC, Zhu YH, Li MQ, He JZ, Zeng TT, Ban XJ, Yuan YF, Li Y, Guan XY. LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics. 2019;9:796–810. doi: 10.7150/thno.28992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Tang X, Li M, Zheng Y. Long noncoding RNA DLX6-AS1 promotes liver cancer by increasing the expression of WEE1 via targeting miR-424-5p. J Cell Biochem. 2019;120:12290–12299. doi: 10.1002/jcb.28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Yang L, Chen T, Liu X, Guo Y, Zhu Q, Tong X, Yang W, Xu Q, Huang D, Tu K. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18:28. doi: 10.1186/s12943-019-0957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Liu W, Zeng XC, Jiang N, Fu BS, Guo Y, Yi HM, Li H, Zhang Q, Chen WJ, Chen GH. Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed Pharmacother. 2016;84:583–591. doi: 10.1016/j.biopha.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 25.Chen JS, Liang LL, Xu HX, Chen F, Shen SL, Chen W, Chen LZ, Su Q, Zhang LJ, Bi J, Zeng WT, Li W, Ma N, Huang XH. miR-338-3p inhibits epithelial-mesenchymal transition and metastasis in hepatocellular carcinoma cells. Oncotarget. 2017;8:71418–71429. doi: 10.18632/oncotarget.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Zhao L, Fang Q, Sun J, Zhang S, Zhan C, Liu S, Zhang Y. MiR-338-3p inhibits hepatocarcinoma cells and sensitizes these cells to sorafenib by targeting hypoxia-induced factor 1alpha. PLoS One. 2014;9:e115565. doi: 10.1371/journal.pone.0115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Mejias A, Tay Y. Competing endogenous RNA networks: tying the essential knots for cancer biology and therapeutics. J Hematol Oncol. 2015;8:30. doi: 10.1186/s13045-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]