Abstract
RhoA GTPase is physiologically involved in the formation of stress fibers, cellular contractility and polarity, maintenance of cell cycle and transcriptional control. During tumorigenesis, it plays roles in cancer cell proliferation, apoptosis, adhesion, invasion and metastasis. While RhoA seems to act as a tumor promotor in most malignancies, data regarding its function in skin melanoma are fragmentary and conflicting. We aimed to clarify the clinical significance of RhoA expression in melanoma by immunohistochemical evaluation of 134 primary tumors and subsequent statistical analysis with clinicopathological profiles of patients. Increased RhoA expression was associated with thinner tumors, higher grade of tumor-infiltrating lymphocytes and lack of disease recurrence. Moreover, we observed a trend towards higher RhoA expression in cases without concurrent metastases. Recurrence-free survival and melanoma-specific survival of patients with high RhoA-expressing tumors were significantly prolonged. Multivariable regression model adjusting for melanoma thickness and status of regional lymph nodes confirmed independent prognostic value of RhoA immunoreactivity. In summary, we found associations between RhoA expression and histopathological phenotype of primary tumors as well as patient survival which suggest a suppressive role of RhoA in skin melanoma.
Keywords: RhoA, malignant melanoma, prognosis, survival
Introduction
Rho family proteins are small GTPases that influence numerous processes including cellular adhesion, polarity, migration as well as cell cycle progression [1]. They operate as molecular switches that become active when the coupled nucleotide GDP is exchanged for GTP [1]. Rho proteins’ activity is dependent on a number of regulators classified as guanine nucleotide exchange factors, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors [1]. Imbalance between these controllers, enhancing Rho signaling, is a frequent finding in cancer [2]. This, as well as overexpression and activating mutations of some Rho GTPases themselves demonstrated in many tumors, is suggestive of their prooncogenic properties [2,3]. Contrarily, other Rho proteins seem to play significant roles in tumor suppression [3].
RhoA is one of the canonical and most studied members of the family. Its activity may be induced by heterogeneous stimuli such as cytokines, hormones and interactions with extracellular matrix proteins [4,5]. Besides its physiological functions, it plays roles in hallmarks of cancer development and progression including proliferation, apoptosis, invasion and metastasis [6-9]. The majority of authors have highlighted cancer-promoting activities of RhoA and associated its overexpression with aggressive tumor phenotype and adverse prognosis [2,3]. However, recent functional studies, notably extensive investigations of colorectal and squamous cell lung cancers, demonstrate engagement of RhoA in important pathways impeding tumorigenesis [10,11]. Thus, the effect of RhoA signaling in cancer is not universal and seems to be context dependent.
In melanoma, in vitro studies documenting the activity of RhoA in the context of selected features of malignancy gave conflicting results. Some authors reported tumor-promoting functions of RhoA related to increased migration, cell survival and regulation of melanoma cell apoptosis [12-15]. Other experiments endorsed mechanisms of opposite significance such as RhoA-dependent immune modulation and inhibition of invasiveness [16-18].
To date there has been no definitive evidence for the clinical relevance of RhoA expression in skin melanoma. We aimed to address this issue by immunohistochemical analysis of RhoA expression in 134 variably advanced primary cutaneous melanomas. Then, we checked for statistical relationships between RhoA reactivity and other histopathological and clinical parameters, including patient survival.
Materials and methods
Patients
Tissue samples from 134 patients with a diagnosis of skin melanoma made between 2005 and 2010 were analyzed. The patients were diagnosed and treated in the Regional Oncology Centre in Opole, Poland. Inclusion criteria were based on the availability of histopathology slides, paraffin blocks and medical documentation, including archival pathology reports and disease staging. Medical records at the outpatient clinic of the Regional Oncology Centre in Opole and the Civil Register Office were the sources of information about diagnostic and therapeutic procedures applied and patient survival. The study was carried out in accordance with the Declaration of Helsinki and was approved by the Bioethics Committee of Wroclaw Medical University (consent No. 478/2017). The need for informed consent was waived by the Bioethics Committee of Wroclaw Medical University.
The patients’ treatment was up-to-date with the prevailing guidelines. If after removal of the primary lesion the histopathological diagnosis was skin melanoma, the scar was excised with a margin of 5, 10 or 20 mm depending on tumor location and Breslow thickness. Sentinel lymph node biopsy was performed in cases with Breslow thickness above 1 mm (>pT1a), but no evidence of metastatic spread (cN0). If metastases in the regional lymph nodes were found (either by sentinel lymph node biopsy or clinically), lymphadenectomy was performed.
Clinicopathological characteristics of the patients included sex, age, primary tumor location, TNM stratification and staging according to the 7th ed. of American Joint Committee on Cancer guidelines, data on disease recurrence and sentinel lymph node biopsies (Table 1).
Table 1.
RhoA immunoreactivity in melanoma cells and clinicopathological parameters
| Clinicopathological parameters | RhoA IRS | ||
|---|---|---|---|
|
| |||
| Low n = 46 | High n = 88 | p value | |
| Age in years (18-87)a | (18-86) | (24-87) | 0.075 |
| mean: 61.6±14.8; median: 64.5 | 64.6±14.0; 68 | 60.0±15.0; 60.5 | |
| Genderb | |||
| Female | 23 | 43 | 1.000 |
| Male | 23 | 45 | |
| Primary tumor locationc | |||
| Head/neck | 5 | 6 | 0.470 |
| Extremities | 17 | 39 | |
| Hand/foot | 2 | 1 | |
| Trunk | 22 | 42 | |
| 7th ed. AJCC stagec | |||
| I | 12 | 36 | 0.052 |
| II | 15 | 34 | |
| III | 11 | 13 | |
| IV | 8 | 5 | |
| Primary tumor (pT)a | |||
| pT1 | 9 | 22 | 0.028 |
| pT2 | 4 | 19 | |
| pT3 | 10 | 25 | |
| pT4 | 23 | 22 | |
| Regional lymph nodes status (pN)b | |||
| Metastases absent (pN-) | 30 | 72 | 0.053 |
| Metastases present (pN+) | 16 | 16 | |
| Distant metastases (pM)b | |||
| Metastases absent (pM-) | 38 | 83 | 0.061 |
| Metastases present (pM+) | 8 | 5 | |
| Sentinel lymph node biopsy statusb (60 patients) | |||
| No metastases | 9 | 28 | 0.091 |
| Metastases present | 11 | 12 | |
| Recurrenceb | |||
| No | 22 | 66 | 0.002 |
| Yes | 24 | 22 | |
p value of Wilcoxon two sample test;
p value of Fisher’s exact test;
p value of chi2 test.
Hematoxylin and eosin-stained sections of formalin-fixed and paraffin-embedded tumor tissue were used for histopathological evaluation. All slides were viewed in a blinded manner by two pathologists (MK and PD). Histologic type, Breslow thickness, Clark level, mitotic rate (counted per 1 mm2), presence of ulceration, lymphangioinvasion, microsatellitosis as well as tumor infiltrating lymphocytes (TILs) were recorded (Table 2). To assess TILs, we applied a semi-quantitative system-TILs absent: no lymphocytes present or lymphocytes are present but do not infiltrate the tumor at all; TILs non-brisk: lymphocytes infiltrate melanoma focally or not along the entire front of invasion; TILs brisk: lymphocytes diffusely infiltrate the base of the tumor or the entire invasive component.
Table 2.
RhoA immunoreactivity in melanoma cells and histopathological parameters
| Histopathological parameters | RhoA IRS | ||
|---|---|---|---|
|
| |||
| Low n = 46 | High n = 88 | p value | |
| Breslow thickness [mm] (0.3-40)a | (0.4-40) | (0.3-23) | 0.028 |
| mean: 4.8±6.1; median: 2.6 | 6.8±7.9; 3.8 | 3.7±4.5; 2.2 | |
| Clark levela | |||
| II | 11 | 29 | 0.058 |
| III | 14 | 29 | |
| IV | 12 | 26 | |
| V | 9 | 4 | |
| Histologic typec | |||
| Superficial spreading melanoma | 16 | 48 | 0.052 |
| Nodular melanoma | 28 | 39 | |
| Acral-lentiginous melanoma | 2 | 1 | |
| Mitotic ratec | |||
| 0 | 11 | 26 | 0.430 |
| 1-2 | 5 | 15 | |
| >2 | 30 | 47 | |
| Ulcerationb | |||
| No | 24 | 55 | 0.270 |
| Yes | 22 | 33 | |
| TILsc | |||
| No | 7 | 2 | 0.006 |
| Non-brisk | 29 | 52 | |
| Brisk | 10 | 34 | |
| Microsatellitosisb | |||
| No | 43 | 84 | 0.690 |
| Yes | 3 | 4 | |
| Lymphatic invasionb | |||
| No | 40 | 85 | 0.063 |
| Yes | 6 | 3 | |
p value of Wilcoxon two sample test;
p value of Fisher’s exact test;
p value of chi2 test;
TILs: tumor-infiltrating lymphocytes.
Immunohistochemistry
Anti-RhoA antibody (mouse monoclonal, clone 26C4; dilution 1:50; Santa Cruz Biotechnology; Dallas, TX, USA) was used to stain sections from 134 analyzed primary tumors. 4 µm paraffin sections cut with microtome were mounted on sialinized slides (code number S 3003; DAKO, Glostrup, Denmark) and subsequently subjected to automated dewaxing, rehydration and heat-induced epitope retrieval, performed in PT Link Pre-Treatment Module for Tissue Specimens (DAKO), using EnVision Target Retrieval Solution (DAKO) for 30 minute incubation at 97°C. Autostainer Link 48 (DAKO) was used for immunohistochemical staining and EnVision FLEX/HRP (DAKO) was used for detection. Human brain tissue was stained as a positive control. Negative controls were processed using FLEX Mouse Negative Control, Ready-to-Use (DAKO) in place of the primary antibody.
Evaluation of immunohistochemistry
RhoA expression was evaluated in the neoplastic compartment, i.e. in tumor cells, by a semi-quantitative method. Two parameters of immunohistochemical reaction were analyzed: the percentage of positive cells (the percentage of reactive tissue) and staining intensity. Scale of Remmele and Stegner modified by the authors was employed to calculate the final reaction score, as described previously [19,20]. In short, 0-10 points were given (0%-0 pts, 1-10%-1 pt, 11-20%-2 pts, etc.) for the percentage of positive cells and 0-3 points for the intensity of reaction. These values were multiplied to produce the final result for each case named ImmunoReactiveScore (IRS) ranging from 0 to 30 points. Light microscope Olympus BX51 (Olympus America, Inc., Melville, NY, USA) was used for evaluation of slides.
Statistical analysis
The R language, version 3.5 [R2018] (https://www.R-project.org/) was used for statistical analysis. The cohort was divided depending on IRS. We used the value of IRS = 14 as a cut-off for the stratification, chosen according to maxstat package in R to maximize rank test statistics. Patients whose specimens scored lower (IRS<14) were grouped as low RhoA expressors, while those with IRS≥14 formed a subgroup with increased RhoA expression. Continuous variables, like patient age at the diagnosis or Breslow thickness, were characterized with the use of mean, median, min and max values. To analyze recurrence-free survival (RFS) and melanoma-specific survival (MSS), we used Kaplan-Meier curves and log-tests; these calculations were conducted with the survminer package in R. To check the relationship between dichotomized RhoA expression and continuous variables, the Wilcoxon two-sample test was used. The association of IRS with binary variables was assessed by Fisher’s exact test and the relationship with other categorical variables was examined by chi-square test. All relations were summarized by a p-value, and the value of 0.05 was used as a threshold of significance.
Results
RhoA expression in skin melanomas
At least weak and focal expression of RhoA was detected in all 134 primary melanomas. The staining pattern was predominantly cytoplasmic. IRS ranged from 3 to 30 with the mean value of 17.7 and median value of 18. High RhoA expression (IRS≥14) characterized 88 melanomas while low RhoA reactivity (IRS<14) was found in the remaining 46 tumors (Figure 1).
Figure 1.
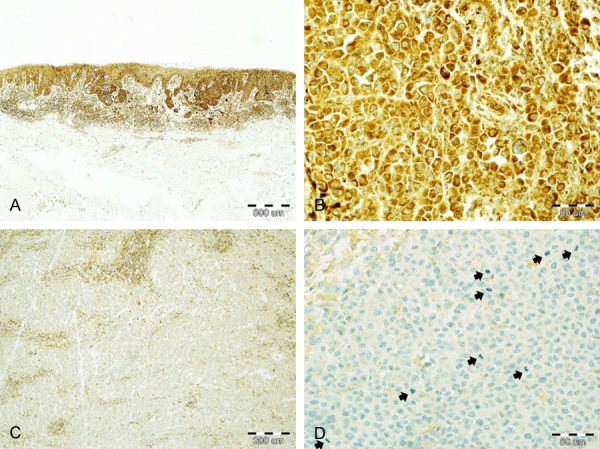
Immunohistochemical staining of skin melanomas with anti-RhoA antibody. Nests of melanoma cells with strong RhoA expression in a case of superficial-spreading melanoma; band-like, brisk infiltrate of lymphocytes is surrounding the tumor base (A: 40×; hematoxylin). High cytoplasmic expression of RhoA in malignant melanocytes (B: 400×; hematoxylin). Weak RhoA immunoreactivity in a case of nodular melanoma; stronger-stained cells are intratumoral lymphocytes (C: 100×; hematoxylin). Highly mitogenic (arrows) melanoma expressing minimal RhoA (D: 400×; hematoxylin).
RhoA expression and clinicopathological characteristics
Clinical characteristics like gender, age and tumor location were not associated with RhoA expression. We observed a shift towards low RhoA reactivity in advancing AJCC stages; this finding was on the borderline of statistical significance. Analogously, there was a relationship between RhoA immunoreactivity and pT variable-while the majority of early tumors expressed high levels of RhoA, slightly over half of pT4 melanomas were low RhoA expressors (P = 0.028). Downregulation of RhoA was more prevalent in cases with concurrent nodal and/or distant metastases compared with non-metastatic primary tumors, but this was another observation without definitive statistical significance. Finally, the disease recurred more frequently among low RhoA-expressing cases (P = 0.002) (Table 1). Considering pathological parameters of the primary tumors, RhoA was inversely associated with Breslow thickness (P = 0.028). Interestingly, RhoA expression was statistically lower in tumors with no or weak lymphocytic reaction compared with melanomas heavily infiltrated by TILs (P = 0.006) (Table 2).
RhoA expression and survival of melanoma patients
Kaplan-Meier analysis revealed significant differences in survival between the groups. High RhoA-expressing tumors were related with longer RFS and MSS (P = 0.00010 and P = 0.00013, respectively; Figure 2). The influence on survival was subsequently tested in a multivariable regression model. High RhoA expression was a protective factor and predicted longer RFS (HR = 0.47, P = 0.0110) and MSS (HR = 0.35, P = 0.0035) independently of Breslow thickness and pN status (Figure 3).
Figure 2.
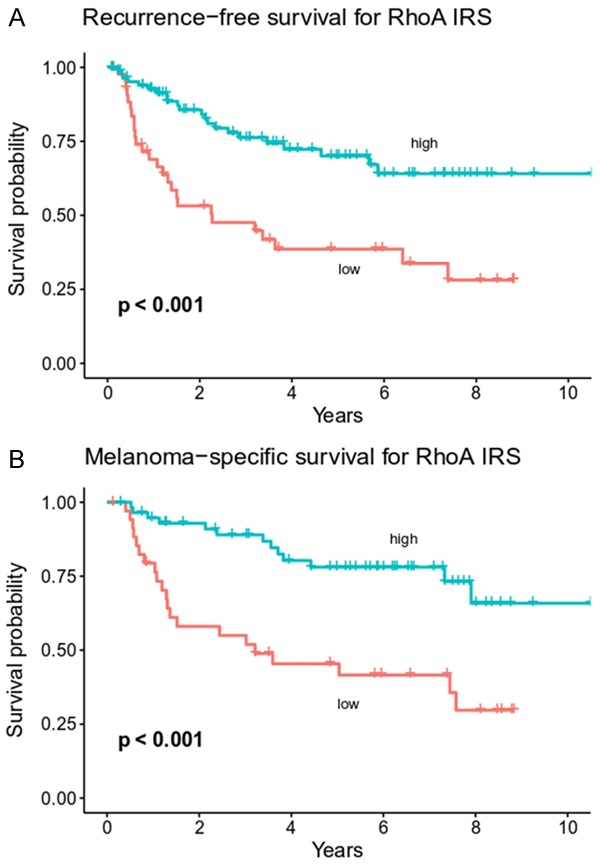
Kaplan-Meier plots of melanoma patient survival in groups stratified according to RhoA expression. Low RhoA reactivity is associated with shorter recurrence-free survival (A) and melanoma-specific survival (B) (p levels of log-rank tests).
Figure 3.
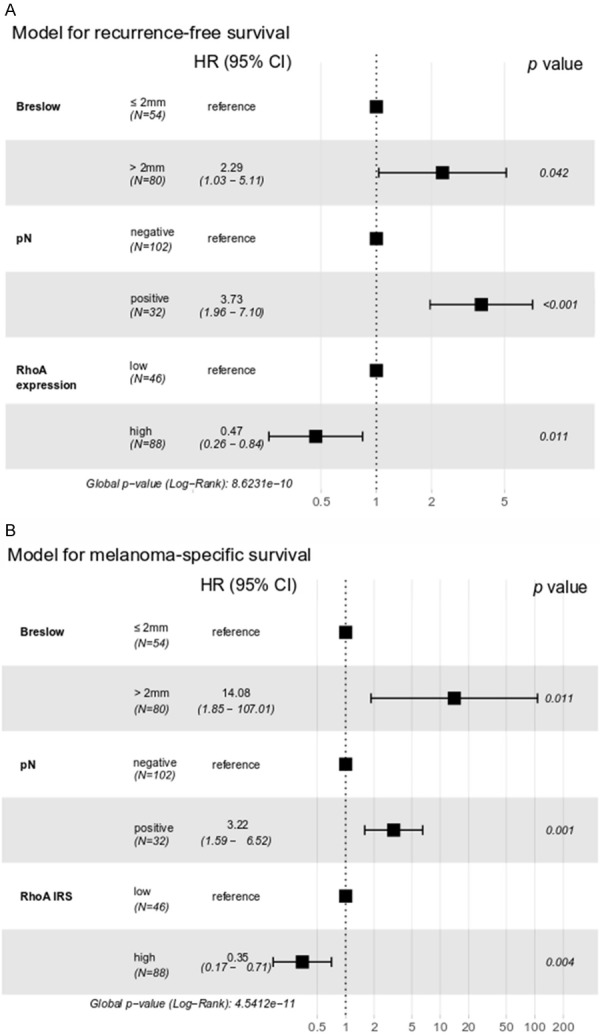
Multivariable regression analysis of recurrence-free survival (A) and melanoma-specific survival (B) in cutaneous melanoma patients. HR: hazard ratio, CI: confidence interval.
Discussion
Cell adhesion, invasiveness, proliferation and metastatic potential are the key features determining the dynamics of cancer progression. RhoA GTPase-physiologically involved in the formation of stress fibers, cellular contractility and polarity, maintenance of cell cycle and transcriptional control-is a crucial regulator of these processes [9,21,22]. The multitude and complexity of molecular pathways affected by RhoA activity account for its pro- or anti-oncogenic role postulated in various cancer settings [2,9-11]. Data concerning the role of RhoA in melanoma are fragmentary and conflicting, and no comprehensive analysis of RhoA expression in patient-derived melanoma tissues has been published so far. To this end, we evaluated RhoA immunoexpression in 134 primary tumors and correlated the results with clinicopathological characteristics of patients. Downregulation of RhoA was more prevalent among thicker and recurring tumors. Moreover, we found a distinct trend towards low RhoA expression in metastatic cases. These findings advocate for tumor-suppressive activity of RhoA in skin melanoma that is diminished in advanced disease.
Data supporting a link between melanoma invasiveness and downregulation of RhoA function come from a study of Díaz-Núñez et al. on histone deacetylase inhibitors [17]. Treatment of melanoma cell lines with these agents showed a pro-invasive effect accompanied by upregulation of N-cadherin and decrease of RhoA function [17]. Moreover, application of Rho inhibitor C3T and transfection with dominant-negative RhoA led to similar results, which confirms that RhoA is functionally involved in modulation of melanoma invasion [17]. On the other hand, Klein and Higgins showed that RhoA signaling was significantly upregulated and determined melanoma invasiveness following treatment with BRAF inhibitor [12]. In the absence of BRAF inhibition, however, depletion of RhoA had no effect on melanoma cell movement [12].
A recent study described a novel, non-immunological role of CD70 molecule in melanoma pathogenesis [23]. Expression of CD70 was high in primary lesions and decreased significantly in metastases [23]. Moreover, high CD70 expression impaired migration, invasiveness and formation of metastases [23]. Interestingly, treatment with anti-CD70 antibody promoted trimerization of CD70 which restored aggressive melanoma phenotype by activation of MAPK pathway [23]. In a follow-up study, the same group reported that RhoA enhances promoter activity of CD70 gene and is a key regulator of CD70 protein expression [24]. Although the authors did not investigate the levels of RhoA over time during melanoma progression, in the light of both studies it seems possible that downregulation of CD70 in aggressive and metastatic melanomas results from low RhoA expression in these tumors. This assumption harmonizes with our observations on clinical samples, in which RhoA immunoreactivity was negatively correlated with Breslow thickness-one of the most important prognostic parameters in malignant melanoma. Although the relation between low RhoA and presence of metastases only formed a trend in our cohort, it would most likely be statistically significant in a larger sample.
Tumor-suppressive activity of RhoA may also be associated with regulation of apoptosis and modulation of immune response. In a murine B16F10 model, RhoA inhibition resulted in membranous FasL expression on melanoma cells [16]. Furthermore, it effectively induced Fas-triggered apoptosis in cocultured B lymphoma cells [16]. Thus, our observation that tumors with low RhoA immunoreactivity are less infiltrated by TILs might be related to increased lymphocyte apoptosis induced by FasL-expressing tumor cells. Conversely, Goundiam et al. showed that inhibition of RhoA activity led to inhibition of tumor growth by stimulation of anoikis in malignant melanocytes [14].
Publications indicating tumor-inhibiting activities of RhoA, including this study, are contrasted by several experiments in which pharmacological inhibition of RhoA led to the suppression of melanoma cell motility and tumor growth [15,25,26]. Usage of different cell lines may be one of the reasons for these discrepancies, but more studies on animal models and human tissue are necessary to elucidate the contribution of RhoA to melanoma pathogenesis.
Epithelial to mesenchymal transition (EMT) is a phenotype switch that promotes dissemination of many epithelial tumors. Similarly, EMT-like process inducing a migratory phenotype of malignant cells plays a crucial role in melanoma progression [27]. Transforming growth factor β (TGFβ) which displays potent prooncogenic activity in advanced cancers, including melanoma, is one of the best studied activators of EMT [28,29]. Although exact mechanisms that trigger EMT in response to TGFβ are not fully understood, RhoA activity appears to be one of the important factors. Inhibiting RhoA or its downstream kinase ROCK blocked TGFβ-induced EMT in mouse mammary epithelial cells [30]. Interestingly, the same group reported that proliferative arrest mediated by TGFβ is associated with signaling through RhoA and ROCK [31]. Dependence on RhoA was also observed in TGFβ-stimulated EMT of rat lens epithelial and mesothelial cells as well as during embryonal development of chicken heart. Conversely, EMT in colon cancer seems to be related with a decrease in RhoA activation [32]. Requirement of RhoA and Cdc45 GTPases for rearrangements of actin cytoskeleton was demonstrated in TGFβ-treated human prostate carcinoma cells [33]. Notably, EMT-related phenotypic changes were at least partly independent of SMAD signaling [30,33]. In our previous study on melanoma we found an association between overexpression of SMAD7, an inhibitor of TGFβ/SMAD pathway, and disease progression [19]. However, levels of RhoA and SMAD7 were not correlated in our cohort (data not shown). The extent to which RhoA regulates EMT-like switch in cutaneous melanoma remains to be established.
In summary, our paper indirectly endorses the significance of RhoA in tumorigenesis. Unlike most data on RhoA expression and function in other cancers, our results argue for its engagement in suppression of melanoma. Previous studies in melanoma gave conflicting conclusions, but their direct comparison is often hindered due to methodological differences and focus on selected, different aspects of malignancy such as invasiveness or apoptosis. To the best of our knowledge this is the first work to demonstrate a more generic, clinical relevance of RhoA in skin melanoma. Therapeutic modulation of Rho/ROCK pathway has been proposed in a number of cancers, but deeper understanding of how it influences the natural history of melanoma progression is prerequisite to its clinical use in this setting.
Acknowledgements
A statutory subsidy by the Polish Ministry of Science and Higher Education as part of grants STM.B131.17.008, ST.B130.18.030 and SUB.C280.19.050 (record numbers in the Simple system); PB was financially supported by the National Science Centre grant 2016/21/B/ST6/02176.
Disclosure of conflict of interest
None.
References
- 1.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Porter AP, Papaioannou A, Malliri A. Deregulation of Rho GTPases in cancer. Small GTPases. 2016;7:123–138. doi: 10.1080/21541248.2016.1173767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–8. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Gordon BS, Kazi AA, Coleman CS, Dennis MD, Chau V, Jefferson LS, Kimball SR. RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells. Cell Signal. 2014;26:461–467. doi: 10.1016/j.cellsig.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjøller L, Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999;253:166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- 6.Pillé JY, Denoyelle C, Varet J, Bertrand JR, Soria J, Opolon P, Lu H, Pritchard LL, Vannier JP, Malvy C, Soria C, Li H. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267–274. doi: 10.1016/j.ymthe.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Xu XT, Song QB, Yao Y, Ruan P, Tao ZZ. Inhibition of RhoA/ROCK signaling pathway promotes the apoptosis of gastric cancer cells. Hepatogastroenterology. 2012;59:2523–2526. doi: 10.5754/hge12147. [DOI] [PubMed] [Google Scholar]
- 8.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3456. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Struckhoff AP, Rana MK, Worthylake RA. RhoA can lead the way in tumor cell invasion and metastasis. Front Biosci. 2011;16:1915–1926. doi: 10.2741/3830. [DOI] [PubMed] [Google Scholar]
- 10.Abraham CG, Ludwig MP, Andrysik Z, Pandey A, Joshi M, Galbraith MD, Sullivan KD, Espinosa JM. ΔNp63α suppresses TGFB2 expression and RHOA activity to drive cell proliferation in squamous cell carcinomas. Cell Rep. 2018;24:3224–3236. doi: 10.1016/j.celrep.2018.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues P, Macaya I, Bazzocco S, Mazzolini R, Andretta E, Dopeso H, Mateo-Lozano S, Bilić J, Cartón-García F, Nieto R, Suárez-López L, Afonso E, Landolfi S, Hernandez-Losa J, Kobayashi K, Cajal SRY, Tabernero J, Tebbutt NC, Mariadason JM, Schwartz S, Arango D. RHOA inactivation enhances Wnt signalling and promotes colorectal cancer. Nat Commun. 2014;5:5458. doi: 10.1038/ncomms6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein RM, Higgins PJ. A switch in RND3-RHOA signaling is critical for melanoma cell invasion following mutant-BRAF inhibition. Mol Cancer. 2011;10:114. doi: 10.1186/1476-4598-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinha G, Osaki JH, Costa ET, Forti FL. Inhibition of the RhoA GTPase activity increases sensitivity of melanoma cells to UV radiation effects. Oxid Med Cell Longev. 2016;2016:2696952. doi: 10.1155/2016/2696952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goundiam O, Nagel M, Vayssade M. Akt and RhoA inhibition promotes anoikis of aggregated B16F10 melanoma cells. Cell Biol Int. 2012;36:311–319. doi: 10.1042/CBI20110069. [DOI] [PubMed] [Google Scholar]
- 15.Dua P, Gude RP. Pentoxifylline impedes migration in B16F10 melanoma by modulating Rho GTPase activity and actin organisation. Eur J Cancer. 2008;44:1587–1595. doi: 10.1016/j.ejca.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Sarrabayrouse G, Synaeve C, Leveque K, Favre G, Tilkin-Mariamé AF. Statins stimulate in vitro membrane FasL expression and lymphocyte apoptosis through RhoA/ROCK pathway in murine melanoma cells. Neoplasia. 2007;9:1078–90. doi: 10.1593/neo.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díaz-Núñez M, Díez-Torre A, De Wever O, Andrade R, Arluzea J, Silió M, Aréchaga J. Histone deacetylase inhibitors induce invasion of human melanoma cells in vitro via differential regulation of N-cadherin expression and RhoA activity. BMC Cancer. 2016;16:667. doi: 10.1186/s12885-016-2693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamura S, Hakomori S, Wada A, Igarashi Y. Sphingosine-1-phosphate inhibits haptotactic motility by overproduction of focal adhesion sites in B16 melanoma cells through EDG-induced activation of Rho. Ann N Y Acad Sci. 2000;905:301–307. doi: 10.1111/j.1749-6632.2000.tb06566.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaczorowski M, Biecek P, Donizy P, Pieniazek M, Matkowski R, Halon A. SMAD7 is a novel independent predictor of survival in patients with cutaneous melanoma. Transl Res. 2018;204:72–81. doi: 10.1016/j.trsl.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 21.Haga RB, Ridley AJ. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JG, Islam R, Cho JY, Jeong H, Cap KC, Park Y, Hossain AJ, Park JB. Regulation of RhoA GTPase and various transcription factors in the RhoA pathway. J Cell Physiol. 2018;233:6381–6392. doi: 10.1002/jcp.26487. [DOI] [PubMed] [Google Scholar]
- 23.Pich C, Sarrabayrouse G, Teiti I, Mariamé B, Rochaix P, Lamant L, Favre G, Maisongrosse V, Tilkin-Mariamé AF. Melanoma-expressed CD70 is involved in invasion and metastasis. Br J Cancer. 2016;114:63–70. doi: 10.1038/bjc.2015.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pich C, Teiti I, Sarrabayrouse G, Gallardo F, Gence R, Tilkin-Mariame AF. Melanoma expressed-CD70 is regulated by RhoA and MAPK pathways without affecting vemurafenib treatment activity. PLoS One. 2016;11:e0148095. doi: 10.1371/journal.pone.0148095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Routhier A, Astuccio M, Lahey D, Monfredo N, Johnson A, Callahan W, Partington A, Fellows K, Ouellette L, Zhidro S, Goodrow C, Smith A, Sullivan K, Simone P, Le L, Vezuli B, Zohni M, West E, Gleason D, Bryan B. Pharmacological inhibition of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth. Oncol Rep. 2010;23:861–867. [PubMed] [Google Scholar]
- 26.Heo JC, Park JY, Woo SU, Rho JR, Lee HJ, Kim SU, Kho YH, Lee SH. Dykellic acid inhibits cell migration and tube formation by RhoA-GTP expression. Biol Pharm Bull. 2006;29:2256–2259. doi: 10.1248/bpb.29.2256. [DOI] [PubMed] [Google Scholar]
- 27.Li FZ, Dhillon AS, Anderson RL, McArthur G, Ferrao PT. Phenotype switching in melanoma: implications for progression and therapy. Front Oncol. 2015;5:31. doi: 10.3389/fonc.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Perrot CY, Javelaud D, Mauviel A. Insights into the transforming growth factor-β signaling pathway in cutaneous melanoma. Ann Dermatol. 2013;25:135–144. doi: 10.5021/ad.2013.25.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhowmick NA, Ghiassi M, Aakre M, Brown K, Singh V, Moses HL. TGF-beta-induced RhoA and p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc Natl Acad Sci U S A. 2003;100:15548–15553. doi: 10.1073/pnas.2536483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005;65:10938–10945. doi: 10.1158/0008-5472.CAN-05-1947. [DOI] [PubMed] [Google Scholar]
- 33.Edlund S, Landström M, Heldin CH, Aspenström P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]


