Abstract
Fibroblast growth factor receptor 2 (FGFR2) amplification has been reported in 5-10% of gastric cancer (GC) and is associated with poor prognosis. In this study, we characterized the anti-tumor effect of PRO-007, a newly developed recombinant monoclonal antibody that targets FGFR2, in GC cell lines KATO III (with FGFR2 amplification) and NCI-N87 (without FGFR2 amplification). Validation was performed in parallel using two patient-derived tumor cells (PDCs) from patients with GC. Cell viability assays were performed using FGFR2-transfected NCI-N87 cells and FGFR2-knockdown KATO III cells that were generated using short hairpin RNA (shRNA). PRO-007 reduced KATO III cell viability (P = 0.0034) but not that of NCI-N87 cells (P = 0.3710). PRO-007 also significantly reduced KATO III cell invasiveness (P < 0.0001) but not NCI-N87 cell invasiveness (P = 0.8136). Immunoblot analysis showed that PRO-007 treatment decreased the levels of phosphorylated AKT and ERK. The FGFR2-inhibitory activity of PRO-007 was confirmed in genetically modified GC cell lines. Cell viability of FGFR2-overexpressing NCI-N87 cells was significantly decreased by PRO-007, while KATO III cells were significantly resistant to the treatment when FGFR2 was knocked down by FGFR2 shRNA transfection. Furthermore, PRO-007 had a synergistic effect with ramucirumab on the invasiveness of cancer cells with FGFR2 amplification. Consistent results were obtained using PDCs from patients with GC. Overall, these preclinical data support the further clinical development of PRO-007 as a potential therapeutic agent for patients with FGFR2-amplified GC.
Keywords: FGFR2-amplified, gastric cancer, PRO-007, patient-derived cell
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer-related death [1]. Most patients with GC present with advanced disease and overall prognosis remains very poor [2] with few molecular-targeted approaches having been successfully incorporated into routine care. To date, only first line trastuzumab combined with chemotherapy for human epidermal growth factor receptor 2 (HER2)-positive tumors [3] and second line ramucirumab targeting vascular endothelial growth factor receptor 2 (VEGFR2) [4,5] have been used for treatment of GC. Over the past decade, molecular and genomic profiling has highlighted the heterogeneity of GC and uncovered potential molecular pathways [6-9]. However, appropriate patient selection for new therapies with predictive biomarkers remains challenging.
The fibroblast growth factor receptor (FGFR) family, consisting of four subtypes of transmembrane tyrosine kinase receptors (FGFR1-FGR4), are deregulated by amplification, point mutations, or translocations and are promising therapeutic targets [10]. Previous preclinical studies have reported that FGFR2 amplification is associated with the proliferation and survival of GC cells and the inhibition of FGFR2 has an anti-tumor effect in FGFR2-amplified GC cell lines [11-14], which supports it as a potential therapeutic target in GC. A few clinical phase I/II trials of various FGFR inhibitors have focused on the FGFR2-amplified subset of GC [15-17]; however, conflicting results have been obtained, including a phase II clinical trial that failed to show a clinical benefit [15]. These findings highlight the need for more potent FGFR2 inhibitors.
PRO-007 is a newly developed recombinant monoclonal antibody that targets FGFR2. In the current study, we investigated the preclinical activity of PRO-007 in both FGFR2-amplified GC cells and patient-derived tumor cells (PDCs). We also validated the role of FGFR2 amplification as a predictive biomarker for PRO-007 efficacy.
Materials and methods
Ethical considerations
Patients were enrolled as part of the SMC Oncology Biomarker Study (NCT#01831609, clinicaltrials.gov), which was described previously [18]. Effusions were collected for therapeutic purposes after obtaining written informed consent. The Samsung Medical Center Institutional Review Board approved the protocol and all procedures were carried out according to the guidelines of the Declaration of Helsinki.
Fluorescence in situ hybridization (FISH) of FGFR2
FISH was performed using a ZytoLight SPEC FGFR2/CEN 10 Dual Color Probe (Z-2122-200; ZytoVision, Bremerhaven, Germany). DNA probe sets were incubated with 1-μm-thick tumor sections overnight at 37°C. Hybridization signals were evaluated for 20 nuclei per sample under a fluorescence microscope. Only nuclei with distinct nuclear borders were evaluated with all overlapping nuclei being excluded. FGFR2 was considered amplified when the FGFR2:CEP17 FISH-signal ratio was ≥ 2.0.
Cell lines and patient-derived tumor cell culture
PRO-007 was evaluated in vitro using two GC cell lines, KATO III and NCI-N87, which were obtained from the Korean Cell Line Bank (KCLB; Seoul, Korea). The cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (Gibco, Carlsbad, CA, USA).
For PDCs, malignant ascites was collected from patients with metastatic GC. Collected effusions (1-5 L) were divided into 50-mL tubes, centrifuged at 1500 rpm for 10 min, and washed twice with phosphate-buffered saline (PBS). Cell pellets were suspended in RPMI 1640 culture medium and added to 75-cm2 culture flasks. The cells were cultured in RPMI 1640 supplemented with 10% FBS, 1% antibiotic-antimycotic solution, 0.5 μg/mL hydrocortisone (Sigma Aldrich, St. Louis, MO, USA), 5 μg/mL insulin (PeproTech, Rocky Hill, NJ, USA), 5 ng/mL epidermal growth factor (EGF), and 2.5 ng/mL FGF (PeproTech). The cells were maintained at 37°C in a humidified 5% CO2 incubator and the medium was changed every three days. PDCs were passaged using TrypLE Express (Gibco BRL) to detach the cells after reaching 80-90% confluence.
Cell treatment and viability assay
To evaluate the effect of PRO-007, cells were seeded at 5 × 103 cells/well in 96-well plates, allowed to adhere overnight, and treated with the indicated drugs for 72 h. Inhibition of cell proliferation was determined using Cell Titer Glo (Promega, Madison, WI, USA), according to the manufacturer’s protocol.
Generation of an FGFR2-modified GC cell line
For FGFR2 overexpression, full-length FGFR2 complementary DNA (cDNA) was generated from the MegaMan cDNA library (Stratagene, La Jolla, CA, USA). The complete FGFR2 cDNA was inserted into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA) using the NheI/XbaI endonuclease restriction sites. The open reading frames of the constructs were verified by sequence analysis performed using an ABI 3730xl DNA Analyzer (Thermo Fisher Scientific, Rockford, IL, USA). Approximately 5 × 106 cells were electroporated with 2.5 μg of pcDNA3.1/FGFR2-construct DNA or parental pcDNA3.1 using the Nucleofector System (Lonza, Basel, Switzerland). The transformed cells were selected in medium containing G418 for 2 wk and expression and translation of the full-length fusion transcripts were confirmed by immunoblotting. In addition, cells were transiently transfected by electroporation with short hairpin FGR2 (shFGFR2) TG318702 (Origene Technologies, Rockville, MD, USA) or scramble RNA.
Immunoblot analysis
To evaluate the effect of PRO-007, cells were seeded at 6 × 105 cells/60-mm dishes, allowed to adhere overnight, and then treated with the indicated drugs for 2 h. Total cell extracts were obtained using lysis buffer (20 mM HEPES [pH 7.4], 1% Triton X-100, 1 mM EDTA, 1 mM MgCl2, 150 mM NaCl, 10% glycerol, protease inhibitor, and phosphatase inhibitor cocktail [Invitrogen]). The protein concentrations of the cell extracts were determined using Micro-BCA Protein Reagent (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of proteins (30 μg per well) from the clarified lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes with a 0.2-μm pore size (Whatman, Maidstone, UK). The membranes were incubated with phospho-AKT (Ser473) antibody (#4060, 1:1,000; Cell Signaling Technology [CST], Danvers, MA, USA), AKT antibody (#9272, 1:1,000; CST), phospho-ERK1/2 (Thr202/Tyr204) antibody (#4370, 1:1,000; CST), ERK1/2 antibody (#9102, 1:1,000; CST), phospho-FGFR (Tyr653/654) antibody (#3471, 1:1,000; CST), FGFR2 antibody (sc-6930, 1:1,000, Santa Cruz Biotechnology, Dallas, TX, USA), phospho-PLCγ1 (Tyr783) antibody (#2821, 1:1,000; CST), PLCγ1 antibody (#5690, 1:1,000; CST), and β-actin antibody (AC-15, 1:5,000; Sigma). The ECL system (Invitrogen) was used for protein detection.
Invasion assay
Cell invasiveness was measured using Matrigel-coated Transwell chambers (BD Biosciences, Mountain View, CA, USA). Cells were treated with PRO-007 for 24 h, trypsinized, counted, and added to the upper well of Transwell chambers. Medium containing 10% FBS was added to the lower chambers as a chemoattractant. After incubation for 24 h, cells in the lower chamber were fixed in 100% methanol and stained with 0.1% crystal violet. Cells in five randomly selected areas of each well were counted using light microscopy. Data from three independent experiments were expressed as means ± SD.
Statistical analysis
For cell viability curves, results are expressed as means. Preclinical data were evaluated by one-way ANOVA using GraphPad Prism version 4.01 (San Diego, CA, USA). All p-values of less than 0.05 were considered statistically significant.
Results
Patients
Two PDCs were used in the current study, PDC#1 and PDC#2. PDC#1 was derived from a 46-year-old male patient with advanced GC and exhibited FGFR2 amplification. Briefly, the patient was initially diagnosed with poorly differentiated tubular adenocarcinoma and a TNM stage of IIIB (T3N3) according to AJCC Cancer Staging Manual, 7th edition. Two years after curative resection, peritoneal seeding was confirmed by exploratory laparotomy. The cancer was refractory to TS-1 plus cisplatin. The FGFR2/CEP10 ratio was 50, as verified by FISH (Figure 1A). The PDC#1 cell line was established from malignant ascites (Figure 1B) after obtaining informed consent from the patient. The PDC#2 cell line was established from malignant ascites derived from a patient with GC. PDC#2 did not exhibit FGFR2 amplification.
Figure 1.
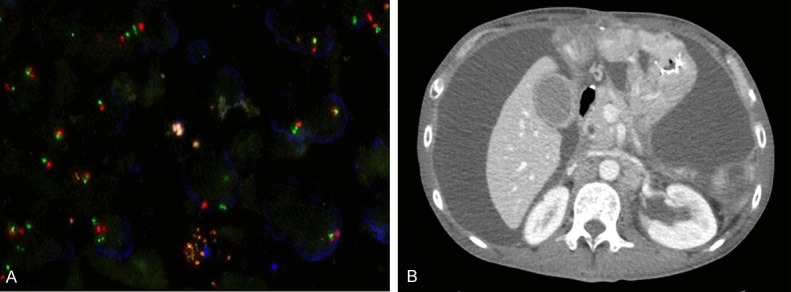
FGFR2 gene amplification in gastric cancer samples. (A) FGFR2 amplification in primary tissues of FGFR2-amplified PDC (PDC1) by FISH showing amplification of the target probe (red signal) to CEP-10 (green signal), (B) CT scan of a patient with FGFR2 amplification; a large amount of ascites was drained and collected for the culture of PDCs.
Confirmation of FGFR2 amplification in PDCs from malignant ascites
We performed targeted sequencing using Archer® VariantPlex assays to confirm FGFR2 amplification in cultured PDC#1 cells. The FGFR2 copy number based on next generation sequencing was 15.39.
Anti-tumor effect of PRO-007 in GC cell lines with and without FGFR2 amplification
We evaluated the anti-tumor effect of PRO-007 on KATO III and NCI-N87 cells. Based on in vitro cell viability assays, PRO-007 reduced the viability of KATO III cells, which exhibit an FGFR2 amplification (P = 0.0034), but not the viability of NCI-N87 cells, which do not exhibit FGFR2 amplification (P = 0.3710) (Figure 2A). PRO-007 also significantly reduced the invasiveness of KATO III cells (P < 0.0001), but not the invasiveness of NCI-N87 cells (P = 0.8136) (Figure 2B).
Figure 2.
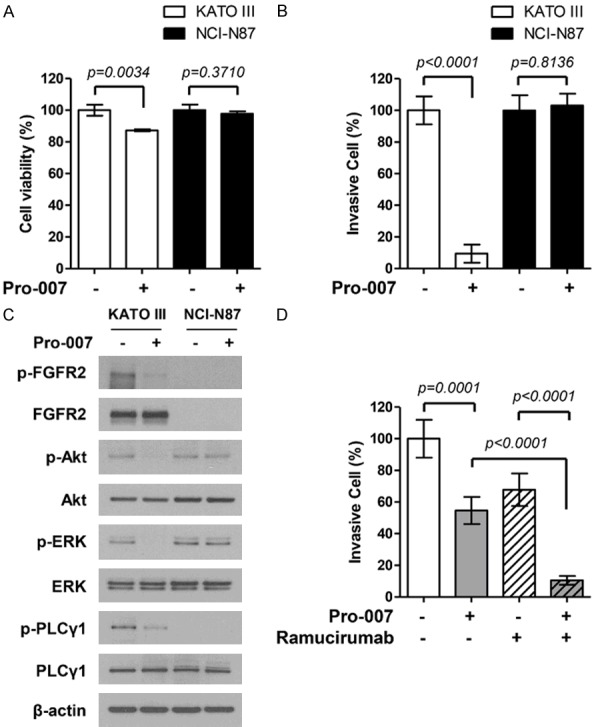
Anti-tumor effect of PRO-007 in the FGFR2-amplified GC cell line. A. Pro-007 suppressed proliferation in FGFR2-amplified KATOIII cells. Cell proliferation was measured by the CTG assay after treatment with PRO-007 at 10 μg/ml for 72 h. Cell viability (%) represents the percentage of growth compared to that of the control (no treatment). B. Pro-007 suppressed KATOIII cell invasion. Cells that invaded the membrane were stained with crystal violet and counted directly under a microscope. Data are presented as mean values ± SD of three independent experiments. C. Pro-007 decreased FGFR phosphorylation and targeted downstream pathways. D. Pro-007 (1 μg/ml) enhances the efficacy of ramucirumab (1 μM) in FGFR2-amplified KATOIII cells.
Next, we analyzed the effects of PRO-007 on FGFR2 pathway markers, such as the phosphorylation of ERK/Akt. KATO III and NCI-N87 cells were evaluated by immunoblot assays after PRO-007 treatment. AKT and ERK phosphorylation levels were clearly reduced in KATO III cells treated with PRO-007 compared to that in untreated cells (Figure 2C), which suggested the inhibition of cell proliferation by PRO-007 was related to a decrease in AKT/ERK phosphorylation. Moreover, we found that the combination of PRO-007 and ramucirumab had a greater inhibitory effect on the invasiveness of KATO III cells than did either PRO-007 or ramucirumab alone (Figure 2D).
Anti-tumor effect of PRO-007 on FGFR2-modified gastric cancer cell lines
To confirm that FGFR2 amplification was an effective target for PRO-007, we transfected NCI-N87 cells with an FGFR2-expressing plasmid and knocked down FGFR2-expression in KATO III cells using FGFR2-specific shRNA. PRO-007 significantly reduced the viability of FGFR2-transfected NCI-N87 cells (Figure 3A). However, the viability of FGFR2-knockdown KATO III cells was not reduced by PRO-007 (Figure 3B).
Figure 3.
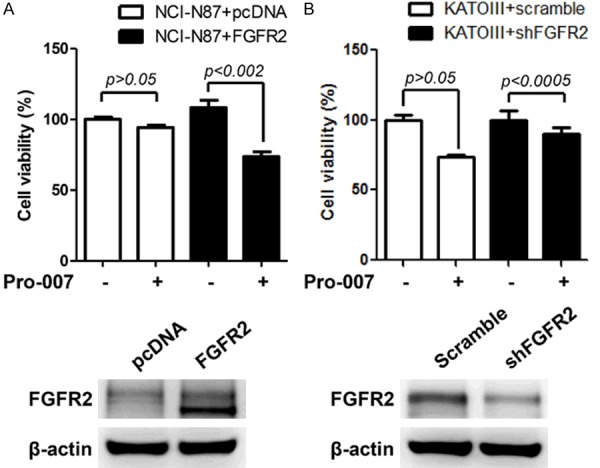
Anti-tumor effect of PRO-007 in FGFR2-modified GC cell lines. A. The viability of NCI-N87 cells transfected with FGFR2 cDNA was measured by the CTG assay after treatment with PRO-007 at 30 μg/ml. FGFR2 expression was confirmed by an immunoblotting assay. B. The viability of KATOIII cells transfected with FGFR2 shRNA was measured by the CTG assay after treatment with PRO-007 at 30 μg/ml. An immunoblotting assay showed decreased FGFR2 expression after transfection with FGFR2 shRNA.
Anti-tumor effect of PRO-007 on PDCs with and without FGFR2 amplification
Consistent with the results obtained for KATO III cells, PRO-007 significantly reduced viability and invasiveness of PDC#1 cells, which exhibited FGFR2 amplification. A decrease in AKT/ERK phosphorylation in response to PRO-007 was also observed (Figure 4A-C). Furthermore, ramucirumab plus PRO-007 had a more significant anti-tumor effect on PDC#1 cells compared to that of either ramucirumab or PRO-007 alone (Figure 4D).
Figure 4.
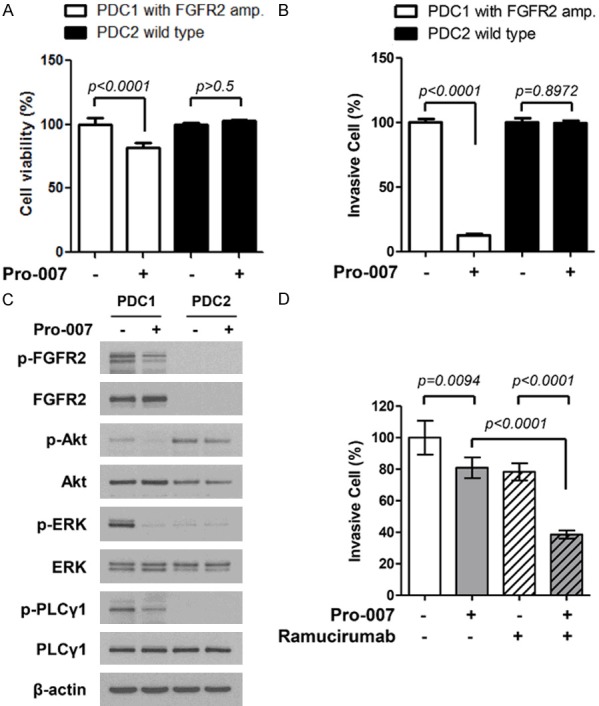
Anti-tumor effect of PRO-007 in FGFR2-amplified GC PDCs. A. Pro-007 suppressed FGFR2-amplified PDC proliferation. Cell proliferation was measured by the CTG assay after treatment with PRO-007 t 30 μg/ml for 72 h. B. Pro-007 suppressed FGFR2-amplified PDC invasion. Cells were treated with PRO-007 at 10 μg/ml for 24 h, and 1 × 105 cells were added to the upper well of Matrigel-coated Transwell chambers. C. Pro-007 decreased FGFR phosphorylation and targeted downstream pathways. D. Pro-007 (1 μg/ml) enhanced the efficacy of ramucirumab (1 μM) in FGFR2-amplified PDC1 cells.
Discussion
FGFR2-amplified GC accounts for approximately 5-10% of all GCs [19,20]. Although the frequency of FGFR2-amplified GC is relatively low, the prognosis of this subset is very poor [4]. The establishment of an effective treatment for FGFR2-amplified GC is currently considered an unmet need. The present study showed that PRO-007 had anti-tumor activity in KATO III and PDC#1 cells, both of which have FGFR2 amplification. In addition, based on immunoblot analysis, PRO-007 clearly reduced the level of phosphorylation of AKT and ERK in KATO III and PDC#1 cells. These findings suggest that PRO-007 may be a promising treatment strategy for patients with FGFR2-amplified GC.
FGFR signaling is considered a critical target in cancer therapy and various FGFR inhibitors have been developed. First-generation FGFR tyrosine kinase inhibitors (TKIs), such as dovitinib, brivanib, pazopanib, and regorafenib, bind to FGFR but are non-selective and also bind to other receptor tyrosine kinases (RTKs) such as VEGFRs and platelet-derived growth factor receptors (PDGFRs). Consequently, these compounds induce a series of toxic effects, including proteinuria, hypertension, cutaneous reactions, and gastrointestinal diseases [21]. Second-generation FGFR TKIs that are selective have been developed to overcome the complications caused by the off-target effects of non-selective FGFR TKIs. Among these, encouraging results have been obtained in vitro and in vivo for the efficacy of AZD4547 in FGFR2-amplified GCs [22]. However, a phase II trial of AZD4547 in patients with GC with FGFR2 polysomy or gene amplification failed to demonstrate an improved outcome compared to that of standard treatments [15]. Monoclonal antibodies (mAbs) to FGFR or FGF are also currently under development. When compared to TKIs, neutralizing anti-FGFR mAbs demonstrate higher specificity, which may result in a reduced toxicity [21]. To date, only one clinical trial of anti-FGFR2 mAbs has been performed [23]. FPA144, a humanized monoclonal IgG1 antibody that specifically binds and blocks FGFR2b, exerts anti-tumor activity by inducing antibody-dependent cell-mediated cytotoxicity. In a phase I study of patients with GC with high FGFR2b overexpression, FPA144 demonstrated promising efficacy with tolerable toxicity [23]. PRO-007 is a newly developed specific monoclonal antibody to FGFR2. The Ab (PRO007) is much more specific and selective to FGFR2 compared to any small molecule inhibitors. These tyrosine kinase inhibitors are usually not selective but inhibit all FGFRs and in many cases also other receptor tyrosine kinases and treatment with these inhibitors are associated with significant side effects. The current preclinical study revealed that PRO-007 was useful for treating FGFR2-amplified GC cells and decreased ERK phosphorylation downstream of FGFR signaling.
Ramucirumab, a monoclonal antibody against VEGF-2, is a standard second-line treatment for patients with GC [4,24]. In the current study, we evaluated the effect of ramucirumab on the invasiveness of both KATO III and PDC#1 cells, which have FGFR2 amplification. The combination of ramucirumab and PRO-007 markedly inhibited the invasiveness of cancer cells with FGFR2 amplification compared that of ramucirumab or PRO-007 alone. This potential combination strategy of PRO-007 and ramucirumab needs to be validated in further clinical studies.
Previous studies have reported a significant relationship between FGFR2 amplification and peritoneal metastasis. PDC#1 cells used in the present study were derived from the ascites of a patient with GC and peritoneal seeding. Peritoneal seeding is common in the progression or recurrence of GC after surgery (30-40%) [25,26]. Approximately 10% of newly diagnosed GC exhibit peritoneal seeding [27]. Considering that patients with CG and peritoneal seeding have the worse prognosis, the effective inhibition of FGFR2-amplified tumors might contribute to improved survival outcomes in this subset of patients.
In summary, PRO-007 is a specific, fully human monoclonal antibody for FGFR2. In this study, we demonstrated that PRO-007 specifically inhibited cell proliferation of a GC cell line and PDCs with amplified FGFR2. The preclinical data reported here supports further development of PRO-007 as a prediction biomarker and potential therapeutic agent for patients with FGFR2-amplified GC.
Acknowledgements
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2750 and HI14C3418).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Maron SB, Catenacci DVT. Update on gastroesophageal adenocarcinoma targeted therapies. Hematol Oncol Clin North Am. 2017;31:511–527. doi: 10.1016/j.hoc.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J Regard Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 5.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 6.Ali SM, Sanford EM, Klempner SJ, Rubinson DA, Wang K, Palma NA, Chmielecki J, Yelensky R, Palmer GA, Morosini D, Lipson D, Catenacci DV, Braiteh F, Erlich R, Stephens PJ, Ross JS, Ou SH, Miller VA. Prospective comprehensive genomic profiling of advanced gastric carcinoma cases reveals frequent clinically relevant genomic alterations and new routes for targeted therapies. Oncologist. 2015;20:499–507. doi: 10.1634/theoncologist.2014-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim ST, Kim KM, Kim NKD, Park JO, Ahn S, Yun JW, Kim KT, Park SH, Park PJ, Kim HC, Sohn TS, Choi DI, Cho JH, Heo JS, Kwon W, Lee H, Min BH, Hong SN, Park YS, Lim HY, Kang WK, Park WY, Lee J. Clinical application of targeted deep sequencing in solid-cancer patients and utility for biomarker-selected clinical trials. Oncologist. 2017;22:1169–1177. doi: 10.1634/theoncologist.2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 10.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 11.Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, Yamada Y, Tamura T, Fukuoka K, Kimura H, Saijo N, Nishio K. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res. 2007;13:3051–3057. doi: 10.1158/1078-0432.CCR-06-2743. [DOI] [PubMed] [Google Scholar]
- 12.Yashiro M, Shinto O, Nakamura K, Tendo M, Matsuoka T, Matsuzaki T, Kaizaki R, Miwa A, Hirakawa K. Synergistic antitumor effects of FGFR2 inhibitor with 5-fluorouracil on scirrhous gastric carcinoma. Int J Cancer. 2010;126:1004–1016. doi: 10.1002/ijc.24763. [DOI] [PubMed] [Google Scholar]
- 13.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C, Lutterbach B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68:2340–2348. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 14.Gozgit JM, Wong MJ, Moran L, Wardwell S, Mohemmad QK, Narasimhan NI, Shakespeare WC, Wang F, Clackson T, Rivera VM. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther. 2012;11:690–699. doi: 10.1158/1535-7163.MCT-11-0450. [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem E, Bang YJ, Mansoor W, Petty RD, Chao Y, Cunningham D, Ferry DR, Smith NR, Frewer P, Ratnayake J, Stockman PK, Kilgour E, Landers D. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol. 2017;28:1316–1324. doi: 10.1093/annonc/mdx107. [DOI] [PubMed] [Google Scholar]
- 16.Pearson A, Smyth E, Babina IS, Herrera-Abreu MT, Tarazona N, Peckitt C, Kilgour E, Smith NR, Geh C, Rooney C, Cutts R, Campbell J, Ning J, Fenwick K, Swain A, Brown G, Chua S, Thomas A, Johnston SRD, Ajaz M, Sumpter K, Gillbanks A, Watkins D, Chau I, Popat S, Cunningham D, Turner NC. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 2016;6:838–851. doi: 10.1158/2159-8290.CD-15-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulos KP, El-Rayes BF, Tolcher AW, Patnaik A, Rasco DW, Harvey RD, LoRusso PM, Sachdev JC, Abbadessa G, Savage RE, Hall T, Schwartz B, Wang Y, Kazakin J, Shaib WL. A Phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer. 2017;117:1592–1599. doi: 10.1038/bjc.2017.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, Kim SY, Park C, Kim NK, Jang J, Park K, Yi JH, Hong M, Ahn T, Rath O, Schueler J, Kim ST, Do IG, Lee S, Park SH, Ji YI, Kim D, Park JO, Park YS, Kang WK, Kim KM, Park WY, Lim HY, Lee J. Patient-derived cell models as preclinical tools for genome-directed targeted therapy. Oncotarget. 2015;6:25619–25630. doi: 10.18632/oncotarget.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto K, Arao T, Hamaguchi T, Shimada Y, Kato K, Oda I, Taniguchi H, Koizumi F, Yanagihara K, Sasaki H, Nishio K, Yamada Y. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer. 2012;106:727–732. doi: 10.1038/bjc.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YJ, Shen D, Yin X, Gavine P, Zhang T, Su X, Zhan P, Xu Y, Lv J, Qian J, Liu C, Sun Y, Qian Z, Zhang J, Gu Y, Ni X. HER2, MET and FGFR2 oncogenic driver alterations define distinct molecular segments for targeted therapies in gastric carcinoma. Br J Cancer. 2014;110:1169–1178. doi: 10.1038/bjc.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghedini GC, Ronca R, Presta M, Giacomini A. Future applications of FGF/FGFR inhibitors in cancer. Expert Rev Anticancer Ther. 2018;18:861–872. doi: 10.1080/14737140.2018.1491795. [DOI] [PubMed] [Google Scholar]
- 22.Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, Xu Y, Gao Z, Liu K, Zhou M, Gao B, Shen D, Zhang L, Ji J, Gavine PR, Zhang J, Kilgour E, Zhang X, Ji Q. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–2583. doi: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 23.Catenacci DVT, Rha SY, Bang YJ, Wainberg ZA, Chao J, Lee KW, Korn WM, Kim YH, Song EK, Chiu CF, Yen CJ, Berlin J, Kim JS, Sikorski RS, Collins H, Clark L, Inamdar SP, Zhang C, Lee J. Updated antitumor activity and safety of FPA144, an ADCC-enhanced, FGFR2b isoform-specific monoclonal antibody, in patients with FGFR2b+gastric cancer. J. Clin. Oncol. 2017;35 abstract 4067. [Google Scholar]
- 24.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol. 1992;1:341–346. doi: 10.1016/0960-7404(92)90034-i. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394–400. doi: 10.1007/BF02573875. [DOI] [PubMed] [Google Scholar]
- 27.Tsujitani S, Oka S, Suzuki K, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Prognostic factors in patients with advanced gastric cancer treated by noncurative resection: a multivariate analysis. Hepatogastroenterology. 2001;48:1504–1508. [PubMed] [Google Scholar]


