Abstract
Digestive system cancers, mainly including gastric cancer, hepatocellular carcinoma, pancreatic cancer, and colorectal cancer, are major public health problems and lead to serious cancer-related deaths worldwide. Clinically, treatment strategies of these cancers include surgery, chemotherapy, and immunotherapy. Although successful resection and chemotherapeutic drugs have improved the treatment level, the survival rate of patients with advanced digestive system cancers remains still low primarily due to tumor metastasis. E-cadherin, the prototypical member of the type-1 classical cadherins, has been characrized as an important molecule in epithelial-mesenchymal transition (EMT) process. Loss of E-cadherin is able to induce EMT process, which is associated with cancer stem cells and drug resistance in human cancer. Therefore, restoring E-cadherin could be a useful strategy for reversal of EMT and overcoming drug resistance. In this review, we describe pharmacological small molecules targeting E-cadherin expression for the treatment of digestive system cancers, which have emerged in the recent 5 years. We hope these compounds could be potentially used for treating cancer in the near future.
Keywords: E-cadherin, EMT, invasion, digestive cancer, natural agents, therapy
Introduction
Digestive system cancer (DSC) is one of the common leading causes for cancer-related death worldwide [1], mainly including gastric cancer (GC), hepatocellular carcinoma (HCC), pancreatic cancer (PC), and colorectal cancer (CRC). Current treatment strategies for these cancers include surgery, chemotherapy, and immunotherapy [2]. Despite successful resection or chemotherapeutic treatment, the survival rate of patients with advanced DSC remains still low primarily due to tumor relapse and metastasis [1-3]. In fact, tumor cell metastasis is a complex procedure in which numerous steps are involved, including local invasion and migration, dissemination of cancer cells through haematogenous or lymphatic pathways [3]. Therefore, it is essential to give a comprehensive understanding on the processes by which malignant tumor cells invade and metastasize to distant positions, which would be helpful to identify some potential therapeutic strategies for DSCs.
E-cadherin, known as the prototypical member of the type-1 classical cadherins, was first discovered as a cell surface molecule that mediated cell-cell adhesion in early mouse embryo blastomeres [4,5]. Structurally, E-cadherin contains a 120 kDa transmembrane glyprotein comprising five Ca2+-dependent extracellular domains (EC1-EC5), a transmembrane domain, and a cytoplasmic domain [6,7]. During the early stage of normal development, cells retain E-cadherin and adherens junctions (AJs) as both are vital for maintaining homeostasis and regulating cell-cell interactions of epithelia tissues. Epithelial-mesenchymal transition (EMT) is a fundamental morphogenetic process whereby epithelial cells acquire a mesenchymal phenotype [8]. Multiple studies have demonstrated that EMT is critically involved in tumorigenesis and cancer progression. Indeed, the expression of epithelial markers, in particular E-cadherin, is lost during the EMT process, leading to destructed cell-cell adhesion, increased cell motility and advanced stages of cancer [9-11] (Figure 1). Furthermore, cancer cells are able to acquire cancer stem cell (CSC) features via induction of EMT [12], resulting in increased drug resistance that limits the success of anticancer therapies [13,14]. Therefore, E-cadherin evolves as a promising therapeutic target for designing antitumor drugs. In this review, we will mainly focus on pharmacological small molecules targeting E-cadherin expression for treatment of DSCs, which have emerged in the recent 5 years.
Figure 1.
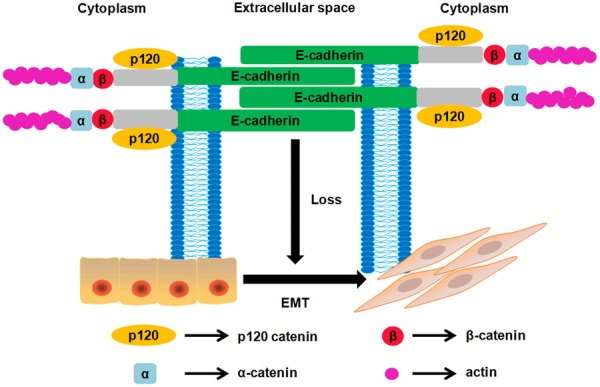
Illustration of E-cadherin structure and how EMT was initiated when E-cadherin expression is lost. E-cadherin is a transmembrane glycoprotein consisting of five calcium-dependent extracellular domains (EC1-EC5) that confer homotypic interactions on the surface of a neighboring cell, a transmembrane domain, and a cytoplasmic domain that binds to members of the catenin protein family to transduce physical and biochemical signals to the cell, including β-catenin and p120 catenin.
Regulation of E-cadherin expression in cells
The expression of E-cadherin is modulated at different levels including gene transcription and post-translational modification [15]. E-cadherin transcription is directly regulated by methylation of promoter activity. Methylation, known as a common modification of DNA induced by a family of DNA methyltransferase enzymes, directly regulates E-cadherin transcription via methylation of its promoter activity [16]. E-cadherin expression is significantly reduced due to promoter methylation, leading to the progression and metastasis in most human malignancies [17-19]. Src tyrosine kinase-mediated phosphorylation is considered as a major post-translational mechanism of E-cadherin expression and promotes the ubiquitination and degradation of E-cadherin [20]. Conversely, phosphorylation of E-cadherin at serine residues by GSK3β substantially increased the affinity of E-cadherin for β-catenin [21]. In tumors, the loss of E-cadherin is induced via activation of several signaling pathways involving oncogenic factors such as TGF-β, EGF, NF-κB, Wnt, and HIF-1α, thereby contributing to the formation of EMT [8,22] (Figure 2).
Figure 2.
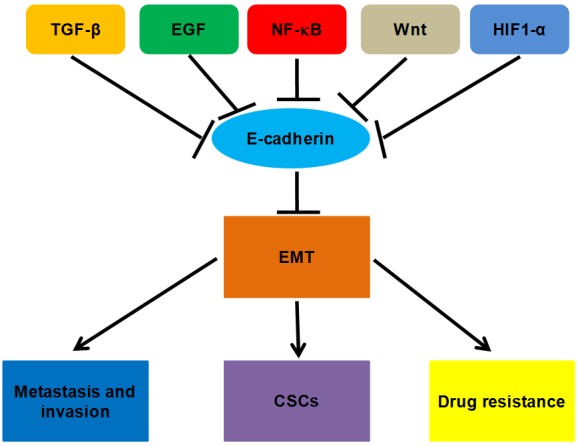
Possible mechanism of E-cadherin in cancer cells. E-cadherin is a core regulator involved in tumorigenesis and cancer progression (metastasis and invasiveness, EMT, CSCs, and drug resistance).
Small molecules targeting E-cadherin expression in DSCs
Numerous emerging small bioactive compounds target E-cadherin expression and enhance the integrity of cell-cell adhesion in DSCs. However, for most of these molecules, clinical trials and applications need to be conducted in the future. In the following sections, we will describe a number of small molecules that could be used for DSC therapies due to restore the expression of E-cadherin, including the name, origin, and molecular mechanism by which these compounds exert their anticancer functions in DSCs (Table 1).
Table 1.
The list of small molecules targeting E-cadherin expression in DSCs
| Compound | Description | Cancer | Mechanism and function | Reference |
|---|---|---|---|---|
| α-mangostin | A major xanthone compound in the pericarp of mangosteen | PC | Downregulates MMP-2 and MMP-9, inhibits the phosphorylation of ERK, increases the expression of E-cadherin, suppresses the ERK signaling pathway, inhibits cell invasion and migration | [59] |
| A novel phytosomal curcumin | A major component of turmeric | CRC | Increases the expression of E-cadherin, suppresses Wnt/β-catenin signaling pathway, inhibits cells growth and invasion | [74] |
| All-trans retinoic acid (ATRA) | An effective drug for the induction therapy of acute promyelocytic leukemia | HCC | Decreases the expression of N-cadherin, vimentin, Snail and Twist, increases the expression of E-cadherin, inhibits cells growth, colony formation, migration and invasion, reverses EMT | [48] |
| Antcin K | An active triterpenoid extract from Antrodia cinnamomea | HCC | Reduces integrin β1, β3, α5, and αv, suppresses phosphorylation of FAK, Src, PI3K, AKT, MEK, ERK, and JNK, reduces the expression and activity of MMP2, MMP9, and vimentin, upregulates E-cadherin, inhibits cell adhesion, migration and invasion | [35] |
| Anthothecol-encapsulated PLGA nanoparticles (Antho-NPs) | Anthothecol: a limonoid isolated from plant Khaya anthotheca; PLGA: poly D, L-lactic-co-glycolic acid | PC | Upregualtes E-cadherin, inhibits N-cadherin and ZEB1, suppress cells proliferation, colony formation, migration, and invasion, induces apoptosis, inhibits self-renewal capacity of CSCs | [62] |
| Arctigenin | A natural lignin compound | CRC | Increases E-cadherin, decreases N-cadherin, vimentin, β-catenin, Snail, MMP-2, and MMP-9, reverses EMT, inhibits cell migration and invasion, induces cell cycle arrest and apoptosis, suppresses lung metastasis | [79] |
| Arsenic trioxide (As2O3) | An anticancer drug for APL | HCC | Increases E-cadherin, decreases the levels of Snail, Slug, vimentin, and MMPs, attenuates the migration, invasion of cells | [49] |
| Berberine | A plant-derived isoquinoline alkaloid | CRC | Inhibits the transcriptional activity and expression of β-catenin, increases E-cadherin, inhibits Wnt/β-catenin signaling pathway | [72] |
| Brusatol | A natural quassinoid isolated from Bruceae Fructus | PC | Downregulates vimentin and Twist, stimulates the expression of E-cadherin, deactivates NF-kB, induces cell cycle arrest at G2/M phase and apoptosis, reduces tumor growth | [65] |
| Casticin | An active compound from Vitex Fructus | HCC | Increases the expression of E-cadherin, decreases the expression of N-cadherin and Twist, inhibits EMT process | [25] |
| Celastrus orbiculatus | A folk medicine in China | GC | Suppresses the NF-kB/Snail, downregulates Hsp27, vimentin, and N-cadherin, upregulates E-cadherin, attenuates TGF-β-induced EMT | [25] |
| Cinnamaldehyde (CA) | A bioactive compound isolated from the stem bark of Cinnamomum cassia | CRC | Upregulates E-cadherin, downregulates MMP-2 and MMP-9, inhibits PI3K/AKT pathway, induces apoptosis, inhibits cell invasion, adhesion, and proliferation | [71] |
| Cladosporol A | A secondary metabolite from Cladosporium tenuissium | CRC | Upregulates p21 and E-cadherin, promotes β-catenin nuclear export and proteasome degradation, inactivates β-Catenin/TCF signaling pathway, inhibits cell growth | [68] |
| Compound 3a | A naphthalimide derivative | HCC | Upregulates cyclin B1, CDK1, p21, and E-cadherin, inhibits migration and tumor growth, interrupts lung metastasis | [39] |
| Compound CH6 | A compound derived from matrine | HCC | Upregulates P21, P27, and E-cadherin, downregulates N-cadherin, induces G1 cycle arrest, inhibits cell proliferation and migration | [37] |
| Sorafenib and vitamin K | Sorafenib: an oral tyrosine kinase inhibitor | HCC | Increases E-cadherin, reduces p-MET and p-ERK, inhibit HGF/c-MET pathway, inhibits proliferation, invasion and migration | [41] |
| Cordycepin | An analogue of adenosine from Cordyceps sinesis | HCC | Reduces FAK, integrin α3, α6 and β1, increases E-cadherin, inhibits cells proliferation, migration, and invasion | [43] |
| Curcumin | A major component of turmeric (Curcuma longa L.) | PC, CRC | Decreases Shh, GLI1 and vimentin, increases the expression of E-cadherin, inhibits cells proliferation, invasion and migration, induces apoptosis, reverses TGF-β-stimulated EMT | [53,77] |
| Curcumin and ulinastatin | Ulinastatin: a urinary trypsin inhibitor | CRC | Decreases MMP-9, increase E-cadherin, inhibits cell migration and invasion, inhibits hepatic metastases | [78] |
| Danusertib (Danu) | A pan-inhibitor of Aurora kinases | GC | Inhibits the PI3K/Akt/mTOR pathway, increases E-cadherin, decreases N-cadherin, induces G2/M arrest and apoptosis, inhibits EMT and cells growth | [26] |
| Diallyl trisulfide (DATS) | A natural compound extracted from garlic | GC | Increases cyclin A2, cyclin B1, JNK, ERK, and p38 phosphorylation, activates MAPK pathway, upregulates E-cadherin, downregulates MMP-9, inhibits cell proliferation, migration and invasion | [31] |
| Ellagic acid (EA) | A polyphenol found in several plants and fruits | PC | Inhibits Snail, MMP-2, and MMP-9, upregulates E-cadherin, suppresses Akt, Shh, and Notch pathways, inhibits cancer growth, angiogenesis and metastasis, reverses EMT | [54,55] |
| Embelin | A compound isolated from Embelia ribes | PC | Suppresses Akt, Shh, COX-2, VEGF, VEGFR, IL-8, MMP-2, and MMP-9, upregulates E-cadherin, inhibits Snail, Slug, and ZEB1, inhibit cancer growth, angiogenesis and metastasis, reverses EMT | [56,57] |
| Emodin | A anthraquinone derivative extracted from the root of rhubarb | CRC | Increases E-cadherin, decreases N-cadherin, Snail, β-catenin, MMP-7, MMP-9, VEGF, TCF4, Cyclin-D1 and c-Myc, inhibits Wnt/β-catenin pathway, tumor growth, cell invasion and migration | [73] |
| Ethanolic extract of bark from Salix aegyptiaca | Willow bark extracts | CRC | Restores E-cadherin, reduces EGFR, Snail1, Snail2, Twist, MMP-9, and MMP-2, inhibits cell growth, migration, adhesion, and EMT | [81] |
| Ethanolic extract of gamboge | A traditional Chinese medicine from gamboge | CRC | Decreases β-catenin, MMP-7, and cyclin D1, increases E-cadherin, inhibits cell proliferation and tumor growth, induces apoptosis. | [75] |
| Gadolinium chloride (GdCl3) | An injectable MRI contrast agent | HCC | Increases E-cadherin, reduces N-cadherin, Twist, Snail, and CD206, inhibits cells invasion, induces cell apoptosis | [50] |
| Galeterone (gal)/analogs (VNPT55, VNPP414, VNPP433-3b) | A multi-target chemotherapeutic agent in phase III clinical development | PC | Downregulates Mnk1/2, peIF4E, NF-κB, N-cadherin, MMP-1/-2/-9, Slug, Snail, CXCR4, β-Catenin, Nanog, BMI-1, Oct-4, and EZH2, upregualtes E-cadherin, induces G1 cell cycle arrest and caspase 3-mediated cell death, inhibits tumor growth, suppresses cell migration, invasion and proliferation | [64] |
| Hexa-D-arginine (D6R) | A suppressor of furin | PC | Upregulates E-cadherin, downregulates N-cadherin and vimentin, reduces the phosphorylation and expression of YAP, inhibits cell proliferation, migration and invasion | [66] |
| Hispidulin | A naturally existing flavonoid | CRC | Upregulates E-cadherin, inhibits Snail, Slug, and Twist, blocks PETN/PI3K/Akt pathway, inhibits cell motility, prevents hypoxia-induced EMT | [83] |
| Holy basil leaf extract | An extract from Holy Basil leaf | PC | Increases E-cadherin and BAD, downregulates ERK-1/2, FAK, p65, Bcl-2, Bcl-xL, AURKA, Chk1, and Survivin, inhibits cell growth, migration and invasion, induces apoptosis | [52] |
| Honokiol | A phenolic compound isolated from Magnolia grandiflora | GC | Increases E-cadherin, cytokeratin-18, and endoplasmic reticulum expression, decreases vimentin, snail, and Tpl2, reverses EMT, inhibits tumor growth and migration | [24] |
| Huaier | An antitumor drug | GC | Increases E-cadherin, decreases N-cadherin, TWIST and vimentin expression, reverses EMT, inhibits cell migration and invasion | [29] |
| Jianpi Huayu decoction (JHD) | A Chinese medicine | HCC | Decreases N-cadherin, Vimentin, and p-Smad3, increases Smad7 and E-cadherin, inhibits cell invasion, metastasis, and EMT process | [44] |
| Jianpi JieDu (JPJD) | An traditional Chinese medicine compound | CRC | Increases E-cadherin, represses vimentin, Snail, and p-Smad2/3, inhibits TGF-β/Smad pathway, inhibits EMT, cell invasion and migration, liver and lung metastases | [89] |
| LBH589 | A new inhibitor of HDAC | HCC | Inhibits gankyrin/STAT3/Akt pathway, downregulate N-cadherin, vimentin, TWIST1, and VEGF, upregulates E-cadherin, inhibit cell growth and metastasis | [32] |
| Luteolin | A flavonoid present in fruits and green plants | GC | Induces E-cadherin, downregulates N-cadherin, vimentin, and snail, suppresses Notch1 signaling, reverses EMT, inhibits cell proliferation, invasion and migration | [27] |
| Matrine | A natural compound from the Sophora plant genus | HCC | Upregulates CAR, E-cadherin, laminin and fibronectin, inhibits the proliferation of cells | [36] |
| Meloxicam | A selective COX-2 inhibitor | HCC | Upregulates E-cadherin, Bax, and Fas-L, downregulates MMP-2, and Mcl-1, inhibits pAkt, inhibits cell invasion and migration | [33] |
| Metformin | An anti-diabetes drug | GC | Reduces vientin and β-catenin, induces E-cadherin, inhibits cell migration and invasion | [28] |
| MLN4924 | An inhibitor of NEDD8-activating enzyme | GC | Activates E-cadherin, represses MMP-9, inhibits cullin 1 neddylation, suppresses tumor growth and migration | [30] |
| Nemopilema nomurai jellyfish venom (NnV) | A venom from Nemopilema nomurai jellyfish | HCC | Suppresses the activation of p-Smad3, Smad4, and p-NF-kB, downregulates N-cadherin and vimentin, upregulates E-cadherin and β-catenin, reverses EMT, inhibits cell invasion and migration | [46] |
| Niclosamide | An antihelminthic drug | CRC | Increases E-cadherin, inhibits Snail, disrupts Axin-GSK3 complex, reverses EMT, suppresses adenoma formation | [76] |
| Norcantharidin (NCTD) | An efficacious anti-cancer drug | CRC | Suppresses integrin αvβ6, MMP-3, MMP-9, F-actin, N-cadherin, vimentin, and ERK, upregulates E-cadherin, inhibits cell migration and invasion, induces MET process | [82] |
| N-trans-feruloyloctopamine (FO) | Originated from garlic skin | HCC | Inhibits the level of Akt, p38 MAPK, and Slug, increases the level of E-cadherin, inhibits cell proliferation and invasion | [38] |
| Nutlin-3 | A small molecule regulator of p53 | HCC | Increases E-cadherin level, decreases the levels of vimentin, Snail, Slug and Smad2, inhibits migration and invasion, suppresses EMT | [45] |
| Obatoclax | A pan-inhibitor of Bcl-2 | CRC | Increases E-cadherin, p21, and p27, downregulates Cyclin D1, induces G1 arrest, inhibits cells migration, invasion and proliferation | [69] |
| Oxymatrine | An alkaloid extracted from the Chinese herb Sophora flavescens Ait | CRC | Increases E-cadherin, decreases PAI-1, TGF-β1, α-SMA, FN, Smad4, pSmad2 and pP38, inhibits TGF-β1/Smad pathway, and EMT | [88] |
| PNEE | Panax notoginseng ethanol extract | CRC | Decreases MMP-9, integrin-1, E-selectin, and ICAM-1, increases E-cadherin, inhibits cell adhesion, migration, and invasion | [70] |
| Physcion | An anthraquinone derivative | CRC | Increases E-cadherin, ROS production and pAMPK and GSK3β, decreases N-cadherin, vimentin, fibronectin, α-SMA, Snail, Slug, Twist, and SOX2, inhibits EMT, adhesion, migration and invasion | [84] |
| Pien Tze Huang (PZH) | A traditional Chinese formula | CRC | Increases E-cadherin, miR-200 fsmily, suppresses N-cadherin, ZEB1, ZEB2, TGF-β1, Smad2/3, and Smad4, modulate TGF-β1/ZEB/miR-200 pathway, inhibits cell proliferation, migration and invasion, induces apoptosis, inhibits tumor growth | [85,86] |
| Propofol | An intravenous anesthetic drug | PC | Decreases miR-21 and Slug, increases E-cadherin, inhibits cell growth and invasion, induces apoptosis | [63] |
| Resveratrol | A natural polyphenilic agent exists in grape and red wine | CRC | Upregulates E-cdherin, reduces vimentin and Snail, inhibits TGF-β1/Smads pathway, cells invasion and migration, inhibits lung and hepatic metastases, inhibits TGF-β1-induced EMT | [87] |
| Rosmarinic acid (RA) | A bioactive constituent from Rosemary | CRC | Upregulates E-cadherin, downregulates N-cadherin, Snail, Twist, vimentin, Slug, MMP-2, MMP-9, ICAM-1, and integrin β1, activates AMPK, inhibits cell adhesion, proliferation, invasion and migration, induce cell cycle arrest and apoptosis, inhibits lung metastasis | [80] |
| Rottlerin | A compound isolated from a medicinal plant Mallotus phillippinensis | PC | Suppresses Akt, Notch and Shh pathways, upregulates E-cadherin, inhibits COX-2, VEGF, VEGFR, IL-8, MMP-2, MMP-9, Snail and Slug, inhibits growth, angiogenesis and metastasis, induces apoptosis, reverses EMT | [58] |
| Sanguinarine | An alkaloid obtained from the root of Sanguinaria canadensis | PC | Upregulates E-cadherin, decreases N-cadherin, Snail, Slug, and ZEB1, suppresses SHH-Gil-Nanog pathway, inhibits cell proliferation, induces apoptosis, inhibits self-renewal capacity of pancreatic CSCs, reverses EMT | [60] |
| Scorpion | A wind calming drug | HCC | Increases E-cadherin, decreases N-cadherin and Snail, induces apoptosis, inhibits cell proliferation, migration, invasion, tumor growth, and metastasis | [51] |
| The anti-hTM4SF5 monoclonal antibody | A mAb of the cell surface transmembrane receptor | HCC | Increases the expression of E-cadherin and RhoA activity | [34] |
| Tunicamycin (TM) | An N-linked glycosylation inhibitor | HCC | Decreases CD44 and alters its glycosylation, Increases E-cadherin, suppresses CD44s- and TGF-β-mediated EMT, inhibits cells proliferation and migration, induce apoptosis and G2/M phase arrest | [42] |
| Ursodeoxycholic acid (UDCA) | A secondary bile acid | PC | Decreases the pSTAT3, inhibits Prx2, N-cadherin, Sox2, upregulates E-cadherin, reverses EMT, diminishes cancer stem cell formation | [61] |
| WZ35 | A derivative of curcumin | HCC | Decreases MMP-2, MMP-9, and N-cadherin, upregualtes E-cadherin, promotes ROS-dependent JNK activation, suppresses proliferation, invasion, and migration | [40] |
Gastric cancer
GC is one of the leading causes of cancer-related death worldwide with a poor response to current chemotherapy [23]. The anticancer effect of honokiol had been studied in GC. One group found that honokiol significantly inhibited invasion and migration of GC cells [24]. Further orthotopic mouse model studies revealed that honokiol-treated human GCs exhibited elevated epithelial biomarkers such as E-cadherin and cytokeratin-18 [24]. Conversely, reduced expression of vimentin, Snail and tumor progression locus 2 (Tpl2) was also noted [24]. Zhu et al. reported that celastrus orbiculatus effectively suppressed TGF-β1-mediated EMT in GC cells, which was further confirmed by upregulation of E-cadherin and downregulation of vimentin and N-cadherin [25]. Danusertib hampered EMT with an improvement in E-cadherin expression and a reduction in expression of N-cadherin in GC cell lines [26]. One group revealed that luteolin reversed EMT by promoting the expression of E-cadherin and reducing the level of N-cadherin, vimentin and snail, leading to inhibition of GC cell growth and metastasis [27]. Metformin ablated both the invasive and migratory ability of GC cells via repression of EMT in a glucose-independent way, as characterized by increased expression of the epithelial signature E-cadherin and decreased expression of the mesenchymal markers vimentin [28]. Huaier, a extensively used antitumor drug in China, presented a potent antimetastatic ability in GC cells partly via reversion of EMT as evidenced by upregulation of E-cadherin and downregulation of TWIST, N-cadherin, and vimentin [29]. A recently discovered inhibitor of NEDD8-activating enzyme (NAE), MLN4924, significantly suppressed neddylation modification activity and abrogated migration in human GC cells via transcriptionally facilitating E-cadherin and repressing MMP-9 [30]. Moreover, diallyl trisulfide (DATS), a natural compound extracted from garlic, suppressed GC growth and metastasis both in vitro and in vivo with no obvious adverse effects through activation of MAPK pathway and elevation of cytokine secretions, as accompanied by an increase of E-cadherin protein expression and a decrease of MMP-9 [31].
Hepatocellular carcinoma
HCC arises in patients as a consequence of long-standing preexisting liver illness, including viral hepatitis, alcohol abuse, or metabolic disease. Current standard practices for the treatment of HCC are unsatisfactory because of recurrence and metastasis [23]. LBH589, a new inhibitor of histone deacetylase, suppressed HCC metastasis both in vitro and in vivo without discernable adverse effects through an upregulation of E-cadherin expression and a downregulation of N-cadherin, vimentin, and TWIST1 expression, which were involved in the gankyrin/STAT3/Akt pathway [32]. Meloxicam, one of the widely used non-steroidal anti-inflammatory drugs (NSAIDs), reduced the level of overexpressed COX-2 in HCC cells, and subsequently promoted E-cadherin expression and curtailed the expression of MMP-2, leading to a repression of migration and invasion [33]. A monoclonal antibody of the cell surface transmembrane receptor (TM4SF5) was developed to inhibit proliferation in HCC cells accompanied with elevation of E-cadherin expression in vitro, which also suppressed tumor growth in vivo [34]. Huang et al. suggested that antcin K mitigated the metastasis of human HCC cells as characterized by upregulation of E-cadherin and downregulation of MMPs [35]. Recently, matrine was reported to repress the growth of HCC stem cells, which was further clarified by elevation of E-cadherin and fibronectin [36]. In fact, one previous study investigated the inhibitory abilities of 15 matrine derivatives. Among these molecules, compound CH6 suppressed migration of HCC cell lines via stimulation of E-cadherin and inhibition of N-cadherin [37]. The reformed N-trans-feruloyloctopamine (FO) that was originated from garlic skin was revealed to remarkably inhibit the viability and motility of HCC cells in part through upreguation of E-cadherin and downregulation of slug [38]. One group synthesized two kinds of naphthalimide derivatives and indicated that compound 3a ablated cell growth and migration in HCC by promoting the expression of E-cadherin without obvious systemic toxicity at the therapeutic dose [39]. WZ35, a derivative of curcumin, dramatically inhibited metastasis of HCC cells via activation of ROS-dependent JNK pathway with upregulation of E-cadherin and downregulation of MMPs and N-cadherin [40]. Ha et al. recommended that combination regime with sorafenib and vitamin K exerted synergistic effects on inhibition of HCC cell invasion and migration via improvement of E-cadherin expression and suppression of HGF/c-MET signaling pathway [41].
A variety of compounds have been found or designed to resist EMT-mediated metastasis through different molecular mechanisms. For instance, tunicamycin (TM) attenuated cell growth and migration in human HCC cells via inhibition of CD44s- and TGF-α-induced EMT [42]. Furthermore, cordycepin [43] reversed EMT by restoring E-cadherin expression and antagonizing integrin/FAK signaling pathway, while Jianpi Huayu decoction (JHD) [44], nutlin-3 [45] and nemopilema nomurai jellyfish venom (NnV) [46] exhibited antimetastatic properties on HCC cell lines by inhibiting Smad-mediated EMT. One group revealed that casticin hampered EMT of HCC stem cells via reducing the expression of TWIST and N-cadherin, and increasing levels of E-cadherin [25]. Retinoic acid is currently used as a differentiation inducer to improve radioiodide uptake and radiosensitivity of TC [47]. Moreover, Cui et al. demonstrated that all-trans retinoic acid (ATRA) impaired EMT with increased E-cadherin and decreased N-cadherin, and vimentin at transcriptional levels, significantly repressed the growth and metastasis of HCC cells [48]. In addition, arsenic trioxide (As2O3), known as the most effective agent for acute paromyelocytic leukemia (APL), also executed a promising antitumor capability on human HCC cells via the demethylation-activated miR-491 [49]. Of note, miR-491 reversed EMT through blockade of NF-κB, transcriptionally upregulating E-cadherin, and downregulating snail, vimentin, and MMP-2 [49]. Gadolinium chloride (GdCl3), which is an injectable MRI contrast agent widely used in the clinic, suppressed EMT on HCC by inducing blockage of tumor associated-macrophages (TAMs) and downregulation of CD206 in TAMs [50]. Scorpion, one of the most important wind calming drugs, suppressed EMT in HCC as verified by upregulation of E-cadherin and downregulation of N-cadherin, and snail [51]. These compounds could be useful for HCC treatment via restoring E-cadherin and reversing EMT.
Pancreatic cancer
PC is known to have a poor prognosis in part due to its high resistance to chemotherapeutic agents. Therefore, new and specific therapeutic strategies are urgently needed. Holy basil leaf extract presented anti-tumorigenic and antimetastatic effects in human PC cells both in vitro and in vivo with upregualtion of E-cadherin [52]. Curcumin reversed TGF-β1-stimulated EMT of PC cells via inhibition of the Hedgehog signaling pathway with an increase in E-cadherin expression level and a decrease in vimentin [53]. Cheng et al. reported that ellagic acid (EA), a polyphenolic compound found in several plants and fruits, suppressed human PC cell growth through reversal of EMT, as evidenced by upregulation of E-cadherin and downregulation of vimentin both in vitro and in vivo [54]. Similarly, one group found that the same dose (40 mg/kg) of EA used by Cheng et al. could inhibit the proliferation of PC in Balb c nude mice [55]. Likewise, embelin was demonstrated to attenuate proliferation and metastasis of human PC in part via repression of EMT by inducing the expression of E-cadherin and reducing the expression of snail, slug, and ZEB1 [56,57]. Furthermore, rottlerin also exerted an inhibitory ability of EMT both in human PC cells and nude mice by restoring E-cadherin expression and mitigating the activity of slug and snail [58]. Yuan et al. provided that α-mangostin was able to thwart the invasion and migration of PC cells through inhibition of ERK signaling pathway, accompanied by upregulation of E-cadherin and downregulation of MMP-2, and MMP-9 [59]. Sanguinarine [60], ursodeoxycholic acid (UDCA) [61], and anthothecol-encapsulated PLGA nanoparticles (Antho-NPs) [62] were suggested to inhibit formation and metastatic properties of PC stem cells via suppression of sonic hedgehog pathway. Notably, the suppressive effect of Antho-NPs was more evident than free anthothecol with the same dose, suggesting that Antho-NPs can be administrated for PC treatment [62]. Furthermore, a significant increase of E-cadherin and a decrease of mesenchymal-related markers such as N-cadherin were corroborated the repression of EMT [60-62]. Propofol, known as an intravenous anesthetic drug, retarded the proliferation and invasion of PC cells by inhibiting the miR-21/slug signaling pathway, leading to upregulation of E-cadherin and slug-dependent PUMA [63]. Galeterone and its analogs exhibited strong antimetastatic effects in PC cells partially through upregulation of E-cadherin and downregulation of N-cadherin, slug and MMPs [64]. One study by Lu et al. had observed that the administration of brusatol could be a potent adjuvant for anticancer therapy in human PC, which was consistent with inhibition of EMT as confirmed by enhancement of E-cadherin expression and decreased expression of vimentin, and TWIST [65]. More importantly, the anticancer effect of brusatol was comparable to some first-line chemotherapeutic agents such as gemcitabine and 5-fluorouracil (5-FU) but with more safe profile. And brusatol exerted a synergistic antitumor function when used together with one of these two agents both in vitro and in vivo. Hexa-D-arginine (D6R), a suppressor of furin, reversed EMT in PC cell lines via regulation of Hippo-YAP signaling pathway [66]. Further, D6R dramatically influenced several key regulatory factors of EMT process with stimulation of E-cadherin expression and downregulation of vimentin, and N-cadherin [66].
Colorectal cancer
CRC is the third most frequent cancer in the Western hemisphere and the incidence is increased with increasing age [67]. Several small compounds that restore E-cadherin expression have been identified to inhibit tumorigenesis and cancer progression in CRC. Cladosporol A, a secondary metabolite from Cladosporium tenuissimum, is considered as a new ligand of PPARγ [68]. Zurlo et al. found that cladosporol A reinforced cell-cell interactions via increasing E-cadherin expression, thereby inhibiting the proliferation in CRC cells [68]. Obatoclax (a pan-inhibitor of the Bcl-2 family), but not ABT-737, recovered E-cadherin expression and resulted in blocking migration and growth of CRC cells [69]. Furthermore, in contrast to ABT-737, obatoclax exhibited its antitumor effects in sublethal doses [69]. Panax notoginseng ethanol extract (PNEE) [70] and cinnamaldehyde (CA) [71] were indicated to enhance the adhesive abilities and simultaneously suppress metastasis in human CRC cells, which were relevant to upregulation of E-cadherin and downregulation of MMPs. In line with this, most recent studies demonstrated that a few natural compounds, such as berberine [72], emodin [73], and novel phytosomal curcumin [74] as well as ethanolic extract of gamboge [75], exhibited inhibitory effects on the growth and metastasis of human CRC through suppression of Wnt/β-catenin signaling pathway, as evidenced by an increase of E-cadherin and concomitant reduction of β-catenin. It was notable that the novel phytosomal curcumin might be more potent than free curcumin in cancer treatment, for the absorption of curcumin was enhanced with application of the former. Furthermore, co-treatment of phytosomal curcumin and 5-FU could significantly reduce the IC50 value of 5-FU [74].
Niclosamide was discovered to block EMT in part via disruption of Axin-GSK3 complex because it succeeded to upsurge E-cadherin amount and reduce the level of snail both in vitro and in vivo [76]. Curcumin was reported to reverse the EMT in colon cancer cells with upregulation of E-cadherin and downregulation of vimentin, and N-cadherin [77]. Notably, co-treatment of curcumin and ulinastatin greatly suppressed hepatic metastases from CRC and significantly extended survival of tumor-bearing mouse models through increased E-cadherin expression and a decrease of MMP-9 expression [78]. Similarly, arctigenin [79] and rosmarinic acid (RA) [80] had been shown to attenuate lung metastasis of CRC via inhibition of EMT in mouse model. Besides, one group showed that the ethanolic extract of bark from Salix aegyptiaca exerted an apparent inhibitory function on EMT of colon cancer cell lines in vitro, which was accompanied by a recovery of E-cadherin levels and a drop of MMPs, snail, and TWIST [81]. Norcantharidin (NCTD), an efficacious anticancer drug that has been used for many years, ablated EMT of colon cancer cells through repression of the αvβ6-ERK-Ets1 signaling pathway [82]. One study suggested that hispidulin, a naturally existing flavonoid, blocked hypoxia-induced EMT in human colon carcinoma cells in part via inhibition of HIF-α expression which was involved in the PTEN/PI3K/Akt pathway [83]. An anthraquinone derivative, physcion, antagonized the migration and invasion dose-dependently and impaired EMT of CRC cells, as manifested by upregulation of E-cadherin and downregulation of N-cadherin and vimentin [84]. Furthermore, numerous plant-derived compounds reversed EMT in human CRC cells through suppressing a series of TGF-β-mediated signaling pathways, containing Pien Tze Huang (PZH) [85,86], resveratrol [87], oxymatrine [88], and JianpiJieDu (JPJD) [89].
Conclusion
In this review article, we summarize the role of E-cadherin in human cancers briefly. We further describe pharmacological small compounds targeting E-cadherin expression for the potential treatment of digestive system cancers. It is well known that EMT is a complex process, which is triggered by numerous factors besides loss of E-cadherin. Therefore, restoring E-cadherin could be not sufficient enough to reverse EMT to MET in human cancer. Targeting multiple EMT inducers may be required to inhibit EMT and tumor metastasis. In addition, although these compounds could upregulate the expression of E-cadherin in digestive system cancers, deeper investigation is necessary to determine whether restoring E-cadherin inhibits tumor metastasis in clinical trials.
Acknowledgements
This work was supported by Major Science and Technology Planning Project of Wenzhou city (No. ZS2017006).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pan R, Zhu M, Yu C, Lv J, Guo Y, Bian Z, Yang L, Chen Y, Hu Z, Chen Z, Li L, Shen H China Kadoorie Biobank Collaborative Group. Cancer incidence and mortality: a cohort study in China, 2008-2013. Int J Cancer. 2017;141:1315–1323. doi: 10.1002/ijc.30825. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Hyafil F, Morello D, Babinet C, Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980;21:927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- 5.Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- 6.Mege RM, Ishiyama N. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- 8.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baulida J, Garcia de Herreros A. Snail1-driven plasticity of epithelial and mesenchymal cells sustains cancer malignancy. Biochim Biophys Acta. 2015;1856:55–61. doi: 10.1016/j.bbcan.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Barkeer S, Chugh S, Batra SK, Ponnusamy MP. Glycosylation of cancer stem cells: function in stemness, tumorigenesis, and metastasis. Neoplasia. 2018;20:813–825. doi: 10.1016/j.neo.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 16.Richards EJ. Inherited epigenetic variation--revisiting soft inheritance. Nat Rev Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 17.Winter JM, Ting AH, Vilardell F, Gallmeier E, Baylin SB, Hruban RH, Kern SE, Iacobuzio-Donahue CA. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abudukadeer A, Bakry R, Goebel G, Mutz-Dehbalaie I, Widschwendter A, Bonn GK, Fiegl H. Clinical relevance of CDH1 and CDH13 DNA-methylation in serum of cervical cancer patients. Int J Mol Sci. 2012;13:8353–8363. doi: 10.3390/ijms13078353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strathdee G. Epigenetic versus genetic alterations in the inactivation of E-cadherin. Semin Cancer Biol. 2002;12:373–379. doi: 10.1016/s1044-579x(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 20.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 21.McEwen AE, Maher MT, Mo R, Gottardi CJ. E-cadherin phosphorylation occurs during its biosynthesis to promote its cell surface stability and adhesion. Mol Biol Cell. 2014;25:2365–2374. doi: 10.1091/mbc.E14-01-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones J, Condeelis J, Merad M, Aguirre-Ghiso JA. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9:21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 24.Pan HC, Lai DW, Lan KH, Shen CC, Wu SM, Chiu CS, Wang KB, Sheu ML. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis. 2013;34:2568–2579. doi: 10.1093/carcin/bgt243. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen J, Guo S, Hisamitsu T. Research on the efficacy of Celastrus Orbiculatus in suppressing TGF-beta1-induced epithelial-mesenchymal transition by inhibiting HSP27 and TNF-alpha-induced NF-kappa B/Snail signaling pathway in human gastric adenocarcinoma. BMC Complement Altern Med. 2014;14:433. doi: 10.1186/1472-6882-14-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X, Wang D, Yang T, Pan SY, Chen XW, Zhou SF. Danusertib, a potent pan-aurora kinase and ABL kinase inhibitor, induces cell cycle arrest and programmed cell death and inhibits epithelial to mesenchymal transition involving the PI3K/Akt/mTOR-mediated signaling pathway in human gastric cancer AGS and NCI-N78 cells. Drug Des Devel Ther. 2015;9:1293–1318. doi: 10.2147/DDDT.S74964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang MD, Hu L, Fan ZY, Wang HX, Zhu ZL, Cao S, Wu XY, Li JF, Su LP, Li C, Zhu ZG, Yan M, Liu BY. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J Transl Med. 2017;15:52. doi: 10.1186/s12967-017-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valaee S, Yaghoobi MM, Shamsara M. Metformin inhibits gastric cancer cells metastatic traits through suppression of epithelial-mesenchymal transition in a glucose-independent manner. PLoS One. 2017;12:e0174486. doi: 10.1371/journal.pone.0174486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Zheng G, Wang Y, Zhang C, Yu J, Teng F, Lv H, Cheng X. Aqueous huaier extract suppresses gastric cancer metastasis and epithelial to mesenchymal transition by targeting twist. J Cancer. 2017;8:3876–3886. doi: 10.7150/jca.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan H, Tang Z, Jin H, Sun Y. Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells. Sci Rep. 2016;6:24218. doi: 10.1038/srep24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Zhu X, Huang W, Xu H, Zhao Z, Li S, Li S, Cai J, Cao J. Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int Immunopharmacol. 2017;48:135–145. doi: 10.1016/j.intimp.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Song X, Wang J, Zheng T, Song R, Liang Y, Bhatta N, Yin D, Pan S, Liu J, Jiang H, Liu L. LBH589 inhibits proliferation and metastasis of hepatocellular carcinoma via inhibition of gankyrin/STAT3/Akt pathway. Mol Cancer. 2013;12:114. doi: 10.1186/1476-4598-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong X, Li R, Xiu P, Dong X, Xu Z, Zhai B, Liu F, Jiang H, Sun X, Li J, Qiao H. Meloxicam executes its antitumor effects against hepatocellular carcinoma in COX-2- dependent and -independent pathways. PLoS One. 2014;9:e92864. doi: 10.1371/journal.pone.0092864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon S, Choi KC, Kim YE, Ha YW, Kim D, Park BK, Wu G, Kim DS, Lee Y, Kwon HJ. Monoclonal antibody targeting of the cell surface molecule TM4SF5 inhibits the growth of hepatocellular carcinoma. Cancer Res. 2014;74:3844–3856. doi: 10.1158/0008-5472.CAN-13-2730. [DOI] [PubMed] [Google Scholar]
- 35.Huang YL, Chu YL, Ho CT, Chung JG, Lai CI, Su YC, Kuo YH, Sheen LY. Antcin K, an active triterpenoid from the fruiting bodies of basswood-cultivated antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J Agric Food Chem. 2015;63:4561–4569. doi: 10.1021/jf5059304. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Liu Y, Jiang J, Cui H. Antitumor effects of matrine on cancer stem like cells isolated from the human liver cancer SMMC-7721 cell line. Oncol Lett. 2018;15:1777–1782. doi: 10.3892/ol.2017.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L, Liu S, Wei J, Li D, Liu X, Wang J, Wang L. Synthesis and biological evaluation of matrine derivatives as anti-hepatocellular cancer agents. Bioorg Med Chem Lett. 2016;26:4267–4271. doi: 10.1016/j.bmcl.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Bai ZT, Wu ZR, Xi LL, Li X, Chen P, Wang FQ, Meng WB, Zhou WC, Wu XA, Yao XJ, Zhang M. Inhibition of invasion by N-trans-feruloyloctopamine via AKT, p38MAPK and EMT related signals in hepatocellular carcinoma cells. Bioorg Med Chem Lett. 2017;27:989–993. doi: 10.1016/j.bmcl.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 39.Ge C, Chang L, Zhao Y, Chang C, Xu X, He H, Wang Y, Dai F, Xie S, Wang C. Design, synthesis and evaluation of naphthalimide derivatives as potential anticancer agents for hepatocellular carcinoma. Molecules. 2017;22 doi: 10.3390/molecules22020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Han L, Tao Z, Zhu Z, Han L, Yang Z, Wang H, Dai D, Wu L, Yuan Z, Chen T. The curcumin derivative WZ35 activates ROS-dependent JNK to suppress hepatocellular carcinoma metastasis. Food Funct. 2018;9:2970–2978. doi: 10.1039/c8fo00314a. [DOI] [PubMed] [Google Scholar]
- 41.Ha TY, Hwang S, Hong HN, Choi YI, Yoon SY, Won YJ, Song GW, Kim N, Tak E, Ryoo BY. Synergistic effect of sorafenib and vitamin K on suppression of hepatocellular carcinoma cell migration and metastasis. Anticancer Res. 2015;35:1985–1995. [PubMed] [Google Scholar]
- 42.Hou H, Ge C, Sun H, Li H, Li J, Tian H. Tunicamycin inhibits cell proliferation and migration in hepatocellular carcinoma through suppression of CD44s and the ERK1/2 pathway. Cancer Sci. 2018;109:1088–1100. doi: 10.1111/cas.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao WL, Ko BS, Liu TA, Liang SM, Liu CC, Lu YJ, Tzean SS, Shen TL, Liou JY. Cordycepin suppresses integrin/FAK signaling and epithelial-mesenchymal transition in hepatocellular carcinoma. Anticancer Agents Med Chem. 2014;14:29–34. doi: 10.2174/18715206113139990305. [DOI] [PubMed] [Google Scholar]
- 44.Zhong C, Zhang YF, Huang JH, Wang ZY, Chen QY, Su LT, Liu ZT, Xiong CM, Tao Z, Guo RP. The Chinese medicine, Jianpi Huayu Decoction, inhibits the epithelial mesenchymal transition via the regulation of the Smad3/Smad7 cascade. Am J Transl Res. 2017;9:2694–2711. [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Q, Wang X, Liu J, Zheng J, Liu Y, Li Y, Su F, Ou W, Wang R. Nutlin-3 reverses the epithelial-mesenchymal transition in gemcitabine-resistant hepatocellular carcinoma cells. Oncol Rep. 2016;36:1325–1332. doi: 10.3892/or.2016.4920. [DOI] [PubMed] [Google Scholar]
- 46.Lee H, Pyo MJ, Bae SK, Heo Y, Choudhary I, Hwang D, Yang H, Kim JH, Chae J, Han CH, Kang C, Yum S, Kim E. Nemopilema nomurai jellyfish venom exerts an anti-metastatic effect by inhibiting Smad- and NF-kappaB-mediated epithelial-mesenchymal transition in HepG2 cells. Sci Rep. 2018;8:2808. doi: 10.1038/s41598-018-20724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmutzler C, Kohrle J. Retinoic acid redifferentiation therapy for thyroid cancer. Thyroid. 2000;10:393–406. doi: 10.1089/thy.2000.10.393. [DOI] [PubMed] [Google Scholar]
- 48.Cui J, Gong M, He Y, Li Q, He T, Bi Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1-6 cells through reversing EMT in vitro. Int J Oncol. 2016;48:349–357. doi: 10.3892/ijo.2015.3235. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Jiang F, Mu J, Ye X, Si L, Ning S, Li Z, Li Y. Arsenic trioxide attenuates the invasion potential of human liver cancer cells through the demethylation-activated microRNA-491. Toxicol Lett. 2014;227:75–83. doi: 10.1016/j.toxlet.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Zhu F, Li X, Jiang Y, Zhu H, Zhang H, Zhang C, Zhao Y, Luo F. GdCl3 suppresses the malignant potential of hepatocellular carcinoma by inhibiting the expression of CD206 in tumorassociated macrophages. Oncol Rep. 2015;34:2643–2655. doi: 10.3892/or.2015.4268. [DOI] [PubMed] [Google Scholar]
- 51.Yan YQ, Xie J, Wang JF, Shi ZF, Zhang X, Du YP, Zhao XC. Scorpion inhibits epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma. Exp Biol Med (Maywood) 2018;243:645–654. doi: 10.1177/1535370218762514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu T, Torres MP, Chakraborty S, Souchek JJ, Rachagani S, Kaur S, Macha M, Ganti AK, Hauke RJ, Batra SK. Holy Basil leaf extract decreases tumorigenicity and metastasis of aggressive human pancreatic cancer cells in vitro and in vivo: potential role in therapy. Cancer Lett. 2013;336:270–280. doi: 10.1016/j.canlet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29:2401–2407. doi: 10.3892/or.2013.2385. [DOI] [PubMed] [Google Scholar]
- 54.Cheng H, Lu C, Tang R, Pan Y, Bao S, Qiu Y, Xie M. Ellagic acid inhibits the proliferation of human pancreatic carcinoma PANC-1 cells in vitro and in vivo. Oncotarget. 2017;8:12301–12310. doi: 10.18632/oncotarget.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao M, Tang SN, Marsh JL, Shankar S, Srivastava RK. Ellagic acid inhibits human pancreatic cancer growth in Balb c nude mice. Cancer Lett. 2013;337:210–217. doi: 10.1016/j.canlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Huang M, Tang SN, Upadhyay G, Marsh JL, Jackman CP, Shankar S, Srivastava RK. Embelin suppresses growth of human pancreatic cancer xenografts, and pancreatic cancer cells isolated from KrasG12D mice by inhibiting Akt and Sonic hedgehog pathways. PLoS One. 2014;9:e92161. doi: 10.1371/journal.pone.0092161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsh JL, Jackman CP, Tang SN, Shankar S, Srivastava RK. Embelin suppresses pancreatic cancer growth by modulating tumor immune microenvironment. Front Biosci (Landmark Ed) 2014;19:113–125. doi: 10.2741/4198. [DOI] [PubMed] [Google Scholar]
- 58.Huang M, Tang SN, Upadhyay G, Marsh JL, Jackman CP, Srivastava RK, Shankar S. Rottlerin suppresses growth of human pancreatic tumors in nude mice, and pancreatic cancer cells isolated from Kras(G12D) mice. Cancer Lett. 2014;353:32–40. doi: 10.1016/j.canlet.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 59.Yuan J, Wu Y, Lu G. alpha-Mangostin suppresses lipopolysaccharide-induced invasion by inhibiting matrix metalloproteinase-2/9 and increasing E-cadherin expression through extracellular signal-regulated kinase signaling in pancreatic cancer cells. Oncol Lett. 2013;5:1958–1964. doi: 10.3892/ol.2013.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Yu W, Shrivastava A, Alemi F, Lankachandra K, Srivastava RK, Shankar S. Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis. 2017;38:1047–1056. doi: 10.1093/carcin/bgx070. [DOI] [PubMed] [Google Scholar]
- 61.Kim YJ, Jeong SH, Kim EK, Kim EJ, Cho JH. Ursodeoxycholic acid suppresses epithelial-mesenchymal transition and cancer stem cell formation by reducing the levels of peroxiredoxin II and reactive oxygen species in pancreatic cancer cells. Oncol Rep. 2017;38:3632–3638. doi: 10.3892/or.2017.6045. [DOI] [PubMed] [Google Scholar]
- 62.Verma RK, Yu W, Singh SP, Shankar S, Srivastava RK. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomedicine. 2015;11:2061–2070. doi: 10.1016/j.nano.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Liu Z, Zhang J, Hong G, Quan J, Zhang L, Yu M. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res. 2016;8:4120–4133. [PMC free article] [PubMed] [Google Scholar]
- 64.Kwegyir-Afful AK, Murigi FN, Purushottamachar P, Ramamurthy VP, Martin MS, Njar VCO. Galeterone and its analogs inhibit Mnk-eIF4E axis, synergize with gemcitabine, impede pancreatic cancer cell migration, invasion and proliferation and inhibit tumor growth in mice. Oncotarget. 2017;8:52381–52402. doi: 10.18632/oncotarget.14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Z, Lai ZQ, Leung AWN, Leung PS, Li ZS, Lin ZX. Exploring brusatol as a new anti-pancreatic cancer adjuvant: biological evaluation and mechanistic studies. Oncotarget. 2017;8:84974–84985. doi: 10.18632/oncotarget.17761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou M, Zhang Y, Wei H, He J, Wang D, Chen B, Zeng J, Gong A, Xu M. Furin inhibitor D6R suppresses epithelial-mesenchymal transition in SW1990 and PaTu8988 cells via the Hippo-YAP signaling pathway. Oncol Lett. 2018;15:3192–3196. doi: 10.3892/ol.2017.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haraldsdottir S, Einarsdottir HM, Smaradottir A, Gunnlaugsson A, Halfdanarson TR. [Colorectal cancer - review] . Laeknabladid. 2014;100:75–82. doi: 10.17992/lbl.2014.02.531. [DOI] [PubMed] [Google Scholar]
- 68.Zurlo D, Assante G, Moricca S, Colantuoni V, Lupo A. Cladosporol A, a new peroxisome proliferator-activated receptor gamma (PPARgamma) ligand, inhibits colorectal cancer cells proliferation through beta-catenin/TCF pathway inactivation. Biochim Biophys Acta. 2014;1840:2361–2372. doi: 10.1016/j.bbagen.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Koehler BC, Scherr AL, Lorenz S, Elssner C, Kautz N, Welte S, Jaeger D, Urbanik T, Schulze-Bergkamen H. Pan-Bcl-2 inhibitor obatoclax delays cell cycle progression and blocks migration of colorectal cancer cells. PLoS One. 2014;9:e106571. doi: 10.1371/journal.pone.0106571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsieh SL, Hsieh S, Kuo YH, Wang JJ, Wang JC, Wu CC. Effects of panax notoginseng on the metastasis of human colorectal cancer cells. Am J Chin Med. 2016;44:851–870. doi: 10.1142/S0192415X16500476. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Teng Y, Liu S, Wang Z, Chen Y, Zhang Y, Xi S, Xu S, Wang R, Zou X. Cinnamaldehyde affects the biological behavior of human colorectal cancer cells and induces apoptosis via inhibition of the PI3K/Akt signaling pathway. Oncol Rep. 2016;35:1501–1510. doi: 10.3892/or.2015.4493. [DOI] [PubMed] [Google Scholar]
- 72.Albring KF, Weidemuller J, Mittag S, Weiske J, Friedrich K, Geroni MC, Lombardi P, Huber O. Berberine acts as a natural inhibitor of Wnt/beta-catenin signaling--identification of more active 13-arylalkyl derivatives. Biofactors. 2013;39:652–662. doi: 10.1002/biof.1133. [DOI] [PubMed] [Google Scholar]
- 73.Gu J, Cui CF, Yang L, Wang L, Jiang XH. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelialmesenchymal transition via the Wnt/beta-catenin pathway. Oncol Res. 2018 doi: 10.3727/096504018X15150662230295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Marjaneh RM, Rahmani F, Hassanian SM, Rezaei N, Hashemzehi M, Bahrami A, Ariakia F, Fiuji H, Sahebkar A, Avan A, Khazaei M. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol. 2018;233:6785–6798. doi: 10.1002/jcp.26538. [DOI] [PubMed] [Google Scholar]
- 75.Wang W, Li Y, Chen Y, Chen H, Zhu P, Xu M, Wang H, Wu M, Yang Z, Hoffman RM, Gu Y. Ethanolic extract of traditional Chinese medicine (TCM) gamboge inhibits colon cancer via the wnt/beta-catenin signaling pathway in an orthotopic mouse model. Anticancer Res. 2018;38:1917–1925. doi: 10.21873/anticanres.12429. [DOI] [PubMed] [Google Scholar]
- 76.Ahn SY, Kim NH, Lee K, Cha YH, Yang JH, Cha SY, Cho ES, Lee Y, Cha JS, Cho HS, Jeon Y, Yuk YS, Cho S, No KT, Kim HS, Lee H, Choi J, Yook JI. Niclosamide is a potential therapeutic for familial adenomatosis polyposis by disrupting Axin-GSK3 interaction. Oncotarget. 2017;8:31842–31855. doi: 10.18632/oncotarget.16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang C, Xu Y, Wang H, Li G, Yan H, Fei Z, Xu Y, Li W. Curcumin reverses irinotecan resistance in colon cancer cell by regulation of epithelial-mesenchymal transition. Anticancer Drugs. 2018;29:334–340. doi: 10.1097/CAD.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 78.Shen F, Cai WS, Li JL, Feng Z, Liu QC, Xiao HQ, Cao J, Xu B. Synergism from the combination of ulinastatin and curcumin offers greater inhibition against colorectal cancer liver metastases via modulating matrix metalloproteinase-9 and E-cadherin expression. Onco Targets Ther. 2014;7:305–314. doi: 10.2147/OTT.S57126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han YH, Kee JY, Kim DS, Mun JG, Jeong MY, Park SH, Choi BM, Park SJ, Kim HJ, Um JY, Hong SH. Arctigenin inhibits lung metastasis of colorectal cancer by regulating cell viability and metastatic phenotypes. Molecules. 2016;21 doi: 10.3390/molecules21091135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han YH, Kee JY, Hong SH. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front Pharmacol. 2018;9:68. doi: 10.3389/fphar.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Enayat S, Banerjee S. The ethanolic extract of bark from Salix aegyptiaca L. inhibits the metastatic potential and epithelial to mesenchymal transition of colon cancer cell lines. Nutr Cancer. 2014;66:999–1008. doi: 10.1080/01635581.2014.936949. [DOI] [PubMed] [Google Scholar]
- 82.Peng C, Li Z, Niu Z, Niu W, Xu Z, Gao H, Niu W, Wang J, He Z, Gao C, Lin P, Agrez M, Zhang Z, Niu J. Norcantharidin suppresses colon cancer cell epithelial-mesenchymal transition by inhibiting the alphavbeta6-ERK-Ets1 signaling pathway. Sci Rep. 2016;6:20500. doi: 10.1038/srep20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie J, Gao H, Peng J, Han Y, Chen X, Jiang Q, Wang C. Hispidulin prevents hypoxia-induced epithelial-mesenchymal transition in human colon carcinoma cells. Am J Cancer Res. 2015;5:1047–1061. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Han YT, Chen XH, Gao H, Ye JL, Wang CB. Physcion inhibits the metastatic potential of human colorectal cancer SW620 cells in vitro by suppressing the transcription factor SOX2. Acta Pharmacol Sin. 2016;37:264–275. doi: 10.1038/aps.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen A, Lin W, Chen Y, Liu L, Chen H, Zhuang Q, Lin J, Sferra TJ, Peng J. Pien Tze Huang inhibits metastasis of human colorectal carcinoma cells via modulation of TGF-beta1/ZEB/miR-200 signaling network. Int J Oncol. 2015;46:685–690. doi: 10.3892/ijo.2014.2772. [DOI] [PubMed] [Google Scholar]
- 86.Lin W, Zhuang Q, Zheng L, Cao Z, Shen A, Li Q, Fu C, Feng J, Peng J. Pien Tze Huang inhibits liver metastasis by targeting TGF-beta signaling in an orthotopic model of colorectal cancer. Oncol Rep. 2015;33:1922–1928. doi: 10.3892/or.2015.3784. [DOI] [PubMed] [Google Scholar]
- 87.Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Liu C, Wang J, Fan Y, Wang Z, Wang Y. Oxymatrine inhibits the migration of human colorectal carcinoma RKO cells via inhibition of PAI-1 and the TGF-beta1/Smad signaling pathway. Oncol Rep. 2017;37:747–753. doi: 10.3892/or.2016.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X, Ji Q, Deng W, Chai N, Feng Y, Zhou L, Sui H, Li C, Sun X, Li Q. JianPi JieDu recipe inhibits epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta/Smad mediated snail/E-cadherin expression. Biomed Res Int. 2017;2017:2613198. doi: 10.1155/2017/2613198. [DOI] [PMC free article] [PubMed] [Google Scholar]


